Abstract
Background
The Japan Clinical Oncology Group (JCOG) 1006 was a phase III trial of patients with clinical T3/T4 colon cancer comparing the no-touch isolation technique (‘No Touch’) with the conventional technique (‘Conventional’). The planned primary analysis at 3 years failed to confirm the superiority of the No Touch over the ‘Conventional’. The present study aimed to compare the ‘No Touch’ and ‘Conventional’ using long-term (6-year) follow-up data.
Methods
Patients aged 20–80 years who had a clinical classification of T3–4, N0–2, and M0 with histologically proven colon cancer were randomly assigned (1 : 1) to undergo open surgery using ‘Conventional’ or ‘No Touch’ techniques. The primary endpoint was disease-free survival.
Results
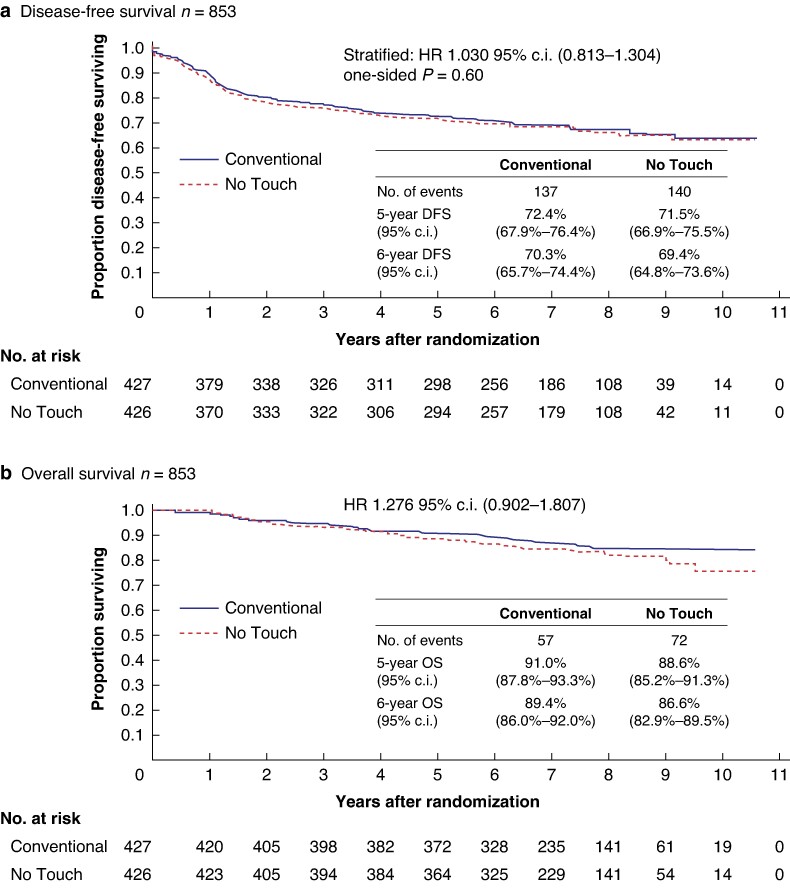
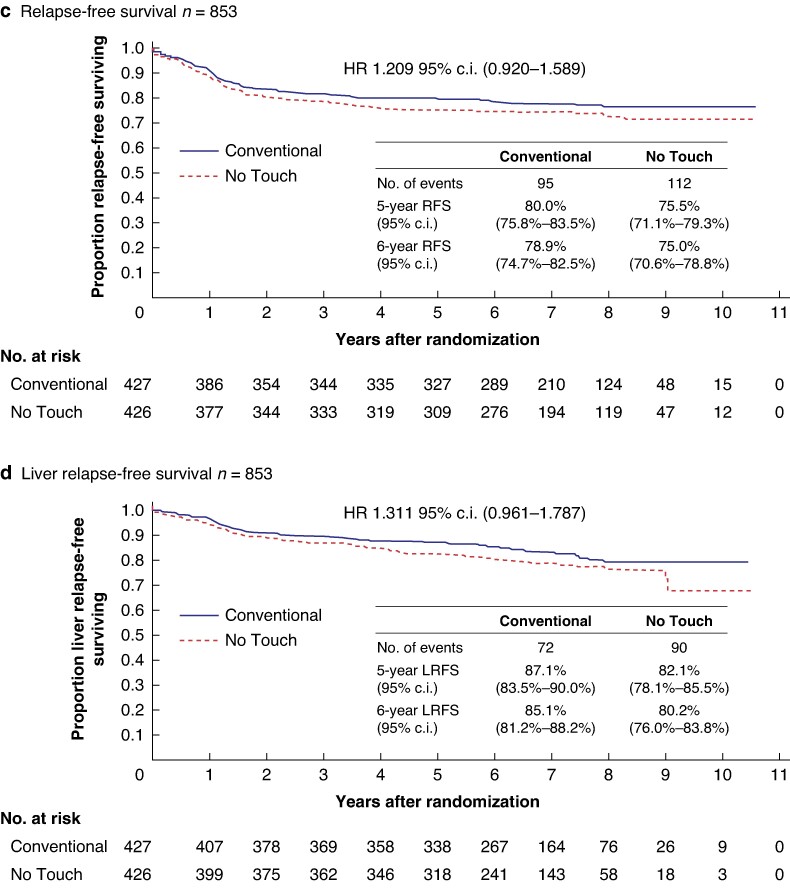
In total, 853 patients from 30 institutions were assigned to the ‘Conventional’ (427) or ‘No Touch’ (426) groups between June 2011 and November 2015. The 6-year disease-free survival was 70.3% and 69.4% for ‘Conventional’ and ‘No Touch’ arms respectively (HR 1.030; 95% c.i. 0.813 to 1.304; one-sided P = 0.60). The 6-year overall survival was 89.4% and 86.6% respectively (HR 1.276; 95% c.i. 0.902 to 1.807). The 6-year relapse-free survival was 78.9% and 75.0% respectively (HR 1.209; 95% c.i. 0.920 to 1.589). The 6-year liver relapse-free survival was 85.1% and 80.2% respectively (HR 1.311; 95% c.i. 0.961 to 1.787).
Conclusion
Long-term follow-up data did not support the superiority of ‘No Touch’ over ‘Conventional’ technique in patients with stages II and III colon cancer. These study findings indicate that the conventional technique is still standard surgery for managing colon cancers.
Trial registration number
UMIN000004957
The present study aimed to compare ‘No Touch’ and ‘Conventional’ techniques using long-term (6-year) follow-up data. Long-term follow-up data did not support the superiority of ‘No Touch’ over ‘Conventional’ in patients with stages II and III colon cancer.
Introduction
