Abstract
Introduction
Cholangiocarcinoma (CCA) is a major contributor to hepatobiliary mortality in the Lao People’s Democratic Republic (Lao PDR). Infection with the carcinogenic trematode Opisthorchis viverrini (OV), acquired through consumption of insufficiently-cooked river fish, is a known risk factor for the development of CCA. Together with OV, other risk factors contribute to the pathogenesis of CCA. We conducted this study to identify the burden of CCA and identify risk factors in high-risk communities in Lao PDR.
Method
A cross-sectional study was performed in Champasack and Savannakhet provinces, southern Lao PDR, where OV infection is highly endemic. We assessed hepatobiliary morbidity with abdominal ultrasound (US). In addition, multiple risk factors known or suspected to be associated with CCA were assessed such as OV infection (examined by Kato-Katz technique for stool examination), lifestyle risks (e.g. smoking and alcohol consumption by face-to-face questionnaire), co-morbidity (e.g. diabetes mellitus) and hepatitis B infection status, both serologically tested.
Results
In 3,400 participants, the overall prevalence of suspected CCA was 7.2% (95% confidence interval [95% CI] 5.4−9.6). The suspected CCA prevalence increased with age, and was higher in men at all ages. Almost all participants (88.3%) were infected with OV. In the multivariate regression analysis, suspected CCA was positively associated with OV infection (adjusted odds ratio [aOR] 3.4, 95% CI 1.7−6.5), and a history of cholecystectomy (aOR 2.7, 95% CI 1.5−4.9).
Conclusion
Our CCA screening in high OV prevalence rural areas of Lao PDR uncovers a high public health burden, primarily driven by elevated OV infection rates. Urgent interventions are needed to curb OV infection in these communities. Age and gender disparities in suspected CCA prevalence highlight the need for targeted efforts. Beyond OV, notable factors like a history of cholecystectomy offer valuable insights for preventive strategies. This research enhances our understanding of hepatobiliary morbidity and informs public health initiatives in Lao PDR.
Author summary
Evidence has long pointed to a significant burden of liver diseases in certain regions of the Lao People’s Democratic Republic (Lao PDR), primarily attributed to the Opisthorchis viverrini (OV) liver fluke. This parasite is transmitted to humans through the consumption of raw or undercooked fish and fish products. Chronic OV infection can lead to cholangiocarcinoma (CCA), a fatal bile duct cancer. The objective of this research was to assess the prevalence of suspected CCA in rural OV-endemic communities in the southern part of Lao PDR. We conducted a cross-sectional study in selected communities within Savannakhet and Champasack provinces.
Adults aged 35 years and older were enrolled and underwent liver disease assessment through abdominal ultrasound. OV infection was diagnosed via fecal examination, while lifestyle risk factors like smoking and alcohol consumption were evaluated through face-to-face interviews. Additionally, diabetes mellitus and hepatitis B infection status were determined serologically.
We found a high prevalence of suspected bile duct cancer (CCA) at 7.2% overall. This rate was found higher in men compared to women, and to increase with age. Most participants (88.3%) were infected with OV, posing the highest risk for suspected CCA (adjusted odds ratio [aOR] 3.4). Additionally, a history of cholecystectomy (aOR 2.7) was a significant predictor of bile duct cancer. These results provide crucial insights for controlling liver morbidity in Lao PDR, emphasizing the urgent need to address OV infection and associated risk factors for CCA in affected communities.
1. Introduction
Cholangiocarcinoma (CCA) is a primary tumor of the extra- and intrahepatic bile ducts with a high fatality rate. Its incidence rates vary around the world, with a much higher incidence in parts of the eastern world than in the west [1]. For example, the annual incidence of intrahepatic CCA (between 1990 and 2000) in the United States of America has been reported at an average of 0.85/100,000 person-years [2]; and, in most European countries, over the recent years, the mortality rate due to intrahepatic CCA has elevated to 1.1/100,000 in men and 0.75/100,000 in women [3]. However, the world’s highest CCA incidence is reported in the northeastern provinces of Thailand where the incidence among individuals 35 years and older varied from 93.8/100,000 to 317.6/100,000 person-years (data collected between 1999 and 2001) [4,5].
CCA is classified in intrahepatic CCA (iCCA), perihilar CCA (pCCA) or distal CCA (dCCA)) [6]. Typically, CCA is diagnosed only at a late stage when a fatal outcome cannot be prevented. The five-year survival rate is less than 10% [7]. Patients diagnosed with an early stage may undergo surgical treatment and have a higher rate of survival [8].
In regions endemic for OV, numerous studies have established a link between OV infection and CCA [1,2,4,5,9–16]. Furthermore, the International Agency for Research on Cancer (IARC) has classified OV as a carcinogenic agent [4,5,17]. In Southeast Asia and particularly in Northeast Thailand, OV infection is highly prevalent [9]. In Lao PDR, approximately 2.5 million people are estimated to carry the infection [17]. Despite interventions aimed at reducing OV related liver morbidity, such as yearly mass-drug administration (MDA) using Praziquantel (PZQ) in Champasack (CPS) province of Lao PDR [18], the reinfection rate remains high due to the Laotians’ raw fish consumption habits.
Chronic inflammation is proposed as the key OV-induced pathway to CCA [17,19,20], which occurs from both fluke’s mechanical damage (feeding and migration) and immunological reactions (parasite secretory-excretory products and mediated immune response) [21]. Such mechanisms have resulted in ulceration and remodeling of the periductal epithelium tissues leading to periductal fibrosis (PDF) and finally CCA [19].
Despite the high endemicity of OV in Lao PDR, there is limited evidence regarding the extent of CCA in the rural communities and OV contributes to the prevalence of hepatic morbidity, such as CCA, across different regions of the country. This is partly due to the absence of collective data collection systems for hepatic morbidity and a clear definitive standard for diagnosing suspected CCA using ultrasound, such as determining the degree of bile duct dilatation. There are, however, some effort revealing the burden, for instance, a retrospective study examining Lao tertiary hospital records from 2006 to 2011 found that 97.4% of 274 patients examined with US and CT scan imaging showed bile duct dilation [22].
This study addresses two evidence gaps. First, there is a lack of population-based and US-based data on the prevalence of suspected CCA in rural parts of Lao PDR with high OV infection and re-infection rates. Second, even though OV infection is a strong CCA risk factor, the likelihood of CCA development is not uniformly distributed among those infected with OV [23]. Additional risk factors may contribute to the pathogenesis of CCA [1]. Consequently, this study aims to explore the extent of suspected CCA in an OV endemic setting of Lao PDR and explore the associations to OV infection and additional risk factors with CCA.
2. Materials and methods
2.1. Ethics statement
This study was approved by the National Ethics Committee for Health Research, Ministry of Health (MoH), Vientiane, Lao PDR (Ref.no.113/2018 NECHR) and the Ethikkommission der Nordwest- und Zentralschweiz (EKNZ, Ref.no. R-2017-00869), Basel, Switzerland. The permission for the fieldwork was obtained from the MoH, the Provincial Health Offices of Champasack (CPS) and Savannakhet (SVK) provinces, the District Health Offices, and the District Office of Education and Sports of Champhone and Khong districts.
Education and Sports authorities of Champhone and Khong districts collaborated in this effort. The village authorities and residents received comprehensive explanations about the study’s objectives, procedures, potential risks, and benefits. A consent form, presented in the Lao language, was carefully read with the participants, addressing any questions they had. Signed informed consent was obtained from all study participants and kept confidential. Furthermore, participants were given the choice to receive the results of their examinations. Those found infected with OV received treatment with PZQ in accordance with the Lao national treatment guidelines [24]. Participants suspected of having CCA were referred for further radiological follow-up. In cases where CCA was confirmed, surgical operations were conducted at Mahosot Hospital in Vientiane, the capital of Lao PDR. All treatments and associated travel expenses to Vientiane were provided to the participants at no cost.
2.2. Study design and sample size
This study is a community-based, cross-sectional study in selected O. viverrini endemic villages in CPS and SVK provinces including adults of 35 years and older. To determine the sample size, we assumed a proportion of exposed participants of 33% and a prevalence of advanced PDF (APF+) in exposed participants of 15% [25]. We estimated that a sample size of 2,100 participants would be sufficient to detect a difference of 5 percentage points with 80% power at the 95% confidence level. Participation in the 21 villages was higher than expected, resulting in a realized sample size of 3,400.
