Abstract
We examined the immunogenicity and induction of inhibitory activity of 19-mer synthetic peptides which contained putative catalytic regions that were associated with the β5 (EAW) and β7 (HDS) strand elements of the suggested (β,α)8 catalytic barrel domain of Streptococcus mutans glucosyltransferase (GTF). Both peptides readily induced serum immunoglobulin G (IgG) and salivary IgA antipeptide activity which was reactive both with the inciting peptide and with intact S. mutans GTF. Antisera to each peptide construct also inhibited the ability of S. mutans GTF to synthesize glucan. These observations support the existence of catalytic subdomains containing glutamate and tryptophan (EAW) or aspartate and histidine (HDS) residues, each of which have been suggested to be involved with the catalytic activity of GTF. Furthermore, the epitopes defined in these sequences have significant immunogenicity and can induce immune responses which interfere with GTF-mediated glucan synthesis.
Glucosyltransferases (GTFs) are important components of the molecular pathogenesis of mutans streptococci, chiefly because of their ability to synthesize extracellular glucan from sucrose (9). Strategies for immunological intervention with the processes leading to dental caries, therefore, have included the development of immune responses to GTF (reviewed in reference 21). Directing these immune responses toward epitopes that include functionally important residues or domains would, theoretically, increase the enzyme inhibitory capacity of the response. Mooser et al. (16) and Funane et al. (7), using labeling techniques with GTF-I from Streptococcus downei and dextransucrase from Leuconostoc mesenteroides, respectively, identified aspartates in two subdomains which participate in catalytic mechanisms. Synthetic peptides constructed to contain either of these subdomains induced immune responses which inhibited GTF enzymatic activity (2, 12, 19) and protected rats from experimental dental caries (20, 26).
Recently, primary and secondary structural comparisons of GTFs with the α-amylase superfamily have provided insights into the structure-function relationships of the GTF catalytic domain. Much of the catalytic activity of α-amylases is contained in a (β,α)8 barrel element (14). Aspartates or glutamates at the C terminis of β strands 4, 5, and 7 have been specifically implicated in amylolytic activity and are invariant in these enzymes (11). The overall homology between α-amylases and GTF is low, except for a 50- to 60-amino-acid sequence stretch near the middle of the GTF molecule (6) for which no catalytically involved residues have been identified. However, sequence alignment techniques (5, 13) have shown significant homologies between GTFs and α-amylase with respect to several invariant residues important to the catalytic activity of the α-amylase family and have suggested that the (β,α)8 barrel element may also be a feature of the GTF catalytic domain. Strengthening this conclusion are site-directed mutagenesis studies (5, 28) which showed that modification of aspartates or glutamates in GTF, which aligned with the catalytically important residues in the β4, β5, and β7 strands of α-amylases drastically reduced GTF catalytic activity.
Since residues in or near the putative β5 and β7 strands of GTF thus appear to be functionally important, it was of interest to determine whether significant antigenic epitopes exist within these sites of GTF catalytic activity and whether antibody to these putative epitopes could inhibit enzyme activity. Under the hypothesis that sequential epitopes within these regions could be mimicked by synthetic peptides, we prepared two synthetic peptide constructs whose sequences contained the β5 and β7 strands as well as adjacent residues that were implicated in catalytic activity by modeling and site-directed mutagenesis techniques (14, 28). These peptide constructs were then explored for their ability to induce serum immunoglobulin G (IgG) and salivary IgA antibody to peptide and to S. mutans GTF, as well as for their ability to inhibit the catalytic activity of mutans streptococcal GTF.
