Abstract
Mirtazapine, an alpha-2 adrenergic receptor, 5-hydroxytryptamine (5-HT)2, and 5-HT3 antagonist, is commonly used in patients for depression and anorexia. Its mechanism disinhibits serotonin and norepinephrine. Though typically a well-tolerated medication, a rare adverse effect is arrhythmia, including ventricular bigeminy. To date, no case report has cited normal dosing of mirtazapine as a cause of premature ventricular or premature atrial contractions. Only cases of mirtazapine overdose have been associated with arrhythmias, including QT prolongation and bradycardia. We report on a unique case of a 64-year-old female who developed sinus tachycardia with premature ventricular and atrial contractions after starting mirtazapine.
Keywords: Mirtazapine, Premature ventricular contraction, Premature atrial contraction, Medication adverse effects, Tachycardia
Introduction
Mirtazapine is an atypical antidepressant that is used in the treatment of major depressive disorder (MDD). This medication acts by inhibiting several different receptors including α2 adrenergic autoreceptors and heteroreceptors as well as antagonizing serotonergic receptors 5-hydroxytryptamine (5-HT)2, 5-HT3. In addition, it also acts as an antagonist to histamine (H1), peripheral α1 adrenergic receptors and muscarinic receptors, which is believed to contribute to the side effects such as somnolence (H1 antagonist) and orthostatic hypotension (peripheral α1 antagonism). Other side effects of mirtazapine include weight gain and increased appetite [1]. When it comes to the treatment of depressive disorders, clinicians have many choices between the selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and other serotonin antagonists and reuptake inhibitors. Mirtazapine is selected as a drug of choice primarily for its ability to treat insomnia and its ability to improve weight gain in underweight patients. Through its antagonistic actions on the H1 receptor mirtazapine provides a favorable side effect on the central nervous system and promotes drowsiness and is useful for patients that may also be suffering from insomnia. Mirtazapine also has multiple side effects on the endocrine and metabolic systems, which include increased appetite, weight gain and increasing total cholesterol/triglyceride levels, which all aid in its ability to improve appetite and promote weight gain in underweight or anorexic patients.
Regarding the potential cardiovascular side effects of mirtazapine, the product labeling for the brand name Remeron® lists hypertension and vasodilation as frequent cardiovascular side effect occurrences [1]. Infrequent occurrences include angina pectoris, myocardial infarction, bradycardia and ventricular extrasystoles. Rare cardiovascular occurrences listed on the product labeling include atrial arrhythmia, bigeminy, vascular headaches, pulmonary embolism, cerebral ischemic, cardiomegaly, phlebitis and left heart failure [1]. A postmarketing case report has demonstrated long QT syndrome in a patient who was started on mirtazapine, which ceased after the discontinuation of the medication. This patient had risk factors that contributed to this presentation, such as having a baseline prolonged QT interval, malnourished and renal insufficiency [2]. Other studies in the literature have associated mirtazapine with increased risk of sudden cardiac death and ventricular arrhythmias in comparison to paroxetine, although the authors contribute this to other residual confounding factors among the patients, such as mirtazapine preferential use in the elderly, less ambulatory patients suffering from chronic diseases [3]. Another paper provides an account of a 60-year-old man with schizophrenia being treated with mirtazapine and risperidone developing several episodes of supraventricular tachycardia and atrial fibrillation while on long-term monitoring [4]. In summary, hemodynamic instability is a rare adverse effect of mirtazapine therapy, and we aim to contribute to the limited literature that cites therapeutic dosing of mirtazapine as a pharmacologic agent that may induce ventricular bigeminy.
Case Report
Investigations
A 64-year-old female with a past medical history of uterine prolapse presented to the Emergency Department due to lower abdominal pain for 5 days’ duration. The pain began radiating to the bilateral flanks and caused her to develop two episodes of non-bloody, non-bilious emesis. She also admitted to increased urinary frequency but denied dysuria. She also denied sick contacts, headache, shortness of breath, chest pain, dizziness, and paresthesia. Her past surgical history was unremarkable. She denied habitual tobacco, alcohol, and illicit drug use. She did not take any medications routinely at home prior to admission.