Surgical resection is the most effective strategy for managing colorectal cancer with curative intent1. Previously, two different approaches to colonic resection were described: the conventional technique (‘Conventional’), which prioritizes the mobilization of the tumour-bearing colon segment followed by central vascular ligation (CVL), and the no-touch isolation technique (‘No Touch’), which prioritizes CVL followed by the mobilization of the tumour-bearing colon segment. The latter aims to reduce the risk of cancer cells spreading to the liver and other organs2.
Turnbull et al.3 and Slanetz4 advocated for the ‘No Touch’ technique to reduce recurrence risk in their retrospective studies. Wiggers et al.5 compared the ‘No Touch’ and ‘Conventional’ techniques in a randomized study with a large number of participants (117 and 119 respectively) and concluded the impossibility of demonstrating the superiority of ‘No Touch’ over ‘Conventional’. However, the sample size was insufficient to elucidate a comparison between the techniques.
JCOG1006 compared ‘No Touch’ and ‘Conventional’ using disease-free survival (DFS) based on the intention-to-treat principle as the primary endpoint. The planned primary analysis at 3 years failed to confirm the superiority of ‘No Touch’ over ‘Conventional’, as evidenced by the absence of significant differences in DFS, overall survival (OS), relapse-free survival (RFS), and liver relapse-free survival (LRFS)6. This failure to confirm the superiority of ‘No Touch’ over ‘Conventional’ may be attributed to some factors. The postoperative survival reported in recent studies has improved markedly compared with that reported in previous reports. Turnbull et al.3 reported a 5-year OS of 52.5% in patients after standard therapy for Dukes A–C disease in the 1960s, which is similar to Stage I–III disease. Wiggers et al.5 reported a 5-year DFS of approximately 60% after standard therapy for Dukes A–C disease in the 1980s. The JCOG1006 revealed a 5-year OS of 90.5% after standard therapy for clinical stage (cStage) II–III disease, even after excluding patients with cStage I disease, because operative procedures such as complete mesocolic excision, CVL or D3 dissection, R0 resection, and adequate lymph node resection were performed in both arms in this trial.
Therefore, in this study, the authors performed a primary analysis at 3-year follow-up and a final analysis at 6-year follow-up according to the initially stipulated protocol to report the final results of the long-term follow-up of the JCOG1006.
