Abstract
We developed an enzyme-linked immunosorbent assay-based assay to assess Haemophilus ducreyi binding to extracellular matrix (ECM) proteins. H. ducreyi 35000HP bound to fibronectin, laminin, and type I and III collagen but not to type IV, V, or VI collagen or elastin. Isogenic strains with mutations in ftpA or losB bound as well as the parent, suggesting that neither pili nor full-length lipooligosaccharide is required for H. ducreyi to bind to ECM proteins.
Haemophilus ducreyi is the causative agent of chancroid, a sexually transmitted genital ulcer disease that facilitates the transmission of human immunodeficiency virus (17). Initiation of H. ducreyi infection requires trauma to the skin (13), indicating that adherence targets may include subsurface skin components, such as the extracellular matrix (ECM). Many bacterial pathogens adhere to ECM proteins via surface structures such as fimbriae and lipopolysaccharide (LPS), as well as afimbrial surface proteins (9, 16, 18). To characterize the binding of H. ducreyi to ECM proteins, we used an enzyme-linked immunosorbent assay (ELISA)-based assay with ECM proteins found in adult human skin, including fibronectin, laminin, several collagens, and elastin. We chose H. ducreyi 35000HP for these studies. Strain 35000HP is a human-passaged variant of 35000 and is fully virulent in the human challenge model of chancroid (2). We also examined the roles of two H. ducreyi surface structures, the fine tangled pili and lipooligosaccharide (LOS), in ECM adherence.
ECM protein binding assay.
Microtiter plates (Immulon 4 HBX; Dynex Technologies, Chantilly, Va.) were coated with ECM proteins by incubating 100 μl of the appropriate protein, diluted in coating buffer (0.25 M NaHCO3, 0.25 M Na2CO3 [pH 9.6]) to 20 μg of protein per ml or the concentrations indicated below, in assay wells overnight at 4°C (for collagens) or 35°C (for fibronectin and laminin) (8, 12). Wells were then washed three times with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 0.69 mM KH2PO4, 6.4 mM Na2HPO4 [pH 7.5]) containing 0.05% Tween 20 (PBST), blocked with 300 μl of 10% fetal calf serum in PBST for 2 h at 35°C, and washed three times in PBS. H. ducreyi 35000HP (2) was grown in broth with fetal calf serum to mid-log phase as described previously (6), harvested by centrifugation, and washed and suspended in PBS to the desired optical density at a wavelength of 660 nm (OD660). Staphylococcus aureus Phillips and PH100 (gift of Joseph Patti) (10) were grown overnight on Luria-Bertani agar (11), harvested, washed, and suspended in PBS. PH100 was supplemented with gentamicin (10 μg/ml). Bacteria were added to each well, and the plates were incubated at 35°C in 5% CO2. For bacterial dose-response assays, bacteria were serially diluted twofold in PBS and tested at concentrations ranging from an OD660 of 0.8 to 0.003. For ECM dose-response assays, bacteria were added to all wells at an OD660 of 0.2.
After 4 h, wells were washed three times with PBST to remove unbound bacteria. Bound H. ducreyi was detected by incubation overnight with a 1:5,000 dilution of rabbit antiserum against whole H. ducreyi cells (6), followed by incubation for 2 to 3 h with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.). Bound S. aureus was detected with a 1:2,000 dilution of polyclonal rabbit antiserum against whole S. aureus cells (supplied by Lech Switalski). ELISA plates were incubated with horseradish peroxidase substrate (SIGMAFAST tablets; Sigma Chemicals, St. Louis, Mo.) for 1 h, and the absorbance at a wavelength of 450 nm (A450) was measured for each well. Signals of bound bacteria were calculated as follows: the average A450 of triplicate test wells − the average A450 of triplicate control wells. For each bacterial dose-response assay, control wells were coated with ECM proteins but received no bacteria; for each protein dose-response assay, control wells received no protein but were incubated with bacteria. To control for bacteria binding nonspecifically to the plates or to protein, assays were performed in tandem with wells coated with bovine serum albumin (BSA) or the highly glycosylated serum protein fetuin at the same concentration as the ECM proteins.
Deposition of ECM proteins and detection of ECM-bound bacteria.
