Summary
Background
Tuberculosis incidence is increasing in Latin America, where the incarcerated population has nearly quadrupled since 1990. We aimed to quantify the impact of historical and future incarceration policies on the tuberculosis epidemic, accounting for effects in and beyond prisons.
Methods
In this modelling study, we calibrated dynamic compartmental transmission models to historical and contemporary data from Argentina, Brazil, Colombia, El Salvador, Mexico, and Peru, which comprise approximately 80% of the region’s incarcerated population and tuberculosis burden. The model was fit independently for each country to incarceration and tuberculosis data from 1990 to 2023 (specific dates were country dependent). The model does not include HIV, drug resistance, gender or sex, or age structure. Using historical counterfactual scenarios, we estimated the transmission population attributable fraction (tPAF) for incarceration and the excess population-level burden attributable to increasing incarceration prevalence since 1990. We additionally projected the effect of alternative incarceration policies on future population tuberculosis incidence.
Findings
Population tuberculosis incidence in 2019 was 29·4% (95% uncertainty interval [UI] 23·9–36·8) higher than expected without the rise in incarceration since 1990, corresponding to 34 393 (28 295–42 579) excess incident cases across countries. The incarceration tPAF in 2019 was 27·2% (20·9–35·8), exceeding estimates for other risk factors like HIV, alcohol use disorder, and undernutrition. Compared with a scenario where incarceration rates remain stable at current levels, a gradual 50% reduction in prison admissions and duration of incarceration by 2034 would reduce population tuberculosis incidence by over 10% in all countries except Mexico.
Interpretation
The historical rise in incarceration in Latin America has resulted in a large excess tuberculosis burden that has been under-recognised to date. International health agencies, ministries of justice, and national tuberculosis programmes should collaborate to address this health crisis with comprehensive strategies, including decarceration.
Funding
National Institutes of Health.
Introduction
Globally, 10·6 million people developed tuberculosis in 2022.1 Although the global tuberculosis incidence has decreased by 8·7% since 2015, in Latin America the tuberculosis incidence increased by 19% over the same period, highlighting the urgent need to address key tuberculosis drivers in the region.1 In Latin America, the incarcerated population has nearly quadrupled over the last 30 years, the most rapid growth of any region in the world. People deprived of liberty, who might already have elevated risk of tuberculosis before incarceration, are further exposed to prison conditions that foster transmission and disease progression, including overcrowding, poor ventilation, malnourishment, and limited access to health care.2 Together these factors contribute to tuberculosis rates that, in South America, are 26 times higher among people deprived of liberty than in the general population.3
Recognising the crisis of tuberculosis in prisons, the Pan American Health Organization (PAHO) began requesting data from member states on case notifications occurring among people deprived of liberty. Between 2014 and 2019, the percent of all notified tuberculosis cases in the region occurring among people deprived of liberty increased from 6·6% to 9·4%.2,4 While alarmingly high, this figure underestimates the tuberculosis burden attributable to incarceration, for several reasons. First, the case detection ratio is lower in prisons than in the general population.4 Second, individuals who acquire infection in prison often do not progress to tuberculosis disease until after release. Indeed, previous studies showed that formerly incarcerated individuals had elevated rates of tuberculosis for up to 7 years following release from prison.5,6 As notifications databases do not record information on incarceration history, these cases are not currently attributed to incarceration.7 Finally, infections acquired in prisons, including among people who work in or visit prisons, can spread in the community. Accordingly, genomic epidemiologic studies have identified tuberculosis transmission chains that span prisons and communities.8-12 Therefore, existing studies that focus on tuberculosis occurring in prisons overlook the role of incarceration as a population-level tuberculosis driver.
Understanding the full contribution of incarceration to the worsening tuberculosis epidemic in Latin America is crucial to inform tuberculosis prevention strategies and resource allocation. Furthermore, the effect of alternative incarceration policies on the tuberculosis epidemic remains unknown, as previous studies have focused on biomedical interventions. In this study, we use mathematical modelling to quantify the population-level burden of tuberculosis attributable to incarceration in six countries: Argentina, Brazil, Colombia, El Salvador, Mexico, and Peru. Specifically, we hypothesise that the rise in incarceration since 1990 has produced a growing excess tuberculosis burden and hindered tuberculosis progress in the region. We additionally simulate alternative incarceration policies and project their effects on future population tuberculosis incidence.