Methods
Study design
The JCOG1006 was a multicentre, open label, randomized, phase III study. The institutional review boards of participating institutions approved the study protocol and details of the trial have been previously reported6. The study’s eligibility criteria included histologically proven Stage II or III colon cancer; most of the lesions located in the caecum (C), ascending colon (A), transverse colon (T), descending colon (D), sigmoid colon (S), or rectosigmoid (RS); clinical tumour depth of T3, T4a or T4b; nodal status of N0–2 based on preoperative endoscopic and radiographic imaging findings; absence of preoperative findings indicating M1 disease; aged between 20 and 80 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; no previous chemotherapy or radiotherapy for any malignancies; sufficient organ function; BMI < 30 kg/m2; no history of intestinal resection, serious obstruction or perforation; and no history of familial adenomatous polyposis, ulcerative colitis or Crohn disease. Tumour staging was performed based on the Japanese Classification of Colon and Rectal Carcinoma (seventh edition)1.
Randomization and masking
Details of randomization have been previously reported6. Briefly, eligible patients were randomly assigned (1 : 1) to undergo ‘Conventional’ or ‘No Touch’. Randomization was performed using a minimization method with a random component based on institution, tumour location (C, A, T versus D, S, RS) and sex. Both the investigators and patients were aware of the patient allocation. The JCOG Data Centre conducted central monitoring of data submission, patient eligibility, protocol compliance, safety and study progress.
Procedure
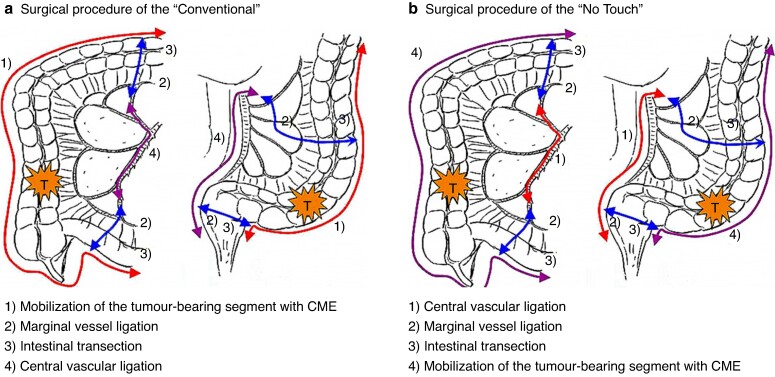
In both arms, the laparoscopic approach was prohibited, as the standard procedure for colon cancer in Japan was open surgery at the time the JCOG1006 commenced. Palpation of the intra-abdominal organs after laparotomy was also not permitted. In the ‘No Touch’ arm, the initial operation step involved mobilization of the tumour-bearing segment before ligating any vessels (Fig. 1a), followed by CVL. The subsequent steps included marginal vessel ligation, intestinal transection and mobilization of the tumour-bearing segment (Fig. 1b). All patients with pathological Stage III disease within 56 days after curative resection were recommended to receive adjuvant capecitabine chemotherapy at a dose of 1250 mg/m2 twice daily on days 1–14 every 21 days for 6 months7.
Fig. 1.
Surgical procedure
a Using the ‘Conventional’ technique; b using the ‘No Touch’ technique. CME, complete mesocolic excision.
Outcomes
The primary endpoint of the JCOG1006 was DFS, defined as the time from randomization to the first evidence of relapse, development of a second primary cancer, or death by any cause. Secondary endpoints included OS (time to death), RFS (time to the first evidence of relapse or death), LRFS (time to the first evidence of liver metastasis or death), mode of recurrence, surgical morbidity, adverse events related to postoperative chemotherapy, serious adverse events and short-term clinical outcomes.
Statistical analysis
The planned sample size was 840 patients (420 patients per arm) assuming a 3-year DFS of 75% versus 81% (HR = 0.732) with a one-sided alpha level of 5%, power of 80%, accrual period of 3 years and follow-up period of 3 years. In total, 259 events were expected to occur within the first 3 years of follow-up. DFS was analysed on an intention-to-treat basis. Survival curves were estimated using the Kaplan–Meier method. DFS was compared using the stratified log-rank test, with tumour location and sex as strata. The HR for DFS was estimated using a stratified Cox regression model with tumour location and sex as strata. Other time-to-event type endpoints including OS, RFS and LRFS were compared using the log-rank test, and HR for OS, RFS and LRFS was estimated using a Cox regression model. The Data and Safety Monitoring Committee of the JCOG independently reviewed the interim analysis reports and had the authority to prematurely terminate the trial if necessary. All analysis was done using SAS 9.4. The present study is registered with the UMIN Clinical Trials Registry under the number UMIN000004957.
