Abstract
Objective
To evaluate the relationship between center volume and inpatient mortality after inter-hospital transfer among patients undergoing pediatric cardiac surgery using contemporary real-world data.
Methods
The Kids’ Inpatient Database (KID) was queried for cardiopulmonary bypass (CPB) cases (CPB) for years 2016 and 2019. Hospitals were divided into three groups based on terciles of volume: “low”: ≤103 cases/year, “mid”:104–194 cases/year, and “high”: >194 cases/year. Multilevel regression models were created to evaluate the association of volume and inpatient mortality for transferred patients for the entire cohort as well as high-complexity cases. (Risk Stratification for Congenital Heart Surgery (RACHS-2) categories 3,4 and 5)
Results
Of 25,749 patients undergoing cases on CPB, 3,511 (13.6%) were preoperative inpatient transfers between hospitals. Compared to direct admissions, unadjusted mortality for patients who were transferred was higher in all groups: 1.7% vs. 5.6% (low-volume), 1.1% vs. 4.6% (mid-volume) and 1.1% vs. 4.9% (high-volume). Compared to low-volume hospitals, inpatient mortality for patients admitted on transfer was not significantly different in mid-volume (OR = 0.85, 95% CI 0.54–1.34, p = 0.483) and high-volume centers (OR = 0.7, 95% CI 0.45–1.12, p = 0.127) for the entire cohort. There was no significant difference in risk-adjusted inpatient mortality for high-complexity cases performed at mid-volume (OR 1.06, p = 0.845, 95% CI (0.62–1.85)) or high-volume hospitals (OR 0.82, p = 0.482, 95% CI (0.48–1.45)).
Conclusion
Annual CPB case volume may not accurately predict risk-adjusted inpatient mortality for children transferred for heart surgery. Annual case volume alone should not dictate transfer practices in pediatric heart surgery.
Keywords: Hospital transfer, annual volume, pediatric cardiac surgery, mortality
Introduction
Contemporary reports within the literature suggest that higher volume is associated with improved outcomes in patients undergoing pediatric cardiac surgery. [1–7] However, volume alone is an imperfect metric to assess quality. Although a volume-outcome relationship may exist, it has been observed that high quality is present in centers of all volume levels. [8, 9] Overall outcomes after pediatric cardiac surgery are excellent and are likely attributable to combination of factors including improved surgical techniques and standardized intensive care unit (ICU) care. [10–16]
Recent expert consensus recommendations suggest the creation of a tiered system for pediatric cardiac surgery centers in the United States and go as far as to suggest that neonatal and high-complexity surgeries should not be performed in low-volume centers. [17] The implementation of such a tiered system may limit access to care and potentially result in the initiation of transfers solely based on differences in annual volume, without more objective correlation to the quality of care provided. This practice may result in increased cost, inefficiency and risks overlooking the utility and pragmatism of providing care lower volume but potentially high-quality center.
To date, there is paucity of literature that investigates the outcomes of patients undergoing inter-hospital transfer for the purpose of pediatric cardiac surgery. Further there is little evidence to support the volume-outcome associations in this subset of patients. Therefore, the goal of this study was to explore the association of hospital volume and inpatient mortality in pediatric patients transferred for cardiac surgery using a national cohort of real-world contemporary data. We hypothesized that there would be no difference in inpatient risk-adjusted all-cause mortality and incidence of post-operative ECMO in patients undergoing pediatric cardiac surgery among hospitals grouped by their annual case volume.
Methods
Data source and inclusion criteria:
Data from the Kids’ Inpatient Database (KID) for years 2016 and 2019 were utilized for this retrospective cohort analysis. KID belongs to the Healthcare Cost and Utilization Project (HCUP). and is the largest publicly available all-payer claims-based pediatric database. KID is an administrative database. This database samples up to 80% of complex newborn and pediatric discharges (< 21 years) from forty-nine US states. [18]
The pertinent congenital heart disease diagnoses and pediatric cardiac surgical procedures were derived using the International Classification of Diseases, 10th Edition, Clinical Modification (ICD-10-CM) and Procedure Coding System (ICD-10-PCS) respectively. The study included pediatric patients (< 21 years) undergoing cardiac surgery under cardiopulmonary bypass (CPB). Only the hospitals performing at least one of the ten STS benchmark procedures were included in the study. Patients were extracted from the database using the algorithm published by Allen et al. [19] Patients being transferred for the surgery from another hospital were extracted as a subgroup. Patients from the hospitals only performing ventricular septal defect as a surgery were excluded as well as those undergoing off-pump coarctation from thoracotomy. The off-pump cases e.g. delayed chest closures, explorations for bleeding, band adjustments, washouts for infections, and ECMO cannulation were excluded from the analysis. Patients undergoing thoracic organ transplantation or ventricular assist device placement were also excluded.
