Abstract
Purpose
The Clinical Genome Resource (ClinGen) Gene Curation Expert Panels (GCEPs) have historically focused on specific organ systems or phenotypes; thus, the ClinGen Syndromic Disorders GCEP (SD-GCEP) was formed to address an unmet need.
Methods
The SD-GCEP applied ClinGen’s framework to evaluate the clinical validity of genes associated with rare syndromic disorders. 111 Gene-Disease Relationships (GDRs) associated with 100 genes spanning the clinical spectrum of syndromic disorders were curated.
Results
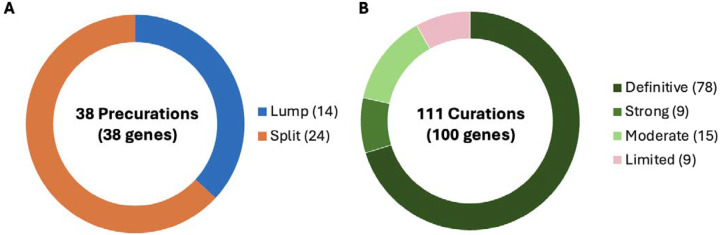
From April 2020 through March 2024, 38 precurations were performed on genes with multiple disease relationships and were reviewed to determine if the disorders were part of a spectrum or distinct entities. 14 genes were lumped into a single disease entity and 24 were split into separate entities, of which 11 were curated by the SD-GCEP. A full review of 111 GDRs for 100 genes followed, with 78 classified as Definitive, 9 as Strong, 15 as Moderate, and 9 as Limited highlighting where further data are needed. All diseases involved two or more organ systems, while the majority (88/111 GDRs, 79.2%) had five or more organ systems affected.
Conclusion
The SD-GCEP addresses a critical gap in gene curation efforts, enabling inclusion of genes for syndromic disorders in clinical testing and contributing to keeping pace with the rapid discovery of new genetic syndromes.
Introduction:
Syndromic disorders are complex conditions involving multiple organs or body systems and are highly enriched among infants with structural birth defects. Combined, genetic disorders and congenital malformations represent the leading cause of infant mortality in the US, responsible for 20% of deaths,1,2 and have a large health economic burden in children and adults.3 Syndromic disorders are one of the largest and fastest growing categories of disorders with multiple new syndromes published every month.4 To ensure the clinical validity of genetic testing for syndromic disorders, it is critical to rigorously evaluate the underlying evidence and classify the validity of purported gene-disease relationships (GDRs).
GDR validity curation is an essential first step for accurate and consistent clinical interpretation across variant triage, classification, and reporting.5 The American College of Medical Genetics and Genomics (ACMG) recommends that diagnostic gene panels include only GDRs that meet the Clinical Genome Resource’s (ClinGen) criteria for Definitive, Strong, or Moderate evidence. GDRs with a classification of Limited should typically only be assessed as part of exploratory exome or genome analysis, while GDRs classified as Disputed or Refuted are not appropriate for diagnostic testing.6 For variant classification,7 variants found in genes with only a Moderate GDR should not exceed a classification of likely pathogenic, and variants in genes with Limited evidence should not be classified above a variant of uncertain significance.6
The Clinical Genome Resource (ClinGen), a National Institutes of Health (NIH) National Human Genome Research Institute (NHGRI)-funded initiative, is building an authoritative central resource to define the clinical relevance of genes and variants for use in precision medicine and research.8 To achieve this goal, ClinGen has developed an evidence-based gene-disease validity curation framework that allows semiquantitative assessment of the strength of evidence underlying GDRs which is then translated into seven qualitative classifications. Four classification categories indicate evidence supporting a GDR (Definitive, Strong, Moderate, and Limited) while two categories indicate contradictory evidence (Disputed, Refuted). A Strong classification has the same level of evidence as a Definitive classification, but less than three years have passed between publications documenting human genetic evidence. No Known Disease Relationship indicates the GDR is not supported by human genetic evidence. Curated evidence is reviewed by gene curation expert panels (GCEPs), with appropriate clinical and laboratory expertise, and the resultant classification confirmed or adjusted based on expert insight.9
ClinGen GCEPs have historically focused on a specific organ system or phenotype. The challenge of the growing number of syndromic disorders published each year not covered by these curation efforts provided an opportunity for a GCEP to take a different approach to address this unmet need. The ClinGen Syndromic Disorders Gene Curation Expert Panel (SD-GCEP; https://clinicalgenome.org/affiliation/40060) was therefore established in March 2020 to classify the clinical validity of GDRs involving multiple body systems not under evaluation by another GCEP and is co-funded by the National Institute of Child Health and Human Development (NICHD) and National Institute of Neurological Disorders and Stroke (NINDS). The SD-GCEP is currently composed of 46 expert and biocurator members (and 25 former members) across 41 institutions and 6 continents. The GCEP meets twice a month plus additional quarterly meetings to accommodate as many time zones as possible. Here, we describe the work of the SD-GCEP, providing the community with outcomes and updates from the group.