Methods
Study design
In this modelling study, we selected countries in Latin America, defined as Mexico, Central America, and South America, based on data availability and to represent regional heterogeneity in incarceration and tuberculosis trends (appendix p 28). Together, the six included countries represent 82·4% of the region’s incarcerated population, 79·7% of total tuberculosis notifications, and 80·1% of tuberculosis notifications in prisons in 2018.
We collected data on incarceration prevalence, prison entries or releases, and recidivism from each country’s penitentiary department or census agency via published reports and information requests for the years 1990–2023 (specific dates were country dependent; appendix p 13). We also referenced reports and articles published by researchers, international agencies, and journalists. Population estimates and projections were obtained from World Population Prospects. Population-wide tuberculosis notifications and incidence estimates were retrieved from the WHO Global Tuberculosis Report.1 Notifications and incidence estimates for people deprived of liberty were sourced from PAHO and a 2023 study (appendix p 14).4
Procedures
We developed a deterministic, meta-population compartmental model to simulate incarceration and tuberculosis transmission (appendix p 29). The model includes a simple representation of the natural history of tuberculosis across five compartments: susceptible, early latent, late latent, infectious, and recovered. These compartments are replicated across four population strata, which individuals traverse via incarceration and release: never incarcerated, currently incarcerated, recent history of incarceration, and distant history of incarceration. We distinguish between recent and distant incarceration history to account for the elevated risk of recidivism, tuberculosis, and mortality in the early period after release.5,6,13 The model does not include HIV, drug resistance, gender or sex, or age structure and excludes children aged 14 years and younger who are assumed to not be at risk of incarceration. We assume that higher tuberculosis risk in prisons results from higher effective contact rates, higher disease progression rates, and lower diagnosis rates compared with outside prison. We include low levels of mixing between incarcerated and non-incarcerated individuals to represent interactions with prison staff and visitors.
The model was fit independently for each country to incarceration and tuberculosis data from 1990 to 2023. Yearly calibration targets included incarceration prevalence, prison entries (admissions), recidivism, and total and within-prison tuberculosis incidence and notification rates (appendix pp 13-14). We accounted for uncertainty by sampling from distributions for calibration targets and for a subset of parameters that were fixed during calibration (appendix pp 15-16). For each sample of calibration targets and fixed parameters, optimisation algorithms were run to calibrate the remaining parameters, obtaining at least 1000 fitted parameter sets per country (appendix pp 6, 32).
For time-varying parameters, we let the model reach equilibrium with baseline values and then applied rates of change starting in the year 1990. Changes in incarceration prevalence over time were obtained through changes in prison entry and release rates; changes in tuberculosis incidence and notification rates were obtained through changes in effective contact rates and diagnosis rates (appendix pp 17, 33). COVID-19 pandemic-related changes were also accounted for (appendix p 18).
Statistical analysis
For each country, we quantified the excess population-level tuberculosis incidence attributable to the rise in incarceration prevalence since 1990 by simulating a counterfactual scenario in which incarceration prevalence and dynamics remained stable at 1990 levels. To operationalise this scenario, the model was rerun for each set of fitted parameters from 1990, with time-dependent changes in prison entry and release rates turned off. Time-dependent changes in the effective contact rate within prison were also eliminated, as they were assumed to be linked to growing prison populations. The excess burden was then calculated as the relative and absolute difference in population tuberculosis incidence between the observed and counterfactual scenarios. In this Article, we report model generated excess burden estimates for the years 2019 and 2022, but we use 2019 for the main estimates due to COVID-19-related uncertainty. We also analyse where excess incident cases arose (ie, where individuals progressed or relapsed to infectious tuberculosis disease) and where cases were diagnosed and the required notification made.
To estimate the transmission population attributable fraction (tPAF) for incarceration among individuals aged 15 years and older, we simulated a scenario where incarceration prevalence was gradually reduced to zero by 2009 (appendix p 9). After 10 years of no new exposure to incarceration, we calculated the tPAF for incident cases in 2019 as follows:14
We compared our estimates of the tPAF for incarceration with WHO’s country-specific estimates of the fraction of all incident cases attributable to each of the five major tuberculosis risk factors in 2019. We note that risk factors might be overlapping, and that WHO’s estimates apply to varying age groups (undernutrition to all ages; HIV to all ages; alcohol use disorders to those aged ≥15 years; smoking to those aged ≥15 years; and diabetes to those aged ≥18 years). For diabetes, the population attributable fraction (PAF) is reported as a fraction of all cases among individuals aged 15 years and older, rather than a true PAF, and therefore might be overestimated.
