Abstract
An outer surface lipoprotein of 22 kDa was identified in the avian pathogen Borrelia anserina Ni-NL by using antibody preparations reactive with bacterial surface-exposed proteins. Amino acid sequence analysis of the 22-kDa protein demonstrated 90% identity with VmpA of B. turicatae, suggesting that the protein belongs to the family of 20-kDa outer surface proteins of the genus Borrelia. All of the 60 chicks intramuscularly treated with antibodies specifically reacting with the 22-kDa protein and infected with strain Ni-NL were completely protected from infection, since no spirochetemia was detected, and from death. Control chicks were treated with immune sera raised against apathogenic strain B. anserina Es, which expresses a prominent 20-kDa polypeptide that is also a member of the Vmp family but does not cross-react immunologically with the 22-kDa protein of the Ni-NL strain. These animals, infected with B. anserina Ni-NL, showed a high degree of spirochetemia 10 days after infection, and all died between 14 and 21 days after infection. The results showed that the 22-kDa surface protein of B. anserina Ni-NL is a determinant of the pathogenic potential of the strain and also confirmed that only strain-specific antibodies are protective against B. anserina infection.
Bacteria of the genus Borrelia cause several human and animal diseases (15). Many studies (3, 20, 23–25) on the pathogenic mechanisms of these spirochetes have been carried out since the early 1970s when Kelly (16) achieved the in vitro cultivation of Borrelia hermsii, and especially since 1982, when the etiologic agent of Lyme disease, B. burgdorferi, was identified (5). B. anserina, which is responsible for avian borreliosis (10), is a worldwide pathogen that is of economic importance for domestic poultry breeding in defined geographic areas (10). Several studies have demonstrated both the presence of different antigenic types (18, 26, 28, 29) and the serotype specificity of the protective immune response (10). Attenuation of the avian pathogenicity of B. anserina has also been obtained by serial culture passage in vitro (18) without observation of morphological differences between virulent and attenuated spirochetes as detected by electron microscopy (14). The protein profile of the low- and high-passage cultures of the strain adapted to grow in vitro showed only one major difference: the presence of an increasingly abundant and highly represented 20-kDa polypeptide in a high-passage strain (18). However, as far as we know, specific pathogenicity determinants in B. anserina have not yet been identified. On the other hand, the importance of the outer surface proteins (OSP) of B. burgdorferi in the determination of Lyme disease is well known. Therefore, we analyzed the surface composition of B. anserina Ni-NL, a strain pathogenic for chickens, in comparison with B. anserina Es, a strain that has lost its avian pathogenicity, and focused on the in vivo protective activity of antibodies reactive with the 22-kDa surface-exposed protein of pathogenic strain Ni-NL.
Bacterial strains and growth conditions.
B. anserina Ni-NL (14), kindly provided by L. Spanjaard, Amsterdam, The Netherlands, was maintained by intravenous passage of infected blood in pathogen-free chicks (18), since the strain does not grow in vitro. Briefly, 2-day-old chicks provided with antibiotic-free food and water ad libitum were intramuscularly injected in the leg with 0.1 ml of infected blood containing approximately 2 × 105 to 3 × 105 bacteria. Spirochetemia was evaluated daily from 3 to 20 days after infection. Spirochetemia reaches a plateau (mean value, 2.8 × 108/ml) 10 days after infection and lasts until the death of the animals within 15 to 21 days of infection. Examination was done by dark-field microscopy of 1 drop of blood collected from the main wing vein as previously reported (9, 11). Ten days after infection, 40 to 50% of the animals died, whereas all of the remaining chicks died within 21 days postinfection.
Since B. anserina Es, obtained from Russell C. Johnson, Minneapolis, Minn., has lost the ability to infect chicks in vivo (18), it was maintained in BSK II medium (2) by serial weekly passage. The other Borrelia strains used in this work, B. turicatae, B. parkeri, B. coriaceae, B. hermsii, B. afzelii, B. burgdorferi sensu stricto, and B. garinii, were similarly grown in BSK II medium as previously described (22).
MIAFs.