Results
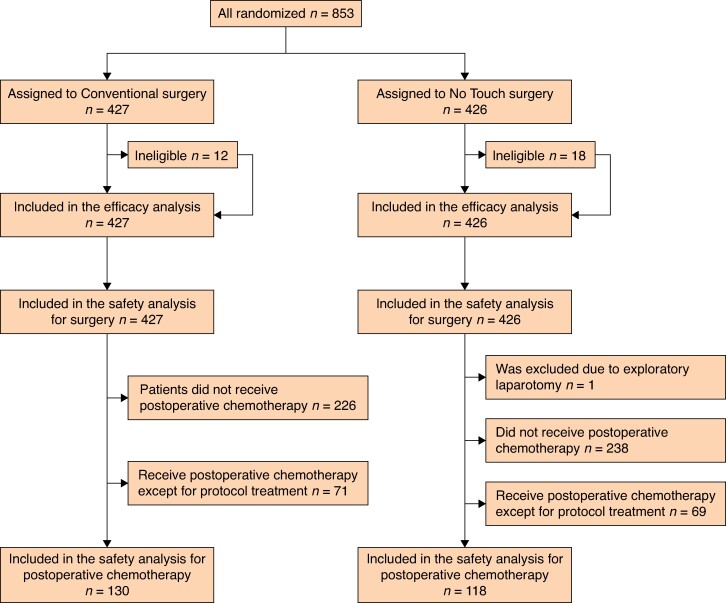
In total, 853 patients were randomized to the ‘Conventional’ (427) or ‘No Touch’ (426) arms between January 2011 and November 2015 (Fig. 2). After randomization, 12 (3%) and 18 (4%) patients were ineligible in the ‘Conventional’ and ‘No Touch’ arms respectively. In the ‘Conventional’ arm, patients were excluded owing to the lower margin of the tumour involving the upper rectum (five patients), detection of other cancers after registration (four patients), confirmed lung metastasis after surgery (one patient) and other ineligibility factors (two patients). In the ‘No Touch’ arm, patients were excluded owing to the lower margin of the tumour involving the upper rectum (four patients), detection of other cancers after registration (three patients), confirmed lung metastases after surgery (five patients) and other ineligibility factors (six patients).
Fig. 2.
CONSORT diagram
Clinical characteristics such as age, sex, ECOG PS, tumour location, nodal status, BMI, pathological results, tumour size, number of harvested lymph nodes, pathological stage and residual tumour were similar between the two arms. In the ‘Conventional’ and ‘No Touch’ groups, 13 and 25 patients were diagnosed with pathological stage IV colon cancer respectively and 6 and 15 patients had macroscopic or microscopic residual tumours respectively (Table 1).
Table 1.
Patient characteristics
| Conventional n = 427 | No Touch n = 426 | |
|---|---|---|
| Age (years), median (range) | 66 (23–80) | 66 (33–80) |
| Sex | ||
| Male | 211 (49.4) | 212 (49.8) |
| Female | 216 (50.6) | 214 (50.2) |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 407 (95.3) | 407 (95.5) |
| 1 | 20 (4.7) | 19 (4.5) |
| Tumour location | ||
| Right | 193 (45.2) | 191 (44.8) |
| Left | 234 (54.8) | 235 (55.2) |
| Tumour depth | ||
| cT3 | 298 (69.8) | 281 (66.0) |
| cT4a | 113 (26.5) | 122 (28.6) |
| cT4b | 16 (3.7) | 23 (5.4) |
| Nodal status | ||
| cN0 | 174 (40.7) | 166 (39.0) |
| cN1 | 177 (41.5) | 183 (43.0) |
| cN2 | 76 (17.8) | 76 (17.8) |
| cN3 | 0 (0.0) | 1 (0.2) |
| BMI (kg/m2), median (range) | 22.4 (12.8–30.0) | 22.5 (13.8–29.8) |
| Tumour size (cm), median (range) | 4.8 (2.0–13.5) | 5.0 (0.7–14.0) |
| Pathological results | ||
| Number of harvested lymph nodes, median (range) | 28 (3–129) | 28 (3–104) |
| Pathological stage | ||
| I | 25 (5.9) | 18 (4.2) |
| II | 181 (42.4) | 188 (44.1) |
| III | 208 (48.7) | 194 (45.9) |
| IV | 13 (3.0) | 25 (5.9) |
| Residual tumour | ||
| R0 | 421 (98.6) | 410 (96.2) |
| R1 | 1(0.2) | 5 (1.2) |
| R2 | 5 (1.2) | 10 (2.3) |
Values are n (%) unless otherwise indicated.
