Abstract
Biofilm production is thought to be a crucial factor in the ability of Staphylococcus epidermidis to produce a biomaterial-based infection. A rat central venous catheter (CVC)-associated infection model was used to assess the importance of biofilm production, mediated by polysaccharide intercellular adhesin/hemagglutinin (PIA/HA), in the pathogenesis of intravascular catheter-associated infection. PIA/HA-positive S. epidermidis 1457 was significantly more likely to cause a CVC-associated infection (71 versus 14%, P < 0.03) resulting in bacteremia and metastatic disease than its isogenic PIA/HA-negative mutant. These results confirm the importance of biofilm production, mediated by PIA/HA, in the pathogenesis of S. epidermidis experimental CVC-associated infection.
Staphylococcus epidermidis is the most prominent cause of intravascular catheter-associated infection (28). According to the Centers for Disease Control and Prevention’s National Nosocomial Infection Surveillance System, S. epidermidis is responsible for 33.5% of nosocomial bloodstream infections (35). These bacteremias are largely due to intravascular catheter-associated infection. Unfortunately, nosocomial bacteremia due to S. epidermidis is a rapidly increasing problem and is responsible for significant morbidity and mortality (1, 22, 28).
Bacterial adherence to biomaterials appears to be a pivotal step in the pathogenesis of S. epidermidis infections. Adherence occurs in a complex, multistep process (16). The later phases of adherence, in which organisms adhere to one another and elaborate biofilm, are mediated by polysaccharide intercellular adhesin (PIA), which is synthesized by products of the four-gene operon ica (12, 17, 21). Recent investigation has revealed that PIA and the hemagglutinin (HA) of S. epidermidis are closely related, if not identical (7, 20). In addition, we recently demonstrated the importance of PIA/HA in the pathogenesis of experimental prosthetic device infections in the mouse foreign body infection model. To more closely mimic conditions found in the human intravascular system, a rat model of central venous catheter (CVC)-associated infection was developed and used to test the importance of PIA/HA in the pathogenesis of CVC-associated infection.
(This work was presented in abstract form at the 35th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., 13 to 16 September 1997 [26a]).
The bacterial strains used for these studies were S. epidermidis 1457 and a PIA/HA-negative isogenic mutant, S. epidermidis 1457-M10. Strain 1457 was isolated from a patient with an infected CVC and has been previously described (21). Strain 1457-M10 is a PIA/HA-negative isogenic mutant of strain 1457 that was produced by insertion of transposon Tn917 at nucleotide 931 in the icaA gene of the icaADBC gene locus (19, 20).
The rat CVC-associated infection model was used to compare the virulence of S. epidermidis 1457 with that of S. epidermidis 1457-M10. Fourteen male Sprague-Dawley rats underwent catheterization as previously described (34). Briefly, the surgical area was prepped and draped in a sterile fashion. The neck was surgically dissected and a Silastic lumen-within-lumen catheter (inside diameter, 0.064 cm [34a]) was inserted in the right external jugular vein and advanced into the superior vena cava. The proximal portion of the catheter was tunneled subcutaneously to exit in the midscapular space. The catheter was held in place by a rodent restraint jacket (34b) which simultaneously protected the catheter and allowed ready access to the vein. Twenty-four hours following CVC placement, blood was obtained from the catheters and cultured to ensure sterility, and 104 CFU of S. epidermidis 1457 or 1457-M10 was injected into the catheters. The catheters were flushed daily with a heparin solution. On day 8, the animals were sacrificed. Blood from the CVC and the periphery was obtained and quantitatively cultured by directly plating 0.1 ml of blood on Trypticase soy agar (Remel, Lenexa, Kans.). The location of the distal tip of the CVC in the superior vena cava was confirmed, and the catheters and surrounding venous tissue were removed aseptically and vigorously vortex washed in phosphate-buffered saline, after which the wash fluid was quantitatively cultured. Previous studies, in which quantitative culture results were confirmed by electron microscopy, documented complete removal of adherent organisms by this procedure (31). In addition, to ascertain the extent of metastatic disease, the heart, lungs, liver, and kidneys were aseptically harvested, weighed, homogenized in 1 ml of phosphate-buffered saline with sterile disposable tissue grinders (Sage Products, Crystal Lake, Ill.), and quantitatively cultured by plating 0.1 ml of the tissue homogenate on Trypticase soy agar. Bacteria recovered from the catheters, blood, or tissues were identified to the species level.