Mouse immune ascitic fluids (MIAFs) to B. anserina Es and Ni-NL were obtained by the method previously reported (9), i.e., by intraperitoneal immunization of BALB/c mice with whole sonicated bacterial cells. Briefly, 0.8-ml volumes of immunogen (0.05 mg of protein) emulsified 1:9 (vol/vol) with complete Freund’s adjuvant were injected intraperitoneally into 8 to 12-week-old mice on days 0, 7, 14, and 21. On day 6, 0.5 ml of pristane (2,6,10,14-tetramethylpentadecane; Sigma, St. Louis, Mo.) was injected intraperitoneally. Ascitic fluid was collected on day 30 by peritoneal paracentesis.
Ab-SEE.
The MIAFs were used to select antibodies reactive with surface-exposed epitopes (Ab-SEE) on living spirochetes as previously reported (21). B. anserina Es in the logarithmic growth phase with no more than 0.5% damaged organisms were used. The preparations of Ab-SEE used were obtained by incubating 4 ml of a B. anserina Es culture (108 cells/ml) with 1 ml (diluted 1:5) of B. anserina Es MIAF for 45 min at 37°C. The bacterial suspension was then pelleted and washed twice with 0.15 M phosphate-buffered saline (PBS). Antibodies bound to the spirochete surface were then recovered by resuspending the bacteria with 0.1 ml of 0.2 M glycine-HCl (pH 2.2) and then incubating them for 10 min at 25°C. The pH of the suspension was then brought to neutrality by adding 120 μl of 3.75 M Tris-HCl (pH 8.8) and the suspension was centrifuged at 13,000 × g for 15 min at 25°C. Ab-SEE of B. anserina Es were then purified by affinity chromatography by using a HiTrap protein A column (Pharmacia-LKB, Uppsala, Sweden) and then concentrated with Centricon 30 tubes (Amicon, Beverly, Mass.). Individual preparations were pooled before any further use, and protein concentrations were determined with the Bradford reagent (Bio-Rad, Richmond, Calif.). The selection of Ab-SEE for strain Ni-NL was done by using infected blood as follows. When chick spirochetemia, evaluated by taking 1 drop of blood from the main wing vein, reached at least 200 microrganisms/field (×400) and spirochete viability was over 99%, the animals were sacrificed by cardiac puncture and blood was collected in vials containing heparin. Afterwards, the homologous MIAF was diluted 1:5 (vol/vol) in heparinized blood, incubated for 1 h at 37°C, and then centrifuged at 500 × g for 10 min to separate erythrocytes from antibody-coated bacteria. Spirochetes were then washed with PBS, and antibodies bound to surface antigens were removed and purified as described above.
Gel electrophoresis, [3H]palmitate labeling, and immunoblotting.
Single-dimension sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by using the buffers of Laemmli (17) as previously described (8). The presence of fatty acid moieties linked to the polypeptides of borrelias was determined by incubating the spirochetes in the presence of [3H]palmitate (22); the radiolabeled proteolipids were then detected by autoradiography as previously described (22). Immunoblot analysis of bacterial proteins separated by SDS-PAGE and transferred to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) was performed by the method of Towbin et al. (27) as described elsewhere (21).
Amino acid sequencing.
The 22- and 24-kDa proteins of strains Ni-NL and Es, respectively, were separated by SDS-PAGE and then transferred to polyvinylidene difluoride membranes (Schleicher & Schuell) by using 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid; Sigma) buffer (pH 11) containing 10% methanol. Blotted proteins were stained for 1 min with 0.025% Coomassie R-250 (Sigma) in 40% methanol–60% H2O and immediately destained in 50% methanol in H2O. The 22- and 24-kDa bands were then excised and in situ digested with trypsin as reported by Blanco and coworkers (4). The resulting peptides were then separated on a capillary high-pressure liquid chromatography system (173A; Perkin-Elmer ABI). Chemical sequencing of peptides was done with a Perkin-Elmer ABI 476A automated sequencer by Suzanne Perry-Riehms at the NAPS Protein Service Laboratory, University of British Columbia, Vancouver, British Columbia, Canada. Each sequence obtained was analyzed by BLASTP 1.4.11 (1) software to perform homology searches with known protein sequences.
PCR analysis.