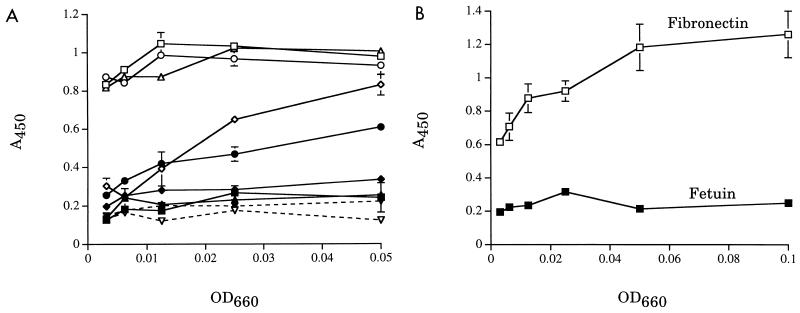
To confirm that ECM proteins had bound to the ELISA plates, we probed wells coated with fibronectin, fetuin, and type I, III, IV, V, and VI collagen (all of human origin; Southern Biotechnology Associates, Inc., Birmingham, Ala., or Sigma Chemicals) with S. aureus Phillips and its isogenic cna mutant PH100. Phillips, known to bind to type I, II, and III collagen (4, 5), bound to all proteins tested except type VI collagen (Fig. 1A) and fetuin (Fig. 1B). This binding was dependent on the amount of ECM protein used to coat the wells (data not shown). PH100, in which the S. aureus collagen adhesin gene, cna, is insertionally inactivated (10), showed greatly reduced binding to the panel of collagens (Fig. 1A). These data indicated that the plates were coated with the ECM proteins and that the assay was able to distinguish between strains on the basis of their ability to bind to ECM proteins.
FIG. 1.
S. aureus strains binding to ECM proteins. Error bars indicate standard deviations of triplicate wells. Data shown are representative of at least three independent assays. (A) S. aureus Phillips (open symbols) and PH100 (closed symbols) binding to collagens. Symbols represent binding to type I (squares), type III (circles), type IV (triangles), type V (diamonds), and type VI (inverted triangles) collagen. (B) S. aureus Phillips binding to fibronectin and fetuin.
S. aureus did not bind to laminin (from Engelbreth-Holm-Swarm mouse sarcoma; Sigma Chemicals), type VI collagen, or elastin (bovine, oxalic acid solubilized; Elastin Products Company, Owensville, Mo.) (data not shown). To confirm deposition of these proteins, we probed ELISA plates coated with serial dilutions of each protein or BSA (as a negative control) with antibodies specific for each ECM protein (Sigma Chemicals or Rockland, Inc., Gilbertville, Pa.). Signals that were dependent on the concentration of coated laminin, type VI collagen, or elastin were obtained (data not shown).
H. ducreyi binds to specific ECM proteins.
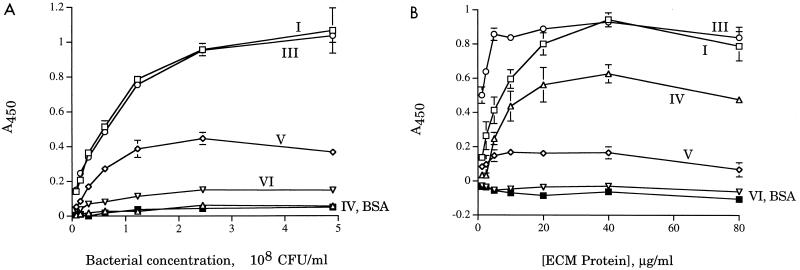
Utilizing the ECM adherence assay, we examined the ability of H. ducreyi 35000HP to bind to human type I, III, IV, V, and VI collagen, with BSA as a negative control. Strain 35000HP bound efficiently to type I and III collagen (Fig. 2). This binding varied with the amount of bacteria (Fig. 2A) and with the amount of collagen (Fig. 2B) used in the assay. Levels of binding to type IV, V, and VI collagen varied somewhat, but they were usually in the range of levels of binding to BSA and were consistently lower than levels of binding to type I and III collagen (Fig. 2).
FIG. 2.
H. ducreyi 35000HP binding to collagens. Error bars indicate standard deviations of triplicate wells. Data shown are representative of at least three independent assays. (A) Bacterial dose-response assays; (B) protein dose-response assays.
