Abstract
The purpose of this work was to assess the immunogenicity of a single nasal or oral administration of recombinant 28-kDa glutathione S-transferase of Schistosoma mansoni (rSm28GST) entrapped by poly(lactide-co-glycolide) (PLG)- or polycaprolactone (PCL)-biodegradable microparticles. Whatever the polymer and the route of administration used, the equivalent of 100 μg of entrapped rSm28GST induced a long-lasting and stable antigen-specific serum antibody response, with a peak at 9 to 10 weeks following immunization. Isotype profiles were comparable, with immunoglobulin G1 being the predominant isotype produced. The abilities of specific antisera to neutralize the rSm28GST enzymatic activity have been used as criteria of immune response quality. Pooled 10-week sera from mice receiving PLG microparticles by the nasal or oral route neutralized the rSm28GST enzymatic activity, whereas sera of mice receiving either PCL microparticles, free rSm28GST, or empty microparticles inefficiently neutralized this enzymatic activity. Finally, this study shows that a single administration of these microparticles could provide distinct and timely release pulses of microencapsulated antigen, which might greatly facilitate future vaccine development.
The 28-kDa glutathione S-transferase of Schistosoma mansoni (Sm28GST) (2), a molecule commonly found in the larval, adult, and egg stages of the schistosome (28), has proven its efficacy as an antigen for protective immunity in several animal models, including rodents and baboons (1, 6). The protection conferred by the humoral immune response to Sm28GST affects worm burden and female fecundity (6). This last effect is of a great interest, as it has the potential to minimize both the pathology and the spread of the disease. The presence of antibodies capable of neutralizing the Sm28GST enzymatic activity has been found to correlate with resistance to reinfection in humans (13). A study of the immunological mechanism underlying the reduction of parasite fecundity and egg viability has revealed the existence of an unsuspected neutralizing activity of immunoglobulin A (IgA) antibodies (13). Involvement of IgA in protection mechanisms has been previously described for a mouse model (12). With regard to the design of a vaccine strategy, mucosal immunization may well enhance secretory IgA production, the most important antibody isotype in external secretions, and favor a Th2-type response which contributes to protective immunity (8, 22).
Frequently, multiple administrations are necessary to generate immune responses sufficient for protection. In developing countries, where access to health care is poor, patient compliance for vaccination schemes requiring repeated immunizations has been notoriously low (5, 7). Thus, a vaccine delivery system which increases both the immunogenicity of mucosally delivered antigens and the required immune profile after a single administration of the antigen is needed.
In this study, we elected to use biocompatible and biodegradable microparticles with entrapped recombinant Sm28GST (rSm28GST), since controlled-release vaccines require only a single mucosal administration. Their uptake into the immunity-inductive tissues of the gut- and bronchus-associated lymphoid tissues (GALT and BALT, respectively) is mediated by M cells which selectively take up particles smaller than 10 μm in diameter (9). To prepare the microparticles, poly(lactide-co-glycolide) (PLG) polymer was selected for its compliance with human application. Indeed, its degradation products (CO2 and H2O) are easily eliminated, and it has received Food and Drug Administration approval for a number of clinical applications in humans (27). In this study, poly(ɛ-caprolactone) (PCL) was also used as a biodegradable polymer because of its hydrophobicity (which would favor the uptake of microparticles by the GALT) (9), its in vitro stability, and its low cost. PCL degrades more slowly than PLG and therefore does not generate an inauspicious acid environment for antigens as the PLG do (17). PCL is seldom used for the microencapsulation of antigens, but its lack of toxicity makes it of interest as a matrix for controlled release.
Preparation of rSm28GST-entrapped microparticles.