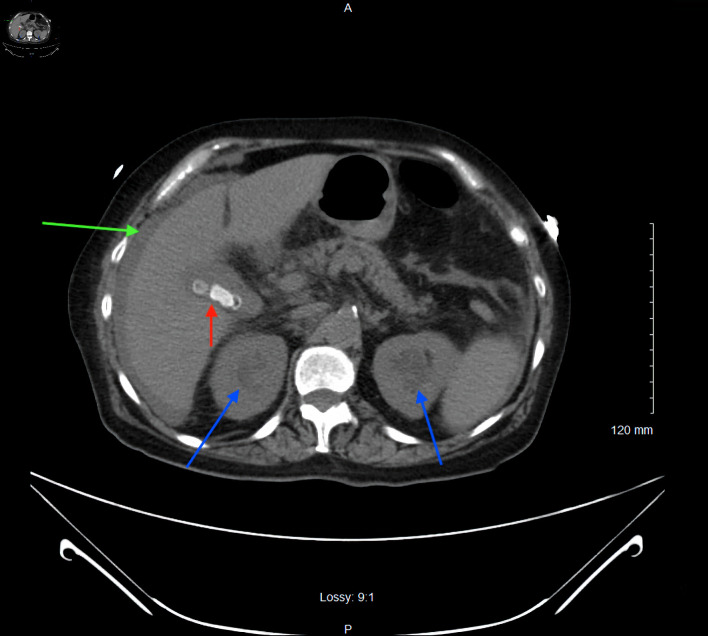
In the Emergency Department, vital signs included a blood pressure of 84/35 mm Hg, heart rate of 84, temperature of 98 °F, respiratory rate of 14, and oxygen saturation of 100% on room air. Physical exam was notable for tachycardia and right lower quadrant abdominal tenderness. She was given 2 L of normal saline via intravenous (IV) bolus, but her blood pressure continued to decline. The patient was subsequently started on norepinephrine and vasopressin infusions and broad-spectrum IV antibiotics (vancomycin 20 mg/kg and piperacillin-tazobactam 3.375 g every 8 h) at this time for management of septic shock. Initial laboratory studies revealed a lactic acidosis, mild hypokalemia, and bandemia (Table 1). A urinalysis and chest X-ray were obtained, and results were unremarkable for findings of a pneumonia. A computed tomography (CT) of the abdomen and pelvis without IV contrast revealed bilateral hydronephrosis, likely secondary to bladder outlet obstruction from the pelvic floor prolapse, cholelithiasis without gallbladder wall thickening, and perihepatic ascites (Fig. 1). Urology was consulted, and the uterine prolapse was reduced. Gynecology was consulted, and a Gellhorn pessary was placed. Following resuscitation and hemodynamic stabilization, the patient was transferred to the intensive care unit (ICU).
Table 1. Initial Laboratory Findings, Demonstrating Mild Hypokalemia, Lactic Acidosis, and Bandemia.
| Laboratory test | Reference values | Measured values |
|---|---|---|
| White blood cells (cells/µL) | 4,300 - 11,100 | 5,600 |
| Hemoglobin (g/dL) | 11.5 - 15.5 | 13.6 |
| Hematocrit (%) | 36 - 46 | 43 |
| Platelet (cells/µL) | 120,000 - 360,000 | 295,000 |
| Neutrophils (%) | 38 - 75 | 45 |
| Lymphocytes (%) | 20 - 48 | 6 |
| Bands (%) | 0 - 5 | 22 |
| Sodium (mEq/L) | 135 - 148 | 135 |
| Potassium (mEq/L) | 3.5 - 5.5 | 3.2 |
| Chloride (mEq/L) | 98 - 110 | 92 |
| Bicarbonate (mmol/L) | 24 - 34 | 12 |
| BUN (mg/dL) | 8 - 20 | 23 |
| Creatinine (mg/dL) | 0.5 - 1.5 | 2.26 |
| Calcium (mg/dL) | 8.5 - 10.5 | 9.1 |
| Phosphorous (mg/dL) | 2.4 - 4.4 | 4.3 |
| Magnesium (mg/dL) | 1.6 - 2.3 | 1.6 |
| AST (U/L) | 5.0 - 40 | 20 |
| ALT (U/L) | 5.0 - 40 | 17 |
| Lactate (mmol/L) | 1 - 1.8 | 4.3 |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; BUN: blood urea nitrogen.
Figure 1.
Axial section of a computed tomography of the abdomen and pelvis without intravenous contrast showing bilateral hydronephrosis (blue arrows), cholelithiasis without gallbladder wall thickening (red arrow), and perihepatic ascites (green arrow).