To limit the number of experimental animals used to the minimum that would reveal a significant difference in pathogenicity, inoculum size experiments were performed. Prior to the conducting of the comparative studies described above, the smallest inoculum of S. epidermidis 1457 that reliably resulted in a CVC-associated infection was determined in dose-ranging inoculum studies. CVCs were placed as described above. Three animals each were challenged with an inoculum of either 103, 104, or 105 CFU of S. epidermidis 1457. Following inoculation, the animals were evaluated for the presence of infection as described above.
The chi-square test was used to assess whether there was a significant difference in infection rate between the two groups of animals. The Wilcoxon signed-rank test or Mann-Whitney test was used to analyze data regarding levels of bacteremia and metastatic disease. Statistical tests were performed with Prism 2.0 (Graphpad, San Diego, Calif.).
The inoculum studies showed that all of the animals inoculated with 104 or 105 CFU of S. epidermidis 1457 developed CVC-associated infection with metastatic disease (data not shown). One of the animals inoculated with 103 CFU of S. epidermidis 1457 had organisms recovered from the CVC, blood, and lungs. The other two animals did not develop a CVC-associated infection. Therefore, the 104-CFU inoculum size, the lowest dose which reproducibly caused CVC-associated infection and metastatic disease, was used in the larger comparative trial.
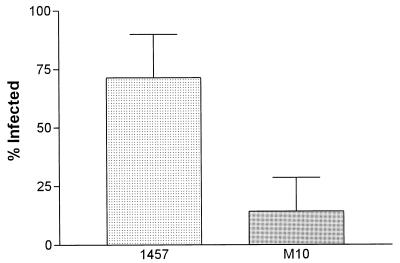
The overall infection rate, defined as recovery of S. epidermidis from the blood, catheter, or organs at the time of sacrifice, is shown in Fig. 1. More rats challenged with the wild-type strain developed CVC-associated infection than did those challenged with the PIA/HA mutant strain (71.4 versus 14.3%; P = 0.03, chi-square test).
FIG. 1.
Rate of CVC-associated infection in animals challenged with S. epidermidis 1457 or 1457-M10. Error bars represent standard errors of the means.
Five of the seven animals challenged with S. epidermidis 1457 had organisms recovered from the CVC at the time of sacrifice (mean ± standard deviation, 195 ± 92 CFU per catheter), compared to none of the seven animals challenged with the mutant strain 1457-M10 (P < 0.0001, chi-square test).
Four of the seven animals challenged with S. epidermidis 1457 had organisms recovered from the peripheral blood at the time of sacrifice (976 ± 413 CFU per ml), compared to one of the seven animals challenged with strain 1457-M10 (100 CFU per ml) (P = 0.02, Wilcoxon signed-rank test).
Table 1 summarizes results from studies defining the burden of metastatic disease in animals challenged with either S. epidermidis 1457 or 1457-M10. For all organ systems, there were more animals with metastatic disease in the group challenged with strain 1457 than in the group challenged with strain 1457-M10. In addition, for all organ systems, the number of organisms recovered per gram of tissue was greater in the animals challenged with 1457 than in those challenged with 1457-M10. However, these differences were not statistically significant.
TABLE 1.
Metastatic disease caused by S. epidermidis 1457 or 1457-M10 in the rat model of CVC-associated infectiona
| Organ |
S. epidermidis 1457
|
S. epidermidis 1457-M10
|
||
|---|---|---|---|---|
| No. of animals infected/total | Mean no. of CFU ± SD/g of tissue | No. of animals infected/total | Mean no. of CFU/g of tissue | |
| Lung | 4/7 | 2,855 ± 1,950 | 1/7 | 1,116 |
| Heart | 3/7 | 10,970 ± 9,394 | 1/7 | 411 |
| Liver | 3/7 | 9,045 ± 4,480 | 1/7 | 1,174 |
| Kidney | 2/7 | 305 ± 197 | 1/7 | 67 |
Differences were not significant, as determined by the Mann-Whitney test.