The West Virginia University Institutional Review Board approved the study with waiver of consent (Protocol #2210660362, approved 3/27/2023).
Statistical methods
Based on total annual number of CPB cases, hospitals were divided into three volume-groups: low, mid, and high-volume hospitals. This was done based on annual volume terciles. The baseline characteristics were compared using chi-square tests and one way analysis of variance (ANOVA) tests. Primary outcome measure was risk-adjusted all-cause inpatient mortality. The secondary measure was risk-adjusted incidence of post-operative extracorporeal membrane oxygenation (ECMO). Multi-level multivariable logistic regression was used for the transfer cohort and high complexity transfers subgroup (Risk Stratification for Congenital Heart Surgery (RACHS-2) category 3, 4, and 5 cases) to investigate the association of center volume and risk-adjusted inpatient mortality and post-operative ECMO. Using the same model, predicted risk-adjusted mortality was derived for each hospital in the study. Separate multi-level models were created for transferred patients to investigate impact of center volume on outcomes for each individual RACHS-2 category. A brief description of multilevel analysis is included in paragraph 1 of the supplementary appendix. Histograms were plotted for predicted inpatient mortality by hospital volume groups for all transfer patients, for high-complexity transfer cases and by each RACHS-2 category transfer cases.
Risk-adjustment
The algorithm for risk stratification for pediatric cardiac surgery by surgical procedure/complexity for administrative data published by Allen et al. was used to calculate the RACHS-2 score for all patients. [19] This algorithm has been verified for its accuracy and validated for the KID database. [20] RACHS-2 was added as an ordinal variable in the regression analysis. Hospital volume was included as an independent variable comparing low-volume centers directly to mid-volume and high-volume centers. Patient age, gender, race, elective status, genetic diagnosis, reoperation, and preoperative length of stay were added as covariables. These variables were chosen based on the previously published literature. [21–24] Please see supplemental table 1 for details of ICD-10 codes used to derive key covariables.
Missing data was handled by imputing the missing variables by the Multiple Imputation by Chained Equations (MICE) method. [25, 26] Regression models were investigated for lack of collinearity between covariables. A p-value of less than 0.05 was considered as a statistically significant value. All tests were two-sided. Statistical package R was used for statistical analysis. [27]
Results
Of the 25,749 children undergoing CPB, 3,511 (13.66%) patients were transferred to the hospital where they received surgery from another hospital. The center volume categories were distributed as follows: 113 low-volume hospitals performing ≤ 103 cases per year; 61 mid-volume hospitals performing 104–194 cases per year; and 31 high-volume hospitals performing > 194 cases per year. The median (interquartile range) number of transferred cases per hospital per year were 4.5 (2–9) for low-volume hospitals, 21 (11–31) for mid-volume hospital and 43 (34–61) for high-volume hospitals. The distribution of STS benchmark procedures by hospital volume groups is displayed in supplemental table 2. The distribution of cases by each hospital is shown in supplemental Fig. 1.
Two-thirds of the transferred patients were neonates. As expected, more than 90% of the procedures performed were urgent or emergent procedures. Low-volume centers had higher number of patients with RACHS-2 category 1 patients and lower number of RACHS-2 category 5 patients compared to mid and high-volume centers. (Table 1).
Table 1.
Baseline characteristics of the cohort of patients transferred in from another hospital.
| Variable | Total (3,511) | Low-volume (669) | Mid-volume (1,266) | High-volume (1,576) | p-value |
|---|---|---|---|---|---|
| Age | |||||
| Neonate | 2,476 (70.5%) | 457 (68.3%) | 929 (73.4%) | 1,090 (69.2%) | 0.023 |
| Infants | 829 (23.6%) | 166 (24.8%) | 262 (20.7%) | 401 (25.4%) | |
| > 1 year | 206 (5.9%) | 46 (6.9%) | 75 (5.9%) | 85 (5.4%) | |
| Female gender | 1,494 (42.6%) | 304 (45.4%) | 537 (42.4%) | 653 (41.4%) | 0.212 |
| Caucasian | 1,730 (49.3%) | 317 (47.4%) | 682 (53.9%) | 731 (46.4%) | < 0.001 |
| Low birth weight | 205 (5.8%) | 37 (5.5%) | 70 (5.5%) | 98 (6.2%) | 0.688 |
| Genetic diagnosis | 234 (6.7%) | 34 (5.1%) | 82 (6.5%) | 118 (7.5%) | 0.106 |
| Heterotaxy | 15 (0.4%) | 1 (0.1%) | 8 (0.6%) | 6 (0.4%) | 0.281 |
| Elective procedures | 197 (5.6%) | 41 (6.13%) | 50 (3.9%) | 106 (6.7%) | 0.005 |
| Non-cardiac congenital anomalies | 170 (4.8%) | 27 (4.0%) | 69 (5.5%) | 74 (4.7%) | 0.362 |
| RACHS-2 score | |||||
| 1 | 377 (10.7%) | 92 (13.8%) | 127 (10%) | 158 (10%) | <0.001 |
| 2 | 928 (26.4%) | 201 (30%) | 340 (26.9%) | 387 (24.6%) | |
| 3 | 543 (15.5%) | 117 (17.5%) | 188 (14.8%) | 238 (15.1%) | |
| 4 | 1,134 (32.3%) | 206 (30.8%) | 400 (31.6%) | 528 (33.5%) | |
| 5 | 529 (15.1%) | 53 (7.9%) | 211 (16.7%) | 265 (16.8%) | |
| Reoperation | 136 (3.9%) | 14 (2.1%) | 48 (3.8%) | 74 (4.7%) | 0.014 |
| Pre-operative length of stay | 9.26 (± 16.3) | 11.1 (± 20) | 9.6 (± 15.3) | 8.19 (± 15.2) | 0.001 |
The unadjusted mortality rate for the entire transfer cohort was 5.2%. The unadjusted inpatient mortality rates for low, mid and high-volume centers were 5.6%, 4.6% and 4.9% respectively. For direct admissions, these rates were 1.7%, 1.13% and 1.1% respectively.