Three peptides were synthesized. Two of the sequences selected for synthesis (EAW and HDS) were based on putative catalytic regions within the predicted (β,α)8 barrel structure of GTF (5, 13). EAW was a 19-mer peptide construct whose sequence contained the β5 strand sequence, as well catalytically implicated Glu-489 and Try-491 (Table 1). HDS was also a 19-mer peptide whose sequence contained the β7 strand sequence, as well as catalytically implicated His-561 and Asp-562 (Table 1). Both EAW and HDS sequences were highly conserved among all mutans streptococcal GTFs and were identical to the respective S. mutans GTF-B sequence (Table 1). A third peptide (PQW) was synthesized to serve as a specificity and immunogencity control. Its sequence (Table 1) had 100% homology with sequence of GTF-I of S. sobrinus and S. downei and 67% homology with S. mutans GTF-B sequence that lay outside the (β,α)8 barrel domain predicted by MacGregor and coworkers (13). Peptides were synthesized (Applied Diagnostics, Foster City, Calif.) by the stepwise solid-phase method of Merrifield (15) on a core matrix of lysines to yield macromolecules with four identical peptides per molecule, after the method of Tam (23). Assessment of purity (>90%) by high-pressure liquid chromatography, amino acid analysis, and molecular weight determination by mass spectrometry were carried out. Enriched preparations of GTF from S. mutans SJ and S. sobrinus 6715 were obtained as previously described (19, 24).
TABLE 1.
Amino acid sequence homology of PQW, EAW, and HDS peptides with S. mutans, S. sobrinus, and S. downei GTFs and association with β5 and β7 strand domains
| Construct | Source (reference) | Sequencea | % Homology with peptide |
|---|---|---|---|
| PQW peptide | PQWNGESEKPYDDHL | ||
| GTF-B | S. mutans (18) | 342-SAWNSDSEKPFDDHL | 67 |
| GTF-C | S. mutans (29) | 368-SAWNSDSEKPFDDHL | 67 |
| GTF-D | S. mutans (10) | 354-PNWNSQTESDTSAGE | 27 |
| GTF-I | S. downei (6) | 342-PQWNGESEKPYDDHL | 100 |
| GTF-S | S. downei (8) | - - | 0 |
| GTF2 | S. sobrinus (1) | 336-PQWNGESEKPYDDHL | 100 |
| † # | |||
| EAW peptide | ANDHLSILEAWSDNDTPYLHD | ||
| GTF-B | S. mutans | 480-ANDHLSILEAWSDNDTPYLHD | 100 |
| GTF-C | S. mutans | 506-ANDHLSILEAWSYNDTPYLHD | 95 |
| GTF-D | S. mutans | 494-AINHLSILEAWSDNDPQYNKD | 68 |
| GTF-I | S. downei | 482-ANNHVSIVEAWSDNDTPYLHD | 90 |
| GTF-S | S. downei | 467-AIDHLSILEAWSGNDNDYVKQ | 63 |
| GTF2 | S. sobrinus | 476-ANNHVSIVEAWSDNDTPYLHD | 84 |
| . .β5. | |||
| ‡† ¤ | |||
| HDS peptide | VPSYSFIRAHDSEVQDLIA | ||
| GTF-B | S. mutans | 549-VPSYSFIRAHDSEVQDLIA | 100 |
| GTF-C | S. mutans | 575-VPSYSFIRAHDSEVQDLIRNII | 95 |
| GTF-D | S. mutans | 571-MANYIFIRAHDSEVQTVIAKII | 63 |
| GTF-I | S. downei | 551-VPSYSFARAHDSEVQDLIRDII | 84 |
| GTF-S | S. downei | 534-VPNYVFIRAHDSEVQTRIAKII | 74 |
| GTF2 | S. sobrinus | 545-VPSYSFARAHDSEVQDIIRDII | 84 |
| .β7. |
†, glutamic and aspartic acids at these positions are catalytic in α-amylases (14, 28); modification of these amino acids in GTF leads to loss of activity (5, 28). ‡, Histidine stabilizes transition states at this position in α-amylases (14, 22); modification of this histidine in GTF leads to loss of activity (28). #, Tryptophan is highly conserved at this position in GTF; activity is lost when mutated (28). ¤, Glucan product type changed when aspartic acid at this position in GTF is mutated (17).