The flagellin gene sequence of B. anserina (flaB gene, GenBank accession no. X75201) was compared with those of B. turicatae (D82862), B. parkeri (D82863), B. coriaceae (D82864), B. hermsii (M86838 and M33839), B. afzelii (X75202), B. burgdorferi sensu stricto (X15661 and X14841), and B. garinii (L29236) to identify a species-specific region to be used as a target of the amplification process. All of the sequences studied were obtained from GenBank, and the accession numbers are in parentheses. The target sequence specific for B. anserina was identified between nucleotides 581 and 1053. The primers used to perform the PCR assay were Bafla1 (5′TAA TAC ACC AGC ATC ACT AT3′ from nucleotide 581 to nucleotide 600) and Bafla3 (5′TTG CGG ATT GTG TAA AAA TA3′, complementary to the sequence from nucleotide 1053 to nucleotide 1034). The amplification product was a sequence of 473 bp. As controls for the species specificity of the PCR experiment, target DNAs extracted as previously described (12) from B. turicatae, B. parkeri M3001, B. coriaceae Co53, B. hermsii HS1 B. afzelii isolate VS461, B. burgdorferi IRS sensu stricto, and B. garinii P/Bi were used. The amplification was performed by using a Perkin-Elmer DNA Thermal Cycler apparatus for 30 cycles, each one consisting of 45 s at 94°C, 45 s at 62°C, and 45 s at 72°C. The reaction mixture and the detection of the amplification products were as previously reported (12).
Infectivity neutralization assay.
To evaluate the ability of the antibodies to neutralize the infectivity of B. anserina Ni-NL in vitro, 0.1 ml of MIAF to whole bacterial cells and preparations of Ab-SEE were incubated with heparinized infected blood (approximately 2 × 105 to 3 × 105 bacteria) for 60 min at 37°C and then injected into animals as described above. Each experiment was repeated six times and performed with 10 animals for each antibody preparation. MIAF and preparations of Ab-SEE raised against B. anserina Es were also tested in a similar way. In addition, MIAF raised against whole elementary bodies of Chlamydia trachomatis (9) was used as a control in one experiment. The outcome of the infection was then evaluated over a period of 3 weeks, and the number of surviving chicks was recorded.
Animal protection test.
The protection of antibodies reactive either with whole B. anserina Ni-NL cells (MIAF) or with B. anserina Ni-NL surface antigens (Ab-SEE) was evaluated in chicks as follows. Chicks were given 0.1-ml doses of different antibody preparations previously adjusted to contain the same titer of immunoglobulins (8). The animals were then challenged within 1 h by intramuscular injection into the opposite leg of an inoculum of approximately 2 × 105 to 3 × 105 borrelias in 0.1 ml of infected blood. Each experiment was performed with 10 animals for each antibody preparation and repeated six times over a period of 180 days. The protective activity of MIAF and preparations of Ab-SEE raised against B. anserina Es was also tested in a similar way by challenging animals with B. anserina Ni-NL. In addition, two groups of chicks treated with MIAF to strains Ni-NL and Es were challenged with blood freshly obtained from uninfected animals to ensure that no mortality was due to this procedure. Spirochetemia was evaluated by dark-field microscopic examination of six different 10-μl samples at a magnification of ×400. Examination of the samples was performed blindly to prevent identification of the samples. To ensure the absence of living spirochetes, a new inoculum of each blood specimen scored as negative for spirochetemia was done by injecting 0.1 ml intramuscularly into a new animal. After 20 additional days, each chick was bled and tested as described above.