Results for all transferred patients
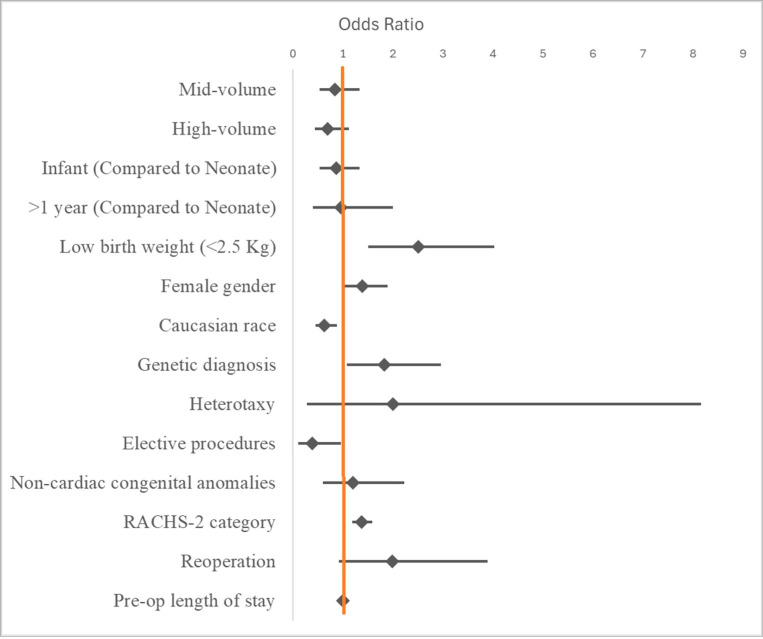
The risk-adjusted inpatient mortality rate for all transferred patients was 4.99%. The risk-adjusted inpatient mortality rates for low, mid and high-volume centers were 5.4%, 5.1% and 4.3% respectively. Following multilevel multivariable regression analysis for all transferred patients, there was no statistically significant difference in risk-adjusted inpatient mortality when comparing low-volume centers to mid-volume centers (OR = 0.85, 95% CI 0.54–1.33, p = 0.469) or to high-volume centers (OR = 0.7, 95% CI 0.45–1.12, p = 0.127). Factors associated with increased inpatient mortality included low birth weight, non-Caucasian race, presence of a genetic diagnosis, higher RACHS-2 score, emergent/urgent procedures and reoperation (Fig. 1, Table 2).
Figure 1.
Forest plot showing Odds ratios of variables included in the multivariable logistic regression model for inpatient mortality.
Table 2.