Microparticles were prepared by the double emulsion-solvent evaporation technique as follows. One milliliter of rSm28GST (expressed in Saccharomyces cerevisiae) in ultrapure water was emulsified with 10 ml of 5% (wt/vol) PLG (polylactide-co-glycolide RG 505; molecular weight, 65,000; Boehringer, Ingelheim, Germany) or 6% (wt/vol) PCL (molecular weight, 80,000; Union Carbide, Versoix, Switzerland) in dichloromethane, using an Ultraturrax model T 25 (IKA Laboratory Technology, Staufen, Germany) at 8,000 rpm for 5 min. The resulting water-in-oil emulsion (2.5 ml) was then emulsified at 8,000 rpm for 5 min in an Ultraturrax with 50 ml of 5% (wt/vol) polyvinyl alcohol (molecular weight, 13,000 to 23,000; 87 to 89% hydrolyzed; Aldrich Chemical Co., Bornem, Belgium) to produce a water-in-oil-in-water emulsion. This emulsion was stirred magnetically overnight under pressure at room temperature to allow evaporation of the organic solvent and formation of microparticles. Microparticles were isolated by centrifugation (10 min at 4,000 × g), washed three times in 10 ml of ultrapure water, and freeze dried.
Microparticle characteristics and antigen loading.
Microparticles were spherical, smooth, and fairly monodispersed, with a surface weakly pitted as previously observed by scanning electron microscopy (3). Microparticles were sized by using a Coulter Multisizer (Coulter Electronics Ltd., Luton, United Kingdom). For evaluation of rSm28GST loading, microparticles were dissolved in 3.0 ml of 1 M NaOH containing 5% (wt/vol) sodium dodecyl sulfate for 24 h at room temperature (15). After centrifugation (4,000 × g for 10 min at room temperature), the supernatant was assayed for antigen concentration by the method of Lowry et al. (20). The percentage (by weight) of antigen loaded per dry weight of microparticles was determined. The entrapment efficiency was expressed by relating the actual antigen loading to the theoretical antigen loading as previously described (18). As shown in Table 1, microparticles produced from PLG were characterized by a higher antigen loading and entrapment efficiency than PCL microparticles, with no significant difference in the mean microparticle size (approximately 10 μm).
TABLE 1.
Characteristics of microparticles from various polymers entrapping rSm28GSTa
| Polymer used (mol wt) | Mean particle size (μm)b ± SEM | Antigen loading (%) | Entrapment efficiency (%) |
|---|---|---|---|
| PLG (65,000) | 9.94 ± 0.71 | 3.33 ± 0.17 | 90.40 ± 2.84 |
| PCL (80,000) | 11.81 ± 0.31 | 0.85 ± 0.18 | 20.00 ± 2.41 |
Each sample was assayed in triplicate.
Despite the relatively adverse chemical and physical stresses imposed upon the antigen during the microencapsulation process, the structural integrity, antigenicity, and immunogenicity of the rSm28GST were conserved (3, 4), confirming the potential of these microparticles for immunization.
Systemic immune response after nasal administration.
Single-dose intranasal administration was performed by the deposition of rSm28GST-entrapped microparticles (100 μg of antigen in 50 μl of phosphate-buffered saline) into the nostrils of anesthetized 6-week-old female BALB/c mice (Iffa Credo, L’Arbresle, France). The control groups received the same amount of either free antigen or empty microparticles by the same route. The pooled sera were analyzed by enzyme-linked immunosorbent assays (ELISA) as previously described (16).