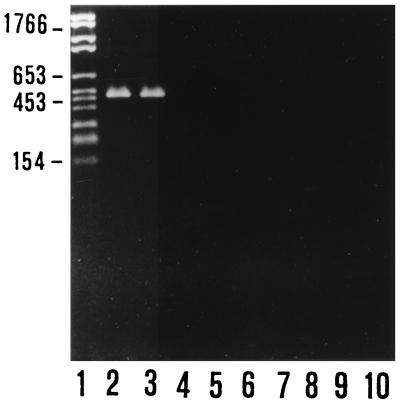
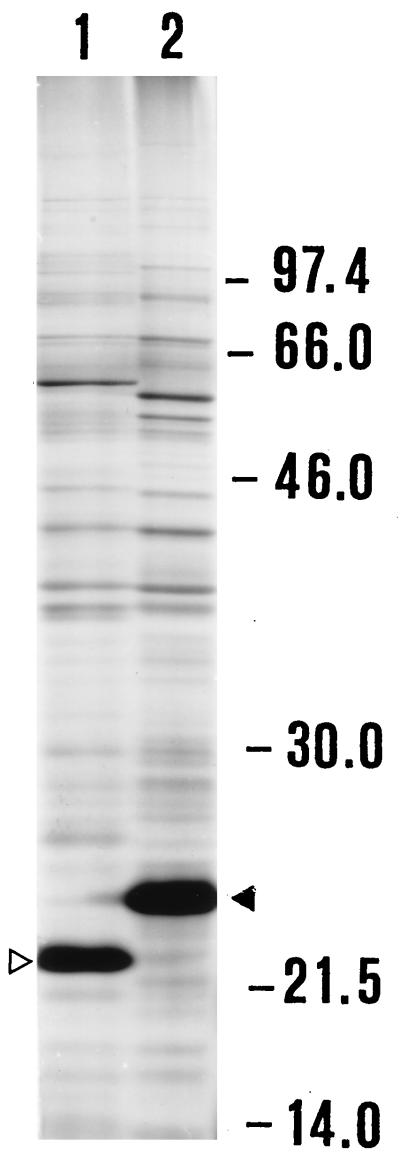
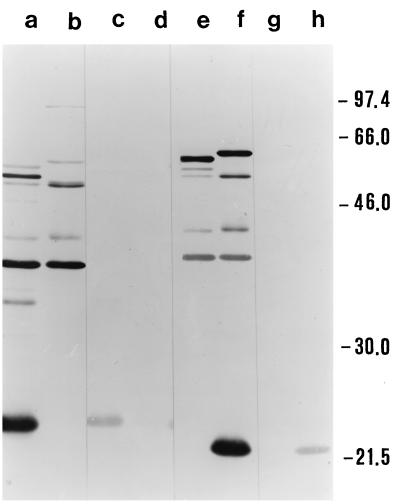
Preliminary PCR typing experiments confirmed that the two strains we were working with were indeed two B. anserina strains, despite their substantial difference in pathogenic potential for chicks. In fact, these strains were the only two of the several Borrelia strains used to be amplified by primers specific for B. anserina (Fig. 1). As expected, SDS-PAGE analysis showed similar protein profiles of the two B. anserina strains (Fig. 2) with a major difference in the 20 to 24-kDa region and with other, minor differences, notably, in the 46- to 66-kDa region. The identification of the OSP of B. anserina Ni-NL and Es performed by using preparations of Ab-SEE indicated the presence of two major OSP of 22 and 24 kDa in Ni-NL and Es, respectively, which were the proteins exclusively recognized by a homologous antibody preparation (Fig. 3). The fluorographs obtained after intrinsic [3H]palmitate labeling of the borrelias showed that the only positive bands were of 22 and 24 kDa for strains Ni-NL and Es (data not shown), respectively. This observation confirmed the previously reported presence of a major lipoprotein of 24 kDa in B. anserina Es (22).
FIG. 1.
Agarose gel electrophoresis analysis of the amplification products obtained by B. anserina flaB gene PCR assay. Lanes: 2 and 3, B. anserina Es and Ni-NL, respectively; 4 to 10, B. afzelii VS461, B. garinii PBi, B. burgdorferi IRS, B. hermsii HS-1, B. parkeri M3001, B. turicatae, and B. coriaceae Co-53, respectively. Lane 1 contained molecular size markers. On the left, molecular sizes are indicated in base pairs.
FIG. 2.
SDS-PAGE analysis of B. anserina strains. Lanes: 1, pathogenic strain Ni-NL; 2, strain Es. The empty arrowhead indicates the 22-kDa protein, and the full arrowhead indicates the 24-kDa protein. Molecular sizes (kilodaltons) are shown on the right.
FIG. 3.
Western blot analysis of B. anserina strains. Lanes: a, c, e, and g, strain Es; b, d, f, and h, pathogenic strain Ni-NL. Lanes were probed as follows: a and b with MIAF to Es, c and d with Ab-SEE to Es, e and f with MIAF to Ni-NL, and g and h with Ab-SEE to Ni-NL. Molecular sizes (kilodaltons) are shown on the right.