Results of multi-level logistic regression models for inpatient mortality as outcome for the entire cohort
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Hospital volume | ||
| Low-volume | 1 | (reference) |
| Mid-volume | 0.85 (0.54–1.34) | 0.483 |
| High-volume | 0.7 (0.45–1.12) | 0.127 |
| Age | ||
| Neonate | 1 | (reference) |
| Infant | 0.87 (0.54–1.34) | 0.535 |
| >1 year | 0.96 (0.4–2) | 0.913 |
| Low birth weight (< 2.5 kg) | 2.51 (1.51–4.03) | < 0.001 |
| Female gender | 1.39 (1.01–1.9) | 0.04 |
| Caucasian race | 0.63 (0.46–0.88) | 0.006 |
| Genetic diagnosis | 1.83 (1.08–2.96) | 0.019 |
| Heterotaxy | 2.01 (0.29–8.16) | 0.388 |
| Elective procedures | 0.39 (0.11–0.96) | 0.071 |
| Non-cardiac congenital anomalies | 1.21 (0.6–2.23) | 0.557 |
| RACHS-2 category | 1.38 (1.19–1.59) | 0.001 |
| Reoperation | 1.99 (0.93–3.89) | 0.056 |
| Pre-op length of stay | 1.01 (1–1.02) | 0.001 |
RACHS-2: Risk Adjustment for Congenital Heart Surgery
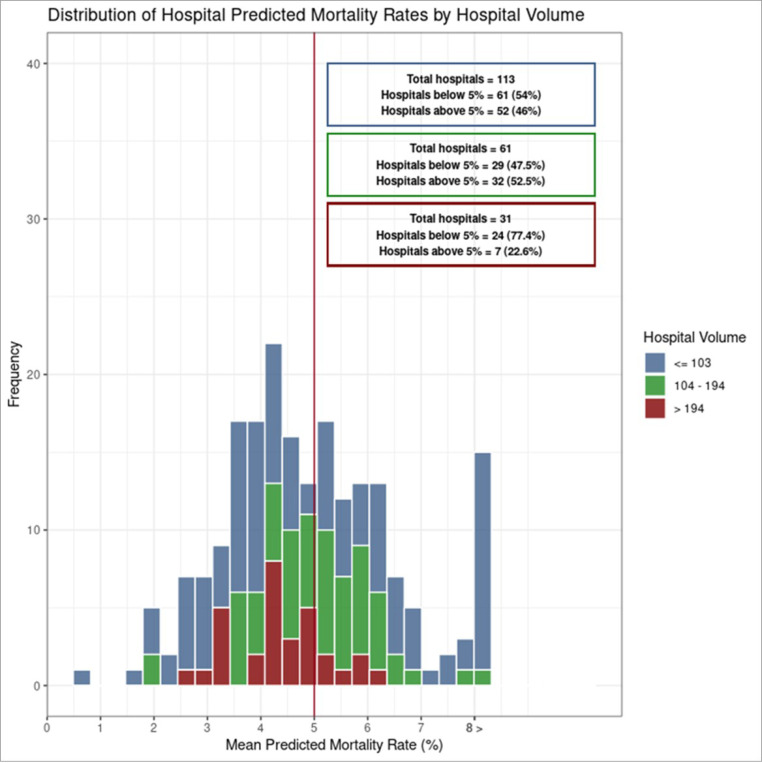
The distribution of risk-adjusted mortality was similar across all volume groups (Fig. 2), highlighting low-mortality and high-mortality hospitals in all three volume categories.
Figure 2.
Histogram of risk-adjusted inpatient mortality by hospital volume groups for the entire cohort
The incidence of ECMO was 3.2% within the transferred cohort. It was 4.0% for low-volume centers, 2.8% for mid-volume centers and 3.2% for high-volume centers. There was no statistically significant difference in the incidence of post-operative ECMO between low-volume centers and mid-volume centers (OR = 0.59, 95% CI 0.26–1.39, p = 0.218) and low-volume centers and high-volume centers (OR = 0.74, 95% CI 0.3–1.89, p = 0.502). Factors associated with increased incidence of post-operative ECMO were low birth weight and female gender (Table 3).
Table 3.
Results of multi-level logistic regression models for post-operative ECMO as outcome for the entire cohort
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Hospital volume | ||
| Low-volume | 1 | (reference) |
| Mid-volume | 0.66 (0.28–1.64) | 0.358 |
| High-volume | 0.8 (0.32–2.15) | 0.632 |
| Age | ||
| Neonate | 1 | (reference) |
| Infant | 0.49 (0.25–0.91) | 0.029 |
| >1 year | 0.53 (0.15–1.47) | 0.274 |
| Low birth weight (< 2.5 kg) | 1.87 (0.88–3.69) | 0.083 |
| Female gender | 1.34 (0.88–2.03) | 0.171 |
| Caucasian race | 0.75 (0.48–1.16) | 0.197 |
| Genetic diagnosis | 1.19 (0.53–2.4) | 0.64 |
| Heterotaxy | 10.83 (1.84–52.67) | 0.004 |
| Elective procedures | 0.95 (0.35–2.21) | 0.918 |
| Non-cardiac congenital anomalies | 1.3 (0.51–2.87) | 0.551 |
| RACHS-2 category | 1.25 (1.04–1.51) | 0.021 |