Bacterial adherence to biomaterials is thought to be a pivotal event in the pathogenesis of intravascular catheter-associated infections and infections with other prosthetic medical devices. It appears that bacterial adherence is a complex multistep process that is influenced by the host, the device, and the microbe. Gristina conveniently subdivided the adherence process into the following stages: attachment, adhesion, and aggregation (9). Aggregation, the final stage of adherence, is characterized by the formation of multicell macrocolonies and elaboration of biofilm. It has long been suspected that staphylococcal biofilm, also known as slime, is important in the pathogenesis of biomaterial-based infections (2, 4, 26). Biofilm appears to function in the later, aggregative stages of adherence and may serve to protect the organism from host phagocytic cells and improve the local nutritional environment (9). Unfortunately, until the development and application of molecular genetic techniques, the data regarding the importance of biofilm conflicted (3). Epidemiologic studies, clinical observations, and animal model studies were contradictory. For example, Christensen and coworkers, using a mouse foreign body infection model, found that a biofilm-producing strain of S. epidermidis caused three times more infections than a non-biofilm-producing strain (5). Conversely, Patrick and colleagues, using the same model, observed that although biofilm-producing S. epidermidis strains adhered to catheters in greater numbers than non-biofilm-producing strains, they were less likely to cause a clinically evident infection (24). Likewise, efforts to biochemically characterize biofilm were fraught with difficulty (6).
More recently, techniques to genetically manipulate S. epidermidis have been improved, and a number of specific factors related to biofilm have been described. PIA, described by Mack and colleagues, is synthesized by the gene products of the icaADBC locus, the genes of which are organized in an operon structure (8, 12, 17, 21). PIA is a polysaccharide that is elaborated by the majority of clinically significant strains of S. epidermidis (17, 18, 36). Isogenic, PIA-negative Tn917 insertional mutants are capable of attachment to biomaterials but are unable to form multilayer macrocolonies or to produce biofilm (19). The HA of S. epidermidis has also been observed in the majority of clinically relevant strains and is composed of carbohydrates (27, 30). HA appears to play a role in adherence (15, 27, 29, 30), and recent phenotypic and genotypic characterizations revealed that PIA and HA are closely related, if not identical (7, 20). In addition, a 140-kDa extracellular protein that appears to be involved in cellular aggregation and biofilm formation has been described (13). A number of investigators have described factors that appear to be significant in the early stages of adherence. These include a polysaccharide adhesin (PSA) described by Tojo and coworkers (33) and a 148-kDa proteinaceous autolysin described by Heilmann and colleagues (10, 11). Recently, data indicating that the ica locus may also encode production of PSA and that PSA mediates both initial adherence and aggregation were reported (23).