Methods:
Membership
The membership of the SD-GCEP is composed of medical geneticists, genetic counselors, clinical molecular geneticists, variant scientists, and basic scientists, as well as staff biocurators from ClinGen. These members are largely volunteers from academic institutes, clinical laboratories, and organizations that provide online gene-level resources. Initial membership was solicited through invitation or self-nomination. New members can volunteer through ClinGen’s Community Curation Database (https://ccdb.clinicalgenome.org/apply) or by reaching out to the SD-GCEP coordinator directly (https://clinicalgenome.org/affiliation/40060).
Identifying Relevant Genes
Five approaches have been used during the existence of the SD-GCEP to identify relevant GDRs for curation. Approach 1 was to identify syndromic GDRs most frequently tested in clinical laboratories, prioritized by the number of tests in the Genetic Testing Registry (GTR)10 and the number of pathogenic or likely pathogenic variants in the gene in ClinVar. Approach 2 identified GDRs detected through clinical genome or exome sequencing performed by diagnostic laboratories within the membership of the SD-GCEP, utilizing and building upon the curation efforts performed as part of this testing. Approach 3 identified new GDRs from research consortia including NHGRI’s Centers of Mendelian Genomics and GREGoR consortium, again utilizing existing internal efforts. Approach 4 included any GDRs requested by other GCEPs for phenotypes requiring the broad expertise of the SD-GCEP. Most recently, Approach 5 was used to capture additional syndromic GDRs that have been curated by groups not using the ClinGen framework by first searching the Gene Curation Coalition (GenCC) database (https://search.thegencc.org/) for disease assertions with “syndrome” in name, then prioritizing Strong or Definitive classifications given these are often included on gene panels. The genes from all approaches were reviewed to ensure the disease assertions were syndromic in nature, rather than pertaining to a single organ system, and the final list reviewed and approved by the SD-GCEP chairs.
Precuration for Genes with Multiple Disease Assertions
ClinGen’s precuration is the process of evaluating available information for GDRs to determine the disease entity and mode of inheritance to be curated.11 When evaluating genes with multiple disease assertions listed within ontologies such as Online Mendelian Inheritance in Man (OMIM),12 Orphanet,13 Mondo Disease Ontology (Mondo),14 or the literature, the SD-GCEP refers to ClinGen’s Lumping and Splitting guidelines15 to precurate the gene. ‘Lumping’ involves combining two or more conditions into a single disease entity, while ‘splitting’ involves separating disease assertions into distinct entities. To inform this decision, the molecular mechanism, phenotypic variability within and across families, and the mode of inheritance are reviewed for each disease assertion, and the decision to lump or split is then voted on by the expert panel. Following ClinGen’s guidelines, disorders are generally lumped into a single entity when the underlying molecular mechanism is consistent, and the clinical features represent a spectrum of the same condition. When there is insufficient data on the molecular mechanism or uncertainty on the phenotypic spectrum, disadvantages of splitting include dilution of evidence across multiple GDRs and underweighting of the final curation strength. The expert panel decides on the most appropriate name for the entity to be curated by applying the dyadic naming convention(s) for lumped conditions, which are defined by the ClinGen Guidance and Recommendations for Monogenic Disease Nomenclature.16 The naming decisions are communicated to OMIM, Mondo, and Orphanet for consideration of inclusion in ontology.
Curation and Expert Panel Review
The SD-GCEP evaluates the clinical validity of GDRs according to ClinGen’s gene curation workflow,9 and curation is performed by SD-GCEP curators using ClinGen’s Gene Curation Interface (GCI)17. Genetic and experimental data, either supporting or disputing the validity of the disease relationship, are examined and classified using the current standard operating procedures document (for this study, versions 7 to 10 depending on the date of curation (https://clinicalgenome.org/docs/?doc-type=curation-activity-procedures&curation-procedure=gene-disease-validity)) by a SD-GCEP biocurator. On average, two biocurators each present one curation per meeting on either the biweekly or quarterly video conference call. The final decision is voted on and approved by the members in attendance at the meeting, including both biocurators and experts. Curators submit evidence summaries for approval by the chairs which are then published to the ClinGen website for broad community access.
SD-GCEP Specific Guidelines
Many GCEPs adopt internal modifications to the ClinGen Gene-Disease Validity Standard Operating Procedures in places where there is a degree of subjectivity. In addition to the formal guidelines, and to help with consistency, the SD-GCEP has created a comprehensive “SD-GCEP Scoring Guidance and Specifications Document” and a short form that includes additional guidance and detailed information specific to the SD-GCEP, including where to look for data and how to score them (Supplemental Material). Of note, considering human genetic evidence, the SD-GCEP specifically downgrades loss-of-function variants for homozygosity and if consanguinity cannot be ruled out, because these individuals are likely have multiple homozygous variants due to runs of homozygosity, and it may be unclear which homozygous variants are causative18 (Table 1). Due to the rarity of many syndromic phenotypes meaning the number of unique pathogenic variants can be limited, the SD-GCEP allows scoring of multiple observations of the same variant when de novo occurrence is proven. Each observation is awarded the default score for the variant type as well as the upgrade for de novo status. Recurrently occurring variants that present with a highly specific phenotype and are known to act via a dominant mechanism are upgraded (Table 1). In considering experimental evidence, non-human animal models are thoroughly evaluated to determine whether the phenotype appropriately recapitulates the syndromic human disease. Models that lack reproducibility of multiple human phenotypes are downgraded to 1 point, instead of the default 2 points (Table 1). Models where a human disease variant has been knocked-in are considered for the maximum 4 points. Morpholino-mediated knock-down models are downgraded to 1 point, unless re-expression of the wild-type allele demonstrates rescue of the phenotype.19
Table 1.