Various incarceration scenarios were simulated over a 10-year period (from the start of 2024 to the start of 2034) and their effects on future population tuberculosis incidence were estimated. Under the reference or stable scenario, prison entry rates and average duration of incarceration remain constant. Under the continue trends scenario, entry rates and duration undergo the same relative net change from 2024 to 2034 as over the previous 10 years (ie, start of 2013 until start of 2023). The decarceration scenarios involved gradual 25% or 50% reductions in entry rates, duration, or both by the beginning of 2034. We computed the percent difference in projected population tuberculosis incidence in the year 2034 under each scenario compared with that expected under the stable scenario.
In El Salvador, the prison population has nearly tripled since March, 2022, under a continued state of emergency.15 We estimated the excess population tuberculosis incidence in 2024 attributable to the state of emergency by simulating a counterfactual scenario without the observed rise in incarceration prevalence since March, 2022. We additionally simulated the following future scenarios for 2024–34: continuation of current entry and release rates under the state of emergency; passive abatement through entry and release rates gradually returning to their pre-emergency levels by 2034; and active cessation of the state of emergency by 2025 and reversion of incarceration prevalence to its approximate pre-emergency level in 10, 5, or 2 years (ie, by the start of 2034, 2029, or 2026), with continued decarceration thereafter (appendix p 11). Reversion of incarceration prevalence to pre-emergency levels under the three different scenarios was done through entry and release rates changing promptly by 2025. Rather than comparing with a reference scenario, the percent change in population tuberculosis incidence was computed for 2034 under each scenario compared with 2021.
We calculated all estimates for each set of sampled calibration targets, sampled parameters, and fitted parameters, yielding outcome distributions with more than 1000 values per country. For each outcome, we report the median and 95% uncertainty interval (UI) from these distributions, representing uncertainty in data and model parameters.
Five sensitivity analyses were done to vary key assumptions around natural history, differences across strata, changes over time, and mixing (appendix pp 9-11). Linear regression meta-modelling was also done using a multi-level model to identify parameters associated with variation in excess burden estimates.16
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Argentina, Brazil, Colombia, El Salvador, Mexico, and Peru exhibited wide variability in the population-wide and within-prison burden of tuberculosis between 1990 and 2019 (table 1). In 2019, the tuberculosis notification rate in prisons was a median 28·7 (IQR 13·1–31·6) times the population-wide notification rate (table 1).
Table 1:
Incarceration-related and tuberculosis-related characteristics by country
| Argentina | Brazil | Colombia | El Salvador | Mexico | Peru | Source | |
|---|---|---|---|---|---|---|---|
| Incarcerated population in 1990* | 21 016 | 90 000 | 32 387 | 5 982 | 93 119 | 17 859 | Data |
| Incarcerated population in 2019 | 109 405 | 755 274 | 123 078 | 38 114 | 200 936 | 95 548 | Data |
| Prison occupancy in 2018 (%) | 112 | 165 | 146 | 333 | 97 | 219 | Data |
| Prevalence of incarceration per 100 000 population in 2019 | 321 | 452 | 316 | 825 | 216 | 399 | Data |
| Percent increase in prevalence of incarceration since 1990 (95% UI) | 239 (229–249) | 397 (393–400) | 120 (107–134) | 372 (331–404) | 28 (19–38) | 190 (171–197) | Model |