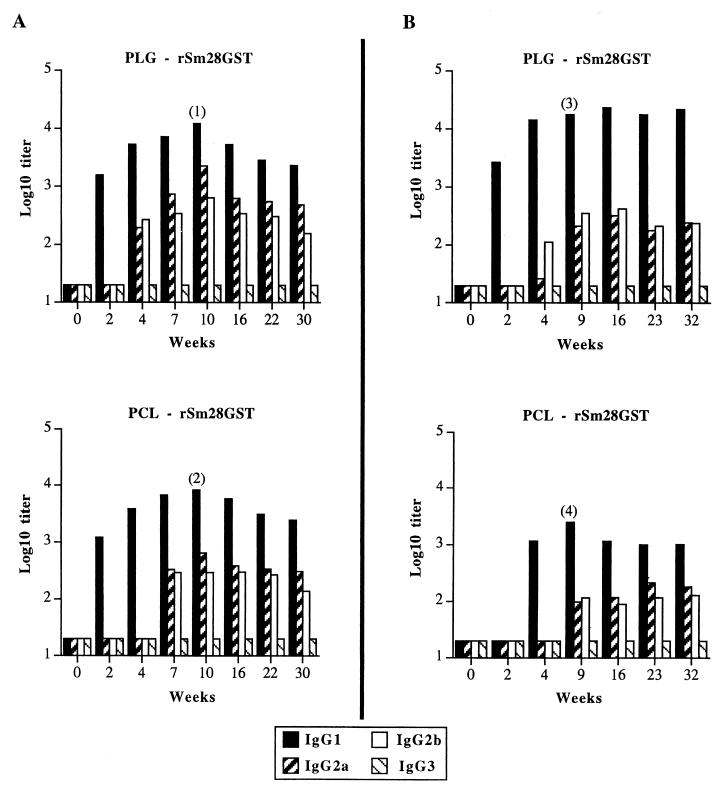
The serum IgG antibody responses to entrapped antigen were detectable, whereas no response was observed after administration of free rSm28GST or empty microparticles (titer < 20; data not shown). As shown in Fig. 1A, the nasal administration of rSm28GST-entrapped microparticles resulted in the coexistence of IgG1, IgG2a, and IgG2b isotypes, with a peak response at 10 weeks. Antibody responses appeared after 2 weeks for the IgG1 isotype and only 4 weeks after administration for the IgG2a and IgG2b isotypes (Fig. 1A). IgG1 antibody response intensities were similar for both types of microparticles (titers of up to 12,300 and 8,100 after 10 weeks for PLG and PCL microparticles, respectively). The IgG2a and IgG2b antibody responses also resulted in a peak after 10 weeks for the both polymers (IgG2a titers up to 2,300 and 700, and IgG2b titers up to 650 and 300, respectively for PLG and PCL microparticles). These responses had weakly decreased after the 30 weeks of the study. The IgG3 isotype was never detected (titer < 20) (Fig. 1A). Finally, the production of IgA was detected in sera (titer of up to 800 after 10 weeks for both of the rSm28GST-entrapped microparticles [Table 2]). After a single nasal administration of microparticles entrapping rSm28GST, the specific antibody response resulted in a mixed Th1-Th2-type response. However, the production of specific IgG1, IgG2b, and IgA in sera revealed a predominantly Th2-like profile. This selective pattern of specific antibodies is likely to be of particular interest in the S. mansoni model discussed above.
FIG. 1.
Antibody isotype profiles elicited after a single intranasal (A) or intragastric (B) administration with rSm28GST-entrapped microparticles. Mice were immunized with either PLG or PCL microparticles. Anti-rSm28GST IgG1, IgG2a, IgG2b, and IgG3 titers were determined at the indicated time points in pooled sera from BALB/c mice (10 mice per group before weeks 9 to 10, 7 mice per group between weeks 9 and 10 and 26, and 4 mice per group after week 26). Titers are given as log10 of maximal dilution of the antisera that gave absorbances threefold higher than the background. Similar results were obtained in two repeated experiments. Individual IgG1 responses: 1, 15,349 ± 1,037; 2, 9,132 ± 806; 3, 31,728 ± 1,817; 4, 2,676 ± 408. These results were expressed as titer mean values obtained from 10 mice ± standard deviation of the mean.
TABLE 2.
IgA antibody response against rSm28GST after nasal administration
| Wks | Antibody levela
|
|
|---|---|---|
| PLG-rSm28GST | PCL-rSm28GST | |
| IgA in serum | ||
| 10 | 830 | 930 |
| 30 | 410 | 530 |
| IgA in BAL fluids | ||
| 9 | 270 | 430 |
| 26 | 120 | 200 |
The results are given for pools of 10 (at week 10) or 4 (at week 30) mice for sera and 3 mice for BAL fluids. Titers are defined as the highest dilution yielding an absorbance three times above the background (no serum added). For free rSm28GST, empty PLG, and empty-PCL, titers were <20 (serum IgA) or <2 (IgA in BAL fluids).