Sprague-Dawley CD strain 42-day-old male rats (Charles River Laboratories, Wilmington, Mass.) were used for injection. Two experiments were performed. In the first experiment, groups of four to seven rats were injected subcutaneously in the vicinity of the salivary glands with 50 μg of either HDS or PQW peptide construct, injected with 10 μg of S. mutans GTF, or sham immunized with buffer alone. In the second experiment, groups of four to six rats were injected with 50 μg of the EAW peptide construct, injected with 10 μg of S. mutans GTF, or sham immunized. The remainder of the protocol was identical to that for the first experiment. The initial injection included complete Freund adjuvant (Difco Laboratories, Detroit, Mich.). Twenty-one days later, animals were again immunized with antigen in incomplete Freund adjuvant. Animals were bled and salivated prior to injection and at days 21 and 42 after the first injection. Sera and clarified saliva samples were stored at −70°C prior to assay.
Serum IgG and salivary IgA antibodies were tested by enzyme-linked immunosorbent assay (ELISA). Polystyrene microtiter plates (Flow Laboratories) were coated with 2.5 μg of each peptide construct or 0.5 μg of S. sobrinus or S. mutans GTF per ml. Antibody activity was then measured by incubation with 1:400 and 1:4,000 dilutions of sera or 1:4 and 1:8 dilutions of saliva. Plates were then developed for IgG antibody with rabbit anti-rat IgG, followed in sequence by alkaline phosphatase goat anti-rabbit IgG (Biosource Inc.) and p-nitrophenylphosphate (Sigma Chemical Co., St. Louis, Mo.). A mouse monoclonal reagent to rat α chain (Zymed, South San Francisco, Calif.) was used with biotinylated goat anti-mouse IgG (Zymed) and avidin-alkaline phosphatase (Cappel) to reveal levels of salivary IgA antibody to peptides. Reactivity was recorded as A405 in a microplate reader (Biotek Instruments, Winooski, Vt.). Data are reported as ELISA units (EU) which were calculated relative to the levels of appropriate reference sera or salivas from Sprague-Dawley rats twice immunized with the respective peptide construct. Dilutions of sera producing an A405 of approximately 1.0 were considered 100 EU for serum IgG antibody measurements. These corresponded to dilutions of 1/51,200, 1:25,000, 1:12,800, or 1:6,400 for serum antibody to S. sobrinus GTF or S. mutans GTF, EAW, or HDS construct, respectively. Dilutions of saliva of 1:4, producing an A405 of approximately 0.8, were considered 100 EU for salivary IgA to both EAW and HDS constructs.
Selected rat sera were evaluated for the ability to inhibit water-soluble glucan synthesis catalyzed by S. mutans GTF, using a filter assay. This GTF preparation contains a mixture of GTFs, including GTF-B, which has complete homology with both peptide constructs in the respective region (Table 1). Ten-microliter volumes of diluted sera (1:10 dilutions in 0.02 M sodium phosphate-buffered saline and 0.2% sodium azide [PBSA], pH 6.5) were preincubated with the GTF for 1 h at 37°C in a total volume of 0.04 ml of PBSA. Then 1.7 mg of sucrose and 24 nCi of [14C-glucose]sucrose (approximately 35,000 cpm) were added in 0.2 ml of PBSA in the absence of primer (26). Incubation proceeded overnight at 37°C, after which water-insoluble glucan was collected on, and water-soluble glucan was collected after passage through, Whatman GF/F glass fiber filters. Water-insoluble glucan collected on filters was washed, and retained radioactivity was determined as previously reported (24). Water-soluble glucan was precipitated with 70% ethanol, and radioactivity was determined as previously described (24). Under the conditions of this assay, approximately 800 cpm was incorporated into water-soluble glucan, and 3,000 cpm was incorporated into water-insoluble glucan, in the presence of sham-immune sera. Percentage inhibition of enzyme activity was calculated by using these mean sham incorporation values as the 100% incorporation levels.