Because of the complexity of the milieu surrounding implanted prosthetic medical devices, it is difficult to duplicate these conditions in in vitro models, and it is desirable to test hypotheses related to pathogenesis in in vivo models that reflect the human condition. We recently demonstrated the importance of PIA/HA in a mouse foreign body infection model (31). However, this model does not accurately reflect the dynamics of the intravascular environment, and thus we developed the rat CVC-associated infection model. This model makes use of a rat CVC and a rodent restraint jacket that allow for long-term venous access and mimic the condition found in surgically implanted CVCs in humans, such as Hickman or Broviac catheters. Our model differs from other models used to study staphylococcal prosthetic device infection pathogenesis in several important respects. As mentioned above, the mouse foreign body model does not involve the vascular space. Consequently, features such as blood flow dynamics, serum proteins and other blood components, and humoral immunity are not accurately reflected. Tissue cages have been used in guinea pigs and other animal species to study the interaction between microbes and biomaterials (37). However, the limitations noted for the mouse foreign body model also apply to the tissue cage models. A number of investigators have used the rabbit model of endocarditis to study putative virulence determinants of coagulase-negative staphylococci. However, the model relies on a foreign body placed across the heart valve to induce endothelial damage and involves the arterial circulation. Coagulase-negative staphylococci are a very rare cause of native valve endocarditis, and therefore, this model may not be the optimum model to study the pathogenesis of infections caused by these organisms. Factors that predispose bacteria to adherence to damaged endothelial cells and contribute to the propagation of cardiac vegetations may not be the same factors that mediate both adherence to biomaterials and the formation of biofilms on prosthetic devices. Also, catheterization of the high-pressure arterial system may not reflect conditions in the venous system. These factors may explain why Perdreau-Remington et al. noted that chemically induced mutants deficient in biofilm formation showed no decreased virulence in the rabbit endocarditis model (25). Also, it has been demonstrated that the mutant M7, used in those studies, is capable of PIA production (13). A model that closely approximates human CVC-associated infection was employed by Kojima and colleagues to study the protective value of antibody directed against PSA (14). In this rabbit CVC-associated infection model, rabbits underwent implantation of jugular venous catheters that were seeded with bacteria. The catheters were connected to subcutaneously implanted osmotic pumps that delivered a continuous flow of heparin solution. Antibody against PSA appeared to protect the animals from the development of bacteremia. PSA is a capsular polysaccharide that appears to play a role in the early stages of adherence (32, 33). PSA-deficient transposon mutants were also less virulent in the rabbit endocarditis model (32).
The rat model utilized in this study appears to mimic the human condition. In this model we demonstrated that PIA/HA is important in the pathogenesis of CVC-associated infection. Isogenic, PIA/HA-deficient mutants caused lower overall infection rates, were recovered in lower numbers from the implanted catheters and the bloodstream, and caused less metastatic disease than the wild-type parent strain. These observations may be due to the inability of the PIA/HA-negative mutant to form macrocolonies in the aggregative stage of adherence or may be secondary to the immunologic or nutritional properties of biofilm. Although the burden of metastatic disease was greater in animals infected with the wild-type strain of S. epidermidis, the differences did not reach statistical significance. Several explanations are possible. First, the number of animals simply may not have been great enough to demonstrate significance in the secondary measures of CVC-associated infection (primary endpoints being organisms recovered from the CVC and blood). Alternatively, once the CVC is infected, there may not be differences in virulence. PIA/HA may not influence metastatic seeding of organs which may be secondary to as-yet-undefined nonspecific or tissue-specific adhesins.
The pathogenesis of prosthetic device infections is a complex process in which bacterial adherence to biomaterials and elaboration of biofilm are crucial. Future work should be directed at increasing the understanding of how the various adhesins and putative virulence determinants interplay to result in a prosthetic device infection.
Acknowledgments
This work was supported by a Grant-in-Aid from the American Heart Association, 96006810 (M.E.R.).
We thank Gordon L. Archer for review of the manuscript and James Anderson for assistance with the statistical analysis.