Systemic immune response after oral administration.
BALB/c mice were intragastrically administered 200 μl of bicarbonate solution containing rSm28GST-entrapped microparticles (100 μg). Control animals received free antigen (100 μg) or empty microparticles via the same route. Pooled sera of immunized mice were analyzed by ELISA as previously described (16) (Fig. 1B).
With microparticles produced from both PLG and PCL, serum IgG antibody responses to entrapped rSm28GST were observed (Fig. 1B), compared with the totally ineffective immunization obtained (titer < 20) with free antigen or empty microparticles (data not shown). This result illustrated the ability of microparticles to protect the orally administered antigen from acidic and proteolytic degradation in the stomach and the intestine. The coexistence of IgG1, IgG2a, and IgG2b isotypes in sera (Fig. 1B) without production of IgG3 and IgA isotypes suggests the induction of a mixed Th1-Th2-type response. These responses did not decrease throughout the time of the experiment (32 weeks). No IgG2a nor IgG2b isotypes appeared before 4 weeks after the onset of the IgG1 immune response (Fig. 1B).
In contrast to nasal administration (Fig. 1A), the use of rSm28GST-entrapped PCL microparticles showed a delayed immune response after oral administration compared with the immune response induced by PLG microparticles. With PCL, the predominant IgG1 immune response appeared 4 weeks after oral administration (Fig. 1B), whereas with PLG, specific IgG1 production started only 2 weeks after administration (Fig. 1B).
In a biodegradable microparticle system, the release of a protein is initially controlled by the desorption of protein from the surface of the microparticle (burst release), followed by diffusion of the protein through porous channels in the polymer matrix. Later, the polymer begins to degrade, and a combined erosion/diffusion-controlled release mechanism occurs (11). A two-step antigen release could explain the delayed immune response observed with PCL microparticles. The surface localization of the antigen associated with PCL microparticles, compared to the uniform localization of the antigen in PLG microparticles, would result in an antigen burst release which would be completed before their expected uptake by M cells from the GALT (4). After this burst release, the delayed immune response observed with PCL microparticles could be explained by the slow antigen release from this polymer caused by the high antigen retention in PCL matrix due to its physicochemical characteristics (high molecular weight and hydrophobicity) (9). These observations showed that the simultaneous administration of the two types of microparticles would be an approach to produce a single-dose vaccine. PLG microparticles would be predicted to induce the primary immune response, while PCL microparticles would be expected to induce the booster immune response.
After 9 weeks, IgG1 antibody immune responses reached plateaus of 24,000 and 2,500, respectively, for PLG and PCL microparticles, whereas the IgG2a and IgG2b antibody responses were constantly below 400 for both polymers. Compared to PLG microparticles, the lower IgG1 antibody titer observed with rSm28GST-entrapped PCL microparticles could be explained both by their slower antigen release rate and by the presumably lower antigen quantity taken up by M cells due to the previous antigen burst release.
Thus, there was a difference in the intensity of the immune response between oral and nasal administration of rSm28GST-entrapped PCL microparticles. This difference could be explained both by a higher and faster absorption of microparticles by the BALT than by the GALT and by a natural loss of microparticles in the intestinal tract. This would result, in part, in an antigen burst release directly in the inductive immune tissues of the BALT.
Mucosal immune response after mucosal administration.
Intestinal lavage and bronchoalveolar lavage (BAL) fluids of mice were collected, and their content in anti-rSm28GST IgA antibody was analyzed by ELISA as previously described (16, 24).