Antibody levels measured in sera collected 42 days after initial antigen injection are presented in Tables 2 and 3. Results are shown for sera tested at 1:400 dilutions. Serum antibody could be detected 21 days after the initial injection in most HDS- and EAW-injected rats (not shown). By day 42, all HDS (Table 2) and EAW (Table 3) peptide-injected rats had high levels of serum IgG antibody to the epitope(s) associated with the respective peptide. In fact, serum antibody could be detected at dilutions greater than 105 in some sera from rats injected with HDS and EAW peptide constructs (not shown). In contrast, injection with PQW induced IgG antibody that could be detected at 1:400 diluted sera in four of five rats but was absent in three of five rat sera at a dilution of 1:1,600 (not shown). No significant reactivity with HDS or EAW was observed with sera from sham-, PQW-, or GTF-injected groups. Also, sera from HDS- or EAW-injected rats did not cross-react with the heterologous peptide (Tables 2 and 3).
TABLE 2.
Serum and salivary immune responses to HDS after subcutaneous injection with PQW, EAW, or HDS peptide constructs or S. mutans GTF
| Injected group | Mean EU ± SE (n = 4–7 rats/group)a
|
|||||
|---|---|---|---|---|---|---|
| Serum IgG
|
Salivary IgA
|
|||||
| Pre | Day 42 | Range | Pre | Day 42 | Range | |
| Sham | 0 ± 0 | 0 ± 0 | 0 | 1 ± 1 | 0 ± 0 | 0 |
| PQW | 0 ± 0 | 0 ± 0 | 0 | 0 ± 0 | 1 ± 1 | 0–1 |
| EAW | ND | 0 ± 0 | 0 | ND | ND | |
| HDS | 0 ± 0 | 118 ± 18 | 99–155 | 1 ± 1 | 142 ± 36 | 16–206 |
| S. mutans GTF | 0 ± 0 | 0 ± 0 | 0 | 0 ± 0 | 1 ± 1 | 0–3 |
Pre, preimmunization; ND, not done.
TABLE 3.
Serum and salivary immune responses to EAW after subcutaneous injection with the PQW, EAW, or HDS peptide construct or S. mutans GTF
| Injected group | Mean EU ± SE (n = 4–7 rats/group)a
|
|||||
|---|---|---|---|---|---|---|
| Serum IgG
|
Salivary IgA
|
|||||
| Pre | Day 42 | Range | Pre | Day 42 | Range | |
| Sham | 0 ± 0 | 0 ± 0 | 0 | 0 ± 0 | 0 ± 0 | 0 |
| PQW | ND | 0 ± 0 | 0 | ND | ND | |
| EAW | 0 ± 0 | 117 ± 40 | 105–126 | 1 ± 1 | 53 ± 19 | 14–78 |
| HDS | ND | 0 ± 0 | 0 | ND | ND | |
| S. mutans GTF | 0 ± 0 | 2 ± 2 | 0–3 | 0 ± 0 | 1 ± 1 | 0–3 |
Pre, preimmunization; ND, not done.
Two immunizations with the HDS and EAW peptide constructs also induced significant levels of salivary IgA antibody that were reactive with the respective peptide in all rats by day 42 (Tables 2 and 3). The HDS peptide construct also induced elevated salivary IgA immune responses in three of seven HDS-injected rats on day 21 after one immunization, although no antibody to EAW could be detected at this time in EAW-injected rats. Thus, both the EAW and HDS peptide constructs have significant systemic and mucosal immunogenicity when given by the subcutaneous route of injection.
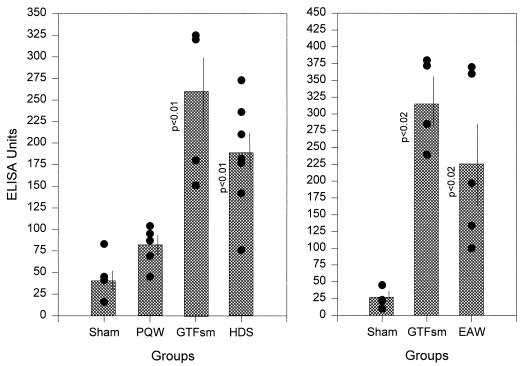
All antisera were evaluated by ELISA for IgG antibody reactive with S. mutans GTF preparations. Sera from all rats injected with S. mutans GTF and EAW had elevated levels of IgG antibody to S. mutans GTF at day 21 (not shown) and day 42 (Fig. 1). Anti-GTF antibody levels in day 42 sera of two of five EAW-injected rats were within the range of those of the GTF-injected rats, suggesting that the epitope(s) presented on the EAW peptide construct is prominent on native GTF. Sera from six of seven rats injected with the HDS peptide construct demonstrated IgG antibody that reacted with S. mutans GTF on day 42 (Fig. 1). At this time, five of seven HDS-injected rats showed serum IgG reactivity to GTF within the range of the GTF-injected rats. In contrast, antibody to PQW-injected rats had significantly lower levels of antibody reactive with S. mutans GTF.