| Reoperation | 1.84 (0.65–4.44) | 0.205 |
| Pre-op length of stay | 1.01 (1–1.02) | 0.191 |
Results for RACHS-2 high-risk category transfer cohort
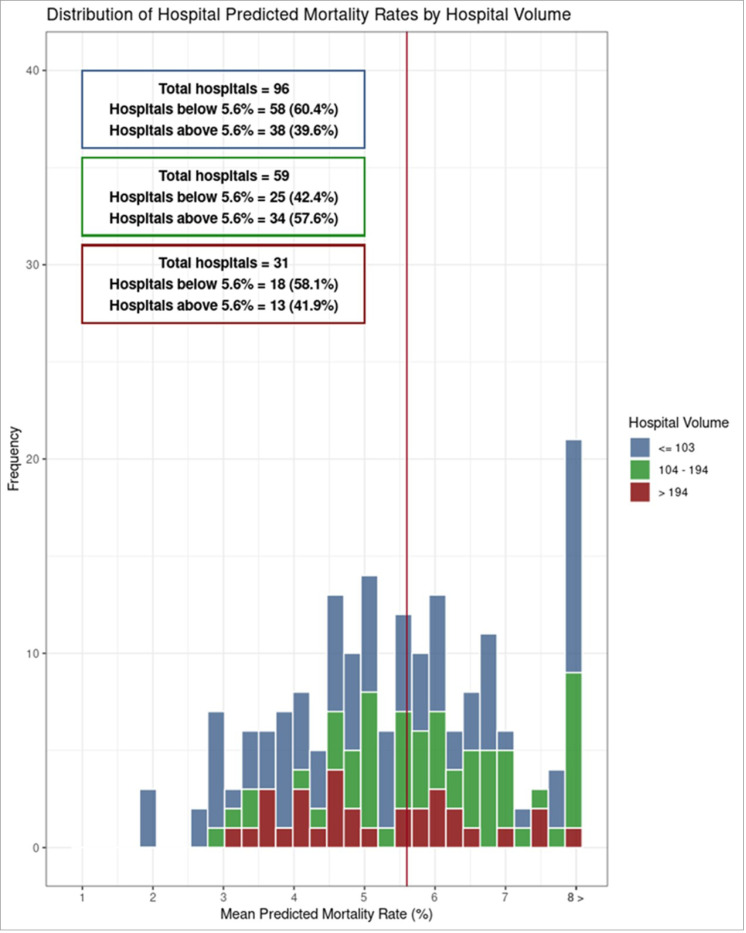
The risk-adjusted inpatient mortality rate for high-risk cohort was 5.6%. The risk-adjusted inpatient mortality rates for low, mid and high-volume centers were 5.5%, 6.1% and 5.2% respectively. There was no statistically significant difference in risk-adjusted inpatient mortality for high-complexity transfer cases (RACHS-2 category 3,4 and 5) between low-volume hospitals and mid-volume hospitals (OR = 1.06, 95% CI 0.62–1.85, p = 0.845) and high-volume hospitals (OR = 0.82, 95% CI 0.48–1.45, p = 0.482) (Supplemental table 3). Similarly, we observed low-mortality and high-mortality hospitals in all three hospital groups (Fig. 3).
Figure 3.
Histogram of risk-adjusted inpatient mortality by hospital volume groups for high-complexity cases (RACHS-2 categories 3, 4 and 5)
There was no statistically significant difference in incidence of post-operative ECMO between low-volume and mid-volume hospitals (OR = 0.51, 95% CI 0.21–1.24, p = 0.127) or high-volume hospitals (OR = 0.58, 95% CI 0.24–1.54, p = 0.243). (Supplemental table 4)
The results of multilevel multivariable logistic regression analyses for individual RACHS-2 categories also showed no statistically significant difference in inpatient mortality for transferred patients (Table 4).
Table 4.
Results of multilevel multivariable logistic regression models for risk-adjusted inpatient mortality by each RACHS-2 category
| RACHS-2 category | Hospital volume group | OR (95% CI) | p-value |
|---|---|---|---|
| 1 | Low-volume | 1 | (reference) |
| Mid-volume | 1.33 (0.44–4.44) | 0.624 | |
| High-volume | 0.94 (0.3–3.28) | 0.92 | |
| 2 | Low-volume | 1 | (reference) |
| Mid-volume | 0.4 (0.13–1.12) | 0.087 | |
| High-volume | 0.4 (0.14–1.08) | 0.072 | |
| 3 | Low-volume | 1 | (reference) |
| Mid-volume | 1.01 (0.27–4.22) | p = 0.986 | |
| High-volume | 2.28 (0.74–8.71) | p = 0.174 | |
| 4 | Low-volume | 1 | (reference) |
| Mid-volume | 1.41 (0.63–3.34) | p = 0.414 | |
| High-volume | 0.81 (0.36–1.98) | p = 0.628 | |
| 5 | Low-volume | 1 | (reference) |
| Mid-volume | 0.72 (0.3–1.78) | p = 0.461 | |
| High-volume | 0.43 (0.17–1.06) | p = 0.056 |
Histograms of the independent RACHS-2 categories showed that the hospital volume did not correlate with the risk-adjusted inpatient mortality with mid-volume hospitals having high mortality in RACHS-2 category 1 cases, low-volume hospitals having high mortality in RACHS-2 category 2 cases, and high-volume hospitals having high mortality in RACHS-2 category 3 cases (Supplemental Figs. 2–6).