For each polymer, a specific mucosal immune response in BAL fluids was observed after the nasal administration of the rSm28GST-entrapped microparticles (Table 2) and was associated with the induction of the specific systemic immune response described above. However, no anti-rSm28GST IgA antibodies were detected in intestinal lavage fluids after nasal administration of rSm28GST-entrapped microparticles (data not shown). This result is in agreement with the findings of Mestecky et al. (23) demonstrating that intranasal administration in human volunteers induces antibody responses in the upper airway mucosae and regional secretions (saliva and nasal secretions) without evoking an immune response in the gut. Finally, no IgA antibody response was observed in wash fluids when free antigen or empty microparticles were used (Table 2).
In contrast to the nasal route, a single dose of orally administered entrapped antigen failed to elicit IgA antibodies against rSm28GST in sera or washes (data not shown), a finding that is in accordance with those of other studies using multiple doses of different antigens (10, 21, 25, 26, 29). Indeed, multiple oral dosing or combinations of oral and parenteral administrations have been necessary to prime mucosal IgA responses. The absence of IgA following a single oral immunization could be correlated to the particular physiological and anatomical features of the GALT. Following oral administration, microparticles which are not taken up by the GALT (microparticles with a size larger than 10 μm) are eliminated in the feces. In contrast to the situation encountered in the GALT, the size of particles does not seem to be a limiting factor for uptake by the BALT. The small particles could induce an immune response immediately after their uptake, and larger ones would accumulate in the upper respiratory tract and be taken up by the BALT after their erosion. This would result in an additional means of storage of the antigen. In this respect, the BALT would be restimulated several times by the microparticles after a single nasal administration of microparticles compared with the single encounter between the GALT and the loaded microparticles.
Antiserum-mediated neutralization of the rSm28GST enzymatic activity.
Previous studies have shown a correlation between neutralization of the rSm28GST enzymatic activity by specific antibodies and protection against schistosomiasis in humans (13). Furthermore, results obtained in vivo for both rats and mice indicate that antibodies which neutralize the enzymatic activity would confer reduction in both female schistosome fecundity and egg viability (30). Glutathione S-transferases (GST) are enzymes catalyzing conjugation reactions in which glutathione acts as a nucleophile. GST from schistosomes is thought to play a key role in detoxification processes involved in their defense against host immune systems. The in vitro addition of glutathione to the substrate bearing an electrophilic carbon atom (e.g., 1-chloro-2,4-dinitrobenzene) results in a thioether formation and thus in a direct change in the absorbance of the substrate measured by spectrophotometry (14). The neutralizing activity of anti-rSm28GST serum was analyzed in 96-well flat-bottomed standard ELISA plates as previously described (13, 19). The enzymatic activity recorded for the rSm28GST in the presence of antisera was related to that measured with sera of nonimmunized mice, which was assigned the 100% value for specific activity.
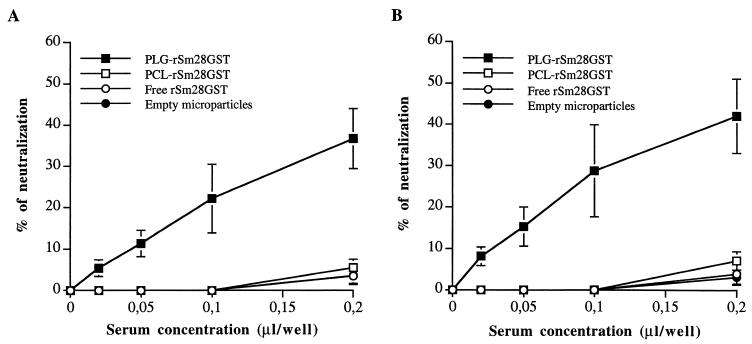
In this study, antisera (week 10) obtained after a single nasal or oral administration of rSm28GST-entrapped PLG microparticles induced a neutralization of the rSm28GST enzymatic activity (Fig. 2). This neutralizing capacity was observed in a dose-dependent manner in the presence of sera. Total neutralization of the rSm28GST (3.5 pmol/well) enzymatic activity was obtained with 1 μl of serum per well (data not shown). It has been shown that anti-Sm28GST IgA is of major importance in the neutralization of enzymatic activity by antisera in infected human populations (13). Our results suggest that in the murine model and following administration by the mucosal route, other neutralizing isotypes may be implicated. Indeed, serum from rSm28GST-entrapped microparticles obtained after oral administration and which did not contain anti-rSm28GST IgA induced a significant neutralization of enzymatic activity at a level similar to that obtained after nasal administration. Similarly, Kremer et al. (19) have recently reported that the main neutralizing isotypes produced in mice after mucosal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing Sm28GST might be the IgG1 and/or IgG2b isotypes. In our PLG microparticle model, two isotypes could be engaged in the serum neutralization capacity observed. The first is IgG1, the predominant isotype obtained; the second is IgG2a, which showed the highest affinity to the antigen (data not shown).