Diagnosis
Blood cultures demonstrated group A streptococcus bacteremia, so the patient was transitioned to IV ceftriaxone 2 g daily. A transthoracic echocardiography (TTE) was performed to evaluate for possible endocarditis. As there were thickened mitral leaflets noted on the TTE, endocarditis could not be ruled out; thus, a transesophageal echocardiography (TEE) was performed, which was negative for vegetations. An abdominal ultrasound was also obtained, revealing cholelithiasis without evidence of cholecystitis and a small amount of perihepatic ascites. At this time, percutaneous sampling of the ascitic fluid was deemed unsafe due to a small pocket of ascites. Following optimization in the ICU, the patient was weaned off pressors and transferred from the ICU to the telemetry unit for further workup.
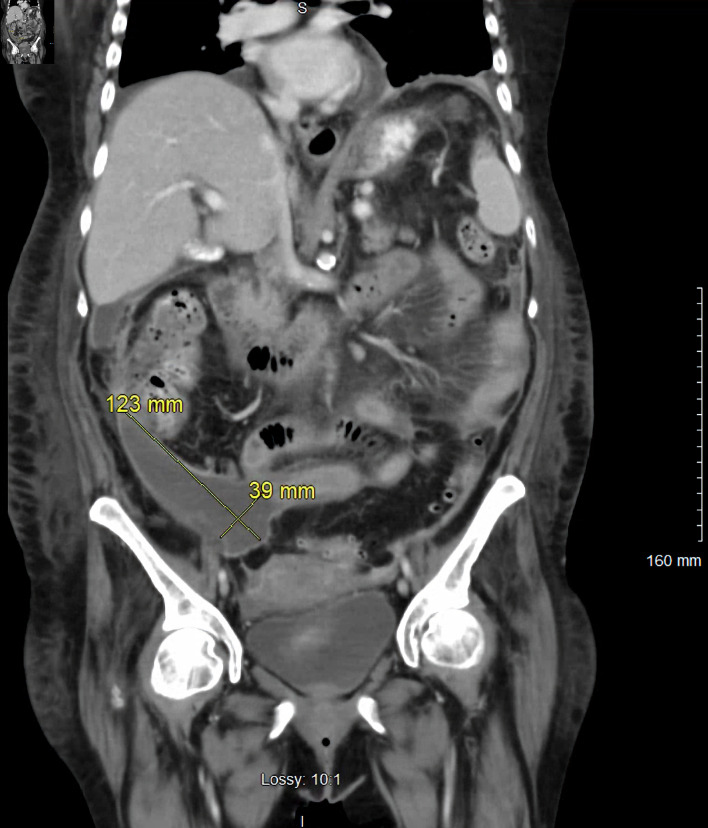
Due to persistent leukocytosis, our infectious disease specialist was consulted for the case, and the etiology of group A streptococcus bacteremia was determined likely to be from peritonitis. At this time, IV metronidazole 500 mg every 8 h was initiated in addition to ceftriaxone, leading to slight improvement in the patient’s leukocytosis. However, in the subsequent days, the patient’s leukocytosis and tachycardia persisted. A repeat CT of the abdomen and pelvis with oral and IV contrast demonstrated thickened enhancing peritoneum surrounding a loculated abdominal fluid collection (12.3 × 3.9 cm), suggesting peritonitis (Fig. 2). A 10-French pigtail catheter was placed under CT guidance in the loculated abdominal fluid collection, draining cloudy fluid without pus. Cultures from this sample were negative, and this finding was attributed to the patient receiving IV antibiotics. Ultimately, the patient’s leukocytosis and tachycardia resolved following this intervention.
Figure 2.
Coronal section of a repeat computed tomography of the abdomen and pelvis with oral and intravenous contrast demonstrated thickened enhancing peritoneum surrounding a loculated abdominal fluid collection measuring 12.3 × 3.9 cm (marked in yellow).
