Abstract
Background
Immunotherapy has demonstrated limited activity in prostate cancer to date. This likely reflects an immune suppressive tumor microenvironment (TME), with previous studies suggesting low PD-L1 expression and a sparse immune cell infiltrate. We aimed to further characterise the immune TME in primary prostate cancer and correlate immune subset densities with clinical outcomes.
Methods
Two distinct cohorts of patients treated with radical prostatectomy were identified, based on the development of biochemical recurrence (BCR), one subgroup with high International Society of Urological Pathologists (ISUP) grade group, recurrent disease and a second with low grade, non-recurrent disease. A prostate immunohistochemical (IHC) antibody cocktail was used to differentiate tumor and peritumoral benign tissue. Specific CD8+, CD4+, FoxP3+, CD20+ and CD68+ cell subsets were identified using IHC staining of consecutive slides. PD-L1 and CD8/PD-L1 dual staining were also performed. Cell subset densities were quantified within tumor and peritumoral regions. We used descriptive statistics to report cell subset densities and T-tests to compare groups by age, grade and the development of BCR. Univariable and multivariable logistic regression were used to analyse risk factors for BCR and the development of metastatic disease.
Results
A total of 175 patients were included, with a median age of 63 years and median pre-operative PSA of 8.2ng/ml. BCR occurred in 115 patients (66%) and 56 (32%) developed metastatic disease. CD68+ cells were the most abundant (median 648.8/mm2 intratumoral, 247.6/mm2 peritumoral), while PD-L1+ and PD-L1/CD8+ cell density was low overall (PD-L1+ median 162.4/mm2 intratumoral, 141.7/mm2 peritumoral; PD-L1/CD8+ (median 5.52/mm2 intratumoral, 3.41/mm2 peritumoral). Overall, grade group and T-stage were independently associated with BCR and metastatic disease. Higher density of peritumoral PD-L1+ cells was an independent risk factor for BCR (OR 5.33, 95%CI 1.31–21.61, p = 0.019).Although higher densities of CD8+ and CD4+ cells were observed in higher grade group 3–5 tumors, these were not associated with the development of BCR or metastasis.
Conclusions
In our cohort of prostate cancer patients who underwent radical prostatectomy, higher grade group and T-stage were independent predictors of BCR and metastasis. Despite higher grade group being associated with higher CD8+ cell density, PD-L1+ and PD-L1/CD8+ cell densities were low overall, suggesting lower T cell receptor recognition of tumor antigens. Further understanding of this phenomenon would influence development of future immunotherapeutic strategies in prostate cancer.
1. Introduction
Prostate cancer is the second-most common cancer among men, with over 1.1 million new cases worldwide each year [1]. Although often considered an indolent cancer, there are over 300,000 deaths from prostate cancer each year, the third highest cause of cancer death [1]. Despite the recent development of multiple life-prolonging therapeutic options for metastatic disease, treatment responses are rarely durable, and the disease remains incurable. Immune cell activation through inhibition of cell surface checkpoint proteins such as PD-1, PD-L1 or CTLA-4 have demonstrated durable responses in several tumor types [2–5]. However, only modest activity has been demonstrated in prostate cancer and durable responses are rare and limited to certain subtypes, such as those with deficient mismatch repair or high tumor PD-L1 levels [6, 7].
Unique characteristics within the prostate cancer tumor microenvironment (TME) are likely to underlie the modest activity of immunotherapy. Whilst some studies have demonstrated a sparse immune cell infiltrate within prostate cancer [8, 9], other studies have demonstrated an inflammatory TME with high proportions of macrophages and T cells within the prostate cancer stroma [10], although a ‘cold’ phenotype with low levels of activation has been observed [11]. Data from The Cancer Genome Atlas described 6 distinct immune subtypes, with prostate cancer being described as inflammatory (C3) defined by increased expression of Th17 and Th1 genes [12].
While higher densities of CD8+ T cells are typically associated with a favourable prognosis in other tumor types, higher densities of both CD8+ and CD4+ cells have been shown to be poor prognostic factors in prostate cancer [8, 13–16]. The interaction between immune cells located within the tumor and those within the surrounding peritumoral benign tissue can also influence cancer progression [17]. In prostate cancer, CD8+, CD4+ and FoxP3+ cells have been shown to be more common in malignant regions compared to benign regions [18]. The PD-1/PD-L1 immune checkpoint axis also plays an important role in cancer pathogenesis by regulating cytotoxic T-cell effector responses. It is a hallmark of T cell exhaustion and leads to immune escape [19, 20]. In advanced prostate cancer, studies have observed low levels of tumor cell PD-L1 expression [21]. This possibly contributes to the limited efficacy observed with checkpoint inhibition in prostate cancer, although other factors within the immune TME are also likely to play a role.
Therefore, greater understanding of the prostate TME will help us to better understand the modest activity of immune checkpoint inhibitors and also guide future development of immunotherapeutic strategies in prostate cancer.
Our study aimed to assess the density of selected immune cell subsets present in both intratumoral and peritumoral benign (non-tumor) regions of primary prostate cancer specimens, and to examine the association of immune cell subsets with biochemical recurrence (BCR) and the development of metastatic disease following radical prostatectomy.