FIG. 1.
IgG antibody reactivities to S. mutans GTF evaluated by ELISA in all rat sera taken 42 days after initial injection (two injections). The left and right panels represent different experiments immunized under identical protocols. Bars indicate the mean absorbance for all rat sera of the indicated group (n = 4 to 7), tested at 1:400 dilutions, at least in duplicate. Vertical bars enclose two standard errors. The levels of significance, compared with the sham group by using the Kruskal-Wallace analysis of variance on ranks, are indicated alongside the bars.
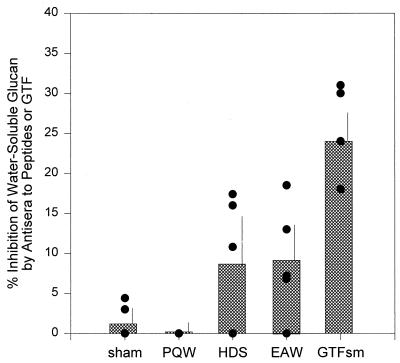
Sera from sham-, S. mutans GTF-, and peptide construct-injected rats were evaluated for the ability to inhibit the formation of water-soluble and water-insoluble glucan by S. mutans GTF. Sera from many but not all EAW and HDS-injected rats inhibited the ability of S. mutans GTF to synthesize water-soluble glucan (Fig. 2). The level of inhibition of water-soluble glucan formation approached 20% in sera of three rats injected with the EAW or HDS peptide construct. Three of the five most inhibitory sera (Fig. 2) also had the highest levels of antipeptide antibody reactive with GTF in ELISA (Fig. 1), although as a group the sera were not significantly correlated for this comparison. In contrast, no serum from rats injected with the PQW peptide control inhibited S. mutans GTF water-soluble glucan synthetic activity. Water-insoluble glucan formation by S. mutans GTF was not found to be inhibited by sera from any peptide-injected rat under the conditions of this assay.
FIG. 2.
Percentage inhibition of the S. mutans GTF-mediated incorporation of [14C]glucose from labeled sucrose into water-soluble glucan by sera from peptide- or GTF-injected rats. Bars indicate the mean inhibition for rat sera (n = 4 to 6) of the indicated peptide-injected groups, tested at 1:10 dilutions, or the S. mutans GTF-injected group, tested at 1:50 dilutions. The vertical bar indicates 1 standard deviation. Closed circles indicate the inhibition levels of individual rat sera. Data are expressed as the percentage [14C]glucose incorporation of individual sera, compared with the mean [14C]glucose incorporation by four sera from sham-injected rats tested under the same protocol (mean incorporation = 830 cpm).
Within the β5-associated strand of α-amylases is a glutamate residue (position 230 in taka-amylase A) which is considered to serve catalytically as a proton donor (13). Site-directed mutagenesis of the analogous residue in S. downei GTF (Glu-489 in S. mutans GTF-B) to glutamine resulted in a catalytically inactive enzyme (5). Mutagenesis of Trp-491 in S. mutans GTF-B, highly conserved in all mutans streptococcal GTFs (Table 1), also eliminated detectable enzyme activity (28). The EAW peptide sequence used in the present study overlapped both of these important residues as well as the complete β5 strand sequence. Not only did antibody induced by the EAW peptide construct bind to S. mutans GTF (Fig. 1), but some sera also significantly inhibited GTF activity, supporting the catalytic function(s) contained within the sequence of EAW.