SD-GCEP scoring guidance.
| Scenario | Recommended Score | |
|---|---|---|
| Genetic Evidence | ||
| Predicted or proven null variants | Other variant types | |
| Variant observed in individual with expected phenotype for the disease | 1.5 points (suggested ClinGen default points) | 0.1 (suggested ClinGen default points) |
| Variant homozygosity and/or parental consanguinity | −0.5 points for each (2.5 or 2.0 points total for proband) | Score each variant at 0.05 (0.1 points total for proband) |
| De novo occurrence of variant (each recurrent de novo can be scored at full strength) | +0.5 points (suggested ClinGen upgrade) | +0.4 points (suggested ClinGen upgrade) |
| Recurrent AD variants with highly specific phenotype | +0.25 points | +0.15 points |
| Variant not predicted to cause a true loss of function | −1.0 points | N/A |
| Mechanism of disease is unknown or hasn’t been properly established | −0.5 points | 0.1 (suggested ClinGen default points) |
| Variant located in hotspot or functional domain | N/A | +0.15 points |
| Functional modeling of variants: can consider increasing score for structural and 3D modeling (but not for in silico predictors alone) | +0.15 points (if the modeling is for specific amino acids N/A (e.g, Cys) that are known to be important to protein structure) | |
| Experimental Evidence | ||
| Animal model recapitulates syndromic human disease | 2 points (suggested ClinGen default points) | |
| Transgenic model that overexpresses variant of interest, with recapitulation of syndromic phenotype | +1 points | |
| Animal model where human pathogenic variant is knocked in, with recapitulation of syndromic phenotype | +2 points maximum | |
| Knockdown animal model, without demonstration of rescue by WT allele | −1 point | |
| Knockdown animal model, with rescue by non-human WT allele | −0.5 points | |
| Animal model that lacks reproducibility of multiple phenotypes associated with human disease | −1 point | |
Results:
Assessing Gene-Disease Validity Across Syndromic Disorders
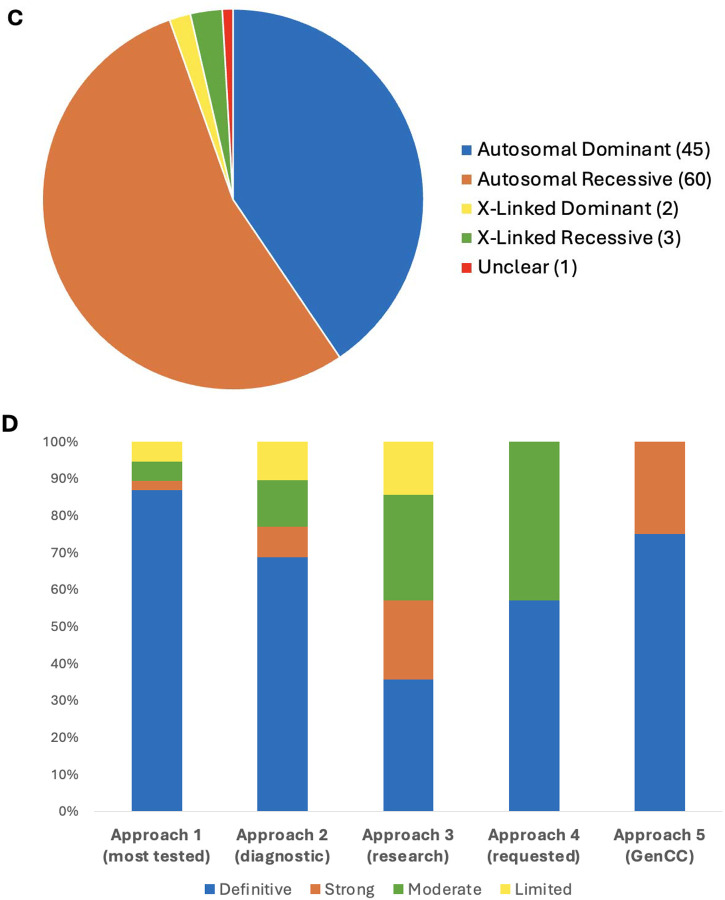
In the first four years, from April 2020 through March 2024, the SD-GCEP performed 38 precurations and 111 curations of GDRs involving 100 genes. For precurations, 14 genes with multiple assertions were lumped and curated as a single entity and 24 were split. For curations, 78 GDRs were classified as Definitive, 9 as Strong, 15 as Moderate, and 9 as Limited (Figure 1A+B). For the 111 GDRs, 60 had an autosomal recessive (AR) mode of inheritance, 45 were autosomal dominant (AD), three were X-linked recessive, two were X-linked dominant, and one had an unclear mode of inheritance (Figure 1C). The unclear mode of inheritance is associated with the GDR for UNC13A and congenital nervous system disorder, which was curated by the SD-GCEP in 2021. At the time, three variants including missense and stop-gained variants had been reported in association with a neurodevelopmental syndrome characterized by variable features of developmental delay, seizures, microcephaly, and myopathy/movement disorders.20–22 Based on the limited number of reported cases, a distinct mode of inheritance, mechanism of disease, and phenotypic spectrum could not be determined,therefore the classification remains limited and provisional until further evidence is published.