2. Materials and methods
2.1 Cohort selection
Patients with invasive prostate cancer who underwent radical prostatectomy were identified from a tumor registry at two sites in Melbourne, Australia. Patients within this registry had confirmed written informed consent to the Urological Biorepository Protocol, which has been approved by the Royal Melbourne Hospital Human Research and Ethics committee (HREC/14/MH/342 approved 3rd February 2015). Two cohorts were selected based on the development of BCR, including one subgroup with high International Society of Urological Pathologists (ISUP) grade group (GG), recurrent disease and a second with low-grade, non-recurrent disease. Clinico-pathological information and outcome data were recorded, including age, pre-operative PSA level, T-stage, ISUP GG, time to BCR and time to the development of metastatic disease, if applicable. Patients were recruited between 15th February 2015 and 1st June 2019. Clinical data were accessed by authorised study staff only between 1st June 2019 and 24th December 2019 through review of medical records and data were recorded in a de-identified manner.
2.2 Immunohistochemistry
Archival formalin fixed paraffin embedded (FFPE) prostatectomy specimens were obtained from TissuPath Specialist Pathologists Laboratory, Mount Waverley, Victoria, Australia. Tissue sections of 4μm thickness were stained from each index tumor region and surrounding benign prostate peritumoral tissue, as identified by a specialist uro-pathologist. Immunohistochemistry (IHC) was performed using the Ventana Benchmark ULTRA autostainer. Tissue sections were deparaffinised using the onboard EZ prep solution, antigen retrieval was performed using the “Cell Conditioning 1” solution (Roche/Ventana) followed by inhibition of endogenous peroxidases. IHC was performed using a Ventana Benchmark ULTRA autostainer. Tissue sections were incubated at 36°C with Confirm anti-CD3 (2GV6) rabbit monoclonal antibody for 24 minutes, anti-CD8 (SP57) rabbit monoclonal antibody for 32 minutes, Confirm anti-CD20 (L26) rabbit monoclonal antibody for 20 minutes, Confirm anti-CD68 mouse monoclonal antibody for 16 minutes and FoxP3 (SP97) rabbit monoclonal antibody for 32 minutes with amplification for 8 minutes. Markers were selected to broadly represent the presence of T-helper cells (CD4+), cytotoxic T cells (CD8+), regulatory T cells (FoxP3+), macrophages (CD68+) and B-cells (CD20+). We also performed PD-L1 (SP263) staining, a common predictive biomarker for response to checkpoint inhibition and dual staining with PD-L1 (SP263)-CD8(SP57). The Ventana Basal Cell Cocktail consisting of 34βE12 and p63 which highlights basal cells present in benign prostate glands, was used to differentiate malignant versus non-malignant areas. Representative images are presented in S1 Fig.
Positive staining for PD-L1 and cell subsets including CD4+, CD20+, FOXP3+ and CD68+ using the Ventana Basal Cell Cocktail was visualized using the Optiview DAB IHC detection kit and CD8+ cells were visualized using the ultraView universal alkaline phosphatase red detection kit.
We assessed the density (# per mm2) for each cell subset in the cohort, within the tumor (intratumoral) and surrounding matched benign peritumoral regions, which were demarcated by a histopathologist. Tissue sections were scanned at x40 magnification using a Roche HT iScan whole slide scanner at a resolution of 0.46 μm/pixel, and imported into Definiens Developer XD (Definiens AG, Munich, Germany). Definiens software was used to quantitate IHC staining on immune cells. Samples where IHC quantification was not achieved due to technical problems were excluded from the analysis for a given cell subtype.
2.3 Statistical analysis
Descriptive statistics were used to report cell subset densities in intratumoral and peritumoral benign regions and between groups based on tumor characteristics and the development of BCR or metastatic disease. BCR was defined as a post-operative PSA level of ≥ 0.2ng/ml, or a rising PSA levels that led to a change in clinical management. Metastasis were detected by conventional imaging. Categorical variables were compared using chi square analyses and continuous variables were compared using the Mann-Whitney U Test. These statistical analyses were performed using Prism software (version 8.3.1, GraphPad Software LLC, La Jolla California, USA). Univariable and multivariable logistic regression analyses were performed to analyse the effect of variables on the development of BCR or metastasis. Variables with a p-value of <0.1 on univariable analysis were included in the multivariable model. Odds ratios (OR) and 95% confidence intervals (CI) were reported for each variable. Logistic regression was conducted using Stata/SE software (version 16.1, StataCorp LLC, Texas, USA). Results with p-values of <0.05 were considered statistically significant.
3. Results
Our study examined a cohort of 175 patients with prostate cancer who met eligibility criteria with available archival primary tumor specimens. After a median follow-up of 82.5 months, 115 patients (66%) had developed BCR, with a median time to recurrence of 9.0 months (Range 1.5–165.4months). Baseline characteristics for each group are recorded in Table 1. Patients who developed BCR had a higher median pre-operative PSA (13.8ng/ml vs 7.9ng/ml, p = 0.001) and were significantly more likely to have ISUP GG ≥4 disease (43% vs 5%, p<0.001).