Disease-free survival
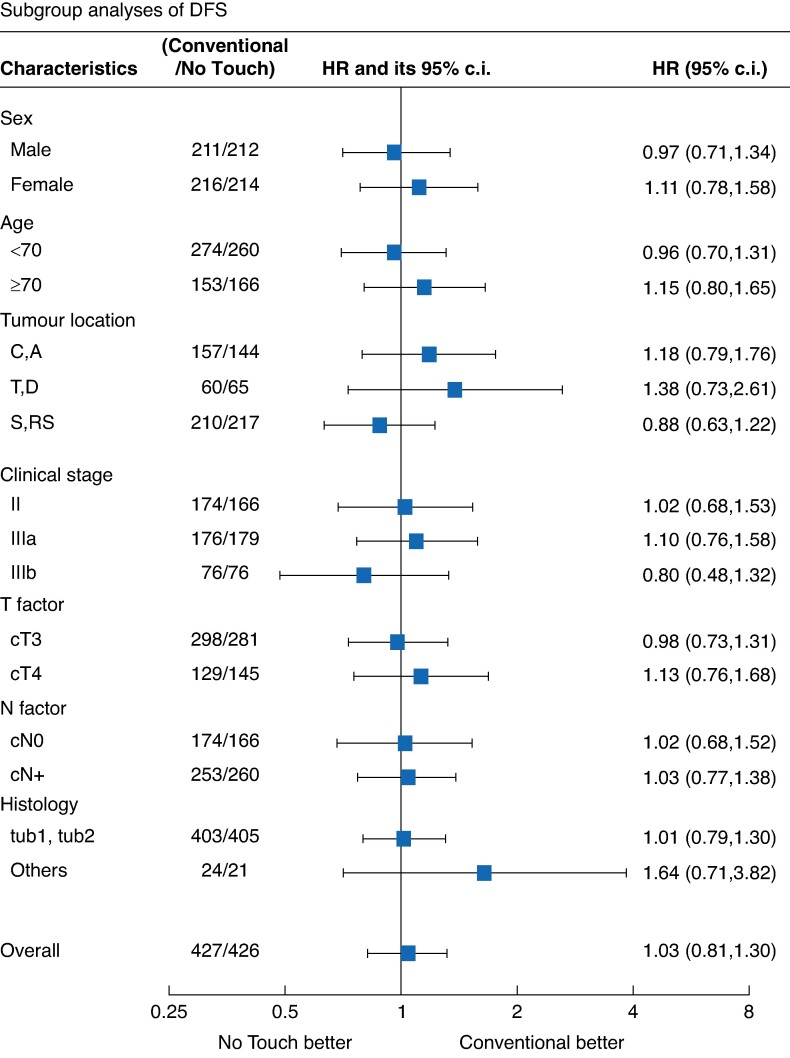
In total, 137 and 140 patients experienced recurrence, secondary cancer or died in the ‘Conventional’ and ‘No Touch’ arms respectively, with 35 developing recurrence, secondary cancer or dying beyond the initially planned 3-year analysis period. The 6-year DFS was 70.3% (95% c.i. 65.7 to 74.4) and 69.4% (95% c.i. 64.8 to 73.6) in the ‘Conventional’ and ‘No Touch’ arms respectively, with an HR of 1.030 (95% c.i. 0.813 to 1.304; one-sided P = 0.60). This finding is consistent with those of the primary analysis, failing to support the superiority of the ‘No Touch’ technique over the ‘Conventional’ technique in patients with Stages II and III colon cancer (Fig. 3a). Subgroup analysis of DFS demonstrated that there was no significant difference between ‘Conventional’ arm and ‘No Touch’ arm in each group (Fig. 4).
Fig. 3.
a Disease-free survival. b Overall survival. c Relapse-free survival. d Liver relapse-free survival.
Fig. 4.
Subgroup analysis of disease-free survival
C, caecum; A, ascending colon; T, transverse colon; D, descending colon; S, sigmoid colon; RS, rectosigmoid; tub1, well differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma.
Overall survival
In total, 57 and 72 patients died in the ‘Conventional’ and ‘No Touch’ arms respectively, with 33 deaths occurring beyond the initially planned 3-year analysis period. The 6-year OS was 89.4% (95% c.i. 86.0 to 92.0) and 86.6% (95% c.i. 82.9 to 89.5) in the ‘Conventional’ and ‘No Touch’ arms respectively, with an HR of 1.276 (95% c.i. 0.902 to 1.807; Fig. 3b).
Relapse-free survival
At the 6-year follow-up, 95 and 112 patients experienced local recurrence or died in the ‘Conventional’ and ‘No Touch’ arms respectively. The 6-year local RFS was 78.9% (95% c.i. 74.7 to 82.5) and 75.0% (95% c.i. 70.6 to 76.8) in the ‘Conventional’ and ‘No Touch’ arms respectively, with an HR of 1.209 (95% c.i. 0.920 to 1.589; Fig. 3c).