Figure 1. Precurations and curations performed from April 2020 - March 2024.
(A) Summary of lumping and splitting decisions for the 38 precurations performed to date. (B) Summary of the final classifications for the 111 approved GDR classifications to date. (C) Summary of mode of inheritance of the 111 approved GDRs to date. (D) Summary of final classifications for GDRs across the five curation approaches. Approach 1 (38 genes - GDRs most frequently tested in clinical laboratories): 33 Definitive, 1 Strong, 2 Moderate, 2 Limited; Approach 2 (47 genes - GDRs through clinical genome or exome sequencing performed by diagnostic laboratories within the membership of the SD-GCEP): 33 Definitive, 4 Strong, 6 Moderate, 5 Limited; Approach 3 (14 genes - new GDRs from research consortia including NHGRI’s Centers of Mendelian Genomics and GREGoR consortium): 5 Definitive, 3 Strong, 4 Moderate, 2 Limited; Approach 4 (7 genes - GDRs requested by other GCEPs for phenotypes requiring the broad expertise of the SD-GCEP): 4 Definitive, 3 Moderate; Approach 5 (4 genes as of March 2024 - syndromic GDRs in GenCC with Strong or Definitive classifications not curated by other GCEPs): 3 Definitive, 1 Strong.
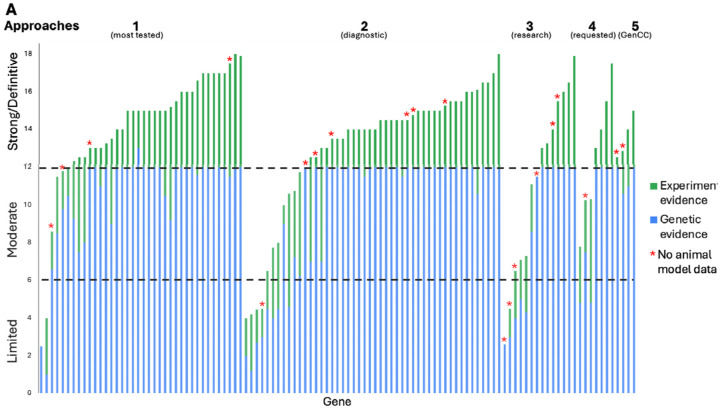
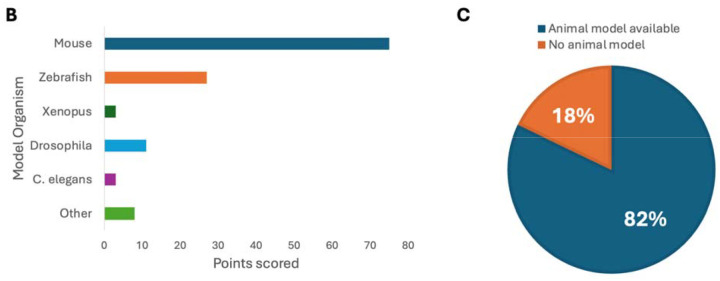
The 38 GDR curations from the most frequently tested genes in clinical laboratories were more often classified as Definitive (“Approach 1”: 33 Definitive, 1 Strong, 2 Moderate, 2 Limited). This was similar to the distribution seen for the 48 GDR curations identified through clinical genomic sequencing performed by diagnostic laboratories by the membership of the SD-GCEP (“Approach 2”: 33 Definitive, 4 Strong, 6 Moderate, 5 Limited). A broader distribution was seen for the 14 GDR curations from within a research setting (“Approach 3”: 5 Definitive, 3 Strong, 4 Moderate, 2 Limited). Seven GDR curations came from collaboration with other GCEPs (Approach 4”: 4 Definitive, 3 Moderate), and 4 GDR curations came from the more recently designed gene list looking at Strong and Definitive GDRs in the GenCC that have not yet been curated by ClinGen (“Approach “5: 3 Definitive, 1 Strong) (Figure 1D). It was an open question whether GDRs without animal models would have sufficient evidence to reach classifications necessary to be included in diagnostic testing panels (Moderate or above); however, of the curations for which there were no animal models available to score, 8 were classified as Definitive, 4 as Strong, 4 as Moderate, and only 4 as Limited (Figure 2A). For example, the GDR for ARSL and X-linked chondrodysplasia punctata 1 is well defined in the literature, with variants in at least 50 probands in seven publications scored in this curation23–29, maxing out the genetic evidence at 12 points and reaching a Definitive classification, but has no model organism. Overall, the presence of an animal model did correlate with the curation classification. 86.1% of curations classified as Definitive, 55.6% of curations classified as Strong, 66.7% of curations classified as Moderate, and 55.6% of curations classified as Limited had an animal model scored. Mouse models were the predominant model organism scored, while zebrafish, Xenopus, Drosophila, C. elegans, and other models have also been scored (Figure 2B). 82% of all curations had animal models counted as experimental data (Figure 2C).