Table 1. Baseline characteristics.
| Characteristic | Total | BCR | No BCR | P-value |
|---|---|---|---|---|
| N = 175 | N = 115 | N = 60 | ||
| Median Age | 62.8 years | 62.9 years | 62.1 years | 0.09 |
| Median | ||||
| Pre-operative PSA | 8.3ng/ml | 13.8 ng/ml | 7.9 ng/ml | 0.001 |
| ISUP Grade Group | ||||
| 1–2 | 74 (42%) | 25 (22%) | 49 (82%) | <0.001 |
| 3 | 48 (27%) | 40 (35%) | 8 (13%) | |
| 4–5 | 53 (31%) | 50 (43%) | 3 (5%) | |
| T-stage | ||||
| T2 | 83 (47%) | 26 (23%) | 57 (95%) | <0.001 |
| T3-4 | 89 (51%) | 86 (75%) | 3 (5%) | |
| unknown | 3 (2%) | 3 (3%) | 0 |
Overall, 56 patients (32%) had developed metastatic disease. Patients who developed metastatic disease had significantly higher median pre-operative PSA (14.8ng/ml vs 10.3ng/ml, p = 0.02), were more likely to have ≥T3 (80% vs 38%; P<0.001) and ISUP GG ≥4 disease (65% vs 55%, p<0.001).
3.1 Median density of multiple immune subsets was significantly higher within intratumoral regions compared to peritumoral regions
Of 175 prostatectomy specimens, successful cell marker IHC analysis for all subtypes was achieved in 108 specimens (62%), including >85% staining for CD20 (100%), CD8 (90%), PD-L1 (86%) and CD8/PD-L1 (86%).
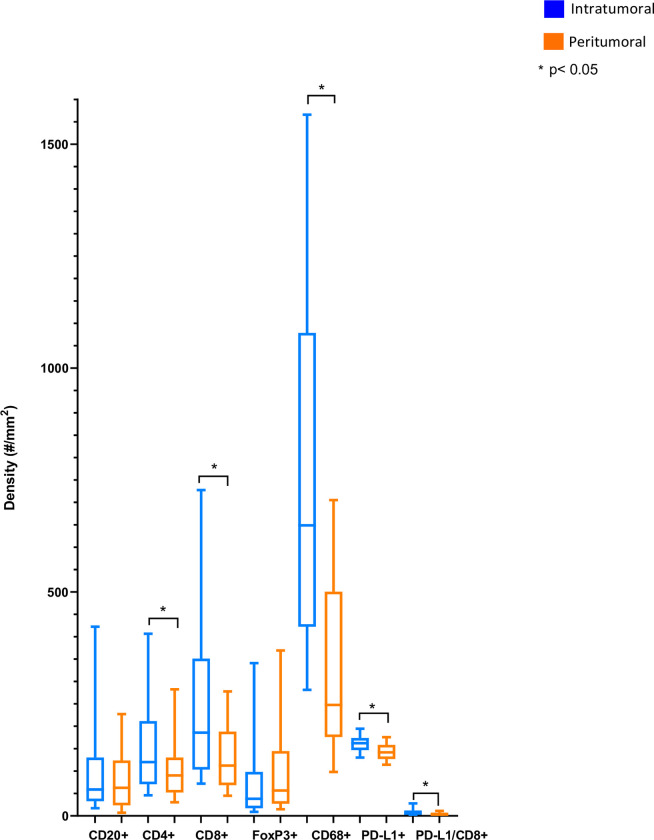
To better understand the immune context of localized prostate cancer, we first evaluated immune subset density. Overall, the most abundant immune subtype was CD68+ cells (median 648.8/mm2 intratumoral, 247.6/mm2 peritumoral) followed by CD8+ cells (median 185.8/mm2 intratumoral, 112.4/mm2 peritumoral) and PD-L1+ cells (median 162.4/mm2 intratumoral, 141.7/mm2 peritumoral) was observed. The density of cells with PD-L1/CD8+ dual staining was very low (median 5.52/mm2 intratumoral, 3.41/mm2 peritumoral) (Fig 1).
Fig 1. Cell subset densities in peritumoral and intratumoral regions.
We then compared the location of localized prostate cancer immune cell subsets by segmenting the tissue into intratumoral and peritumoral benign regions and quantitated each immune cell density within those histological regions. Median intratumoral density was significantly higher than peritumoral densities for multiple cell types (Fig 1), including PD-L1+ cells (p = 0.003), CD8+ cells (p<0.001), Dual CD8/PD-L1+ cells (p<0.0001), CD4+ cells (p = 0.0011) and CD68+ cells (p<0.001).
Table 2 demonstrates that the significant differences between intratumoral and peritumoral cell densities were also observed within both the cohort who developed BCR and the cohort who did not.