2.3. Sampling population and study sites
The eligibility criteria encompassed (i) all residents of the selected villages and (ii) individuals aged 35 years and above. Exclusion criteria included (i) pregnancy and (ii) residents who had moved to the study villages within the last 5 years.
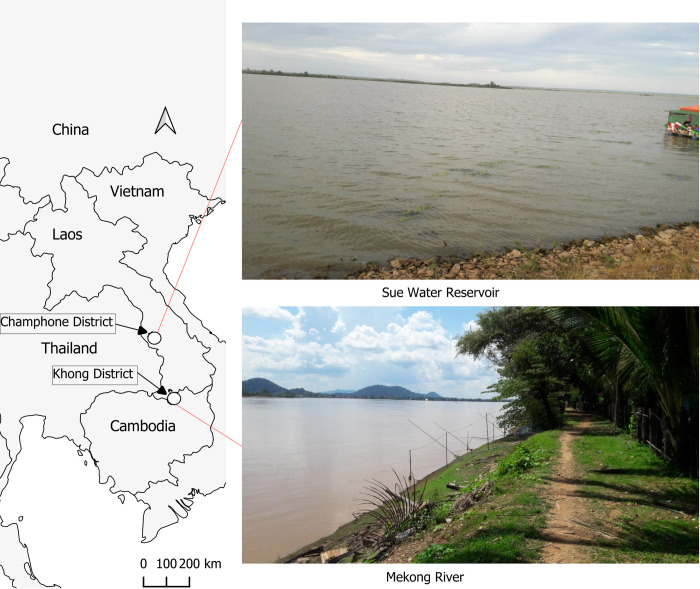
We utilized cluster sampling of villages based on logistical feasibility and the available data on known OV infection intensity, in collaboration with district health authorities, with the goal of including all eligible individuals in the selected villages. In total, we selected 21 villages comprising 12 from Khong district (CPS) and 9 from Champhone district (SVK). These villages were strategically situated along major natural water bodies, such as the Mekong River in CPS, and the “Sue” water reservoir, a substantial water body with aquaculture ponds in SVK (Fig 1). The field study was conducted in the villages of Khong and Champhone districts from December 2017 to February 2019.
Fig 1. Map of study area.
The country boundary base layer is taken from the World Bank Official Boundary (https://datacatalog.worldbank.org/search/dataset/0038272/World-Bank-Official-Boundaries) under the Creative Commons Attribution 4.0 (CC-BY 4.0) license. The photos from the field were taken by one of our research members.
2.4. Fieldwork procedure
The process was divided into three sequential days: (i) consenting participants were registered and provided with plastic containers labeled with participant IDs for stool collection. (ii) Participants brought their filled fecal containers to the designated study point for processing and examination, which took place on the same day. Additionally, face-to-face interviews were conducted. Participants were also given a second stool collection container along with instructions to fast in preparation for the third day’s examination. (iii) Participants arrived in the morning to undergo all remaining examinations, including abdominal ultrasound, physical examination, and blood drawing. The second fecal sample was also processed on this same day. Around 25–50 participants were recruited daily until all participants in the village had been included.
A 5 ml venous blood sample was collected from each participant and stored in EDTA containers. Serum was separated from the whole blood samples through centrifugation at 1,500 rpm for 10 minutes at room temperature. These blood aliquots were promptly stored at -20°C at the study sites for a maximum of 4 weeks before being transported to the laboratory at the Lao Tropical and Public Health Institute (Lao TPHI) in Vientiane. To ensure proper storage conditions, we maintained the cold chain using portable sample freezers designed for vehicle use. Upon arrival at Lao TPHI, both serum and ETDA blood were preserved at -80°C until further testing.
2.5. Research tools and measurements
Abdominal ultrasound examination
Six radiologists, each with at least three years of clinical experience following residency at Lao PDR tertiary hospitals under the Lao PDR University of Health Sciences curriculum, conducted abdominal US. Additionally, an international specialist with six years of experience in US in infectious diseases also performed the US examination. To minimize errors, radiologists constantly exchanged working procedures and result assessments.
A mobile US device (Mindray Z6, Shenzhen Mindray Bio-Medical Electronics, Shenzhen, China), equipped with a convex (model 3C5P) and a linear (model 7L4P) probe, was used.
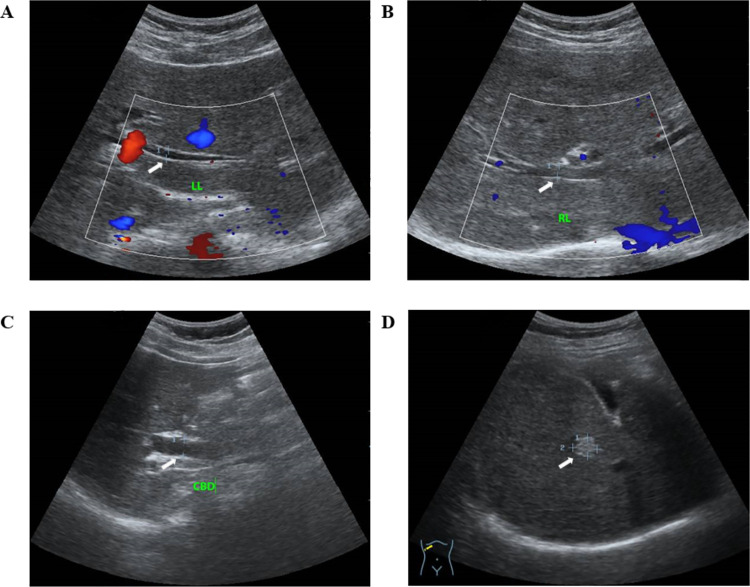
The abdominal ultrasound assessment tools used to evaluate liver pathology associated with OV infection were developed by independent research groups: Mairiang et al. [23] and Chamadol et al. [20] both from Khon Kaen University, Thailand. Intrahepatic bile ducts diameter were measured for dilatation (at the left (LLL) and/or right liver lobes (RLL) > 2 millimeters (mm) was considered as present) [20]. Intrahepatic bile duct dilatation was also considered present when the bile duct diameter exceeds 40% of the diameter of the corresponding portal vein, also known as the "shotgun sign" [20,23]. Common bile duct (CBD) dilatation was defined as a measured diameter exceeding 6 mm (Fig 2). For participants over 60 years of age, this threshold was adjusted upward by 1 mm per decade. Additionally, in patients who had undergone cholecystectomy, only a CBD diameter of more than 10 mm was considered to indicate dilatation [26,27]. Fibrosis of the bile duct walls (PDF) was assessed using the adapted increased periportal echo protocol used in schistosomiasis proposed by Chamadol and colleagues [20].
Fig 2. Ultrasound images for bile duct dilatation and liver masses.
These ultrasound images, obtained from two different study participants (A-C from a 60-year-old male and D from a 63-year-old male), were classified into four categories for suspected CCA diagnosis: (A) left liver lobe (LL) dilatation (3.7 mm), (B) right liver lobe (RL) dilatation (4.8 mm), (C) common bile duct (CBD) dilatation (9.9 mm), and (D) a single hyperechoic liver mass with an ill-defined margin (9.3*11.9 mm). White arrows indicate areas of pathology, while red areas in A indicate blood flow toward the probe, and blue areas in A and B indicate blood flow away from the probe.
Liver parenchyma was screened for masses [23]. The liver was examined with regard to shape, size and surface, and the liver parenchyma with regard to structure. A fatty liver was graded according to Strauss and colleagues [28], defining fatty liver into three categories: (i) a slight increase in liver echogenicity, (ii) intrahepatic vessels and diaphragm slightly impaired and increased echogenicity, and (iii) a marked increase in intra-hepatic echogenicity with poor penetration to the posterior segment of the right liver lobe, and poor or no visualization of hepatic vessels and diaphragm.
Gallbladder sludge referred to hyperechoic mobile-like images with or without posterior acoustic shadow, and gallbladder stones defined as hyperechoic structure with an acoustic shadow [16]. Post cholecystectomy was noted when the gallbladder did not appear on the ultrasound imaging and if the patient had a history of cholecystectomy.
Questionnaires
The participants were interviewed using a standardized questionnaire with details on socio-demographic characteristics, household assets, and livestock (such as fish pond, chicken, cow, pig, water buffalo, rice cooker, radio, fan, refrigerator, television, bicycle, motorbike, farm vehicle, place to grow vegetables, cement floor, and tap water). Smoking (current smoker and ever smoker) and alcohol consumption were also recorded as consumption frequency in days per week. Additionally, food consumption behaviors such as eating raw fish and fish products (i.e., koi-pa, pickled fish) were also asked. Medical history information included self-reported diagnoses or medication intake for type 2 diabetes mellitus (T2DM), and anthelminthic treatment (e.g. PZQ). The interviews lasted between 15 and 20 minutes.