Liver relapse-free survival
At the 6-year follow-up, 72 and 90 patients experienced liver recurrence or died in the ‘Conventional’ and ‘No Touch’ arms respectively. The 6-year LRFS was 85.1% (95% c.i. 81.2 to 86.2) and 80.2% (95% c.i. 76.0 to 83.8) in the ‘Conventional’ and ‘No Touch’ arms respectively, with an HR of 1.311 (95% c.i. 0.961 to 1.787; Fig. 3d).
Subgroup analysis of DFS
Figure 4 depicts the subgroup analysis of DFS. Among patients with tumours in the transverse or descending colon, DFS tended to be better in the ‘Conventional’ arm than in the ‘No Touch’ arm (HR 1.38; 95% c.i. 0.73 to 2.61). Among patients with histological findings other than tub1 or tub2, DFS was better in the ‘Conventional’ arm than in the ‘No Touch’ arm (HR 1.64; 95% c.i. 0.71 to 3.82). Among patients with clinical Stage III disease, the improved DFS in the ‘Conventional’ arm was not significant (HR 0.80; 95% c.i. 0.48 to 1.32). Compared with the ‘Conventional’ arm, the ‘No Touch’ arm tended to be associated with worse DFS among patients who were female, aged >70 years and had cT4 disease.
Late complications
At the 6-year follow-up, in the case of late complications except adverse effects of blood/bone marrow in Common Terminology Criteria for Adverse Events (CTCAE v3.0), 25 (5.9%) and 20 (4.7%) patients experienced more than Grade 2 and 16 (3.7%) and 11 (2.6%) patients experienced more than Grade 3 in the ‘Conventional’ and ‘No Touch’ arms respectively. There was no Grade 4 in either arm (Table 2).
Table 2.
Late complications
| Conventional n = 427 | No Touch n = 426 | |
|---|---|---|
| Grade 2 or more | 25 (5.9) | 20 (4.7) |
| Grade 3 or more | 16 (3.7) | 11 (2.6) |
| Grade 4 | 0 (0.0) | 0 (0.0) |
Values are n (%) unless otherwise indicated.
Discussion
The present study aimed to determine whether the ‘No Touch’ technique is superior to the ‘Conventional’ technique using long-term (6-year) follow-up data from the JCOG1006. The JCOG1006 reported a 3-year OS of >90% and DFS of >75% in both arms. An analysis at 6 years after enrolling the last patient was needed as defined in the original protocol. However, the present 6-year follow-up analysis also could not demonstrate the superiority of the ‘No Touch’ technique over the ‘Conventional’ technique in terms of long-term DFS, OS, RFS and LRFS.
Many investigators reported laparoscopic surgery as the best approach for colon cancer, considering the short-term (operative time, blood loss, duration of hospital stay, number of harvested lymph nodes, and morbidity) and long-term (prognosis) outcomes8–15. Two main surgical approaches for colon cancer have been identified in the literature: ‘a ligation first followed by mobilization of the tumour approach’ or ‘mobilization followed by ligation approach’. These approaches have been described as ‘a medial-to-lateral group’ and ‘lateral-to-medial group’ or ‘caudal-to-cranial plus artery first’ and ‘conventional medial approach.’ The former is comparable to the ‘No Touch’ and the latter to the ‘Conventional’ techniques used in this study8–15.
The consensus from previous reports suggested that the short-term outcomes of the ‘No Touch’ technique were better than or equal to those of the ‘Conventional’ technique, indicating procedural convenience and therapeutic benefits. Nevertheless, the evidence supporting this conclusion was not robust because most previous reports were retrospective studies that had a limited number of participants, ranging from 40 to 450 cases8–15. The number of participants in these studies was lower than in the present study. Xu et al. reported on 450 patients (150 cases of ‘No Touch’ and 300 cases of ‘Conventional’), which was the largest number reported among previous retrospective studies on approaches for colon cancers13. The second-largest number of participants was reported by Hussain et al., with 137 patients11.
Liang et al. reported a prospective study on laparoscopic procedures for sigmoid colon lesions; however, the number of participants was small (36 cases for the ‘medial-to-lateral (No Touch)’ group and 31 for the ‘lateral-to-medial group’), totalling 67 cases9. Liang et al. also investigated right-sided colon cancer in a randomized, double-blind clinical trial, mirroring the approach for sigmoid colon lesions, and deduced favourable short-term outcomes without reference to long-term outcomes (prognosis). However, the number of the participants was only 104 cases9. Therefore, the current study outperformed previous studies in terms of participant numbers (n = 853) and being a prospective study for prognosis.