Table 2. Intratumoral and peritumoral median densities by BCR.
| Cell Subtype | BCR | Peritumoral density (#/mm2) | p-value | Non-BCR | Peritumoral density (#/mm2) | p-value |
|---|---|---|---|---|---|---|
| Intratumoral density (#/mm2) | Intratumoral density (#/mm2) | |||||
| CD8+ | 193.3 | 115.0 | <0.0001 | 147.0 | 93.6 | 0.0024 |
| CD4+ | 125.8 | 99.45 | 0.007 | 96.5 | 61.9 | 0.034 |
| FOXP3+ | 37.6 | 56.5 | 0.042 | 51.2 | 61.9 | 0.35 |
| CD68+ | 617.6 | 240.8 | <0.0001 | 734.5 | 321.2 | 0.0007 |
| CD20+ | 52.5 | 58.7 | 0.54 | 85.9 | 64.8 | 0.11 |
| PD-L1+ | 162.0 | 144.4 | <0.0001 | 163.7 | 137.4 | <0.0001 |
| PD-L1/CD8+ | 5.7 | 3.3 | <0.0001 | 5.1 | 3.6 | 0.0089 |
3.2 Immune cell infiltrates differ between high and low GG localized prostate cancer
To investigate whether the ISUP grade influenced the density of immune infiltrating cells, we analyzed immune subset data according to ISUP category. Patients with higher ISUP GG (3–5) disease had a significantly greater density of CD8+ cells in both intratumoral and peritumoral regions within their prostate cancer specimen compared to patients with lower grade disease (intratumoral median: 207.4/mm2 versus 142.3/mm2; p = 0.01; peritumoral median: 118.0/mm2 vs 78.5/mm2; p = 0.02) as demonstrated in Table 2. Density of CD4+ cells in both intratumoral and peritumoral regions were also higher in those with GG 3–5 (intratumoral median: 138.2/ mm2 versus 84.8/mm2; p = 0.002; peritumoral median: 103.5/mm2 vs 61.7/mm2; p = 0.002). Median densities of all other immune cell subsets did not significantly differ between high GG (3–5) and low GG (1–2) disease, nor did dual stained PD-L1/CD8+ cells.
3.3 Peritumoral PD-L1+ cells were an independent predictor of BCR
On univariable analysis, higher pre-operative PSA (OR = 1.11, 95%CI 1.04–1.17, p = 0.001), ISUP GG ≥ 3 (OR = 16.11, 95%CI 7.55–34.37, p<0.001) and T-stage ≥T3a (OR = 14.07, 95%CI 6.55–30.23, p<0.001) were associated with the development of BCR (Tables 3 and 4). When examining subsets of peritumoral immune infiltrates, higher density of PD-L1+ cells (≥ median) (OR = 2.28, 95% CI 1.17–4.44, p = 0.016) was also associated with BCR. Within subsets of intratumoral immune infiltrates, a lower density of CD20+ cells (< median) was associated with BCR (OR = 0.49, 95%CI 0.26–0.91, p = 0.024) on univariable analysis but not in the multivariable model. On multivariable analysis, higher density of peritumoral PD-L1+ cells remained an independent predictor of BCR (OR 5.33, 95%CI 1.31–21.61, p = 0.019), as did higher ISUP GG ≥ 3 (OR = 10.17, 95%CI 2.55–40.59, p = 0.004) and T-stage ≥T3a (OR = 7.18, 95%CI 1.88–27.39, p = 0.001).
Table 3. Median cell densities by Grade Group (GG).
| Cell Subset | Low GG 1–2 | High GG 3–5 | p-value |
|---|---|---|---|
| #/mm2 | #/mm2 | ||
| CD8+ intratumoral | 142.3 | 207.4 | 0.01 |
| CD8+ peritumoral | 78.5 | 118.0 | 0.02 |
| CD20+ intratumoral | 60.8 | 57.9 | 0.49 |
| CD20+ peritumoral | 63.8 | 58.7 | 0.82 |
| CD4+ intratumoral | 84.8 | 138.2 | 0.002 |
| CD4+ peritumoral | 61.7 | 103.5 | 0.002 |
| FOXP3+ intratumoral | 31.7 | 40.15 | 0.22 |
| FOXP3+ peritumoral | 49.7 | 58.0 | 0.55 |
| CD68+ intratumoral | 565.1 | 684.8 | 0.50 |
| CD68+ peritumoral | 212.8 | 254.2 | 0.64 |
| PD-L1+ intratumoral | 163.4 | 161.0 | 0.75 |
| PD-L1+ peritumoral | 137.5 | 147.4 | 0.12 |
| PD-L1+/CD8+ intratumoral | 5.6 | 2.3 | 0.73 |
| PD-L1/CD8+ peritumoral | 3.0 | 3.6 | 0.46 |
Table 4. Univariate and multivariate logistic regression model for development of BCR.