Recording the helminth infection status
The Kato-Katz technique was used for parasitological examination [29]. Participants provided two separate samples collected on different days. For each participant’s sample, two glass smears were prepared according to the producer’s instructions and allowed to clear for 30 to 60 minutes before examination under light microscopes by experienced microscopists from Lao TPHI. All OV and other helminth eggs were counted separately by species.
Blood examinations
All laboratory examinations were conducted at Lao TPHI. The hemoglobin A1c (HbA1c %) values were measured in EDTA blood samples, liver biochemistry parameters were measured in serum (Liver biochemical included alanine aminotransferase—ALT IU/L, aspartate aminotransferase—AST IU/L, gamma-glutamyl transferase—GGT (IU/L), alkaline phosphatase—ALP (IU/L), direct bilirubin—D-bil (mg/dL), total bilirubin—T-bil (mg/dL)). An enzymatic assay method on an automated commercially available clinical chemistry analyzer (Mindray, model: BS-240, Mindray Corporation, Shenzhen, China), following the manufacturer’s instructions was used for the analysis. The testing process employed ready-to-use reagents, calibrators, and control sets for each parameter, all of which were sourced from the same manufacturer as the analyzer. Hepatitis B (HBV) infection was determined by the HBV rapid diagnostic test (Vikia HbsAg, bioMérieux, France) using serum sample.
Physical examination
The examination performed on each participant encompassed measurements of weight (rounded to the nearest 0.1 kg; Seca weighting scale, model: M 877, Hammer Steindamm 3–2522089 Hamburg, Germany) and height (rounded to the nearest 0.5 cm; Seca, model: 206, Hammer Steindamm 3–2522089 Hamburg, Germany). Furthermore, physical examinations were performed by our doctors to assess signs and symptoms associated with liver disease (S4 Appendix). This included measurement of body temperature, inspection of the skin (jaundice, spider angiomas, and abdominal collateral veins), and palpation (ascites, right upper quadrant tenderness of the abdomen, liver edge, and peripheral edema).
2.6. Data management, variables management and statistical analysis
A Commcare ODK mobile database was developed and installed on tablets utilizing Commcare server (www.commcarehq.org, version 3.4). Recoded forms were downloaded to Microsoft Excel where data cleaning and checking was performed. All data analysis was performed using STATA software, version 16.0 (StataCorp, College Stata, TX, USA).
The social economic status (SES) was calculated using assets (see section 1.5) and deriving a component scores formed by principle factor analysis. The SES score was categorized into tertile as poor, middle, and wealthy [30]. The prevalence of OV was calculated using the OV’s egg seen in any microscopic slides, and the egg per 1g of stool (EPG) was calculated by multiplying the mean of OV egg counts (total OV eggs divided by 4 slides) with 24 (as single fecal sample applied in a slide is equal to 41.7mg). Four categories of OV infection intensity were calculated (no-infection; light, EPG 1 to 999; moderate, EPG 1.000 and 9,999; and heavy, EPG ≥ 10,000) [31]. Body mass index (BMI) was calculated using the formula weight in kg/m2, and classified into lean (BMI < 23) and not lean (BMI ≥ 23) [32]. Participants were categorized as having "present" clinical symptoms for liver disease if they exhibited at least one symptom detailed in Section 1.5 (refer to S4 Appendix for a comprehensive examination).
Suspected CCA was defined based on the presence of at least one of the following US findings: (i) liver mass, (ii) dilated intrahepatic and/or extrahepatic (i.e. CBD) bile ducts, and (iii) shotgun sign [20,23]. Typical benign liver lesions, such as cysts or hemangioma, or bile duct dilation due to identified stones were not classified as suspicious for CCA (details regarding the US examination protocol described in section 2.5).
Frequencies, percentages (%), and mean (standard deviation [SD]) were used to describe categorical and numerical variables, respectively. Pearson’s chi-squared (x2) test was used to assess the independence of categorical variables. Confidence intervals of the estimated prevalence were adjusted for potential within-village correlation using random-effects models, as described below.
Logistic regression random-effects model (incorporating the variable “village” as a random effect to account for potential clustering within villages) was used to calculate crude (cOR), and adjusted odds ratio (aOR), and 95% confidence intervals (95% CI) of being suspicious for a CCA. Based on the literature on the presumed pathway to developing CCA, the primary predictors of interest (OV and HBV infection, T2DM, cholecystectomy, smoking and heavy alcohol consumption) [1,2,4,5,9–16] and a priori selected confounders (socio-economic and -demographic status) were included in the models. Models building included (i) bivariate analysis, (ii) all predictors model adjusted for age and gender, (iii) all predictors model adjusted for socio-economic and demographic confounders, (iv) all predictors model adjusted for co-morbidity (fatty liver disease, and obesity), and (v) fully adjusted model. Gallbladder co-morbidities, including gallbladder stones, gallbladder sludge, and periductal fibrosis, were excluded from the adjusted models because they are thought to be intermediate effects in the pathway between OV and suspected CCA [33,34].
Sensitivity analysis was performed to assess the robustness of the findings by excluding individuals with the following categories: (i) with T2DM (excluded due to potential ambiguity regarding its direct link to CCA pathogenesis), (ii) had high OV infection intensity (potential intrahepatic bile obstruction caused by physical blockage from adult worms), and (iii) had undergone cholecystectomy (to minimize potential bias from altered CBD physiology after surgery).
3. Results
3.1. Demographic characteristics of the participants
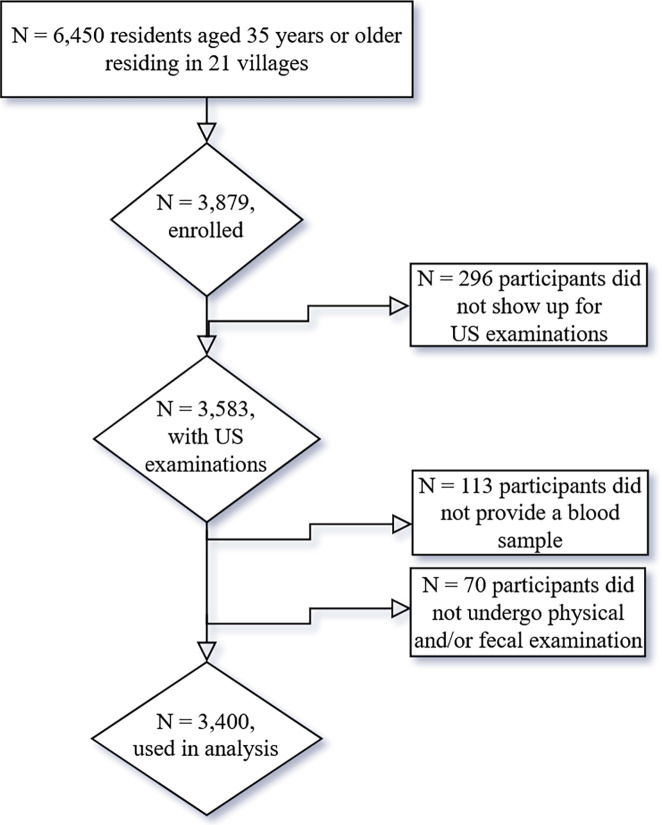
Out of 6,450 residents from 21 villages (aged 35 years old and older), 3,583 participants took part in the US examination, resulting in an average participation rate of 55.6% for the US examination (see S1 Appendix). In total, 3,400 study participants were included in the final analysis. 133 individuals had to be excluded because they did not provide a blood sample, and 55 individuals because they did not undergo physical examination and/or did not provide a complete set of stool samples (Fig 3).
Fig 3. Study diagram.
An almost equal proportion of participants originated from CPS and SVK province (50.3% vs. 49.7%, respectively). About 60% of study participants were women. The mean age of the participants was 50.0 (SD 10.7) years. More than half of the participants (52.5%) were aged between 35 and 49 years. Almost all participants (98.8%) belonged to Lao-Thai ethnic group. About half (47.2%) of participants had completed primary school education. More than two-thirds (79.4%) were farmers and/or laborer and/or fishermen (Table 1).