A preliminary amino acid sequence analysis of the major OSP of B. anserina strains was undertaken in an attempt to identify the NH2-terminal sequences of both the 22- and 24-kDa proteins. As expected, the sequencing failed, confirming the previous lipidation results suggesting that the polypeptides were N terminally blocked. Consequently, using the tryptic digestion and peptide mapping technique, several different peptides were generated and subjected to amino acid sequencing. In particular, a BLAST-P analysis (1) of 11-base peptide E (IGANGLEADAG) obtained by digestion of the 22-kDa protein of strain Ni-NL revealed the highest homology (identity 90%) with the VmpA protein of B. turicatae (accession no. U85413) and the second highest homology score (identity 72%) with OspC of B. afzelii (accession no. AB000348). On the other hand, the analysis of two different and nonoverlapping peptides of the 24-kDa protein of B. anserina Es showed that peptide 6 (15 amino acid residues: IQNSDTLATEANHHG) has 73 and 60% identity with the OspC sequences of B. japonica (accession no. AB000358) and B. burgdorferi (accession no. L42890), respectively. The second peptide derived from the 24-kDa protein (peptide 3 [22 bases: VLMGSVSTLLEEAINELTTPAP]) demonstrated 45% identity with the VmpA sequence of B. turicatae (accession no. U85413). The tryptic peptide analysis of these proteins, in particular, the pairwise comparison of peptide E from the 22-kDa antigen that was 90% identical to VmpA of B. turicatae and the very high (73 to 60%) identity of the 24-kDa protein of strain Es with the OspC sequence of B. japonica and B. burgdorferi, strongly suggested that these major OSP of the B. anserina isolates used in this study belong to the family of 20-kDa exposed OSP described by Carter et al. (7) in the genus Borrelia.
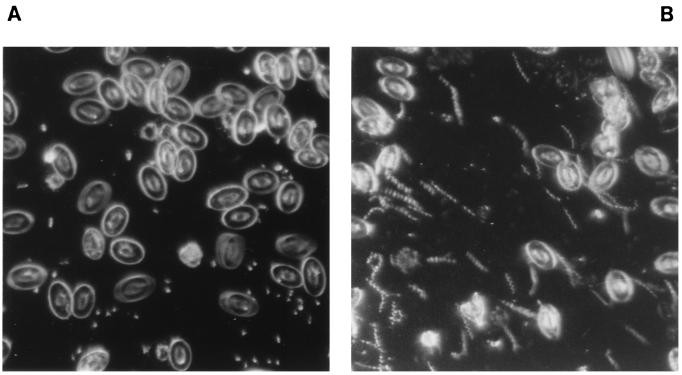
The effects of the in vitro reaction of Ni-NL spirochetes with different antibody preparations on the infectivity for chicks are reported in Table 1. All of the animals that received borrelias pretreated with either MIAF or Ab-SEE raised to B. anserina Ni-NL showed no spirochetemia during the follow-up period, and all of them survived after 3 weeks. On the contrary, chicks infected with spirochetes pretreated with antibody preparations to strain Es were found to be spirochetemic 5 days postinfection with a mean number of 2.75 × 108 organisms/ml at 10 days postinfection. Similar values were obtained with control chicks pretreated both with PBS and with antibodies to C. trachomatis. The mortality rate in these groups of animals reached 100% 3 weeks after infection (Table 1). The results obtained by infectivity neutralization in vitro were confirmed when animal protection tests were performed in vivo by treating chicks with anti-Ni-NL antibodies before infection. The results are reported in Table 2. Two groups of animals treated with antibodies (either MIAF or Ab-SEE) to pathogenic strain Ni-NL were completely protected from infection; all of the chicks were alive 3 weeks after infection and showed no spirochetemia during this period (Fig. 4A). On the contrary, animals treated with MIAF and Ab-SEE to nonpathogenic strain Es were not protected, and all died by 3 weeks after infection, with a high level of spirochetemia starting 3 and 5 days postinfection, respectively (Fig. 4B).
TABLE 1.
Results of in vitro B. anserina Ni-NL infectivity neutralization assay using specific antibodies reacting with B. anserina Ni-NL or Es
| Antibody prepn | Mean spirochetemia after 10 days (108 organisms/ml) | No. of surviving chicks after 3 weeks/ no. of chicks injected |
|---|---|---|
| MIAF to Ni-NL | 0 | 60/60 |
| Ab-SEE to Ni-NL | 0 | 60/60 |
| MIAF to Es | 2.7 | 0/60 |
| Ab-SEE to Es | 2.8 | 0/60 |
| MIAF to C. trachomatis LGV2 | 3.0 | 0/60 |
| None (PBS) | 2.8 | 0/60 |
TABLE 2.