FIG. 2.
Neutralization of rSm28GST enzymatic activity by specific antisera. Neutralizing activity was analyzed for sera obtained 10 weeks after a single intranasal (A) or intragastric (B) administration of either rSm28GST-entrapped PLG or PCL microparticles, free rSm28GST, or empty microparticles. The results were expressed as the mean values ± standard deviations S.D. of the neutralizing activity (percent) of sera for five mice in each group. The catalytic neutralization of rSm28GST (3.5 pmol/well) was measured in the absence or presence of increasing concentrations of antisera. Sera expressing less than 10% of neutralization at a concentration of 0.2 μl/well are considered inefficient sera. Results are from one representative experiment of three.
No significant neutralization was observed with sera obtained after nasal or oral administration of PCL microparticles entrapping rSm28GST, free antigen, or empty microparticles (Fig. 2). The difference in the neutralization level of the rSm28GST enzymatic activity according to the type of polymer used could be attributed to the antigen presentation or folding inside the microparticles. Neutralization was observed with microparticles from PLG, the more hydrophilic polymer. As discussed above, the uniform localization of the rSm28GST in PLG microparticles compared to the antigen surface localization on PCL microparticles indicates that the antigen was probably entrapped in PLG microparticles more efficiently without significant modification of its folding. Moreover, the absence of neutralization of the rSm28GST enzymatic activity by serum from PCL-immunized mice which contained high level of specific IgA after nasal administration showed again the weak neutralizing activity of this class of antibody in our experimental model.
In conclusion, this study confirms the potential of microparticles produced from biodegradable polymers as single-dose vaccine carriers. Both nasal and oral administration of rSm28GST-entrapped microparticles led to high and long-lasting humoral immune responses, while only nasal administration elicited a vigorous mucosal response (long-term IgA production in secretions). This study describes for the first time the use of microparticles to induce a mixed Th1-Th2-type immune response after a single mucosal administration with an antigen from a multicellular and extracellular parasite. PLG appears to be an appropriate polymer to produce rSm28GST-entrapped microparticles for protection studies against schistosomiasis. Indeed, after a single nasal administration, these complexes are able to induce a Th2-type humoral immune response which is able to neutralize the Sm28GST enzymatic activity. In contrast, PCL microparticles entrapping rSm28GST, which were able to induce comparable immune response, were inefficient for generating neutralizing antibodies. Thus, both types of microparticles represent valuable material for verifying our model concerning the effects of neutralizing activities of antibodies induced by vaccination in reducing schistosome fecundity and egg viability. Large-scale experiments regarding the ability of the single-dose immunization to protect against a schistosomal infection are in progress in Niger with a relevant monkey model, Erythrocebus patas.
Finally, the combination of microparticles produced from different polymers presenting their own kinetics of degradation and characterized by different sizes could provide a multidose pulsatile release system. This system might be able to convert a vaccine requiring multiple-booster administrations to a single-dose vaccine with the same or increased efficacy.