Table 1. Socio-demographic and -economic characteristics of the study participants stratified by suspected cholangiocarcinoma (data presented as row percentage, except in the overall column).
| Variables | Overall (N = 3,400) | Suspected CCA | No suspected CCA |
Pearson χ2 | |||
|---|---|---|---|---|---|---|---|
| N (%) | N = 274 | (%) | N = 3,126 | (%) | |||
| Province | Champasack | 1,711 (50.3) | 127 | 7.4 | 1,584 | 92.6 | 1.88 |
| Savannakhet | 1,689 (49.7) | 147 | 8.7 | 1,542 | 91.3 | ||
| Gender | male | 1,368 (40.2) | 131 | 9.6 | 1,237 | 90.4 | 7.11** |
| female | 2,032 (59.8) | 143 | 7.0 | 1,889 | 93.0 | ||
| Age in years | mean age (±SD) | 50.02 (±10.73) | 52.1 (±10.7) | 49.8 (±10.7) | |||
| Age groups | 35–49 year | 1,784 (52.5) | 120 | 6.7 | 1,664 | 93.3 | -3.35*** |
| 50–59 year | 909 (26.7) | 79 | 8.7 | 830 | 91.3 | ||
| ≥ 60 year | 707 (20.8) | 75 | 10.6 | 632 | 89.4 | ||
| Ethnicity | Lao-Thai | 3,357 (98.8) | 272 | 8.1 | 3,085 | 91.9 | 0.58 |
| Mon-Khmer/Mong-Mein | 43 (1.3) | 2 | 4.7 | 41 | 95.4 | ||
| Education | illiterate | 591 (17.4) | 56 | 9.5 | 535 | 90.5 | 4.94 |
| up to primary school | 1,606 (47.2) | 137 | 8.5 | 1,469 | 91.5 | ||
| secondary school & above | 1,203 (35.4) | 81 | 6.7 | 1,112 | 93.3 | ||
| Profession | housewife/elderly/retired | 306 (9.0) | 27 | 8.8 | 279 | 91.2 | 3.83 |
| farmer/laborer/fisherman | 2,699 (79.4) | 225 | 8.3 | 2,474 | 91.7 | ||
| civil servant/trader | 395 (11.6) | 22 | 5.6 | 373 | 94.4 | ||
| SES | poor tertile | 1,079 (31.7) | 73 | 6.8 | 1,006 | 93.3 | 5.39 |
| middle tertile | 1,072 (31.5) | 84 | 7.8 | 988 | 92.2 | ||
| wealthy tertile | 1,249 (36.7) | 117 | 9.4 | 1,132 | 90.6 | ||
Note. CCA–cholangiocarcinoma; ±SD: ± standard deviation; SES–social-economic status. Pearson x2 compares the independence between suspected CCA prevalence and each sociodemographic and–economic variables. *p-value < 0.05, **p-value < 0.01, ***p-value < 0.001
3.2. Prevalence of liver morbidity according to demographic characteristics and health risks
Ultrasound examination showed bile duct dilatation in the left liver lobe (LLL), right liver lobe (RLL), and common bile duct (CBD) at rates of 4.5%, 4.6%, and 3.3%, respectively. The shotgun sign was observed in 5.9% of participants and atypical liver masses found in 1.5% of them. When all the criteria for suspected CCA were considered, the overall prevalence of suspected CCA was determined to be 7.2% (95% CI 5.4−9.6) (Table 2). Suspected CCA prevalence in CPS was 7.4% (127 out of 1,711), and in Savannakhet, it was 8.7% (147 out of 1,689). The prevalence of suspected CCA was significantly higher in men (9.6%, 131/1,368) compared to women (7.0%, 143/2,032).
Table 2. Prevalence of liver morbidities.
| Variables | Overall (N = 3,400) | |
|---|---|---|
| N | % | |
| Bile duct dilatation | ||
| At left liver lobe (LLL) | 153 | 4.5 |
| At right liver lobe (RLL) | 157 | 4.6 |
| At common bile duct (CBD) | 113 | 3.3 |
| Double-barrel shotgun sign | 200 | 5.9 |
| Any combined | 249 | 7.3 |
| Liver mass | 52 | 1.5 |
| Suspected CCA | 274 | 7.2 (5.4−9.6)* |
*Prevalence rate and 95% confidence interval were derived from logistic regression random-effect model (village as a random effect); N–number of observation.
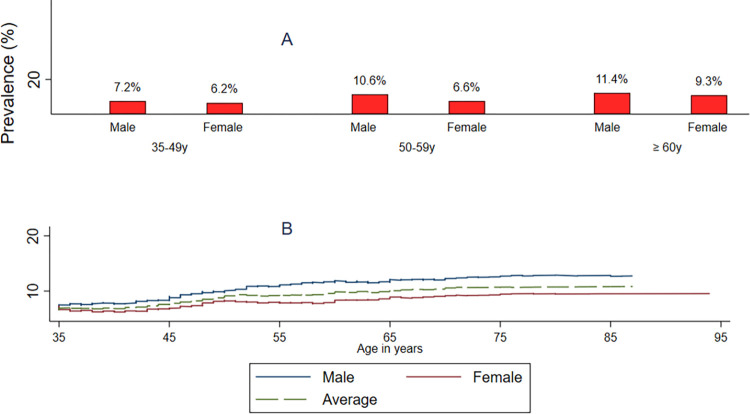
The prevalence of suspected CCA increased significantly with age, reaching a high of 10.6% among participants 60 years or older. The age-related increase was observed in both genders, with a higher overall mean prevalence among men across all age groups (Fig 4).
Fig 4. A indicates average prevalence by age group and gender; B indicates average prevalence by ages and gender.
Upon examining the prevalence distributions across various risk factors, it appears that a significantly higher prevalence of suspected CCA is likely to be found in the group that exhibited a higher prevalence of OV infection (8.8% vs 2.5%) and in higher OV infection intensity group (heavy: 21.9% vs moderate: 11.6% vs non/light: 4.8%). It was significantly higher in individuals with periductal fibrosis (12.3% vs 4.5%), absence/mild fatty liver (8.5% vs 3.9%), gallbladder stones (11.1% vs 7.7%), gallbladder sludge (26.2% vs 6.6%), and a history of cholecystectomy (16.7% vs 7.8%), compared with the group without suspected CCA (Table 3). The distribution of other factors did not differ between those two groups.
Table 3. OV infection status and other covariates stratified by suspected cholangiocarcinoma status (data presented as row percentage, except in the overall column).