| Incarceration growth driven by increasing entry rates or duration | Entry rates | Entry rates | Both | Both | Both | Duration | Model |
| Average duration of incarceration in 2019 (years [95% UI]) | 2·6 (2.2–3·4) | 1·3 (1·1–1·6) | 3·2 (2·7–3·8) | 6·0 (4·8–8·0) | 1·9 (1·6–2·3) | 4·7 (3·9–5·8) | Model |
| Within-prison prevalence of incarceration history | 30% (21–37) | 52% (41–59) | 21% (15–27) | 18% (12–22) | 25% (17–32) | 25% (18–32) | Model |
| Community prevalence of incarceration history | 1·3% (1·0–1·7) | 2·8% (2·0–3·9) | 4·2% (3·0–5·6) | 2·9% (2·2–3·6) | 6·0% (4·7–7·6) | 3·5% (2·8–4·3) | Model |
| Population-level tuberculosis notifications in 2019 | 11 446 | 85 523 | 14 292 | 3009 | 23 702 | 31 764 | Data |
| Population tuberculosis notification rate per 100 000 in 2019 | 25·7 | 40·5 | 28·7 | 47·9 | 19·0 | 97·6 | Data |
| Prison tuberculosis notification rate per 100 000 in 2019 | 214 | 1303 | 784 | 3484 | 144 | 2945 | Data |
| Percent of all tuberculosis notifications occurring in prisons in 2019 | 2·0 | 11·5 | 6·8 | 44·1 | 1·2 | 8·9 | Data |
Data are n or % (95% uncertainty interval), unless otherwise specified. For model outputs, medians are shown with 95% uncertainty intervals in parentheses. All population-wide prevalence estimates are for the population aged 15 years and older. Data sources are detailed in the appendix (pp 13-14). UI=uncertainty interval. *Data are from 1992 for Argentina.
Calibrated parameter values differed in prisons compared with the community: effective contact rates in prison were a median 6·8 (IQR 2·5–11·9) times those in the community, disease progression rates were a median 2·3 (2·0–2·9) times those in the community, and diagnosis rates were a median 0·55 (0·44–0·59) times those in the community (appendix p 19). Between 1990 and 2019, the prevalence of incarceration among the population aged 15 years and older more than doubled in all countries except Mexico; across all studied countries the median reached 360 (IQR 317–439) per 100 000 population in 2019 (table 1). This historical rise was driven by an increase in prison entry rates (Argentina and Brazil), an increase in average duration of incarceration (Peru), or both (El Salvador, Colombia, and Mexico; appendix p 20). By 2019, the average duration of incarceration ranged from 1·3 years (95% UI 1·1–1·6) in Brazil to 6·0 years (4·8–8·0) in El Salvador (table 1). The percent of the prison population with previous incarceration history ranged from 18% (12–22) in El Salvador to 52% (41–59) in Brazil. Such differences in incarceration dynamics contribute to the heterogeneity in community prevalence of incarceration history among the population aged 15 years and older, which ranged from 1·3% (1·0–1·7) in Argentina to 6·0% (4·7–7·6) in Mexico (table 1). Across all six countries in 2019, 1·3 million people were incarcerated at any given time and an estimated additional 13·4 million (11·4–15·7 million) people had an incarceration history.
Compared with a counterfactual scenario in which incarceration prevalence remained stable since 1990, the observed rise in incarceration prevalence since 1990 resulted in an estimated 34 393 (95% UI 28 295–42 579) excess incident cases in 2019 across the six countries or 29·4% (95% UI 23·9–36·8) more than expected under the counterfactual scenario (figure 1A, B, table 2). The excess population tuberculosis incidence in 2019 varied widely across countries, ranging from 6% or 1·3 (95% UI 0·8–2·1) cases per 100 000 person-years in Mexico to 134% or 32·2 (25·5–41·3) cases per 100 000 person-years in El Salvador (table 2). Estimates for the year 2022 were similar to 2019 (appendix p 21). Sensitivity analyses varying several assumptions did not substantively change our results (appendix p 37).
Figure 1: Excess population tuberculosis incidence attributable to the rise in incarceration prevalence since 1990.