Indeed, PLG microparticles would be able to initiate and direct the primary immune response in order to obtain a long-term neutralizing boost immune response with PCL microparticles. The ability of a single administration of microparticles to provide distinct and timely release pulses of microencapsulated antigen might greatly facilitate future vaccine development.
Acknowledgments
This work was supported by grant BIO4-CT96-0374 from European Economic Community. B.B. holds a fellowship from the Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture.
The size analysis of microparticles was kindly carried out by P. Rombaut (Catholic University of Leuven, Leuven, Belgium). We thank M. Mekranfar and C. Leportier (Pasteur Institute of Lille, Lille, France) for technical assistance with immunological methods and Jean Sabatier (Transgène S.A., Strasbourg, France) for providing rSm28GST.
REFERENCES
- 1.Balloul J M, Grzych J M, Pierce R J, Capron A. A purified 28,000 daltons protein from Schistosoma mansoni, adult worms protects rats and mice against experimental schistosomiasis. J Immunol. 1987;138:3448–3453. [PubMed] [Google Scholar]
- 2.Balloul J M, Sondermayer P, Dreyer D, Capron M, Grzych J M, Pierce R J, Carvallo D, Lecocq J P, Capron A. Molecular cloning of a protective antigen against schistosomiasis. Nature. 1987;326:149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- 3.Benoit M-A, Baras B, Poulain-Godefroy O, Schacht A-M, Capron A, Gillard J, Riveau G. Evaluation of the antibody response after oral immunization by microparticles containing an antigen from Schistosoma mansoni. In: Hincal A A, Kas H S, editors. Biomedical science and technology: recent developments in pharmaceutical and medical science. New York, N.Y: Plenum Press; 1998. pp. 137–144. [Google Scholar]
- 4.Benoit M-A, Poulain-Godefroy O, Baras B, Youan B B C, Riveau G, Gillard J, Capron A. Study on the antigenicity of microencapsulated Sm28-GST from Schistosoma mansoni. Proc Int Symp Control Release Bioact Mater. 1997;24:817–818. [Google Scholar]
- 5.Bloom B R. Vaccines for the third world. Nature. 1989;342:115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger D, Reid G D F, Sturrock R F, Wolowczuk I, Balloul J M, Grezel D, Pierce R J, Otieno M F, Guerret S, Grimaud J A, Butterworth A E, Capron A. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991;13:473–490. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 7.Cleland J L. Design and production of single-immunization vaccines using polylactide polyglycolide microsphere system. In: Powell M P, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 439–462. [DOI] [PubMed] [Google Scholar]
- 8.Delgado V, McLaren D J. Evidence for enhancement of IgG1 subclass expression in mice polyvaccinated with radiation-attenuated cercariae of Schistosoma mansoni and the role of this isotype in serum-transferred immunity. Parasite Immunol. 1990;12:15–32. doi: 10.1111/j.1365-3024.1990.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 9.Eldridge J H, Hammond C J, Meulbroek J A. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11:205–214. [Google Scholar]
- 10.Eldridge J H, Staas J K, Meulbroek J A, McGhee J R, Tice T R, Gilley R M. Biodegradable microspheres as a vaccine delivery system. Mol Immunol. 1991;28:287–294. doi: 10.1016/0161-5890(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 11.Gombotz W R, Pettit D K. Biodegradable polymers for protein and peptide drug delivery. Bioconj Chem. 1995;6:332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 12.Grezel D, Capron M, Grzych J M, Fontaine J, Lecocq J P, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23:454–460. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 13.Grzych J-M, Grezel D, Xu C B, Neyrinck J-L, Capron M, Ouma J H, Butterworth A E, Capron A. IgA antibodies to a protective antigen in human Schistosomiasis mansoni. J Immunol. 1993;150:527–535. [PubMed] [Google Scholar]