Results of in vivo experiments testing protection of chicks from B. anserina Ni-NL challenge by use of specific antibodies reacting with B. anserina Ni-NL or Es
| B. anserina strain used for challenge | Antibody prepn | Mean spirochetemia after 10 days (108 organisms/ml) | No. of surviving chicks after 3 weeks/ no. of chicks injected |
|---|---|---|---|
| Ni-NL | MIAF to Ni-NL | 0 | 60/60 |
| Ni-NL | Ab-SEE to Ni-NL | 0 | 60/60 |
| Ni-NL | MIAF to Es | 2.9 | 0/60 |
| Ni-NL | Ab-SEE to Es | 3.1 | 0/60 |
| Nonea | MIAF to Ni-NL | 0 | 60/60 |
| Nonea | MIAF to Es | 0 | 60/60 |
Uninfected blood.
FIG. 4.
Dark-field (×400) photomicrographs of blood samples from chicks. (A) Blood from a chick protected with Ab-SEE to Ni-NL and infected with pathogenic strain Ni-NL (no spirochetemia). (B) Blood from a chick protected with Ab-SEE to Es and inoculated with pathogenic strain Ni-NL (high spirochetemia).
Animals injected with uninfected blood from pathogen-free chicks and treated with MIAF to Ni-NL and Es showed 100% survival, and no spirochetes were detected in their blood samples at the end of the follow-up period of 3 weeks.
In conclusion, the results of the present study confirm the lack of protective cross-immunity between different isolates of B. anserina and demonstrate that the principal difference in the polypeptide profile between pathogenic strain Ni-NL and apathogenic, culture-adapted strain Es is due to the presence of two immunogenic, surface-exposed proteins of the family of 20-kDa OSP of Borrelia, of 22 and 24 kDa, respectively, for strains Ni-NL and Es.
The outstanding role of the family of 20-kDa exposed OSP as immunodominant antigens in Borrelia infections has emerged from results of in vivo studies with both gerbils (19) and mice (13) for Lyme disease and with mice (6) for relapsing fever infection with B. turicatae. The results reported here add consistency to these data by suggesting that the 22-kDa OSP is a determinant of the pathogenic potential of B. anserina Ni-NL and demonstrating the protective role of strain-specific antibodies in a chick infection model.
Acknowledgments
This work was supported in part by MURST (co-finaziamento 1997 grant) and by the University of Bologna (ex-quota 60% grant).
We thank Suzanne Perry-Riehm, NAPS Protein Service Laboratory, Biotechnology Department, University of British Columbia, Vancouver, British Columbia, Canada, for performing amino acid analysis and for helpful discussion of the sequence data. We also thank Aldo Farencena for his early contribution to this study.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Barbour A G. Isolation and cultivation of Lyme disease spirochete. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco D R, Champion C I, Exner M M, Edrjument-Bromage H, Hancock R E W, Tempst P, Miller J N, Lovett M A. Porin activity and sequence analysis of a 31-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1) J Bacteriol. 1995;177:3556–3562. doi: 10.1128/jb.177.12.3556-3562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Cadavid D, Pennington P M, Kerentseva T A, Bergström S, Barbour A G. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter C J, Bergström S, Norris S J, Barbour A G. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun. 1994;62:2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cevenini R, Sambri V, Massaria F, La Placa M, Jr, Brocchi E, De Simone F. Complement mediated in vitro bactericidal activity of monoclonal antibodies reactive with outer-surface protein OspB of Borrelia burgdorferi. FEMS Microbiol Lett. 1992;90:147–152. doi: 10.1016/0378-1097(92)90619-y. [DOI] [PubMed] [Google Scholar]
- 9.Cevenini R, Sambri V, Pileri S, Ratti G, La Placa M. Development of transplantable ascites tumours which continuously produce polyclonal antibodies in pristane primed Balb/C mouse immunized with bacterial antigens and complete Freund’s adjuvant. J Immunol Methods. 1991;140:111–118. doi: 10.1016/0022-1759(91)90132-y. [DOI] [PubMed] [Google Scholar]