| Covariates | Overall (N = 3,400) | Suspected CCA | No suspected CCA | Pearson x2 | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | N = 274 | (%) | N = 3,126 | (%) | ||||
| Infection | ||||||||
| Opisthorchis viverrini | Negative | 397 (11.7) | 10 | 2.5 | 387 | 97.5 | 18.62*** | |
| Positive | 3,003 (88.3) | 264 | 8.8 | 2,739 | 91.2 | |||
| OV infection intensity | Non to light intensity | 2,130 (62.7) | 102 | 4.8 | 2,028 | 95.2 | 110.48*** | |
| Moderate intensity | 1,028 (30.2) | 119 | 11.6 | 909 | 88.4 | |||
| Heavy intensity | 242 (7.1) | 53 | 21.9 | 189 | 78.1 | |||
| Hepatitis B status | HBsAg- | 3,295 (96.9) | 264 | 8.0 | 3,031 | 92.0 | 0.31 | |
| HBsAg+ | 105 (3.1) | 10 | 9.5 | 95 | 90.5 | |||
| Health risk | ||||||||
| Obesity | lean | 1,873 (55.1) | 163 | 8.7 | 1,710 | 91.3 | 2.33 | |
| not lean | 1,527 (44.9) | 111 | 7.3 | 1,416 | 91.7 | |||
| T2DM | no | 3,093 (91.0) | 256 | 8.3 | 2,837 | 91.7 | 2.20 | |
| yes | 307 (9.0) | 18 | 5.9 | 289 | 94.1 | |||
| No. of PZQ cycles | Never | 2,029 (59.7) | 177 | 8.7 | 1,852 | 91.3 | 6.37* | |
| 1–2 times | 682 (20.1) | 39 | 5.7 | 643 | 94.3 | |||
| ≥ 3 times | 689 (20.3) | 58 | 8.4 | 631 | 91.6 | |||
| Lifestyle | ||||||||
| Smoking | Never smokers | 2,210 (65.0) | 171 | 7.7 | 2,039 | 92.3 | 0.88 | |
| Past or current | 1,190 (35.0) | 103 | 8.7 | 1,087 | 91.3 | |||
| Alcohol consumption | Less | 3,181 (93.6) | 258 | 8.1 | 2,923 | 91.9 | 0.18 | |
| ≥ 3 times per week | 219 (6.4) | 16 | 7.3 | 203 | 92.7 | |||
| Ever eaten raw fish | No | 409 (12.0) | 31 | 7.6 | 378 | 92.4 | 0.14 | |
| Yes | 2,991 (88.0) | 243 | 8.1 | 2,748 | 91.9 | |||
| Co-morbidity/Intermediate symptoms | ||||||||
| Liver cirrhosis | Absence | 3,343 (98.3) | 268 | 8.0 | 3,075 | 92.0 | 0.48 | |
| Presence | 57 (1.7) | 6 | 10.5 | 51 | 89.5 | |||
| Periductal fibrosis | Absence | 1,852 (54.5) | 84 | 4.5 | 1,768 | 95.5 | 68.15*** | |
| Presence (all stages) | 1,548 (45.5) | 190 | 12.3 | 1,358 | 87.7 | |||
| Fatty liver | Absence or mild | 3,045 (89.6) | 260 | 8.5 | 2,785 | 91.5 | 9.06*** | |
| Moderate or severe | 355 (10.4) | 14 | 3.9 | 341 | 96.1 | |||
| Gallbladder stones | Absence | 3,067 (90.2) | 237 | 7.7 | 2,830 | 92.3 | 4.64* | |
| Presence | 333 (9.8) | 37 | 11.1 | 296 | 88.9 | |||
| Gallbladder sludge | Absence | 3,148 (92.6) | 208 | 6.6 | 2,940 | 93.4 | 120.8*** | |
| Presence | 252 (7.4) | 66 | 26.2 | 186 | 73.8 | |||
| Cholecystectomy | No | 3,310 (97.4) | 259 | 7.8 | 3,051 | 92.2 | 9.24** | |
| Yes | 90 (2.7) | 15 | 16.7 | 75 | 83.3 | |||
| Clinical symptoms* | No | 3,068 (90.2) | 247 | 8.1 | 2,821 | 92.0 | 0.01 | |
| Yes | 332 (9.8) | 27 | 8.1 | 305 | 92.0 | |||
Notes. N indicates the number of participants; HBsAg–hepatitis B surface virus antigen; T2DM–type 2 diabetes mellitus; *clinical symptoms mentioned in method section; CCA: cholangiocarcinoma; PZQ: Praziquantel. OV–Opisthorchis viverrini; HBsAg–hepatitis B virus surface antigen; light OV infection intensity, EPG between 0 and 999; moderate, EPG between 999 and 10,000; and heavy, EPG>10,000
*p-value < 0.05
**p-value < 0.01
***p-value < 0.001
3.3. Regression analysis
By comparing the results of the analysis of the bivariate model with different multivariate models, we observed only slight variations of the results (Table 4). In the fully adjusted model, only two predictors showed statistically significant associations with suspected CCA, including OV infection (aOR 3.4, 95% CI 1.7−6.5), and a history of cholecystectomy (aOR 2.7, 95% CI 1.5−4.9). HBV infection (aOR 1.1, 95% CI 0.6−2.2) and regular alcohol consumption (aOR 1.0, 95% CI 0.6−1.8) were associated with a statistically non-significant increased risk of suspected CCA, while T2DM (aOR 0.7, 95% CI 0.4−1.2) and smoking (aOR 0.9, 95% CI 0.7−1.3) were associated with a statistically non-significant decreased risk of suspected CCA (Table 4).
Table 4. Multivariate analysis of covariates associated with suspected cholangiocarcinoma (N = 3,400).
| Covariates | Bivariate model | Model adjusted for gender and age | Model adjusted for all socioeconomic & demographic | Model adjusted for co-morbidity | Fully adjusted model |
|---|---|---|---|---|---|
| cOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Opisthorchis viverrini | |||||
| Negative | Ref | Ref | Ref | Ref | Ref |
| Positive | 3.53 (1.84−6.77)*** | 3.60 (1.87−6.92)*** | 3.39 (1.76−6.53)*** | 3.47 (1.81−6.66)*** | 3.35 (1.74−6.45)*** |
| Hepatitis B status | |||||
| HBsAg- | Ref | Ref | Ref | Ref | Ref |
| HBsAg+ | 1.07 (0.54−2.12) | 1.11 (0.56−2.21) | 1.10 (0.55−2.20) | 1.08 (0.55−2.15) | 1.10 (0.55−2.19) |
| T2DM | |||||
| Non-DM | Ref | Ref | Ref | Ref | Ref |
| DM | 0.69 (0.42−1.14) | 0.63 (0.38−1.05) | 0.65 (0.34−1.08) | 0.80 (0.48−1.32) | 0.73 (0.43−1.22) |
| Post cholecystectomy | |||||
| No | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.46 (1.37−4.41)*** | 2.60 (1.43−4.74)*** | 2.70 (1.48−4.93)*** | 2.66 (1.47−4.83)*** | 2.67 (1.46−4.89)*** |
| Smoking | |||||
| Never smokers | Ref | Ref | Ref | Ref | Ref |
| Past & current | 1.18 (0.90−1.55) | 0.99 (0.73−1.35) | 0.94 (0.69−1.30) | 1.14 (0.86−1.51) | 0.93 (0.68−1.28) |
| Alcohol consumption | |||||
| Less | Ref | Ref | Ref | Ref | Ref |
| ≥ 3 times per week | 1.13 (0.66−1.95) | 1.01 (0.57−1.77) | 1.02 (0.58−1.80) | 1.06 (0.61−1.84) | 1.03 (0.58−1.82) |
Notes. CI: confidence intervals; cOR: crude odds ratio; aOR: adjusted odds ratio; DM diabetes mellitus; HBsAg hepatitis B virus surface antigen; PZQ Praziquantel
*p-value < 0.05
**p-value < 0.01
***p-value < 0.001.
In the sensitivity analysis, all associated variables retained their association with only some changes in the estimated odds ratios. When all individuals with T2DM were excluded, no relevant changes were observed (OV infection: aOR 3.4, 95% CI 1.7−6.8; cholecystectomy: aOR 2.5, 95% CI 1.3−4.9). When individuals with heavy OV infection intensity were excluded, no relevant changes were observed (OV infection: aOR 3.0, 95% CI 1.5−5.8; cholecystectomy: aOR 2.8, 95% CI 1.4−5.3). Exclusion of all individuals who had cholecystectomy done slightly increased the magnitudes of associations between OV infection and the likelihood of having suspected CCA (OV infection: aOR 3.9, 95% CI 1.9−8.1) (Table 5).
Table 5. Sensitivity analysis.
| Covariates | Exclude individuals with T2DM | Exclude individuals with heavy OV intensity | Exclude individuals who have undergone cholecystectomy |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Risk factors | |||
| Opisthorchis viverrini infection | |||
| Negative | Ref | Ref | Ref |
| Positive | 3.41 (1.71−6.80)*** | 3.00 (1.54−5.78)*** | 3.91 (1.89−8.10)*** |
| Hepatitis B status | |||
| HBsAg- | Ref | Ref | Ref |
| HBsAg+ | 1.15 (0.57−2.31) | 0.99 (0.46−2.13) | 1.13 (0.56−2.26) |
| T2DM | |||
| Non-DM | - | Ref | Ref |
| DM | - | 0.78 (0.45−1.35) | 0.71 (0.41−1.22) |
| Post cholecystectomy | |||
| No | Ref | Ref | - |
| Yes | 2.54 (1.33−4.86)*** | 2.76 (1.44−5.27)*** | - |
| Smoking | |||
| Never smokers | Ref | Ref | Ref |
| Past & current | 0.91 (0.66−1.27) | 1.01 (0.71−1.43) | 0.95 (0.69−1.30) |
| Alcohol consumption | |||
| Less | Ref | Ref | Ref |
| ≥ 3 times per week | 0.85 (0.46−1.58) | 1.03 (0.54−1.96) | 1.00 (0.57−1.77) |
Notes. CI: confidence intervals; aOR: adjusted odds ratio; OV Opisthorchis viverrini; DM diabetes mellitus; HBsAg hepatitis B virus surface antigen; PZQ-Praziquantel
*p-value < 0.05
**p-value < 0.01
***p-value < 0.001
4. Discussion
Due to the high burden of helminth infection like OV in Lao PDR, and the significant role of it in causing bile duct disease in humans, we hypothesize that the burden of liver disease in Lao populations is likely underestimated. Furthermore, focusing on OV infection alone might not capture the whole picture. Additionally, the complex interplay of multiple risk factors for hepatobiliary diseases raises the possibility that other factors besides OV infection might also significantly contribute to the disease. To address this hypothesis, we conducted a population-based investigation in southern Lao PDR to assess liver morbidity and its determinants, including both infectious and non-infectious risk factors. Our most important finding revealed an alarmingly high burden of deadly CCA in Lao PDR.