Figure 2. SD-GCEP Curation Evidence.
(A) The number of points awarded for genetic and experimental evidence for each gene represented by the bar height (blue = genetic evidence; green = experimental evidence). GDRs where no animal model was available are starred. (B) The majority of genes (82%) had an animal model with 92% of sufficient quality and overlap with the human phenotype to be scored in the ClinGen curation framework. (C) Mouse models were the predominant model organism scored.
Phenotypic Diversity in Syndromic Disorders
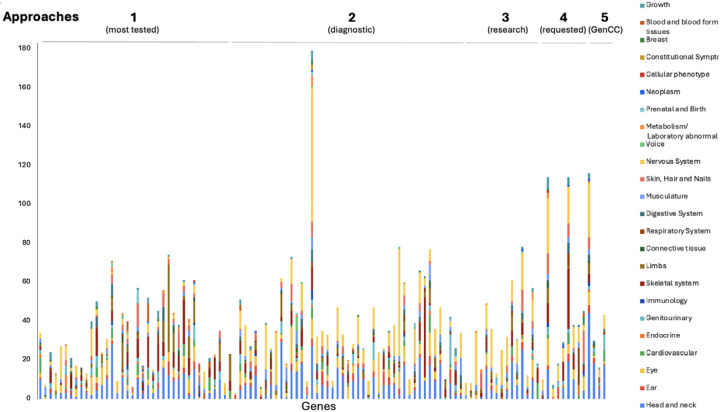
GDRs curated by the SD-GCEP are syndromic in nature, demonstrated by the diseases involved affecting more than one organ system. The number of HPO terms under each of the 23 top-level terms by organ system (direct descendants of “Phenotypic abnormality, “HPO:0000118”) for each of the disease assertions for GDRs under curation was counted (Figure 3). All of the 23 different organ systems were affected in at least one of the curated GDRs. Of the 111 GDRs curated, a median of 8 organ systems were affected per disease. All diseases involved two or more organ systems, while the majority (88/111 GDRs, 79.2%) had five or more organ systems affected (Figure 3). The most commonly involved organ systems included the nervous system, head and neck, eye, and the skeletal system.
Figure 3. GDRs curated are highly syndromic in nature.
HPO higher order terms representing the phenotypic features associated with each disease assertion were graphed by counting the number of HPO terms under each higher order term. For terms that were lumped, HPO terms for all of the assertions were combined and counted. All diseases involved two or more organ systems, while the majority (88/111 GDRs, 79.2%) had five or more organ systems affected.
Collaboration Between GCEPs
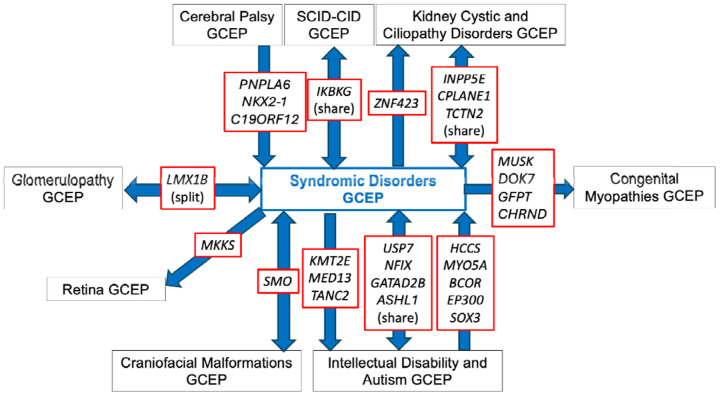
The overlapping phenotypic features between syndromic disorders and other disease areas necessitated communication and collaboration between the SD-GCEP and other GCEPs. Genes are transferred to different GCEPs based on the specific expertise needed. For example, if a disease assertion is more syndromic than a GCEP is comfortable reviewing, they may transfer the gene to be curated by the SD-GCEP. Conversely, if a disease assertion originally thought to be syndromic mostly affects one organ system, it will be transferred to another GCEP if there is a specific GCEP for that disease area. Transfer to a more suitable GCEP also happened when new GCEPs were started after the SD-GCEP planned GDR lists were composed. Over the past four years, the SD-GCEP has collaborated and exchanged genes with eight GCEPs: Cerebral Palsy, Congenital Myopathies, Craniofacial Malformations, Glomerulopathy, Intellectual Disability and Autism, Kidney Cystic and Ciliopathy Disorders, Retina, and Severe Combined Immune Deficiency and Combined Immune Deficiency GCEPs (Figure 4). Through this collaboration, 10 genes have been shared between the SD-GCEP and another GCEP (USP7, NFIX, GATAD2B, ASHL1, SMO, LMX1B, IKBKG, INPP5E, CPLANE1, TCTN2). For example, the GDR CPLANE1 and Joubert syndrome 17 was shared between the SD-GCEP from the Kidney Cystic and Ciliopathy Disorders (KCCD) GCEP due to overlapping phenotypes. The KCCD GCEP has curated several forms of Joubert syndrome because of the renal manifestations; however, patients with Joubert syndrome 17 and variants in CPLANE1 were not found to have kidney involvement,30 so the SD-GCEP and KCCD GCEPs shared the curation and both are acknowledged as contributors on the ClinGen website. Additionally, eight genes have been transferred from the SD-GCEP to another GCEP (KMT2E, MED13, TANC2, MKKS, MUSK, DOK7, GFPT, CHRND), and nine genes have been transferred from another GCEP to the SD-GCEP (HCCS, MYO5A, BCOR, EP300, SOX3, PNPLA6, NKX2-1, C19ORF12, ZNF423).