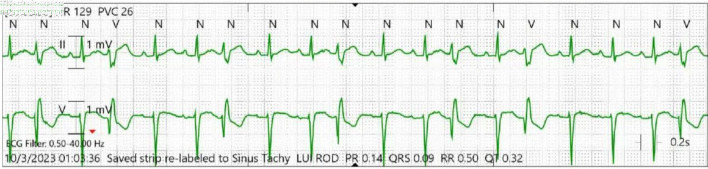
During her prolonged hospital course, the patient had lost 32 pounds in body weight due to poor appetite in the setting of acute illness. Oral mirtazapine 7.5 mg was prescribed on a nightly schedule, and the patient developed narrow complex tachycardia 72 h after initiating this therapy. Telemetry monitoring demonstrated persistent premature ventricular contractions (PVCs) with an underlying sinus tachycardia (Fig. 3). Despite optimizing the patient’s volume status, correcting electrolyte abnormalities, and ruling out infection, the patient had persistent tachycardia, PVCs, and premature atrial contractions (PACs). Upon cessation of mirtazapine, telemetry monitoring demonstrated normal sinus rhythm. Her corrected QT interval was 432 ms prior to initiation of mirtazapine and 380 ms while on mirtazapine.
Figure 3.
Telemetry monitoring showing persistent premature ventricular contractions in bigeminy and trigeminy. N: normal beat; V: ventricular beat.
Treatment
Mirtazapine was suspected to be the offending agent, so it was discontinued. Within 24 h of stopping the medication, the patient’s heart rate normalized, and she no longer demonstrated evidence of ventricular and atrial arrhythmias.
Follow-up and outcomes
The patient was discharged after her arrhythmias stopped. She was set to follow-up with her primary care doctor in the outpatient setting. At 3-month follow-up, she is alive and well.
Discussion
Though mirtazapine is selected over tricyclic antidepressants (TCAs) for its relatively lower risk profile in treating MDD, it may induce arrhythmias in rare instances. Beach et al (2013) noted that in 103 patients who overdosed on mirtazapine, 16% experienced QTc prolongation of over 440 ms, with none over 500 ms [5]. Nordgaard et al (2022) reported that a 60-year-old man with schizophrenia being treated with therapeutic doses of mirtazapine and risperidone developed several episodes of supraventricular tachycardia and atrial fibrillation while being monitored [4]. However, in another case of mirtazapine overdose of 530 ng/mL (therapeutic level 20 - 50 ng/mL), there were no reported tachyarrhythmias in this patient [6]. As can be seen, the literature is limited and inconsistent regarding cardiac side effects of mirtazapine, and the side effects of mirtazapine may not necessarily be dose dependent.
Among antidepressant medications, TCAs are most known for prolonging the QTc interval through sodium channel blockade. Additionally, a case report has noted arrhythmias related to venlafaxine therapy [5]. Therefore, it is important to recognize that psychotropic medications may also exhibit unwanted cardiac side effects. There are many causes for the occurrence of PVCs. A majority of cases occur spontaneously; however, PVCs can occur due to excess caffeine consumption, excess catecholamines, high levels of anxiety, and electrolyte abnormalities. Of electrolyte abnormalities, notably low serum potassium, low serum magnesium, and high serum calcium are common triggers. Alcohol, tobacco, illicit drugs, and sleep deprivation can also trigger PVCs. Of non-cardiac pathologies, hyperthyroidism, anemia, and hypertension may induce PVCs [7]. Of cardiac pathologies, any structural heart disease that alters the conduction pathways can cause PVCs, including thoracic skeletal abnormalities, which may commonly be associated with mitral valve prolapse [7, 8].
As stated earlier, mirtazapine’s mechanism of action includes antagonism of the α2-adrenergic receptor, leading to increased norepinephrine from the presynaptic cleft [1]. In our case, we suspect the PVCs noted were due to excess catecholamines due to disinhibition of norepinephrine by mirtazapine in the setting of acute illness. Electrolyte abnormalities and structural heart disease had been ruled out with an echocardiogram. No other new medications were initiated surrounding the onset of PVCs, and the patient denied taking any stimulant drugs. The only notable factor was that the onset and termination of PVCs and PACs was associated with the initiation and cessation of mirtazapine therapy. This is consistent with the half-life of the medication, which is listed in the package insert as 20 - 40 h [1]. The patient’s heart rate improved with a likely dose-dependent elimination of the drug. Thus, based on our workup and the temporal relationship between mirtazapine and PVCs, we concluded that mirtazapine was the etiology of her narrow complex tachycardia, PVCs, and PACs.