- 14.Habig W H, Jakoby W B. Assays for differentiation of glutathione S-transferase. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 15.Hora M S, Rana R K, Nunberg J H, Tice T R, Gilley R M, Hudson M E. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res. 1990;7:1190–1194. doi: 10.1023/a:1015948829632. [DOI] [PubMed] [Google Scholar]
- 16.Ivanoff N, Phillips N, Schacht A-M, Heydari C, Capron A, Riveau G. Mucosal vaccination against schistosomiasis using liposome-associated Sm28 kDa glutathione S-transferase. Vaccine. 1996;14:1123–1131. doi: 10.1016/0264-410x(96)00048-5. [DOI] [PubMed] [Google Scholar]
- 17.Jameela S R, Suma N, Misra A, Raghuvanshi R, Ganga S, Jayakrishnan A. Poly(ɛ-caprolactone) microspheres as vaccine carrier. Curr Sci. 1996;70:569–671. [Google Scholar]
- 18.Jeffery H, Davis S S, O’Hagan D T. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. 1993;10:362–367. doi: 10.1023/a:1018980020506. [DOI] [PubMed] [Google Scholar]
- 19.Kremer L, Riveau G, Baulard A, Capron A, Locht C. Neutralizing antibody responses elicited in mice immunized with recombinant Bacillus Calmette-Guérin producing the Schistosoma mansoni glutathione S-transferase. J Immunol. 1996;156:4309–4317. [PubMed] [Google Scholar]
- 20.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Marx P A, Compans R W, Gettie A, Staas J K, Gilley R M, Mulligan M J, Yamshchikov G V, Chen D, Eldridge J H. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 22.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 23.Mestecky J, Moldoveanu Z, Novak M, Compans R W. Mucosal immunity and strategies for novel microbial vaccines. Acta Paediatr Jpn. 1994;36:537–544. doi: 10.1111/j.1442-200x.1994.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 24.Mielcarek N, Cornette J, Schacht A-M, Pierce R J, Locht C, Capron A, Riveau G. Intranasal priming with recombinant Bordetella pertussis for the induction of a systemic immune response against a heterologous antigen. Infect Immun. 1997;65:544–550. doi: 10.1128/iai.65.2.544-550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldoveanu Z, Novak M, Huang W, Gilley R M, Staas J K, Schafer D, Compans R W, Mestecky J. Oral immunization with influenza virus in biodegradable microspheres. J Infect Dis. 1993;167:84–90. doi: 10.1093/infdis/167.1.84. [DOI] [PubMed] [Google Scholar]
- 26.O’Hagan D T, McGee J P, Holmgren J, Mowat A M, Donache A M, Mills K H G, Gaisford W, Rahman D, Challacombe S J. Biodegradable microparticles for oral immunization. Vaccine. 1993;11:149–154. doi: 10.1016/0264-410x(93)90011-l. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Inoue Y, Heya T, Ueno H, Ogawa Y, Toguchi H. Pharmacokinetics of once-a-month injectable microspheres of leuprolide acetate. Pharm Res. 1991;8:787–791. doi: 10.1023/a:1015818504906. [DOI] [PubMed] [Google Scholar]
- 28.Taylor J R, Vidal A, Torpier G, Meyer D J, Roitsch C, Balloul J M, Southan C, Sondermeyer P, Pemble S, Lecocq J P, Capron A, Ketterer B. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988;7:465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng J, Komisar J L, Trout R N, Hunt R E, Yok-Jen Chen J, Johnson A J, Pitt L, Ruble D L. Humoral immunity to aerosolized staphylococcal enterotoxin B (SEB), a superantigen, in monkeys vaccinated with SEB toxoid-containing microspheres. Infect Immun. 1995;63:2880–2885. doi: 10.1128/iai.63.8.2880-2885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C B, Verwaerde C, Grzych J M, Fontaine J, Capron A. A monoclonal antibody blocking the Schistosoma mansoni 28-kDa glutathione S-transferase activity reduces female worm fecundity and egg viability. Eur J Immunol. 1991;150:940–949. doi: 10.1002/eji.1830210804. [DOI] [PubMed] [Google Scholar]