The HDS peptide construct contained several residues which have been implicated in GTF function. His-561 and Asp-562 in S. mutans GTF-B are invariant in mutans streptococcal GTFs. The analogous histidine in α-amylases helps to stabilize transition states (22), while the aspartate stabilizes the reaction intermediate carbonium cation (14). Site-directed mutagenesis of the equivalent histidine and aspartic acid residues in mutans streptococcal GTFs catalytically inactivated the enzyme (5, 28). Also contained within the HDS peptide sequence is an aspartate, equivalent to Asp-567 in GTF-B, which has been shown to influence the solubility of the glucan synthesized by GTF (17). Aspartic acid is invariant at this position in all mutans streptococcal GTFs, although it is not conserved in α-amylases, presumably because its function is irrelevant to amylolytic activity. Thus, antibody directed to the HDS peptide construct could be expected to influence several aspects of GTF activity. In the present study, most rats responded to HDS peptide construct immunization with levels of antibody to GTF that were within the range of sera from rats injected with intact S. mutans GTF. Many of these sera also inhibited the water-soluble glucan synthetic activity of S. mutans GTF, which is consistent with the presence of putative functional residues within this sequence.
Peptide-injected rat sera did not detectably inhibit water-insoluble glucan synthesis under the conditions of the assay. This lack of water-insoluble glucan inhibition may be related to the expected lower affinity and avidity of the antipeptide antibody or be a consequence of assay conditions, such as the mixture of S. mutans GTF isotypes used for synthesis or the lack of primer dextran. Interestingly, antisera to intact S. mutans GTF also were less effective as inhibitors of water-insoluble, compared with water-soluble, glucan synthesis under these conditions (data not shown).
The PQW peptide construct, selected primarily as a peptide control in this experiment, was much less immunogenic than the HDS or EAW construct. The absence of detectable S. mutans GTF-inhibitory activity induced by this peptide construct could also be attributed to the relatively low homology of the PQW sequence with corresponding sequences from S. mutans GTFs (Table 1). Interestingly, a similar peptide sequence, identical to residues 342 to 356 of S. mutans GTF-B (reference 6 and Table 1), was immunogenic and induced GTF-inhibitory activity when fed (4) or injected (3) as a protein chimera when fused to the sequence of the B subunit of cholera toxin (CTB). The addition of CTB undoubtedly influenced the immunogenicity of the protein chimera, while its sequence identity with S. mutans GTF-B would have increased the level of specificity for this enzyme isotype.
Thus, these data indicate that sequences containing functionally important residues associated with the β5 and β7 barrel elements are immunogenic and can induce systemic and mucosal antibody responses that can lead to loss of enzyme function. We have shown that antibody and GTF-inhibitory levels (including inhibition of water-insoluble glucan formation) induced by other catalytically associated peptides can be increased by combination with functionally associated GTF peptides that also contain a strong T-cell epitope (27). Combination of HDS and or EAW with such peptides may also enhance immune responses to these important epitopes and may increase the amount of water-insoluble glucan inhibitory activity induced. Since both EAW and HDS peptide constructs also gave rise to significant levels of salivary IgA antibody in many animals, such di- or multiepitopic constructs could also be expected to increase mucosal immunity, thus potentiating their application as subunit vaccines for dental caries.