Discussion
This study presents a risk-adjusted real-world analysis of patients transferred from one hospital to another for pediatric cardiac surgery with four main findings. First, the overall mortality of the cohort was approximately 5%, highlighting the high-risk nature of this cohort of patients. Second, after risk adjustment, there was no significant difference in inpatient mortality or incidence of ECMO based on hospital case volume. Third, when assessing the high-complexity subgroup (RACHS-2 categories 3–5) hospital case volume did not predict inpatient mortality in transfer cases. Finally, we highlight there are high performing low volume centers and low performing high volume centers when assessing risk-adjusted inpatient mortality by each RACHS-2 category.
Our analysis highlights the fact that hospital quality may be a more important determinant of inpatient mortality or incidence of post-operative ECMO than annual case volume in transferred patients undergoing pediatric heart surgery, even for high-complexity cases and hospital volume may not play a role in it. This is in contradistinction to recent expert consensus for centers performing pediatric cardiac surgery in the US that suggests transfers of neonates and patients requiring complex congenital cardiac surgery should only be to higher volume centers. [17] Access to care for patients with congenital heart disease remains a hurdle in the US, especially in socially disenfranchised populations. [28–30] If a high-quality low-volume center is closer to the patient and caregiver(s), they may not need to travel farther to a higher volume center. This may translate into increased access to care, increased compliance in post-operative follow-up and limited extended caregiver life disruption. This might be particularly beneficial, as they may be spared from having to take time away from their work, ensuring a stable income and normalcy in day-to-day life. In cases where the local center is within the same state as the patient/caregiver(s) residence and the larger center is outside the state, it may also have significant implications in insurance coverage.
These data demonstrate that there are also underperforming high volume centers, suggesting that transfer to larger programs does not uniformly guarantee superior outcomes. These findings align with previous work supporting the creation of a tiered system based solely on annual hospital volume may not necessarily be the correct sole arbiter of quality. 9,17 The incidence of mortality in these transferred patients was strikingly high at all recipient volume levels. Although a definite cause cannot be identified through our analysis, the plausible causes may include higher comorbidities, possible errors and delays in diagnosis or management, and a higher possibility of complications at the initial hospital encounter. Most of the transferred patients were neonates, and perhaps some may not have received standard prenatal care, their diagnosis was possibly delayed, or they presented to a hospital without resources to manage complex congenital heart disease.
Our subgroup analysis of hospital volume and inpatient mortality by each RACHS-2 category showed that in each category, there was significant variation and inconsistency without a clear association between higher annual case volume and improved mortality. For the entire cohort and for high-complexity cases, there existed high-mortality and low-mortality hospitals in every volume group. This reveals that higher hospital volume does not guarantee a better result after pediatric cardiac surgery, even when considering the complexity of the heart lesion or the technique of surgery.
There are several important limitations to this analysis including the retrospective nature of the study which precludes demonstration of causality. The administrative data may be subject to errors during coding; however, KID shares a robust quality control audit process with the HCUP family of databases. Regression models are limited to the data available in the database and therefore lack some key variables including surgeon experience, time on cardiopulmonary bypass and other comorbidities that could not be captured by the database. The KID database does not have the information on whether the referring hospital had a congenital heart surgery service or not. Even though in a real-world scenario, majority of the transfers are from hospitals without the surgical services, there might be some patients transferred from a hospital with surgical services for higher level of care. Our hierarchal models and multilevel analysis correct this bias to a certain extent, however without the information about referent hospital origin, it cannot be removed completely. Finally, although our analysis had sufficient statistical power to identify difference in in-patient mortality for the cohorts we reported, results for individual complex operations could not be analyzed due to their small sample size.
Conclusions
Pediatric patients transferred from one hospital to another for \ cardiac surgery are of higher risk, regardless of the volume of the receiving institution. Hospital quality may be a more important metric than hospital volume to predict inpatient mortality or incidence of post-operative ECMO. Volume of the reference hospital alone should not be considered as the sole decision-making metric to refer patients for congenital heart surgery.
Supplementary Material
Acknowledgement
The authors thank Ricardo Pietrobon, MD, PhD, Aline Machiavelli, MS, and Livia Eslabao, MS, for their statistical support. The development of the RACHS-2 code used in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH), under award number R01 HL150044 (principal investigator: Anderson) and supported by the New York Congenital Heart Surgery-Collaborative for Longitudinal Outcomes and Utilization of Resources (CHS-COLOUR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or of CHS-COLOUR.
Footnotes
Declarations
Additional Declarations: No competing interests reported.
Contributor Information
Dhaval Chauhan, West Virginia University.
J. Hunter Mehaffey, West Virginia University.
J. W. Awori Hayanga, West Virginia University.
Pieter Alex Verhoeven, West Virginia University.
Margaret Mathewson, West Virginia University.