This case is unique because the patient developed tachyarrhythmias, PVCs, and PACs while receiving a therapeutic dose of mirtazapine. The major strength of this case is the thorough investigations that were done to rule out all other causes of the patient’s arrhythmias. Another strength of this case is that we were able to monitor the patient on 24-h telemetry to visualize exactly when the arrhythmias began and stopped. The primary limitation of this case is that there is a very small body of evidence that cites mirtazapine as a cause of cardiac arrhythmias; despite this limitation, we believe that our findings contribute to our limited understanding of this medication adverse effect.
Conclusions
Mirtazapine is a commonly utilized medication for depression and appetite stimulation. Though typically well tolerated, cardiac side effects may arise. Our case report focuses on the clinical course of a patient who developed tachycardia with premature ventricular and atrial contractions after initiating mirtazapine therapy for anorexia secondary to poor appetite. Following a thorough cardiac and infectious negative workup, mirtazapine was suspected as a causal agent. Upon cessation of the offending agent, the patient returned to normal sinus rhythm. In our case, we suspect that the mechanism by which PVCs were noted was due to the disinhibition of norepinephrine by mirtazapine, which superimposed the patient’s acute illness. Clinicians should be aware of this clinically significant side effect of mirtazapine, especially in hospitalized patients.
Learning points
The limited available literature reports rare cardiac events related to both supratherapeutic and therapeutic doses of mirtazapine, and we believe that our case contributes to the limited body of evidence citing mirtazapine as an etiology for arrhythmias. We recommend caution in prescribing mirtazapine in the setting of acute illness. We also recommend considering mirtazapine as an etiology for arrhythmias if all other workup is negative, as mirtazapine-induced arrhythmia is a diagnosis of exclusion. Future studies may aim to definitively evaluate the underlying mechanism for mirtazapine-related arrhythmias and isolate patient populations at higher risk for developing this condition
Acknowledgments
The authors would like to thank and express their gratitude to Arrowhead Regional Medical Center’s exceptional nursing staff and unit managers for their expert clinical support.
Funding Statement
The authors have no financial or funding disclosures.
Conflict of Interest
The authors declare there is no conflict of interest.
Informed Consent
Informed consent was obtained from the family of the patient. The patient was also appropriately deidentified for this manuscript.
Author Contributions
SN, DR, ATP, and HA contributed to the initial manuscript write-up, literature review, and editing of the manuscript. DV attended on the case and contributed to decision-making, management of the patient, literature review, and editing of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
- 5-HT
5-hydroxytryptamine
- H1
histamine 1
- MDD
major depressive disorder
- SSRI
selective serotonin reuptake inhibitor
- SNRI
serotonin and norepinephrine reuptake inhibitor
- ICU
intensive care unit
- TTE
transthoracic echocardiography
- TEE
transesophageal echocardiography
- CT
computed tomography
- PVC
premature ventricular contraction
- PAC
premature atrial contraction
- IV
intravenous
- TCA
tricyclic antidepressant
References
- 1. Remeron (mirtazapine) [package insert]. Jersey City, NJ: Organon & Co. Pharmaceutical Company; 2021. Accessed June 12, 2024.
- 2.Matsuda Y, Furukawa Y, Yamazaki R, Inamura K, Kito S, Nunomura A, Shigeta M. Mirtazapine-induced long QT syndrome in an elderly patient: a case report. Psychogeriatrics. 2020;20(4):536–537. doi: 10.1111/psyg.12520. [DOI] [PubMed] [Google Scholar]
- 3.Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf. 2011;20(9):903–913. doi: 10.1002/pds.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordgaard J, Melchior T. Long-term Arrhythmia Detection Using an Implantable Loop Recorder in Patients Receiving Psychotropic Medication. JAMA Psychiatry. 2022;79(1):77–78. doi: 10.1001/jamapsychiatry.2021.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13. doi: 10.1016/j.psym.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez C, Carlson A, Stokes KA, Leikin JB. Relative safety of mirtazapine overdose. Vet Hum Toxicol. 2001;43(6):342–344. [PubMed] [Google Scholar]
- 7.Farzam K, Richards JR. StatPearls. Treasure Island (FL): 2024. Premature ventricular contraction. [PubMed] [Google Scholar]
- 8.Sonaglioni A, Nicolosi GL, Lombardo M. The relationship between mitral valve prolapse and thoracic skeletal abnormalities in clinical practice: a systematic review. J Cardiovasc Med (Hagerstown) 2024;25(5):353–363. doi: 10.2459/JCM.0000000000001614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.