Acknowledgments
This study was supported by Public Health Service grants DE-04733 and DE-06153 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Abo H, Masumura T, Kodama T, Ohta H, Fukui K, Kato K, Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase) J Bacteriol. 1991;173:989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia J-S, Lin R-H, Lin S-W, Chen J-Y, Yang C-S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993;61:4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dertzbaugh M T, Macrina F L. Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect Immun. 1990;58:1509–1513. doi: 10.1128/iai.58.6.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dertzbaugh M T, Peterson D L, Macrina F L. Cholera toxin B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect Immun. 1990;58:70–79. doi: 10.1128/iai.58.1.70-79.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devulapalle K S, Goodman S D, Gao Q, Hemsley A, Mooser G. Knowledge-based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci. 1997;6:2489–2493. doi: 10.1002/pro.5560061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferretti J J, Gilpin M L, Russell R R B. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987;169:4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funane K, Shiraiwa M, Hashimoto K, Ichishima E, Kobayashi M. An active-site peptide containing the second essential carboxyl group of dextransucrase from Leuconostoc mesenteroides by chemical modification. Biochemistry. 1993;32:13696–13702. doi: 10.1021/bi00212a039. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore K S, Russell R R B, Ferretti J J. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun. 1990;58:2452–2458. doi: 10.1128/iai.58.8.2452-2458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda T, Kato C, Kuramitsu H K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990;136:2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins J, Leggio L L, Harris G, Pickersgill R. Beta-glucosidase, beta-galactosidase, family A cellulases, family F xylanases and two barley glycanases form a superfamily of enzymes with 8-fold beta/alpha architecture and with two conserved glutamates near the carboxy-terminal ends of beta-strands four and seven. FEBS Lett. 1995;362:281–285. doi: 10.1016/0014-5793(95)00252-5. [DOI] [PubMed] [Google Scholar]
- 12.Laloi P, Munro C L, Jones K R, Macrina F L. Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose-binding site peptide-cholera toxin B-subunit chimeric protein. Infect Immun. 1996;64:28–36. doi: 10.1128/iai.64.1.28-36.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGregor E A, Jespersen H M, Svensson B. A circularly permuted alpha-amylase-type alpha/beta barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett. 1996;378:263–266. doi: 10.1016/0014-5793(95)01428-4. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura Y, Kusunoki M, Harada W, Kakudo M. Structure and possible catalytic residues of taka-amylase S. J Biochem. 1984;95:697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- 15.Merrifield R B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]
- 16.Mooser G, Hefta S A, Paxton R J, Shively J E, Lee T. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus glucosyltransferases. J Biol Chem. 1991;266:8916–8922. [PubMed] [Google Scholar]
- 17.Shimamura A, Nakano Y J, Mukasa H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J Bacteriol. 1994;176:4845–4850. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiroza T, Ueda S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith D J, Taubman M A, King W F, Eida S, Powell J R, Eastcott J W. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect Immun. 1994;62:5470–5476. doi: 10.1128/iai.62.12.5470-5476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith D J, Shoushtari B, Heschel R L, King W F, Taubman M A. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect Immun. 1997;65:4424–4430. doi: 10.1128/iai.65.11.4424-4430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith D J, Taubman M A. Vaccines against dental caries infection. In: Levine M M, Woodrow G C, Kaper J B, Coban G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 914–930. [Google Scholar]
- 22.Sogaard M, Kadziola A, Haser R, Svensson B. Site-directed mutagenesis of histidine 93, aspartic acid 180, glutamic acid 205, histidine 290, and aspartic acid 291 at the active site and tryptophan 279 at the raw starch binding site in barley alpha-amylase 1. J Biol Chem. 1993;268:22480–22484. [PubMed] [Google Scholar]
- 23.Tam J P. Synthetic peptide vaccine design: synthesis and properties of high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taubman M A, Smith D J, King W F, Eastcott J W, Bergey E J, Levine M J. Immune properties of glucosyltransferases from Streptococcus sobrinus. J Oral Pathol. 1988;17:466–470. doi: 10.1111/j.1600-0714.1988.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 25.Taubman M A, Holmberg C, Smith D J, Eastcott J. T and B cell epitopes from peptide sequences associated with glucosyltransferase function. Clin Immunol Immunopathol. 1995;76:S95. [Google Scholar]
- 26.Taubman M A, Holmberg C J, Smith D J. Immunization of rats with synthetic peptide constructs from the glucan binding or catalytic regions of mutans streptococcal glucosyltransferase protects against dental caries. Infect Immun. 1995;63:3088–3093. doi: 10.1128/iai.63.8.3088-3093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taubman M A, Holmberg C J, Smith D J. Diepitopic construct of functionally relevant peptides enhances immunogenicity and reactivity with glucosyltransferase. J Dent Res. 1997;76:347. doi: 10.1128/IAI.69.7.4210-4216.2001. . (Abstr. 2666.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. Infect Immun. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda S, Shiroza T, Kuramitsu H K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988;69:101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]