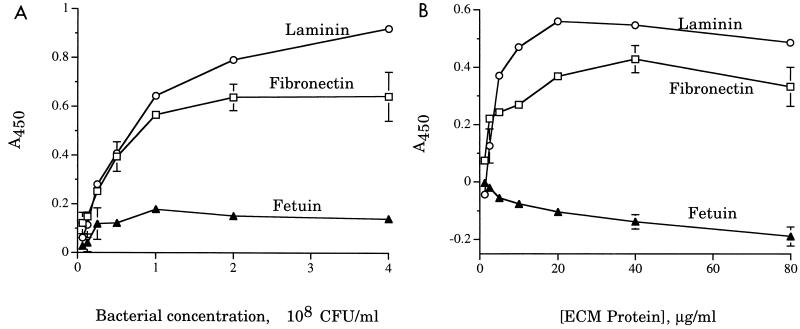
We next tested the ability of 35000HP to bind to fibronectin and laminin, using fetuin as a negative control. Strain 35000HP bound to both fibronectin and laminin in a dose-dependent fashion, while binding to fetuin was uniformly low, regardless of the amount of bacteria or fetuin in the assay (Fig. 3). We also tested 35000HP for adherence to elastin and observed no binding (data not shown).
FIG. 3.
H. ducreyi 35000HP binding to ECM proteins. Error bars indicate standard deviations of triplicate wells. Data shown are representative of at least three independent assays. (A) Bacterial dose-response assays; (B) protein dose-response assays.
We tested several parameters in optimizing the assay for H. ducreyi. Protein dose-response assays showed that coating wells with 2 μg of ECM glycoprotein per well was sufficient for maximal binding signals (Fig. 2B and 3B). We also tested 35000HP binding to each ECM protein over time, with incubation periods ranging from 1 to 8 h. Binding signals peaked at 4 h and were not enhanced by further incubation (data not shown). We compared ECM binding signals of broth-grown cells, harvested at mid-logarithmic, late logarithmic, and stationary phases, with those of cells grown overnight on agar plates. While the results were qualitatively identical, broth-grown cells yielded much higher overall signal levels than plate-grown cells (data not shown). No differences were observed among the differentially harvested broth-grown cells, indicating that the adhesin(s) responsible appears to be constitutively expressed under the growth conditions tested. Mid-log phase, broth-grown cells were used for all subsequent assays.
In a previous study by Abeck et al., a panel of H. ducreyi strains was tested for the ability to agglutinate latex beads coated with fibronectin, type III collagen, or laminin (1). The researchers reported that a single concentration of H. ducreyi agglutinated beads coated with a single concentration of each of these proteins but not with ovalbumin. There was little difference among the strains tested. However, H. ducreyi forms tight intercellular junctions that cause autoaggregation, which could affect the interpretation of the results of a particle agglutination assay. The ELISA format provides a more objective readout than the agglutination assay and allows for processing many samples at once, thus permitting multiple strains and ECM proteins to be tested concurrently in dose-response assays.
The observed pattern of H. ducreyi 35000HP binding to collagens (Fig. 2) indicates some specificity for H. ducreyi adherence to type I and III collagen. Strain 35000HP also bound to fibronectin and laminin but not elastin. In this assay, we detected binding only to immobilized ECM proteins, so we cannot exclude the possibility that H. ducreyi binds to type IV, V, or VI collagen or elastin either in solution or in vivo.
ECM binding is not mediated by FtpA.
H. ducreyi expresses a fine tangled pilus, the major subunit of which is FtpA (3). Pili of several bacterial species have been shown to bind specifically to ECM proteins (7, 15, 19), and one report indicated a correlation between piliation of H. ducreyi and binding to laminin (1). To examine the role of the H. ducreyi pilus in ECM binding, we tested the ability of 35000HP-SMS1 [an isogenic mutant of 35000HP in which ftpA, which encodes the major subunit of the fine tangled pili, is insertionally inactivated by mTn3(Cm)] to bind to fibronectin, laminin, and type I and III collagen. Strain 35000HP-SMS1 bound as well as 35000HP to each of these proteins (data not shown). Neither strain bound to type IV collagen or fetuin. The kinetics of ECM binding were also unaffected by the mutation in ftpA (data not shown). These data indicate that FtpA is not required for adherence to ECM proteins, although we cannot rule out a contributory role for FtpA in conjunction with other adhesins. These data confirm those of Brentjens et al., who reported that both strain 35000 and an isogenic ftpA derivative bind to laminin (3), and contradict those of Abeck et al., who reported that strain 35000 does not bind to laminin and that piliation is required for laminin binding (1). These discrepancies could be due to differences in the assays or in the strains tested.