Veronica Godsey, West Virginia University.
Alyssa Fazi, West Virginia University.
Jai P. Udassi, West Virginia University
Vinay Badhwar, West Virginia University.
Christopher E. Mascio, West Virginia University
Data Availability
The KID database is available through Healthcare Cost & Utilization Project (HCUP) website. Citation: KID Database Documentation. Healthcare Cost and Utilization Project (HCUP). March 2024. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/db/nation/kid/kiddbdocumentation.jsp
References
- 1.Kansy A, Zu Eulenburg C, Sarris G, Jacobs JP, Fragata J, Tobota Z, Ebels T, Maruszewski B (2018) Higher Programmatic Volume in Neonatal Heart Surgery Is Associated With Lower Early Mortality. Ann Thorac Surg 105:1436–1440 [DOI] [PubMed] [Google Scholar]
- 2.Pasquali SK, Li JS, Burstein DS, Sheng S, O’Brien SM, Jacobs ML, Jaquiss RD, Peterson ED, Gaynor JW, Jacobs JP (2012) Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics 129:e370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinocur JM, Menk JS, Connett J, Moller JH, Kochilas LK (2013) Surgical volume and center effects on early mortality after pediatric cardiac surgery: 25-year North American experience from a multi-institutional registry. Pediatr Cardiol 34:1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welke KF, Karamlou T, O’Brien SM, Dearani JA, Tweddell JS, Kumar SR, Romano JC, Backer CL, Pasquali SK (2023) Contemporary Relationship Between Hospital Volume and Outcomes in Congenital Heart Surgery. Ann Thorac Surg 116:1233–1239 [DOI] [PubMed] [Google Scholar]
- 5.Welke KF, O’Brien SM, Peterson ED, Ungerleider RM, Jacobs ML, Jacobs JP (2009) The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg 137:1133–1140 [DOI] [PubMed] [Google Scholar]
- 6.Williamson CG, Tran Z, Kim ST, Hadaya J, Biniwale R, Benharash P (2022) Center Volume Impacts Readmissions and Mortality after Congenital Cardiac Surgery. J Pediatr 240:129–135 e122 [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura N, Hirata Y, Inuzuka R, Tachimori H, Hirano A, Sakurai T, Shiraishi S, Matsui H, Ayusawa M, Nakano T, Kasahara S, Hiramatsu Y, Yamagishi M, Miyata H, Yamagishi H, Sakamoto K (2023) Effect of procedural volume on the outcomes of congenital heart surgery in Japan. J Thorac Cardiovasc Surg 165:1541–1550 e1543 [DOI] [PubMed] [Google Scholar]
- 8.Anagnostopoulos PV, Cartmill RS, Yang Q, Schumacher JR, Fernandes-Taylor S, Hermsen JL, DeCamp MM (2023) Systems of Care Factors Should Be Considered in Regionalization of Congenital Cardiac Surgery. Ann Thorac Surg 116:517–523 [DOI] [PubMed] [Google Scholar]
- 9.Chauhan D, Mehaffey JH, Hayanga JWA, Udassi JP, Badhwar V, Mascio CE (2024) Volume Alone Does Not Predict Quality Outcomes in Hospitals Performing Pediatric Cardiac Surgery. Ann Thorac Surg [DOI] [PubMed] [Google Scholar]
- 10.Brown KL, Crowe S, Franklin R, McLean A, Cunningham D, Barron D, Tsang V, Pagel C, Utley M (2015) Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2:e000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JP, Mayer JE Jr., Mavroudis C, O’Brien SM, Austin EH 3rd, Pasquali SK, Hill KD, He X, Overman DM, St Louis JD, Karamlou T, Pizarro C, Hirsch-Romano JC, McDonald D, Han JM, Dokholyan RS, Tchervenkov CI, Lacour-Gayet F, Backer CL, Fraser CD, Tweddell JS, Elliott MJ, Walters H 3rd, Jonas RA, Prager RL, Shahian DM, Jacobs ML (2016) The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2016 Update on Outcomes and Quality. Ann Thorac Surg 101:850–862 [DOI] [PubMed] [Google Scholar]
- 12.Jeffries HE, Soto-Campos G, Katch A, Gall C, Rice TB, Wetzel R (2015) Pediatric Index of Cardiac Surgical Intensive Care Mortality Risk Score for Pediatric Cardiac Critical Care. Pediatr Crit Care Med 16:846–852 [DOI] [PubMed] [Google Scholar]
- 13.Kumar SR, Gaynor JW, Jones LA, Krohn C, Mayer JE Jr., Nathan M, O’Brien JE Jr, Pizarro, Wellnitz C, Nelson JS(2023) The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2022 Update on Outcomes and Research. Ann Thorac Surg 115:807–819 [DOI] [PubMed] [Google Scholar]
- 14.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH (2009) An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 138:1139–1153 [DOI] [PubMed] [Google Scholar]