Figure 4.
Exchange and collaboration on GDR curations between SD-GCEP and other GCEPs to date.
Discussion:
The SD-GCEP was created to address a gap in evaluating the clinical validity of GDRs involving multiple body systems and not under the purview of existing GCEPs. Additionally, the work of the SD-GCEP highlights the strength of the ClinGen framework. Not every expert has expertise in every syndrome, but the efforts of these “expert generalists” combined with the ClinGen Framework led to consistent gene-disease classifications across a wide range of conditions. Accurate GDR classifications are necessary for reporting, since ACMG recommends that diagnostic gene panels include all GDRs that meet criteria for Definitive, Strong, or Moderate evidence as defined by ClinGen.6
The SD-GCEP also highlights the importance of collaborating with other GCEPs. These collaborations are necessary for the best-informed gene-disease classifications. For example, the Craniofacial Malformations GCEP performed a precuration on the gene SMO and recommended keeping the disease assertions for Curry-Jones syndrome and Pallister-Hall-like syndrome as split. Curry-Jones syndrome is a syndromic craniosynostosis, so the Craniofacial Malformations GCEP curated that assertion, but referred the Pallister-Hall-like syndrome assertion to be curated under the expertise of the SD-GCEP since it involves multiple body systems. Similarly, the SD-GCEP forwarded the MKKS gene to be curated under the expertise of the Retina GCEP due to the retinal phenotypes present. The SD-GCEP has also collaborated with the Primary Immune Regulatory Disorders (PIRD) GCEP, where the PIRD GCEP performed a secondary curation of RAB27A and Griscelli syndrome type 2, a rare autosomal recessive disease characterized by cutaneous hypopigmentation, immunodeficiency, and hemophagocytic lymphohistiocytosis31, adding additional phenotypic information on immunodeficiency to the evidence summary. Effective communication and collaboration between GCEPs is optimal for accurate gene-disease classifications.
Lumping multiple disease assertions after a precuration adds to the challenge of naming the curated disease entity. Rare diseases, including many syndromic disorders, are commonly named after clinicians and patients. This approach does not provide information about the expected clinical features as a result of variation within the gene of interest. The SD-GCEP follows dyadic naming convention as defined by the ClinGen’s guidance and recommendations for monogenic disease nomenclature for GDRs that reach a classification of Moderate, Strong, or Definitive.16 The new label for the entity contains the HGNC gene symbol with the related phenotype. Out of the 14 lumped curations, seven have required the creation of a new name and identifier for the disease entity. These discussions are held in collaboration with relevant ClinGen expert panels, and OMIM and Mondo nomenclature expert members of the SD-GCEP. For example, there were six disease assertions associated with the gene PNPLA6. During the precuration, the GCEP decided to lump the assertions cerebellar ataxia and spastic paraplegia into the term “PNPLA6-related spastic paraplegia with or without ataxia” and lump the terms Boucher-Neuhauser syndrome, Gordon Holmes syndrome, Laurence-Moon syndrome, and Oliver-McFarlane syndrome into the term “retinal dystrophy-ataxia-pituitary hormone abnormality-hypogonadism syndrome.” The GCEP worked closely with Mondo to have these new terms created (MONDO:0100149, MONDO:0100155).