| Variable | Univariate OR | P-Value | Multivariate OR | P-Value |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Age1 | 1.10 (1.00–1.10) | 0.051 | 0.96 (0.87–1.06) | 0.448 |
| ISUP Grade (Ref <3) | 16.11 (7.55–34.37) | <0.001 | 10.17 (2.55–40.59) | 0.004 |
| 3–5 | ||||
| T-stage (Ref. ≤ T2c) | 14.07 (6.55–30.23) | <0.001 | 7.18 (1.88–27.39) | 0.001 |
| ≥T3a | ||||
| Pre-operative PSA1 | 1.11 (1.04–1.17) | 0.001 | 1.02 (0.94–1.12) | 0.575 |
| CD20+ density (intratumoral) | 0.49 (0.26–0.91) | 0.024 | 0.41 (0.10–1.64) | 0.208 |
| (Ref < median) | ||||
| ≥ median | ||||
| CD20+ density (peritumoral) | 0.85 (0.46–1.56) | 0.598 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| CD4+ density (intratumoral) | 1.33 (0.58–3.03) | 0.496 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| CD4+ density (peritumoral) | 1.48 (0.65–3.40) | 0.351 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| CD8+ density (intratumoral) | 1.83 (0.95–3.53) | 0.071 | 1.30 (0.29–5.76) | 0.728 |
| (Ref < median) | ||||
| ≥ median | ||||
| CD8+ density (peritumoral) | 2.12 (0.90–4.96) | 0.085 | 3.22 (0.63–16.40) | 0.159 |
| (Ref < median) | ||||
| ≥ median | ||||
| CD68+ density (intratumoral) | 1.17 (0.51–2.68) | 0.704 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| CD68+ density (peritumoral) | 0.89 (0.39–2.04) | 0.790 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| FoxP3+ density (intratumoral) (Ref < median) | 0.49 (0.20–1.16) | 0.105 | ||
| ≥ median | ||||
| FoxP3+ density (peritumoral) | 0.57 (0.24–1.36) | 0.208 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| PD-L1+ density (intratumoral) | 1.21 (0.63–2.33) | 0.570 | ||
| (Ref < median) | ||||
| ≥ median | ||||
| PD-L1+ density (peritumoral) | 2.28 (1.17–4.44) | 0.016 | 5.33 (1.31–21.61) | 0.019 |
| (Ref < median) | ||||
| ≥ median | ||||
| PD-L1/CD8+ density (intratumoral) (Ref < median) | 1.51 (0.78–2.91) | 0.218 | ||
| ≥ median | ||||
| PD-L1/CD8+ density (peritumoral) | 1.08 (0.56–2.08) | 0.814 | ||
| (Ref < median) | ||||
| ≥ median |
1 Continuous variable.
3.4 Peritumoral CD20+ cells were an independent predictor of metastatic disease
We hypothesised that the presence of a suppressed immune response within localised prostate cancer would be associated with reduced immune surveillance and a propensity to metastasis. In our cohort, higher density of peritumoral CD20+ cells (≥ median) (OR = 2.41, 95%CI 1.25–4.66, p = 0.009) was significantly associated with metastatic disease. In addition, we evaluated more conventional parameters of disease progression. Univariate analyses demonstrated higher pre-operative PSA (OR = 1.11, 95%CI 1.00–1.06, p = 0.031), ISUP GG ≥ 3 (OR = 9.06, 95%CI 3.79–21.67, p<0.001) and T-stage ≥T3a (OR = 7.47, 95%CI 3.42–16.30, p<0.001) (Table 5) were associated with the development of metastatic disease. Interestingly, multivariate analysis demonstrated higher density of peritumoral CD20+ cells (OR = 4.54, 95%CI 1.78–11.60, p = 0.002), ISUP GG ≥ 3 (OR = 4.98, 95%CI 1.43–17.32, p = 0.012) and T-stage ≥T3a (OR = 5.00, 95%CI 1.68–14.86, p = 0.004) as independent predictors for the development of metastatic disease.
Table 5. Univariate and multivariate logistic regression model for development of metastases.