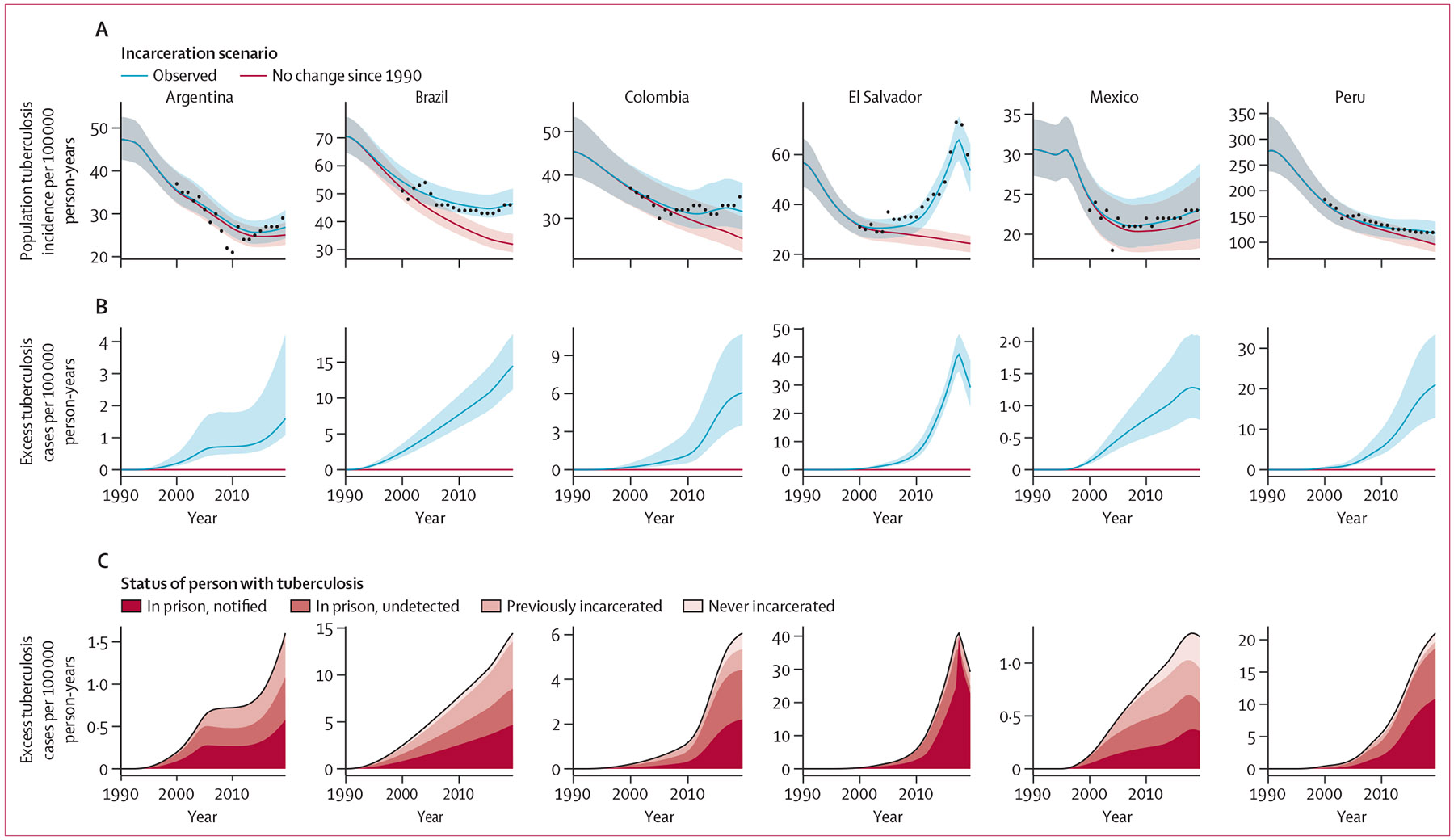
Solid lines represent medians and shaded bands represent 95% UI. (A) Population tuberculosis incidence per 100 000 person-years under the observed and counterfactual (no rise in incarceration since 1990) scenarios. Black points represent population tuberculosis incidence estimates from WHO, which are available from 2000. (B) Excess population-wide incident tuberculosis cases per 100 000 person-years. (C) Median estimates of excess cases, stratified by population subgroup in which they occurred, and for incident cases occurring in prison, additionally stratified by whether the disease was notified or undetected during incarceration. All model results are for the population aged 15 years and older. UI=uncertainty interval.