Full-length LOS is not required for ECM binding.
Another surface structure that can mediate ECM binding is LPS. Helicobacter pylori LPS binds specifically to laminin (16). This activity is most likely mediated by the core sugars, although the specific saccharides involved are unknown. We examined the role of full-length H. ducreyi LOS in ECM binding by testing 35000HP-RSM2 (gift of Robert S. Munson, Jr.), an isogenic losB mutant of 35000HP, for binding to the panel of ECM proteins. The major LOS saccharide chain produced by 35000HP-RSM2 consists of a single glucose attached to a heptose trisaccharide core and 2-keto-deoxyoctulosonic acid and cannot be sialylated. The mutation in losB in 35000HP-RSM2 had no effect on binding to collagens, fibronectin, or laminin (data not shown). Thus, neither the terminal oligosaccharide nor sialylation is required for the observed ECM binding by H. ducreyi. However, these data do not rule out a role for the remaining core sugars expressed in the LOS moiety of the losB mutant.
In summary, we demonstrated that H. ducreyi 35000HP binds to fibronectin, laminin, and type I and III collagen in a dose-dependent manner. These ECM proteins are found throughout the skin and may serve as attachment and colonization sites for H. ducreyi in infection. These results suggest that H. ducreyi may express multiple ECM-binding adhesins. Alternatively, H. ducreyi may express a multifunctional adhesin, such as YadA of Yersinia enterocolitica (14), in which different domains bind to different ECM proteins. The observed binding does not require FtpA or full-length LOS. Work to identify a specific ECM-binding adhesin(s) in H. ducreyi is currently under way.
Acknowledgments
We thank Steven Clegg and Tricia Sebghati for technical advice and prepublication sharing of protocols. We thank Joseph Patti for supplying the S. aureus strains, Robert S. Munson, Jr., for supplying 35000HP-RSM2, and Lech Switalski for supplying S. aureus-specific antiserum. We thank Byron Batteiger for helpful critique of the manuscript.
This work was supported by Public Health Service grant AI27863 from the National Institute of Allergy and Infectious Diseases. M.E.B. was supported by Public Health Service grant AI09971 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Abeck D, Johnson A P, Mensing H. Binding of Haemophilus ducreyi to extracellular matrix proteins. Microb Pathog. 1992;13:81–84. doi: 10.1016/0882-4010(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 3.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hienz S A, Palma M, Flock J-I. Insertional inactivation of the gene for collagen-binding protein has a pleiotropic effect on the phenotype of Staphylococcus aureus. J Bacteriol. 1996;178:5327–5329. doi: 10.1128/jb.178.17.5327-5329.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hienz S A, Schennings T, Heimdahl A, Flock J. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis. 1996;174:83–88. doi: 10.1093/infdis/174.1.83. [DOI] [PubMed] [Google Scholar]
- 6.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 7.Kukkonen M, Raunio T, Virkola R, Lahteenmaki K, Makela P H, Klemm P, Clegg S, Korhonen T K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type 1 fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993;7:229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 8.Ljungh A, Wadstrom T. Binding of extracellular matrix proteins by microbes. Methods Enzymol. 1995;253:501–514. doi: 10.1016/s0076-6879(95)53041-x. [DOI] [PubMed] [Google Scholar]
- 9.Ljungh A, Wadstrom T. Interactions of bacterial adhesions with the extracellular matrix. In: Kahane I, Ofek I, editors. Toward anti-adhesion therapy for microbial diseases. New York, N.Y: Plenum Press; 1996. pp. 129–140. [Google Scholar]
- 10.Patti J M, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, Höök M. The Staphylococcus aureus collagen adhesion is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 12.Sebghati T A S, Korhonen T K, Hornick D B, Clegg S. Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect Immun. 1998;66:2887–2894. doi: 10.1128/iai.66.6.2887-2894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 14.Tamm A, Tarkkanen A M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 15.Tarkkanen A M, Allen B L, Westerlund B, Holthofer H, Kuusela P, Risteli L, Clegg S, Korhonen T K. Type V collagen as the target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990;4:1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 16.Valkonen K H, Wadström T, Moran A P. Interaction of lipopolysaccharides of Helicobacter pylori with basement membrane protein laminin. Infect Immun. 1994;62:3640–3648. doi: 10.1128/iai.62.9.3640-3648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasserheit J N. Epidemiological synergy. Interrelationship between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 18.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 19.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen T K. The 075X adhesion of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]