| Variable | Univariate OR | P-Value | Multivariate OR | P-Value |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Age1 | 1.05 (1.00–1.10) | 0.051 | 1.01 (0.93–1.09) | 0.820 |
| ISUP Grade (ref <3 | 4.98 (1.43–17.32) | 0.012 | ||
| 3–5 | 9.06 (3.79–21.67) | <0.001 | ||
| T-stage (Ref. ≤ T2c) | 5.00 (1.68–14.86) | 0.004 | ||
| ≥T3a | 7.47 (3.42–16.30) | <0.001 | ||
| Pre-operative PSA1 | 1.03 (1.00–1.06) | 0.031 | 1.01 (0.97–1.04) | 0.749 |
| CD20+ density (intratumoral) | ||||
| (Ref < median) | 0.98 (0.52–1.86) | 0.958 | ||
| ≥ median | ||||
| CD20+ density (peritumoral) | ||||
| (Ref < median) | 2.41 (1.25–4.66) | 0.009 | 4.54 (1.78–11.60) | 0.002 |
| ≥ median | ||||
| CD4+ density (intratumoral) | ||||
| (Ref < median) | 1.82 (0.87–3.82) | 0.203 | ||
| ≥ median | ||||
| CD4+ density (peritumoral) | ||||
| (Ref < median) | 1.22 (0.58–2.54) | 0.598 | ||
| ≥ median | ||||
| CD8+ density (intratumoral) | ||||
| (Ref < median) | 1.77 (0.91–3.45) | 0.094 | 0.93 (0.33–2.64) | 0.891 |
| ≥ median | ||||
| CD8+ density (peritumoral) | ||||
| (Ref < median) | 1.95 (0.93–4.08) | 0.077 | 1.07 (0.38–3.06) | 0.894 |
| ≥ median | ||||
| CD68+ density (intratumoral) | ||||
| (Ref < median) | 1.06 (0.50–2.23) | 0.879 | ||
| ≥ median | ||||
| CD68+ density (peritumoral) | ||||
| (Ref < median) | 0.57 (0.27–1.22) | 0.149 | ||
| ≥ median | ||||
| FoxP3+ density (intratumoral) (Ref < median) | 0.72 (0.33–1.57) | 0.409 | ||
| ≥ median | ||||
| FoxP3+ density (peritumoral) | ||||
| (Ref < median) | 1.02 (0.47–2.20) | 0.968 | ||
| ≥ median | ||||
| PD-L1+ density (intratumoral) | ||||
| (Ref < median) | 1.50 (0.76–2.99) | 0.246 | ||
| ≥ median | ||||
| PD-L1+ density (peritumoral) | ||||
| (Ref < median) | 1.57 (0.79–3.12) | 0.203 | ||
| ≥ median | ||||
| PD-L1/CD8+ density (intratumoral) (Ref < median) | 1.33 (0.67–2.64) | 0.416 | ||
| ≥ median | ||||
| PD-L1/CD8+ density (peritumoral) | 1.18 (0.59–2.33) | 0.641 | ||
| (Ref < median) | ||||
| ≥ median |
1 Continuous variable.
4. Discussion
Our study examined the density of selected immune cell subsets within prostate cancer and peritumoral tissue and correlated these with the development of both BCR and metastatic disease. We demonstrated an abundant immune cell infiltrate within the prostate cancer TME, dominated by CD68+ cells, with substantial numbers of CD8+ and CD4+ T cells. In our cohort, higher peritumoral PD-L1+ cell density was an independent risk factor for BCR, while higher peritumoral CD20+ cell density was independently associated with the development of metastatic disease.
Our findings suggest that the prostate cancer TME is not an “immune dessert”. Immune cell densities within the TME in our cohort are comparable with those previously reported in melanoma, a cancer responsive to immunotherapy, with the exception of PD-L1+ cells, which were less abundant than in melanoma [22, 23]. Median CD8+ and CD4+ cell densities were higher in our cohort than those reported in colorectal cancer [24]. Like other studies, we observed a higher density of CD8+ and CD4+ cells in higher GG prostate cancer. CD8+ T cells are associated with cytotoxicity and in several cancers, are associated with superior outcomes [25–28]. In prostate cancer however, CD8+ T-cells have been documented in lower densities, are more refractory to activation and have been linked to poorer outcomes [8, 13, 29, 30]. For example, Sfanos et al. demonstrated that CD8+ prostate-infiltrating T-lymphocytes exhibited a restricted T-cell receptor repertoire [31]. A previous study also demonstrated non-functional CD8+ T cells in mice with prostate cancer, using a double transgenic model, which regained function after removal from the tumor-bearing mice, indicating a prostate-cancer specific phenomenon that leads to CD8+ T-cell inactivation [30].
Our study also demonstrated an association between higher density of peritumoral PD-L1+ cells and BCR. This is consistent with findings from another study, in which higher numbers of PD-L1+ peritumoral cells were associated with poorer clinical outcomes [32, 33]. In our study, median PD-L1+ cell density was significantly higher in intratumoral regions compared to peritumoral regions. PD-L1+ cells have been associated with poor prognosis in many tumor types, including higher risk of BCR in prostate cancer, however, correlation with long term survival remains unclear [21, 32, 34, 35]. PD-L1 expression is uncommon in metastatic prostate cancer tissue samples [21]. The reported expression in primary prostate cancer also varies significantly (7–92%) [32, 34, 36].
Tumor-infiltrating B-lymphocytes also play an important role in cancer biology but have been studied less extensively compared to T-lymphocytes [37]. In our cohort, a higher density of peritumoral CD20+ cells was an independent risk factor for development of metastatic disease. While higher density of CD20+ cells have been associated with improved prognosis in some tumor types, they have demonstrated poorer prognosis in prostate cancer, consistent with our findings [38]. Preclinical models suggest B cells promote castration-resistance through cytokine production [39] and the anti-CD20 antibody Rituximab has been shown to modulate the prostate TME [40]. Further studies are required to validate these findings, which have potentially important therapeutic implications.
Tumor associated macrophages are known to play an immune inhibitory role, differentiating from myeloid-derived suppressor cells (MDSC) and suppressing T-cells [41, 42]. In our study, CD68+ macrophages were the most abundant immune subset and were more abundant within intratumoral regions compared to peritumoral regions. This may support our hypothesis of an immune-suppressive TME, given their inhibitory potential on other immune cells. This is consistent with results from another study demonstrating the importance of monocytes and macrophages in disease recurrence and progression, based on differential transcript abundance analysis and multiplex immunohistochemistry [43]. Our results were limited by the lack of macrophage markers that specifically indicate an inhibitory function and correlate with poor clinical outcomes, particularly CD163 and VSIG4 [44]. Although beyond the scope of our study, further analysis of differential gene expression would potentially enable a more robust assessment of immune cell function and activation within our cohort and validation with an independent dataset would enable confirmation of our study findings.