Conversely, splitting multiple disease assertions during the precuration phase poses a challenge at the variant curation level. Gene curation and variant curation are closely connected because the final clinical validity classification can impact the expected variant classification. One way that the GDR classification is impacted is through splitting disease assertions into separate entities based on the present data. For the 38 genes associated with multiple conditions precurated by the SD-GCEP, 66% (25/38) have been curated as split entities with two or more separate entities. For example, the ENPP1 gene has three gene-disease assertions for arterial calcification generalized of infancy 1; hypophosphatemic rickets autosomal recessive 2; and hypopigmentation-punctate palmoplantar keratoderma syndrome or Cole disease. A precuration identified that biallelic loss-of-function is the underlying mechanism of pathogenicity for all entities. However, based on the differences in inheritance pattern and clinical phenotype found in hypopigmentation-punctate palmoplantar keratoderma syndrome, the SD-GCEP decided to split this entity and lump arterial calcification generalized of infancy 1, and hypophosphatemic rickets autosomal recessive 2 as one disease entity. The final clinical validity classification for the lumped entity was Definitive while the split entity only reached Limited classification. Although splitting is justified utilizing a framework that examines the molecular mechanism, phenotypic spectrum, and inheritance patterns of the asserted disease entities, it may result in a reduction in the final evidence scores because the case reports and functional evidence are restricted to a specific condition rather than a broader “lumped” entity.15 If the final classification is Limited, No evidence, Disputed, or Refuted, it can reflect a lack of sufficient evidence supporting the syndrome and therefore potentially reducing the diagnosis rate.6 The SD-GCEP recognizes this limitation and plans to reconcile the problem by reevaluating these GDRs as defined by the ClinGen Standard Gene-Disease Relationship Recuration Procedure.32 Reevaluation of these curations every 2–3 years allows the consideration of new data and the possibility of upgrading the classification.
Moving forward, the ClinGen SD-GCEP will continue to evaluate the clinical validity of GDRs, prioritizing genes listed as Strong or Definitive for a syndrome in GenCC and those recommended by our expert panel members. The GCEP will begin re-curating genes previously classified as Strong, Moderate, or Limited whilst continuing to collaborate with other relevant GCEPs. By defining the clinical validity of GDRs involved in syndromic disorders, the SD-GCEP enables the incorporation of more clinically relevant genes in genetic testing panels and provides a critical resource to the community to improve diagnostic rates and patient outcomes.
Supplementary Material
Acknowledgements
Thank you to Jennifer Goldstein for her very valuable contribution and input into the early stages of establishing the SD-GCEP.
Funding Statement
ClinGen is primarily funded by the NHGRI with co-funding from the National Cancer Institute (NCI), through the following grants: U24 HG009649 (to Baylor/Stanford), U24 HG006834 (to Broad/Geisinger), and U24 HG009650 (to UNC/Kaiser). The SD-GCEP curation efforts along with AODL, EB, YG and SD were supported by NICHD/NINDS U24HD 104591. JXC is funded by R35HG011297 and U01HG011744. SSJ is supported by the National Medical Research Council Clinician Scientist Award (NMRC/CSAINVJun21-0003). GL was supported by Fonds de recherche en santé du Québec. ShM is funded by the South African Medical Research Council through its Division of Research Capacity Development under the Early Investigators Programme from funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the National Institutes of Health. ST and NV were supported by NHGRI RM1 HG010860 and NIH OD R24 OD011883
Footnotes
Conflict of Interest
K.B., M.P.B., B.T.B., N.J.B., An.C., Ad.C., A.R.C, K.L.G., A.K., A.M., R.R., Z.Sc., J.P.T., A.J.C. are current or former employees and shareholders of Illumina Inc. K.B., J.M.H., D.L.T., B.W. are employees of Ambry Genetics. K.L.G. is an employee of Rady Children’s Institute for Genomic Medicine. SSJ is the co-founder of Global Gene Corporation Pte Ltd. J.P.T is an employee of Blueprint Genetics (a Quest company). AODL was a paid consultant for Tome Biosciences, Ono Pharma USA, and Addition Therapeutics and receives research funding from Pacific Biosciences. All other authors declare no conflicts of interest.
Data Availability
The data used in this publication are available in the Supplemental File and the online version of this article. The Clinical Genome Resource Syndromic Disorders Gene Curation Expert Panel makes all curations publicly available on the Clinical Genome Resource website (https://search.clinicalgenome.org/kb/gene-validity/).