- 10.DaMassa A J, Adler H E. Avian spirochetosis: natural transmission by Argas (Persicargas) sanchezii (Ixodoidea: Argasidae) and existence of different serologic and immunologic types of Borrelia anserina in the United States. Am J Vet Res. 1979;40:154–157. [PubMed] [Google Scholar]
- 11.Fabbi M, Sambri V, Marangoni A, Magnino S, Solari-Basano F, Cevenini R, Genchi C. Borrelia in pigeons: no serological evidence of Borrelia burgdorferi infection. J Vet Med B: 1995;42:503–507. doi: 10.1111/j.1439-0450.1995.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 12.Farencena A, Comanducci M, Donati M, Ratti G, Cevenini R. Characterization of a new isolate of Chlamydia trachomatis which lacks the common plasmid and has properties of biovar trachoma. Infect Immun. 1997;65:2965–2969. doi: 10.1128/iai.65.7.2965-2969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovind-Hougen K. A morphological characterization of Borrelia anserina. Microbiology. 1995;141:79–83. doi: 10.1099/00221287-141-1-79. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R C. The spirochetes. Annu Rev Microbiol. 1977;31:89–106. doi: 10.1146/annurev.mi.31.100177.000513. [DOI] [PubMed] [Google Scholar]
- 16.Kelly R. Cultivation of Borrelia hermsii. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Levine J F, Dykstra M Y, Nicholson W L, Walker R L, Massey G, Barnes H J. Attenuation of Borrelia anserina by serial passage in liquid medium. Res Vet Sci. 1990;48:64–69. [PubMed] [Google Scholar]
- 19.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutscheck E, Reinhardt S, Lehnert G, Klockmann E, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against Borrelia burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 20.Radolf J D. Role of outer membrane architecture in immune evasion by Treponema palidum and Borrelia burgdorferi. Trends Microbiol. 1994;2:307–311. doi: 10.1016/0966-842x(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 21.Sambri V, Marangoni A, Massaria F, Farencena A, La Placa M, Jr, Cevenini R. Functional activities of antibodies directed against surface lipoproteins of Borrelia hermsii. Microbiol Immunol. 1995;39:623–627. doi: 10.1111/j.1348-0421.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 22.Sambri V, Stefanelli C, Rossoni C, La Placa M, Cevenini R. Acylated proteins in Borrelia hermsii, Borrelia parkeri, Borrelia anserina, and Borrelia coriaceae. Appl Environ Microbiol. 1993;59:3938–3940. doi: 10.1128/aem.59.11.3938-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambri V, Aldini R, Massaria F, Montagnani M, Casanova S, Cevenini R. Uptake and killing of Lyme disease and relapsing fever borreliae in the perfused rat liver and by isolated Kupffer cells. Infect Immun. 1996;64:1858–1861. doi: 10.1128/iai.64.5.1858-1861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambri V, Armati S, Cevenini R. Animal and human antibody reactive with the outer surface protein A and B of Borrelia burgdorferi are borreliacidal. FEMS Immunol Med Microbiol. 1993;7:67–72. doi: 10.1111/j.1574-695X.1993.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaible U E, Wallich R, Kramer M D, Museteanu C, Rittig M, Moter S, Simon M M. Role of the immune response in Lyme disease: lessons from the mouse model. In: Schutzer S E, editor. Lyme disease—molecular and immunological approaches. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 243–262. [Google Scholar]
- 26.Soni J L, Joshi A G. A note on strain variation in Akola and Jabalpur strains of Borrelia anserina. Zentrabl Veterinaermed Reihe B. 1980;27:70–72. doi: 10.1111/j.1439-0450.1980.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker R L, Greene R T, Nicholson W L, Levine J F. Shared flagellar epitopes of Borrelia burgdorferi and Borrelia anserina. Vet Microbiol. 1989;19:361–371. doi: 10.1016/0378-1135(89)90101-6. [DOI] [PubMed] [Google Scholar]
- 29.Wouda W, van Veen T W S, Barnes H J. Borrelia anserina in chickens previously exposed to Borrelia theileri. Avian Dis. 1974;19:209–210. [PubMed] [Google Scholar]