Despite the limitations of our case-control cohort, which include inherent selection bias, our findings confirmed the significance of the established poor prognostic factors of higher GG and T-stage, which were independently associated with BCR and metastasis. Our study examines the TME within primary prostate cancer, which may differ from that of metastatic lesions, as demonstrated by the differences in reported PD-L1 levels [32, 34, 36]. However, metastatic biopsies are not routinely performed due to the predominance of bone-only disease and the high failure rate. Furthermore, understanding the TME in earlier disease is relevant to the development of future immunotherapeutic and immunomodulatory strategies.
5. Conclusions
Our study of primary prostate cancer demonstrates an abundant immune cell infiltrate dominated by CD68+ cells with higher density of peritumoral PD-L1+ cells being an independent risk factor for BCR and of peritumoral CD20+ cells being an independent risk factor for metastatic disease. Greater understanding of the immune TME, particularly mechanisms of immune cell signalling and inactivation, will provide guidance for the development of future therapeutic strategies.
Supporting information
(TIF)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–46. Epub 20190928. doi: 10.1056/NEJMoa1910836 . [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–51. Epub 20190722. doi: 10.1016/S1470-2045(19)30388-2 . [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. Epub 20161008. doi: 10.1056/NEJMoa1606774 . [DOI] [PubMed] [Google Scholar]
- 5.Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi: 10.1136/esmoopen-2020-001079 ; PubMed Central PMCID: PMC7703447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35(1):40–7. doi: 10.1200/JCO.2016.69.1584 . [DOI] [PubMed] [Google Scholar]
- 7.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–7. doi: 10.18632/oncotarget.10547 ; PubMed Central PMCID: PMC5288150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasner A, Karin M. Immune Infiltration and Prostate Cancer. Front Oncol. 2015;5:128. doi: 10.3389/fonc.2015.00128 ; PubMed Central PMCID: PMC4495337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin D, Wang X, Choi SYC, Ci X, Dong X, Wang Y. Immune phenotypes of prostate cancer cells: Evidence of epithelial immune cell-like transition? Asian J Urol. 2016;3(4):195–202. doi: 10.1016/j.ajur.2016.08.002 ; PubMed Central PMCID: PMC5730833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348(1–2):9–17. doi: 10.1016/j.jim.2009.06.004 . [DOI] [PubMed] [Google Scholar]
- 11.Keam SP, Halse H, Nguyen T, Wang M, Van Kooten Losio N, Mitchell C, et al. High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot. J Immunother Cancer. 2020;8(1). Epub 2020/06/26. doi: 10.1136/jitc-2020-000792 ; PubMed Central PMCID: PMC7319782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity. 2018;48(4):812–30 e14. Epub 2018/04/10. doi: 10.1016/j.immuni.2018.03.023 ; PubMed Central PMCID: PMC5982584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeigler-Johnson C, Morales KH, Lal P, Feldman M. The Relationship between Obesity, Prostate Tumor Infiltrating Lymphocytes and Macrophages, and Biochemical Failure. PLoS One. 2016;11(8):e0159109. doi: 10.1371/journal.pone.0159109 ; PubMed Central PMCID: PMC4972345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25(6C):4435–8. . [PubMed] [Google Scholar]
- 15.Davidsson S, Ohlson AL, Andersson SO, Fall K, Meisner A, Fiorentino M, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol. 2013;26(3):448–55. doi: 10.1038/modpathol.2012.164 . [DOI] [PubMed] [Google Scholar]
- 16.Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014;74(14):1452–61. Epub 2014/08/12. doi: 10.1002/pros.22862 . [DOI] [PubMed] [Google Scholar]
- 17.Seager RJ, Hajal C, Spill F, Kamm RD, Zaman MH. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg Sci Phys Oncol. 2017;3. doi: 10.1088/2057-1739/aa7e86 ; PubMed Central PMCID: PMC6070160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdman A, Jaraj SJ, Comperat E, Charlotte F, Roupret M, Pisa P, et al. Distribution of Foxp3-, CD4- and CD8-positive lymphocytic cells in benign and malignant prostate tissue. APMIS. 2010;118(5):360–5. doi: 10.1111/j.1600-0463.2010.02604.x . [DOI] [PubMed] [Google Scholar]