This US screening survey revealed a high overall prevalence of US-derived suspected CCA at 7.2% in the OV-endemic rural southern region of Lao PDR. Males and older individuals showed a higher prevalence, highlighting a critical public health concern for these groups within the already high disease burden. Furthermore, this rate was observed no significant difference in the distribution of suspected CCA between areas that received PZQ treatment and those that did not (7.4% in Champasack vs. 8.7% in Savannakhet), indicating the diminished role of PZQ treatment in the situation of repeated OV infection. Although our study focused on two provinces in southern Lao PDR, by considering the similarity of OV endemic regions characterized by high OV endemicity, shared raw-fish eating habits, and ecological features such as the main Mekong River, common to most southern provinces of Lao PDR, we can speculate that the rate of hepatic morbidity due to suspected CCA might be similarly high in other areas.
In comparison, two earlier studies conducted in Lao PDR reported lower US-derived suspected CCA rates of only 1.2% [25], and a similar rate documented in a previous US screening from 2007 to 2011 in several Lao PDR provinces, where 9.7% of 6,113 participants exhibited intrahepatic bile duct dilatation [35]. Additionally, data from an ongoing CCA screening and treatment program in Thailand (2015) revealed a prevalence of 5.6%. While not directly comparable due to geographical differences, this study from Thailand identified 2,661 cases of CCA among 47,285 individuals screened via US [8]. The notable discrepancy in suspected CCA rates could be attributed to various factors, including differences in population demographics or OV infection intensities under the population of studies. Furthermore, as the standard practice for CCA screening in Lao PDR has not been available, there might be some discrepancy of diagnostic definition among studies. For instance, in our study, all individuals with bile duct dilatation (> 2mm) were classified as suspected CCA, while other studies might considered using other criteria (e.g., Aye et al. reported a bile duct dilatation of 1.6%, but suspected CCA rate was at 1.2%) [25]. In the study by Kim et al., a suspected case classification based on the numbers of dilated intrahepatic branches termed "dilated intrahepatic bile duct grading" was used [35].
The results of this study focus on the pre-clinical manifestation of CCA examined within the community using ultrasound (US) examination. Extending this into a long-term follow-up study, preliminary results revealed that out of 274 initially suspected CCA cases (from this baseline US screening), a two-year follow-up study involving further radiological examinations and surgical treatment was conducted. During this period, 174 patients underwent a CT scan at Mahosot Hospital, Vientiane, Lao PDR. Among these individuals, 22 were highly suspicious for CCA based on imaging. Of these confirmed cases, 5 declined surgery, 5 underwent successful surgical procedures, and 12 died during the follow-up period. Importantly, all 5 patients who received surgery had histologically confirmed CCA from the surgical resection samples.
OV infection in this study was strongly associated with suspected CCA, reaffirming its role in pathogenesis. This cause-effect relationship has been extensively documented, particularly in the northeastern region of Thailand, where OV prevalence is moderately high and has been corroborated by both experimental and epidemiological investigations [9–11]. In southern Lao PDR, OV infection rates remain alarmingly elevated. Several key factors contribute to this situation: (i) the persistent consumption of raw or insufficiently cooked fish dishes and a lack of awareness about OV infection [36], akin to observations in northeastern Thailand [37]; (ii) the absence of regular information, education, and communication (IEC) initiatives, including mass drug administrations (MDA) currently limited to Champasack; and (iii) environmental factors conducive to OV’s life cycle, such as the Mekong River, various rivers, large man-made reservoirs, poor hygienic practices in rural communities, and the presence of animal reservoirs like dogs and cats [38].
OV infection is not only linked directly to CCA but also contributes to various bile duct morbidities. This is because of the parasite ability to interfere with bile stasis, the ability of the parasite eggs of stones formation, multiple hepatobiliary morbidities are associated including gallbladder sludge, gallbladder stone, hepatomegaly, cholangitis, periductal fibrosis, cholecystitis, and gallstones [33,34]. Our study identified a high prevalence of some of these morbidities, confirming the link between OV infection and morbidities throughout the bile systems. Periductal fibrosis (PDF) was the most prevalent morbidity observed in our study, affecting 45.5% of participants. PDF is a known intermediate step in the pathway to CCA development, caused by chronic irritation and inflammation from long-term OV infection [9,16,17,19,20]. This finding highlights the potential impact of chronic OV infection on our study population.
This study suggest a possible link between cholecystectomy and suspected CCA, however, interpretation of this association is challenging. This is because of a potential false positive result by defining CBD at >10mm, where this threshold might be questionable because the CBD can naturally widen after a cholecystectomy [26,27]. Therefore, the observed association and the interpretation of US screening results for CCA diagnosis require further investigation with additional follow-up and more sensitive techniques. Importantly, in areas with a high prevalence of OV infection, individuals with past OV infection remain at risk for developing CCA regardless of cholecystectomy status. This is supported by evidence from other studies, such as a meta-analysis of mixed study types showing an increased risk of confirmed CCA in patients who underwent cholecystectomy (pooled OR 1.5, 95% CI 1.2−1.9 [39], outside of OV endemic areas). These findings emphasize the need for careful clinical judgment when diagnosing CCA in patients with a history of cholecystectomy, especially in OV-endemic areas. Here, a comprehensive evaluation should consider (i) natural CBD dilatation after cholecystectomy, (ii) potential additional risk from cholecystectomy itself, and (iii) presence of OV infection.
In areas endemic for chronic HBV or HCV infection, such viruses are known as the main risk factors for common liver malignancies, such as HCC [40]. For CCA, there have been some links between viral hepatitis and CCA. For instance, Palmer et al., reported an increased combined OR for both viral HBV infection (OR 5.1, 95% CI 2.9−8.9) and HCV infection (OR 4.8, 95% CI 2.4−9.7) with increased risk for iCCA [41]. Furthermore, a cohort study conducted in Korea also reported HBV infection and an increased risk of CCA (HR 2.7, 95% CI 1.6−4.6), but not for HCV infection [42]. Despite the high prevalence of HBV among Lao adult population, such as 8.7% in blood adult donors according to a survey conducted in 2008 [13], this study found a lower prevalence of viral infection (<3%) and did not show a significant association with suspected CCA among the studied individuals. Although strong evidence from other studies support the link between HBV infection and CCA, our results suggest that HBV infection does not play a role in the pathogenesis of suspected CCA in Lao PDR.
An alcohol dose-response relationship has been observed elsewhere with the occurrence of CCA (e.g. consuming ≥3 alcoholic drinks/day) [43]. In a meta-analysis by Palmer et al., a combined OR of 2.8 (95% CI, 1.5–5.2) for the risk of iCCA was reported for heavy alcohol consumption (>80 g/day) [41], and, in a cohort study conducted in Korea, heavy alcohol consumption (≥40 g/day) was associated with an increased risk of CCA (HR 1.5, 95% CI 1.3–1.9) [42]. Furthermore, a large meta-analysis of 26 prospective studies revealed that high frequency of alcohol consumption, such as those who consumed five or more drinks a day, was associated with iCCA (HR 2.4, 95% CI 1.5–3.8) [44]. In our study, we did not find a significant association with high alcohol consumption and suspected CCA, likely due to a low prevalence of heavy drinker among our participants.
The association between smoking and CCA has been inconclusive in the literature. Different observational studies have reported no significant associations as example given in a matched case-control study from Thailand, by Poomphakwaen et al. reported no significant association (OR 0.9, 95% CI 0.5–2.8) between the two terms [10,14], and, similarly, in a case-control study by Songsorm et al., (aOR 1.2, 95% CI 0.5–2.8) [15]. In contrast, in a Korean cohort study, smoking was associated with an increased risk of CCA showing that those who currently smoke (less than 1 pack/day) had an increased risk for CCA, with a HR of 1.3 (95% CI 1.1–1.5) and those who currently smoke more than 1 pack/day had a HR increase to 1.5 (95% CI 1.1–1.9) [42]. A further example was reported in a meta-analysis of 26 prospective studies revealed a significant association between ever, former, and current smoking and CCA, with a HR of 1.7 (95% CI 1.3–2.1) [44]. In our study, we arrived at similar findings as other observational studies, indicating no associations between smoking and suspected CCA.