The planned primary analysis at 3-year and long-term (6-year follow-up, the present study) failed to confirm the superiority of the ‘No Touch’ technique over the ‘Conventional’ technique. Operative procedures, including complete mesocolic excision, CVL or D3 dissection, R0 resection, and adequate lymph node resection, were performed in both arms, confirming no differences between them. In total, 17 and 18 patients in the ‘Conventional’ and ‘No Touch’ arms respectively developed recurrence, secondary cancer or died during the additional long-term follow-up of 3 years. The number of events, including recurrences, secondary cancer or deaths, was small. Thus, the results of DFS were the same as those of the authors' previous report. Similarly, for OS, RFS and LRFS, the number of events, including recurrence or death, death, local recurrence or death and liver recurrence or death, remained small. Furthermore, the results of OS, RFS and LRFS were the same as those of the previous report.
Most operative colon cancer cases undergo laparoscopic or robotic surgery, with laparotomy (open surgery) being less frequent. Previous studies focused on laparoscopic procedures; however, the authors' study included participants who exclusively underwent open surgery. Nonetheless, the outcomes could be applied to laparoscopic and robotic surgeries because the approach in both laparoscopic and robotic surgeries for colon cancer was ‘No Touch’. From the results of the prospective study, including a substantial number of participants, no significant oncological prognostic differences were observed between the ‘No Touch’ and ‘Conventional’ techniques. Therefore, both approaches can be used for managing colon cancers.
Long-term follow-up data did not support the superiority of the ‘No Touch’ approach over the ‘Conventional’ approach in patients with stages II and III colon cancer. The study findings indicate that the conventional technique (‘Conventional’) is still the standard approach for managing colon cancers.
Supplementary Material
Acknowledgements
The authors are grateful to all patients, their families, caregivers and investigators for their cooperation in JCOG1006 and the members of the JCOG Data Center and JCOG Operations Office for their support and data management (Ms Ayaka Nakano). Participating institutions (descending order of number of registrations): Aichi Cancer Center Hospital, Kanagawa Cancer Center, National Cancer Center Hospital, Niigata Cancer Center Hospital, Osaka International Cancer Institute, Saitama Cancer Center, Hiroshima Prefectural Hospital, Chiba Cancer Center, National Hospital Organization Shikoku Cancer Center, Saiseikai Yokohama-shi Nanbu Hospital, Yamagata Prefectural Central Hospital, Hyogo College of Medicine, Yokohama City University Medical Center, Kyorin University Faculty of Medicine, Hiroshima City Asa Hospital, Sapporo-Kosei General Hospital, National Defense Medical College, Nagaoka Chuo General Hospital, National Cancer Center Hospital East, Sakai City Medical Center, Kumamoto University Hospital, Tokyo Medical University Hospital, Shizuoka Cancer Center, Toho University Ohashi Medical Center, Okayama Saiseikai General Hospital, Gunma Prefectural Cancer Center, Osaka National Hospital, Miyagi Cancer Center, Nagano Municipal Hospital, Suita Municipal Hospital.
Contributor Information
Koji Komori, Department of Gastroenterological Surgery, Aichi Cancer Center Hospital, Nagoya, Japan.
Yasumasa Takii, Department of Gastroenterological Surgery, Niigata Cancer Center Hospital, Niigata, Japan.
Junki Mizusawa, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Yukihide Kanemitsu, Department of Colorectal Surgery, National Cancer Center Hospital, Tokyo, Japan.
Manabu Shiozawa, Department of Gastrointestinal Surgery, Kanagawa Cancer Center, Yokohama, Japan.
Masayuki Ohue, Department of Gastroenterological Surgery, Osaka International Cancer Institute, Osaka, Japan.
Satoshi Ikeda, Department of Gastroenterological Surgery, Hiroshima Prefectural Hospital, Hiroshima, Japan.
Takaya Kobatake, Department of Gastroenterological Surgery, National Hospital Organization Shikoku Cancer Center, Matsuyama, Japan.
Tetsuya Hamaguchi, Department of Gastroenterological Oncology, Saitama Medical University International Medical Center, Saitama, Japan.
Hiroshi Katayama, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Haruhiko Fukuda, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Funding
This study was supported in part by the National Cancer Center Research and Development Funds (26-A-4, 29-A-3, 2020-J-3, 2023-J-03).