- 15.O’Brien SM, Jacobs JP, Pasquali SK, Gaynor JW, Karamlou T, Welke KF, Filardo G, Han JM, Kim S, Shahian DM, Jacobs ML (2015) The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 1-Statistical Methodology. Ann Thorac Surg 100:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L (2018) Trends in Long-Term Mortality After Congenital Heart Surgery. J Am Coll Cardiol 71:2434–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer CL, Overman DM, Dearani JA, Romano JC, Tweddell JS, Kumar SR, Marino BS, Bacha EA, Jaquiss RDB, Zaidi AN, Gurvitz M, Costello JM, Pierick TA, Ravekes WJ, Reagor JA, St Louis JD, Spaeth J, Mahle WT, Shin AY, Lopez KN, Karamlou T, Welke KF, Bryant R, Husain SA, Chen JM, Kaza A, Wells WJ, Glatz AC, Cohen MI, McElhinney DB, Parra DA, Pasquali SK (2023) Recommendations for centers performing pediatric heart surgery in the United States. J Thorac Cardiovasc Surg 166:1782–1820 [DOI] [PubMed] [Google Scholar]
- 18.Kids’ Inpatient Database (KID) Database Documentation. https://hcup-us.ahrq.gov/db/nation/kid/kiddbdocumentation.jsp [Google Scholar]
- 19.Allen P, Zafar F, Mi J, Crook S, Woo J, Jayaram N, Bryant R 3rd, Karamlou T, Tweddell J, Dragan K, Cook S, Hannan EL, Newburger JW, Bacha EA, Vincent R, Nguyen K, Walsh-Spoonhower K, Mosca R, Devejian N, Kamenir SA, Alfieris GM, Swartz MF, Meyer D, Paul EA, Billings J, Anderson BR, New York State C-C (2022) Risk Stratification for Congenital Heart Surgery for ICD-10 Administrative Data (RACHS-2). J Am Coll Cardiol 79:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaram N, Allen P, Hall M, Karamlou T, Woo J, Crook S, Anderson BR (2023) Adjusting for Congenital Heart Surgery Risk Using Administrative Data. J Am Coll Cardiol 82:2212–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal HS, Wolfram KB, Saville BR, Donahue BS, Bichell DP (2014) Postoperative complications and association with outcomes in pediatric cardiac surgery. J Thorac Cardiovasc Surg 148:609–616 e601 [DOI] [PubMed] [Google Scholar]
- 22.Chang RK, Rodriguez S, Lee M, Klitzner TS (2006) Risk factors for deaths occurring within 30 days and 1 year after hospital discharge for cardiac surgery among pediatric patients. Am Heart J 152:386–393 [DOI] [PubMed] [Google Scholar]
- 23.Kang N, Cole T, Tsang V, Elliott M, de Leval M (2004) Risk stratification in paediatric open-heart surgery. Eur J Cardiothorac Surg 26:3–11 [DOI] [PubMed] [Google Scholar]
- 24.Karamlou T, Jacobs ML, Pasquali S, He X, Hill K, O’Brien S, McMullan DM, Jacobs JP (2014) Surgeon and center volume influence on outcomes after arterial switch operation: analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg 98:904–911 [DOI] [PubMed] [Google Scholar]
- 25.Van Buuren S, Groothuis-Oudshoorn K (2011) mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 45(3):1–67. 10.18637/jss.v045.i03.: [DOI] [Google Scholar]
- 26.Liu Y, De A (2015) Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study. Int J Stat Med Res 4:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [Google Scholar]
- 28.Insaf TZ, Sommerhalter KM, Jaff TA, Farr SL, Downing KF, Zaidi AN, Lui GK, Van Zutphen AR (2021) Access to cardiac surgery centers for cardiac and non-cardiac hospitalizations in adolescents and adults with congenital heart defects-a descriptive case series study. Am Heart J 236:22–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommerhalter KM, Insaf TZ, Akkaya-Hocagil T, McGarry CE, Farr SL, Downing KF, Lui GK, Zaidi AN, Van Zutphen AR (2017) Proximity to Pediatric Cardiac Surgical Care among Adolescents with Congenital Heart Defects in 11 New York Counties. Birth Defects Res 109:1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wamala I, Gongwer R, Doherty-Schmeck K, Jorina M, Betzner A, Zheleva B, Gauvreau K, Baird CW, Jenkins K (2021) Infrastructure Availability for the Care of Congenital Heart Disease Patients and Its Influence on Case Volume, Complexity and Access Among Healthcare Institutions in 17 Middle-Income Countries. Glob Heart 16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The KID database is available through Healthcare Cost & Utilization Project (HCUP) website. Citation: KID Database Documentation. Healthcare Cost and Utilization Project (HCUP). March 2024. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/db/nation/kid/kiddbdocumentation.jsp