- 19.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–76. Epub 2015/03/24. doi: 10.1016/j.it.2015.02.008 ; PubMed Central PMCID: PMC4393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. Epub 2008/01/05. doi: 10.1146/annurev.immunol.26.021607.090331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fankhauser CD, Schuffler PJ, Gillessen S, Omlin A, Rupp NJ, Rueschoff JH, et al. Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer. Oncotarget. 2018;9(12):10284–93. doi: 10.18632/oncotarget.22888 ; PubMed Central PMCID: PMC5828186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gide TN, Silva IP, Quek C, Ahmed T, Menzies AM, Carlino MS, et al. Close proximity of immune and tumor cells underlies response to anti-PD-1 based therapies in metastatic melanoma patients. Oncoimmunology. 2020;9(1):1659093. Epub 2020/02/01. doi: 10.1080/2162402X.2019.1659093 ; PubMed Central PMCID: PMC6959449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–80. Epub 2012/01/24. doi: 10.1158/0008-5472.CAN-11-3218 ; PubMed Central PMCID: PMC3306813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara T, Hazama S, Suzuki N, Yoshida S, Tomochika S, Nakagami Y, et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br J Cancer. 2019;121(8):659–65. Epub 2019/09/07. doi: 10.1038/s41416-019-0559-6 ; PubMed Central PMCID: PMC6889292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–34. Epub 2017/07/26. doi: 10.1038/nrclinonc.2017.101 . [DOI] [PubMed] [Google Scholar]
- 26.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–95. doi: 10.1016/j.immuni.2013.10.003 . [DOI] [PubMed] [Google Scholar]
- 27.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66. doi: 10.1056/NEJMoa051424 . [DOI] [PubMed] [Google Scholar]
- 28.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–39. Epub 2018/05/15. doi: 10.1016/S0140-6736(18)30789-X . [DOI] [PubMed] [Google Scholar]
- 29.Steele KE, Tan TH, Korn R, Dacosta K, Brown C, Kuziora M, et al. Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer. 2018;6(1):20. doi: 10.1186/s40425-018-0326-x ; PubMed Central PMCID: PMC5839005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno TC, Rothwell C, Grosso JF, Getnet D, Yen HR, Durham NM, et al. Anti-tumor effects of endogenous prostate cancer-specific CD8 T cells in a murine TCR transgenic model. Prostate. 2012;72(5):514–22. doi: 10.1002/pros.21453 ; PubMed Central PMCID: PMC3248615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009;69(15):1694–703. doi: 10.1002/pros.21020 ; PubMed Central PMCID: PMC2782577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ness N, Andersen S, Khanehkenari MR, Nordbakken CV, Valkov A, Paulsen EE, et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget. 2017;8(16):26789–801. doi: 10.18632/oncotarget.15817 ; PubMed Central PMCID: PMC5432297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kather JN, Suarez-Carmona M, Charoentong P, Weis CA, Hirsch D, Bankhead P, et al. Topography of cancer-associated immune cells in human solid tumors. Elife. 2018;7. doi: 10.7554/eLife.36967 ; PubMed Central PMCID: PMC6133554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin Cancer Res. 2016;22(8):1969–77. doi: 10.1158/1078-0432.CCR-15-2042 . [DOI] [PubMed] [Google Scholar]
- 35.Xian P, Ge D, Wu VJ, Patel A, Tang WW, Wu X, et al. PD-L1 instead of PD-1 status is associated with the clinical features in human primary prostate tumors. Am J Clin Exp Urol. 2019;7(3):159–69. ; PubMed Central PMCID: PMC6627547. [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18(4):325–32. doi: 10.1038/pcan.2015.39 ; PubMed Central PMCID: PMC4641011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo FF, Cui JW. The Role of Tumor-Infiltrating B Cells in Tumor Immunity. J Oncol. 2019;2019:2592419. doi: 10.1155/2019/2592419 ; PubMed Central PMCID: PMC6778893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. Epub 2014/01/31. doi: 10.1186/1479-5876-12-30 ; PubMed Central PMCID: PMC3914187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–5. Epub 2010/03/12. doi: 10.1038/nature08782 ; PubMed Central PMCID: PMC2866639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan ST, Zhang J, Burner DN, Liss M, Pittman E, Muldong M, et al. Neoadjuvant rituximab modulates the tumor immune environment in patients with high risk prostate cancer. J Transl Med. 2020;18(1):214. Epub 2020/05/30. doi: 10.1186/s12967-020-02370-4 ; PubMed Central PMCID: PMC7257145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–9. Epub 2018/06/29. doi: 10.1038/s41586-018-0266-0 ; PubMed Central PMCID: PMC6461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–19. Epub 2018/01/20. doi: 10.1038/s41590-017-0022-x ; PubMed Central PMCID: PMC5854158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangiola S, McCoy P, Modrak M, Souza-Fonseca-Guimaraes F, Blashki D, Stuchbery R, et al. Transcriptome sequencing and multi-plex imaging of prostate cancer microenvironment reveals a dominant role for monocytic cells in progression. BMC Cancer. 2021;21(1):846. Epub 20210722. doi: 10.1186/s12885-021-08529-6 ; PubMed Central PMCID: PMC8296706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi-Frias D, Damodarasamy M, Hernandez SA, Gil da Costa RM, Vakar-Lopez F, Coleman IM, et al. The Aged Microenvironment Influences the Tumorigenic Potential of Malignant Prostate Epithelial Cells. Mol Cancer Res. 2019;17(1):321–31. Epub 2018/09/19. doi: 10.1158/1541-7786.MCR-18-0522 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.