While studies from non-OV-endemic areas suggest a link between non-communicable diseases (NCDs) like type 2 diabetes (T2DM) and increased CCA risk (e.g., Korean cohort study: HR 1.4, 95% CI 1.2–1.6 [42]; meta-analysis: combined OR 1.9, 95% CI 1.7–2.1 for iCCA in T2DM patients [41]), and even higher risks in OV endemic areas (e.g., Thai study: 2.4-fold increased risk for CCA with both OV and T2DM [45]), our study did not find a significant association between these conditions. Studies in rodents suggest that liver fat deposition, a factor commonly seen in insulin resistance and T2DM patients, may worsen with OV infection and a high-fat, high-fructose diet (as shown by histological examination) [46]. This finding suggests a possible link between OV infection’s inflammatory effects and increased liver fat deposition in these animals. However, more evidence is needed to determine how interactions between T2DM and OV infection might influence the severity of liver disease and potentially contribute to CCA. Despite the lack of association between T2DM and CCA in this study, our previous findings suggest interactions between OV infection and certain NCDs. For example, we observed a lower steatotic liver disease in lean individuals infected with the OV liver fluke [47].
5. Conclusion
In summary, this population-based US screening for suspected CCA conducted in two rural area with high OV prevalence in Lao PDR has unveiled a significant burden of suspected CCA. The primary contributor to this burden is the high OV infection prevalence, highlighting the urgent need for intensified interventions to reduce OV infection and reinfection in these communities. Age and gender disparities in suspected CCA prevalence underscore the importance of targeted interventions. Beyond OV infection, known for its association with CCA, our study identified notable associated factors such as a history of cholecystectomy, providing valuable insights for early warning signs that require attention in preventive strategies. This research enhances our understanding of hepatobiliary morbidity in high-risk communities and can guide public health initiatives aimed at curbing the incidence of CCA in Lao PDR.
6. Limitation
Although mobile US is a very useful screening tool for suspected CCA, the complexity of CCA diagnostics can lead to misdiagnosis of early-stage tumors, as many liver morbidities can mimic similar conditions. Additionally, US cannot differentiate between liver mass types, such as distinguishing HCC from CCA. It is therefore important that patients with suspicious lesions undergo regular follow-up ultrasound examinations in order to monitor the changes of the bile duct dilatation and, in the event of suspected precancerous lesions, to undergo further diagnostics to confirm the diagnosis. Due to the nature of clustered sampling implemented in this study, the prevalence estimate may not be representative of the entire study population. Furthermore, the use of the Kato-Katz technique in detecting OV eggs might have encountered bias, as the egg morphology of OV is similar to that of other minute intestinal flukes (MIF). Moreover, there were suspicions that mixed species of liver fluke might be present in Lao PDR, such as Clonorchis sinensis, which is indistinguishable from OV based solely on egg morphology. The cross-sectional nature of the study does not allow interpretation the direction of associations and the causal inference.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the contribution of staffs from the Lao Tropical and Public Heaths Institute, Vientiane, Lao PDR, the National Center of Mariology, Parasitology and Entomology, Ministry of Health, Vientiane, Lao PDR, The University of Health Science, Vientiane, Lao PDR and the Provincial Health Department of Champasack and Savannakhet. We thank the District Health Office of Khong and Champhone districts for helping in the data collection, Kato-Katz examination, questionnaires administrations, and biospecimens collections. We are grateful for the study participants and authorities at all levels for their supports and contribution to our study.
Data Availability
The clinical data for this study contain potentially identifying and sensitive patient information, including baseline disease characteristics, ongoing clinical follow-up and treatment, and biosamples collected from patients in specific geographical areas. These biosamples are preserved for future research, which could potentially lead to patient identification. Due to these ethical concerns, as imposed by the Institutional Review Board of the National Ethics Committee for Health Research (NECHR), Ministry of Health, Lao PDR, and Lao TPHI, the data cannot be shared publicly. However, de-identified data can be made available upon reasonable request. Requests for access to the data should be directed to the Data Access Committee at NECHR, Samsenthai Road, Ban Kaognot, Sisattanak District, Vientiane Capital, Lao PDR. Tel: +856 21 250670; Fax: +856 21 214012; PO: 01000; email: nechr2021@gmail.com.
Funding Statement
The Swiss Agency for Development and Cooperation (SDC) and the Swiss National Science Foundation (SNSF) funded our study (project #IZ07Z0-160930, R4D to PO). Die Freiwillige Akademische Gesellschaft Basel (FAG Basel, www.fag-basel.ch) provided funding for mobile ultrasound machines (KK and PO). AH and PS received scholarship allowances for PhD study supported by Amt für Ausbildungsbeiträge (AFA, www.hochschulen.bs.ch) and SNF NEWTONIAN (project #IZJFZ3_185644). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver International. 2019;39:19–31. doi: 10.1111/liv.14095 [DOI] [PubMed] [Google Scholar]
- 2.Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. Journal of carcinogenesis. 2015;14. doi: 10.4103/1477-3163.151940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Annals of Oncology. 2013;24(6):1667–74. doi: 10.1093/annonc/mds652 [DOI] [PubMed] [Google Scholar]
- 4.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–25. doi: 10.1055/s-2004-828889 [DOI] [PubMed] [Google Scholar]
- 5.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9(5):588–94. doi: 10.1111/j.1365-3156.2004.01234.x [DOI] [PubMed] [Google Scholar]
- 6.Calvisi DF, Boulter L, Vaquero J, Saborowski A, Fabris L, Rodrigues PM, et al. Criteria for preclinical models of cholangiocarcinoma: scientific and medical relevance. Nature Reviews Gastroenterology & Hepatology. 2023:1–19. doi: 10.1038/s41575-022-00739-y [DOI] [PubMed] [Google Scholar]
- 7.Pattanathien P, Khuntikeo N, Promthet S, Kamsa-Ard S. Survival rate of extrahepatic cholangiocarcinoma patients after surgical treatment in Thailand. Asian Pac J Cancer Prev. 2013;14(1):321–4. doi: 10.7314/apjcp.2013.14.1.321 [DOI] [PubMed] [Google Scholar]
- 8.Khuntikeo N, Chamadol N, Yongvanit P, Loilome W, Namwat N, Sithithaworn P, et al. Cohort profile: cholangiocarcinoma screening and care program (CASCAP). BMC Cancer. 2015;15:459. doi: 10.1186/s12885-015-1475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201. doi: 10.1371/journal.pmed.0040201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poomphakwaen K, Promthet S, Kamsa-Ard S, Vatanasapt P, Chaveepojnkamjorn W, Klaewkla J, et al. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac J Cancer Prev. 2009;10(2):251–8. [PubMed] [Google Scholar]
- 11.Manwong M, Songserm N, Promthet S, Matsuo K. Risk factors for cholangiocarcinoma in the lower part of Northeast Thailand: a hospital-based case-control study. Asian Pac J Cancer Prev. 2013;14(10):5953–6. doi: 10.7314/apjcp.2013.14.10.5953 [DOI] [PubMed] [Google Scholar]
- 12.Alsaleh M, Leftley Z, Barbera TA, Sithithaworn P, Khuntikeo N, Loilome W, et al. Cholangiocarcinoma: a guide for the nonspecialist. Int J Gen Med. 2019;12:13–23. doi: 10.2147/IJGM.S186854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitbounlang P, Marchio A, Deharo E, Paboriboune P, Pineau P. The Threat of Multiple Liver Carcinogens in the Population of Laos: A Review. Livers. 2021;1(1):49–59. doi: 10.3390/livers1010005 [DOI] [Google Scholar]
- 14.Intajarurnsan S, Khuntikeo N, Chamadol N, Thinkhamrop B, Promthet S. Factors Associated with Periductal Fibrosis Diagnosed by Ultrasonography Screening among a High Risk Population for Cholangiocarcinoma in Northeast Thailand. Asian Pac J Cancer Prev. 2016;17(8):4131–6. [PubMed] [Google Scholar]