Disclosure
J.M. received honoraria from Chugai Pharmaceutical and Taiho Pharmaceutical; his spouse is an employee of Pfizer. T.H. received honoraria from Chugai Pharma, Merck Serono, Takeda, Taiho Pharma, Ono Pharma, Yakult Pharma, Lilly, Bristol-Myers Squibb Japan, Bayer and Daiichi Sankyo, and also received research funding from Taiho Pharm, Ono Pharm, Chugai Pharm, BeiGene Astellas Pharm, AstraZeneca, Pfizer, Brsitol-Myers Squbb Foundation and Incyte Japan. H.F. received honoraria from Chugai Pharmaceutical and Kyowa Kirin. We certify that part of this paper was presented as a poster at the ESMO Congress 2022 (Paris, 10 September 2022), but the entire paper has not been published previously.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary materials.
Author contributions
Koji Komori (Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing), Yasumasa Takii (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing—review & editing), Junki Mizusawa (Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review & editing), Yukihide Kanemitsu (Conceptualization, Formal analysis, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Manabu Shiozawa (Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Masayuki Ohue (Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Satoshi Ikeda (Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Takaya Kobatake (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Tetsuya Hamaguchi (Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing—review & editing), Hiroshi Katayama (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Haruhiko Fukuda (Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review & editing) and Colorectal Cancer Study Group Japan Clinical Oncology Group (JCOG) (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization)
References
- 1. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes JP. Physiologic resection of the right colon. Surg Gynecol Obstet 1952;94:722–726 [PubMed] [Google Scholar]
- 3. Turnbull RB Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg 1967;166:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slanetz CA Jr. Effect of no touch isolation on survival and recurrence in curative resections for colorectal cancer. Ann Surg Oncol 1998;5:390–398 [DOI] [PubMed] [Google Scholar]
- 5. Wiggers T, Jeekel J, Arends JW, Brinkhorst AP, Kluck HM, Luyk CI et al. No-touch isolation technique in colon cancer: a controlled prospective trial. Br J Surg 1988;75:409–415 [DOI] [PubMed] [Google Scholar]
- 6. Takii Y, Mizusawa J, Kanemitsu Y, Komori K, Shiozawa M, Ohue M et al. The conventional technique versus the no-touch isolation technique for primary tumour resection in patients with colon cancer (JCOG1006): a multicenter, open-label, randomized, phase III trial. Ann Surg 2022;275:849–855 [DOI] [PubMed] [Google Scholar]
- 7. Twelves C, Wong A, Nowacki MP, Abt M, Burris H III, Carrato A et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–2704 [DOI] [PubMed] [Google Scholar]
- 8. Liang JT, Shieh MJ, Chen CN, Cheng YM, Chang KJ, Wang SM. Prospective evaluation of laparoscopy-assisted colectomy versus laparotomy with resection for management of complex polyps of the sigmoid colon. World J Surg 2002;26:377–383 [DOI] [PubMed] [Google Scholar]
- 9. Liang JT, Lai HS, Huang KC, Chang KJ, Shieh MJ, Jeng YM et al. Comparison of medial-to-lateral versus traditional lateral-to-medial laparoscopic dissection sequences for resection of rectosigmoid cancers: randomized controlled clinical trial. World J Surg 2003;27:190–196 [DOI] [PubMed] [Google Scholar]
- 10. Liang JT, Lai HS, Lee PH. Laparoscopic medial-to-lateral approach for the curative resection of right-sided colon cancer. Ann Surg Oncol 2007;14:1878–1879 [DOI] [PubMed] [Google Scholar]
- 11. Hussain A, Mahmood F, Torrance AW, Tsiamis A. Impact of medial-to-lateral vs lateral-to-medial approach on short-term and cancer-related outcomes in laparoscopic colorectal surgery: a retrospective cohort study. Ann Med Surg (Lond) 2018;26:19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yi X, Li H, Lu X, Wan J, Diao D. “Caudal-to-cranial” plus “artery first” technique with beyond D3 lymph node dissection on the right midline of the superior mesenteric artery for the treatment of right colon cancer: is it more in line with the principle of oncology? Surg Endosc 2020;34:4089–4100 [DOI] [PubMed] [Google Scholar]
- 13. Xu P, Ren L, Zhu D, Lin Q, Zhong Y, Tang W et al. Open right hemicolectomy: lateral to medial or medial to lateral approach? PLoS One 2015;10:e0145175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banchini F, Luzietti E, Romboli A, Palmieri G, Conti L, Capelli P. Could the top-down right hemicolectomy be an easier alternative to the classic medial-to-lateral approach in obese patients? A case report with video example. Int J Surg Case Rep 2022;100:107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poon JT, Law WL, Fan JK, Lo OS. Impact of the standardized medial-to-lateral approach on outcome of laparoscopic colorectal resection. World J Surg 2009;33:2177–2182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary materials.