REFERENCES
- 1.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J the National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91(Suppl. 3B):3B–86S. doi: 10.1016/0002-9343(91)90349-3. 3B89S. [DOI] [PubMed] [Google Scholar]
- 2.Bayston R, Penny S R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonization of Holter shunts. Dev Med Child Neurol. 1972;14(Suppl. 27):25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- 3.Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 45–78. [Google Scholar]
- 4.Christensen G D, Parisi J T, Bisno A L, Simpson W A, Beachey E H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983;18:258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drewry D T, Galbraith L, Wilkinson B J, Wilkinson S G. Staphylococcal slime: a cautionary tale. J Clin Microbiol. 1990;28:1292–1296. doi: 10.1128/jcm.28.6.1292-1296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fey P D, Ulphani J S, Heilmann C, Gotz F, Mack D, Rupp M E. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. Polysaccharide intercellular adhesin (PIA) mediates hemagglutination (HA) in Staphylococcus epidermidis, abstr. B-40; p. 62. [Google Scholar]
- 8.Gerke C, Krafts A, Süßmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18594. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 9.Gristina A G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 12.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima Y, Tojo M, Goldmann D A, Toseteson T D, Pier G B. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter-related bacteremia due to coagulase-negative staphylococci. J Infect Dis. 1990;162:435–441. doi: 10.1093/infdis/162.2.435. [DOI] [PubMed] [Google Scholar]
- 15.Limoges J, Han J, Rupp M E. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Adherence dynamics of hemagglutination (HA)-positive and hemagglutination-negative Staphylococcus epidermidis, abstr. B-436; p. 241. [Google Scholar]
- 16.Mack, D. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect., in press. [DOI] [PubMed]
- 17.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 19.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack D, Riedewald J, Rohde H, Magnus T, Feucht H H, Elsner H-A, Laufs R, Rupp M E. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect Immun. 1999;67:1004–1008. doi: 10.1128/iai.67.2.1004-1008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin M A, Pfaller M A, Wenzel R P. Coagulase-negative staphylococcal bacteremia. Ann Intern Med. 1989;110:9–16. doi: 10.7326/0003-4819-110-1-9. [DOI] [PubMed] [Google Scholar]
- 23.McKenney D, Hübner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick C C, Plaunt M R, Hetherington S V, May S M. Role of the Staphylococcus epidermidis slime layer in experimental tunnel tract infections. Infect Immun. 1992;60:1363–1367. doi: 10.1128/iai.60.4.1363-1367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perdreau-Remington F, Sande M A, Peters G, Chambers H F. The abilities of a Staphylococcus epidermidis wild-type strain and its slime-negative mutant to induce endocarditis in rabbits are comparable. Infect Immun. 1998;66:2778–2781. doi: 10.1128/iai.66.6.2778-2781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- 26a.Rupp M E, Ulphani J S, Fey P D, Mack D. Abstracts of the 35th Annual Meeting of the Infectious Diseases Society of America. 1997. Characterization of Staphylococcus epidermidis 1457 and an isogenic PIA-negative/HA-negative mutant in a rat CVC-associated infection model, abstr. 388; p. 143. [Google Scholar]
- 27.Rupp M E, Archer G L. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect Immun. 1992;60:4322–4327. doi: 10.1128/iai.60.10.4322-4327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–245. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 29.Rupp M E, Han J. Inhibition of Staphylococcus epidermidis hemagglutination and adherence to intravenous catheters by β-lactose. Clin Infect Dis. 1993;17:554. [Google Scholar]
- 30.Rupp M E, Sloot N, Meyer H G W, Han J, Gatermann S. Characterization of the hemagglutinin of Staphylococcus epidermidis. J Infect Dis. 1995;172:1509–1518. doi: 10.1093/infdis/172.6.1509. [DOI] [PubMed] [Google Scholar]
- 31.Rupp M E, Ulphani J S, Lewis M, Mack D. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Characterization of Staphylococcus epidermidis 1457 and an isogenic PIA-negative/HA-negative mutant in a mouse foreign body infection model, abstr. B-405; p. 98. [Google Scholar]
- 32.Shiro H, Muller E, Gutierrez N, Boisot S, Grout M, Tosteson T D, Goldmann D, Pier G B. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J Infect Dis. 1994;169:1042–1049. doi: 10.1093/infdis/169.5.1042. [DOI] [PubMed] [Google Scholar]
- 33.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- 34.Ulphani, J. S., and M. E. Rupp. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab. Anim. Sci., in press. [PubMed]
- 34a.University of Nebraska Board of Reagents. June 1998. U.S. patent 5,762, 636.
- 34b.University of Nebraska Board of Reagents. November 1998. U.S. patent 5, 839,393.
- 35.U.S. Department of Health and Human Services, Public Health Service. National nosocomial infections surveillance (NNIS) report, data summary from October 1986–April 1997. Am J Infect Control. 1997;25:477–487. [PubMed] [Google Scholar]
- 36.Ziebuhr W, Heilmann C, Götz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerli W, Waldvogel F A, Vaudaux P, Nydegger U E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]