- 15.Songserm N, Promthet S, Sithithaworn P, Pientong C, Ekalaksananan T, Chopjitt P, et al. Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol. 2012;36(2):e89–94. doi: 10.1016/j.canep.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 16.Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitology international. 2012;61(1):208–11. doi: 10.1016/j.parint.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24(11):497–501. doi: 10.1016/j.pt.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khieu V, Sayasone S, Muth S, Kirinoki M, Laymanivong S, Ohmae H, et al. Elimination of schistosomiasis mekongi from endemic areas in Cambodia and the Lao People’s Democratic Republic: Current status and plans. Tropical medicine and infectious disease. 2019;4(1):30. doi: 10.3390/tropicalmed4010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, et al. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol. 2012;28(10):395–407. doi: 10.1016/j.pt.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamadol N, Pairojkul C, Khuntikeo N, Laopaiboon V, Loilome W, Sithithaworn P, et al. Histological confirmation of periductal fibrosis from ultrasound diagnosis in cholangiocarcinoma patients. J Hepatobiliary Pancreat Sci. 2014;21(5):316–22. doi: 10.1002/jhbp.64 [DOI] [PubMed] [Google Scholar]
- 21.Hughes T, O’Connor T, Techasen A, Namwat N, Loilome W, Andrews RH, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227–37. doi: 10.2147/IJGM.S133292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aye Soukhathammavong P, Vonghachack Y, Hatz C, Akkhavong K, Odermatt P. Suspected cases of cholangiocarcinoma seen in reference hospitals in Lao People’s Democratic Republic. Parasitol Int. 2017;66(4):510–4. doi: 10.1016/j.parint.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Mairiang E. Ultrasonographic features of hepatobiliary pathology in opisthorchiasis and opisthorchiasis-associated cholangiocarcinoma. Parasitol Int. 2017;66(4):378–82. doi: 10.1016/j.parint.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health Lao PDR. A diagnosis and treatment guideline for the district hospital in Lao PDR. 2nd ed ed. Vientiane Ministry of Health; 2004. [Google Scholar]
- 25.Aye Soukhathammavong P, Rajpho V, Phongluxa K, Vonghachack Y, Hattendorf J, Hongvanthong B, et al. Subtle to severe hepatobiliary morbidity in Opisthorchis viverrini endemic settings in southern Laos. Acta Trop. 2015;141(Pt B):303–9. doi: 10.1016/j.actatropica.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 26.Park SM, Kim WS, Bae I-H, Kim JH, Ryu DH, Jang L-C, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. Journal of the Korean Surgical Society. 2012;83(2):97. doi: 10.4174/jkss.2012.83.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson CJ, Lisanti CJ, Schwope RB, Ramsey D, Dinh T, Cochet A, et al. Mild asymptomatic intrahepatic biliary dilation after cholecystectomy, a common incidental variant. Abdominal Radiology. 2017;42:1408–14. doi: 10.1007/s00261-016-1017-z [DOI] [PubMed] [Google Scholar]
- 28.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. American Journal of Roentgenology. 2007;189(6):W320–W3. doi: 10.2214/AJR.07.2123 [DOI] [PubMed] [Google Scholar]
- 29.Katz N, Chaves A, Pellegrino J. A simple, device for quantita tive stool thick-smear technique in schistosomiasis mansoni. Revista do Instituto de medicina tropical de Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 30.Vonghachack Y, Odermatt P, Taisayyavong K, Phounsavath S, Akkhavong K, Sayasone S. Transmission of Opisthorchis viverrini, Schistosoma mekongi and soil-transmitted helminthes on the Mekong Islands, Southern Lao PDR. Infect Dis Poverty. 2017;6(1):131. doi: 10.1186/s40249-017-0343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayasone S, Meister I, Andrews JR, Odermatt P, Vonghachack Y, Xayavong S, et al. Efficacy and Safety of Praziquantel Against Light Infections of Opisthorchis viverrini: A Randomized Parallel Single-Blind Dose-Ranging Trial. Clin Infect Dis. 2017;64(4):451–8. doi: 10.1093/cid/ciw785 [DOI] [PubMed] [Google Scholar]
- 32.Xu R, Pan J, Zhou W, Ji G, Dang Y. Recent advances in lean NAFLD. Biomed Pharmacother. 2022;153:113331. doi: 10.1016/j.biopha.2022.113331 [DOI] [PubMed] [Google Scholar]
- 33.Sripa B, Kanla P, Sinawat P, Haswell-Elkins MR. Opisthorchiasis-associated biliary stones: light and scanning electron microscopic study. World Journal of Gastroenterology: WJG. 2004;10(22):3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mairiang E, Haswell-Elkins MR, Mairiang P, Sithithaworn P, Elkins DB. Reversal of biliary tract abnormalities associated with Opisthorchis viverrini infection following praziquantel treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(2):194–7. doi: 10.1016/0035-9203(93)90489-d [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Yong TS, Rim HJ, Chai JY, Min DY, Eom KS, et al. Ultrasonographic investigation of cholangiocarcinoma in Lao PDR. Acta Trop. 2018;182:128–34. doi: 10.1016/j.actatropica.2018.02.031 [DOI] [PubMed] [Google Scholar]
- 36.Yoon HJ, Ki M, Eom K, Yong TS, Chai JY, Min DY, et al. Risk factors for Opisthorchis viverrini and minute intestinal fluke infections in Lao PDR, 2009–2011. Am J Trop Med Hyg. 2014;91(2):384–8. doi: 10.4269/ajtmh.13-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saengsawang P, Promthet S, Bradshaw P. Reinfection by Opisthorchis Viverrini after Treatment with Praziquantel. Asian Pac J Cancer Prev. 2016;17(2):857–62. doi: 10.7314/apjcp.2016.17.2.857 [DOI] [PubMed] [Google Scholar]
- 38.Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, et al. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101(1):40–7. doi: 10.1016/j.trstmh.2006.02.018 [DOI] [PubMed] [Google Scholar]
- 39.Xiong J, Wang Y, Huang H, Bian J, Wang A, Long J, et al. Systematic review and meta-analysis: cholecystectomy and the risk of cholangiocarcinoma. Oncotarget. 2017;8(35):59648–57. doi: 10.18632/oncotarget.19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1198–203. doi: 10.1158/1055-9965.EPI-05-0811 [DOI] [PubMed] [Google Scholar]
- 41.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. doi: 10.1016/j.jhep.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho IR, Yi SW, Choi JS, Yi JJ. Comparison of Risk Factors for Cholangiocarcinoma and Hepatocellular Carcinoma: A Prospective Cohort Study in Korean Adults. Cancers (Basel). 2022;14(7). doi: 10.3390/cancers14071709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25(8):1526–35. doi: 10.1093/annonc/mdu020 [DOI] [PubMed] [Google Scholar]
- 44.McGee EE, Jackson SS, Petrick JL, Van Dyke AL, Adami HO, Albanes D, et al. Smoking, Alcohol, and Biliary Tract Cancer Risk: A Pooling Project of 26 Prospective Studies. J Natl Cancer Inst. 2019;111(12):1263–78. doi: 10.1093/jnci/djz103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thinkhamrop K, Khuntikeo N, Laohasiriwong W, Chupanit P, Kelly M, Suwannatrai AT. Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl Trop Dis. 2021;15(9):e0009741. doi: 10.1371/journal.pntd.0009741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaidee A, Onsurathum S, Intuyod K, Haonon O, Pannangpetch P, Pongchaiyakul C, et al. Opisthorchis viverrini Infection Augments the Severity of Nonalcoholic Fatty Liver Disease in High-Fat/High-Fructose Diet-Fed Hamsters. Am J Trop Med Hyg. 2019;101(5):1161–9. doi: 10.4269/ajtmh.19-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homsana A, Southisavath P, Kling K, Hattendorf J, Vorasane S, Paris DH, et al. Steatotic liver disease among lean and non-lean individuals in Southern Lao PDR: a cross-sectional study of risk factors. Annals of Medicine. 2024;56(1):2329133. doi: 10.1080/07853890.2024.2329133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The clinical data for this study contain potentially identifying and sensitive patient information, including baseline disease characteristics, ongoing clinical follow-up and treatment, and biosamples collected from patients in specific geographical areas. These biosamples are preserved for future research, which could potentially lead to patient identification. Due to these ethical concerns, as imposed by the Institutional Review Board of the National Ethics Committee for Health Research (NECHR), Ministry of Health, Lao PDR, and Lao TPHI, the data cannot be shared publicly. However, de-identified data can be made available upon reasonable request. Requests for access to the data should be directed to the Data Access Committee at NECHR, Samsenthai Road, Ban Kaognot, Sisattanak District, Vientiane Capital, Lao PDR. Tel: +856 21 250670; Fax: +856 21 214012; PO: 01000; email: nechr2021@gmail.com.