References:
- 1.Christianson Arnold, Howson Christopher P., Modell Bernadette. March of Dimes Global Report on Birth Defects. March of Dimes Birth Defects Foundation; 2006. https://www.prevencioncongenitas.org/wp-content/uploads/2017/02/Global-report-on-birth-defects-The-hidden-toll-of-dying-and-disabled-children-Full-report.pdf [Google Scholar]
- 2.Mathews TJ, Driscoll AK. Trends in infant mortality in the United States, 2005–2014. NCHS Data Brief. 2017;(279):1–8. [PubMed] [Google Scholar]
- 3.Chung CCY, Ng NYT, Ng YNC, et al. Socio-economic costs of rare diseases and the risk of financial hardship: a cross-sectional study. Lancet Reg Health West Pac. 2023;34(100711):100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamshad MJ, Nickerson DA, Chong JX. Mendelian gene discovery: Fast and furious with no end in sight. Am J Hum Genet. 2019;105(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clause AR, Taylor JP, Rajkumar R, et al. Reactive gene curation to support interpretation and reporting of a clinical genome test for rare disease: Experience from over 1,000 cases. Cell Genom. 2023;3(2):100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean LJH, Funke B, Carlston CM, et al. Diagnostic gene sequencing panels: from design to report-a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(3):453–461. [DOI] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehm HL, Berg JS, Brooks LD, et al. ClinGen--the clinical genome resource. N Engl J Med. 2015;372(23):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strande NT, Riggs ER, Buchanan AH, et al. Evaluating the clinical validity of gene-disease associations: An evidence-based framework developed by the Clinical Genome Resource. Am J Hum Genet. 2017;100(6):895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinstein WS, Maglott DR, Lee JM, et al. The NIH genetic testing registry: a new, centralized database of genetic tests to enable access to comprehensive information and improve transparency. Nucleic Acids Res. 2013;41(Database issue):D925–D935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical Genome Resource. Gene-Disease Validity Training Materials - ClinGen. Accessed October 2, 2024. https://clinicalgenome.org/curation-activities/gene-diseasevalidity/training-materials/
- 12.Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–D517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Home - Ontology Lookup Service. Accessed August 21, 2024. https://www.ebi.ac.uk/ols4/
- 15.Thaxton C, Goldstein J, DiStefano M, et al. Lumping versus splitting: How to approach defining a disease to enable accurate genomic curation. Cell Genom. 2022;2(5):100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaxton C, Biesecker LG, DiStefano M, et al. Implementation of a dyadic nomenclature for monogenic diseases. Am J Hum Genet. 2024;111(9):1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright MW, Thaxton CL, Nelson T, et al. Generating clinical-grade gene-Disease Validity classifications through the ClinGen data platforms. Annu Rev Biomed Data Sci. 2024;7(1):31–50. [DOI] [PubMed] [Google Scholar]
- 18.The Clinical Genome Resource Gene Curation Working Group. Gene-Disease Validity Curation Process Standard Operating Procedure Version 11. Published online September 2024. https://clinicalgenome.org/site/assets/files/9851/gene-disease_validity_standard_operating_procedures-_version_11_docx.pdf
- 19.Eisen JS, Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135(10):1735–1743. [DOI] [PubMed] [Google Scholar]
- 20.Engel AG, Selcen D, Shen XM, Milone M, Harper CM. Loss of MUNC13–1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia. Neurol Genet. 2016;2(5):e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipstein N, Verhoeven-Duif NM, Michelassi FE, et al. Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder. J Clin Invest. 2017;127(3):1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lionel AC, Costain G, Monfared N, et al. Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test. Genet Med. 2018;20(4):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheffield LJ, Osborn AH, Hutchison WM, et al. Segregation of mutations in arylsulphatase E and correlation with the clinical presentation of chondrodysplasia punctata. J Med Genet. 1998;35(12):1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parenti G, Buttitta P, Meroni G, et al. X-linked recessive chondrodysplasia punctata due to a new point mutation of the ARSE gene. Am J Med Genet. 1997;73(2):139–143. [DOI] [PubMed] [Google Scholar]
- 25.Garnier A, Dauger S, Eurin D, et al. Brachytelephalangic chondrodysplasia punctata with severe spinal cord compression: report of four new cases. Eur J Pediatr. 2007;166(4):327–331. [DOI] [PubMed] [Google Scholar]
- 26.Nino M, Matos-Miranda C, Maeda M, et al. Clinical and molecular analysis of arylsulfatase E in patients with brachytelephalangic chondrodysplasia punctata. Am J Med Genet A. 2008;146A(8):997–1008. [DOI] [PubMed] [Google Scholar]
- 27.Franco B, Meroni G, Parenti G, et al. A cluster of sulfatase genes on Xp22.3: mutations in chondrodysplasia punctata (CDPX) and implications for warfarin embryopathy. Cell. 1995;81(1):15–25. [DOI] [PubMed] [Google Scholar]
- 28.Brunetti-Pierri N, Andreucci MV, Tuzzi R, et al. X-linked recessive chondrodysplasia punctata: spectrum of arylsulfatase E gene mutations and expanded clinical variability. Am J Med Genet A. 2003;117A(2):164–168. [DOI] [PubMed] [Google Scholar]
- 29.Matos-Miranda C, Nimmo G, Williams B, et al. A prospective study of brachytelephalangic chondrodysplasia punctata: identification of arylsulfatase E mutations, functional analysis of novel missense alleles, and determination of potential phenocopies. Genet Med. 2013;15(8):650–657. [DOI] [PubMed] [Google Scholar]
- 30.Srour M, Schwartzentruber J, Hamdan FF, et al. Mutations in C5ORF42 cause Joubert syndrome in the French Canadian population. Am J Hum Genet. 2012;90(4):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra K, Singla S, Sharma S, Saxena R, Batra VV. Griscelli syndrome type 2: a novel mutation in RAB27A gene with different clinical features in 2 siblings: a diagnostic conundrum. Korean J Pediatr. 2014;57(2):91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinGen. Standard Gene-Disease Relationship Recuration Procedure(s). https://www.clinicalgenome.org/site/assets/files/2164/clingen_standard_gene-disease_validity_recuration_procedures_v1.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this publication are available in the Supplemental File and the online version of this article. The Clinical Genome Resource Syndromic Disorders Gene Curation Expert Panel makes all curations publicly available on the Clinical Genome Resource website (https://search.clinicalgenome.org/kb/gene-validity/).