Table 2:
Estimates of population tuberculosis incidence attributable to incarceration in 2019
| Incidence rate ratio for observed vs counterfactual (95% UI) |
Excess cases per 100 000 person-years relative to counterfactual (95% UI) |
Absolute excess cases relative to counterfactual (95% UI) |
Transmission population attributable fraction (95% UI) |
|
|---|---|---|---|---|
| Argentina | 1·06 (1·04–1·16) | 1·5 (1·0–3·9) | 506 (344–1344) | 8·4% (6·0–18·6) |
| Brazil | 1·44 (1·32–1·59) | 14·1 (10·9–18·4) | 23 497 (18 160–30 739) | 36·9% (29·5–45·1) |
| Colombia | 1·23 (1·13–1·42) | 6·0 (3·4–10·6) | 2337 (1338–4135) | 21·8% (14·1–34·7) |
| El Salvador | 2·34 (2·03–2·69) | 32·2 (25·5–41·3) | 1489 (1178–1907) | 58·1% (51·6–64·1) |
| Mexico | 1·06 (1·04–1·09) | 1·3 (0·8–2·1) | 1180 (740–1957) | 7·5% (4·8–11·6) |
| Peru | 1·21 (1·13–1·34) | 20·6 (12·6–33·0) | 4922 (3028–7899) | 23·3% (16·7–34·4) |
All estimates are at the population level among individuals aged 15 years and older. Incidence rate ratios and excess burden estimates were obtained from comparing incident tuberculosis cases in 2019 between the observed scenario of the historical rise in incarceration and the counterfactual scenario of no change in incarceration prevalence since 1990. The transmission population attributable fraction in 2019 was estimated using a scenario where incarceration prevalence was reduced to zero by 2009. UI=uncertainty interval.
The burden of excess incident cases that arose (ie, progression to disease or relapse) in prisons in 2019 exceeded that of excess cases diagnosed within prisons by a median of 81% across studied countries, ranging from 10% in El Salvador to 102% in Colombia (figure 1C). Furthermore, a considerable fraction of the excess burden in 2019 was comprised of incident cases arising among individuals who had been formerly incarcerated, particularly in countries with a shorter average duration of incarceration (figure 1C). For instance, the percent of excess cases arising in the community among individuals who had been formerly incarcerated was 34% (95% UI 24–45) in Argentina, 34% (26–42) in Brazil, and 26% (16–36) Mexico (appendix p 23). In all countries, the estimated tuberculosis incidence rates among individuals with recent or incarceration history were much higher than population-wide incidence rates (appendix pp 24, 38).
Collectively across countries, incarceration was the leading determinant compared with other key tuberculosis risk factors, accounting for an estimated 27·2% (95% UI 20·9–35·8) of incident cases in 2019 among the population aged 15 years and older. The country-specific tPAF of incarceration in 2019 was 58·1% (51·6–64·1) in El Salvador, 36·9% (29·5–45·1) in Brazil, 23·3% (16·7–34·4) in Peru, 21·8% (14·1–34·7) in Colombia, 8·4% (6·0–18·6) in Argentina, and 7·5% (4·8–11·6) in Mexico (table 2). Despite this variability, the country-specific tPAF for incarceration was consistently greater than or commensurate with PAFs for other major risk factors (figure 2). Moreover, the median tPAF estimate was 1·3 to 6·3 times the percent of all tuberculosis notifications occurring in prisons in 2019 (figure 2).
Figure 2: Population attributable fraction for incarceration and other tuberculosis risk factors.
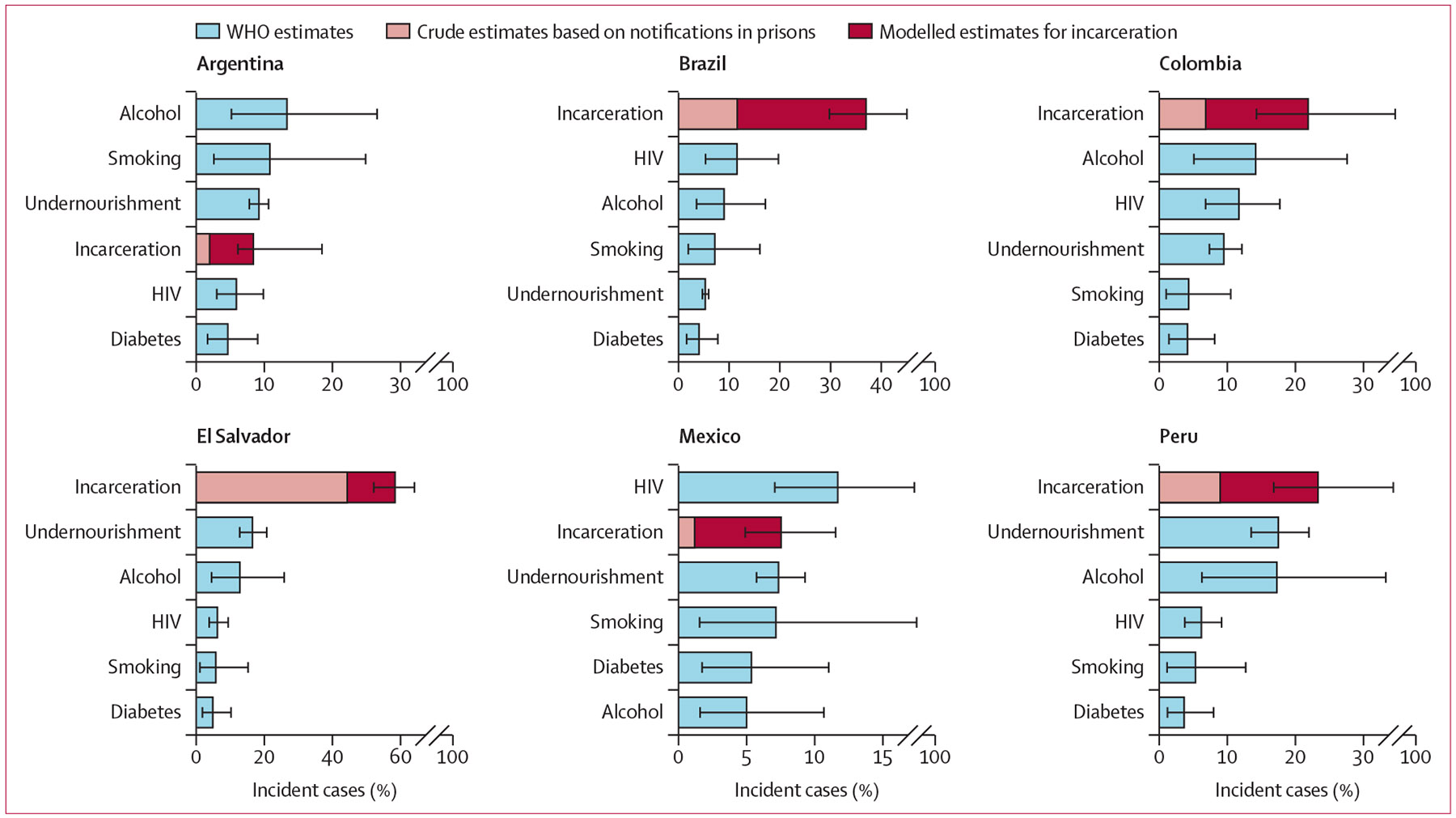
Median estimates and uncertainty intervals for the percent of population-level incident tuberculosis cases in 2019 that can be attributed to each risk factor. The crude estimate of the population attributable fraction for incarceration is based on the percent of all notified tuberculosis cases that occurred in prisons. Estimates for all other risk factors are from WHO. Risk factors are listed in descending order by PAF for each country. Estimates correspond to different age groups: incarceration for age ≥15 years; undernutrition for all ages; HIV for all ages; alcohol for age ≥15 years; smoking for age ≥15 years; and diabetes for age ≥18 years. PAF=population attributable fraction.
We projected the impact of future incarceration policies, implemented from 2024 to 2034, on population tuberculosis incidence in 2034 (figure 3A; appendix p 25). If recent incarceration trends continue, the projected population tuberculosis incidence in 2034 would be slightly (<3%) higher in Peru, Argentina, and Mexico, and slightly lower in Colombia and Brazil (figure 3B). More active decarceration interventions—for instance, a 50% decrease in prison entry rates and duration of incarceration—could reduce population tuberculosis incidence in 2034 by an estimated 28·9% (95% UI 22·0–36·7) in Brazil, 16·4% (11·4–23·3) in Peru, 13·7% (8·9–21·3) in Colombia, 10·3% (7·1–16·9) in Argentina, and 3·0% (1·3–5·7) in Mexico.
Figure 3: Projected impacts of incarceration-related interventions on future population tuberculosis incidence.
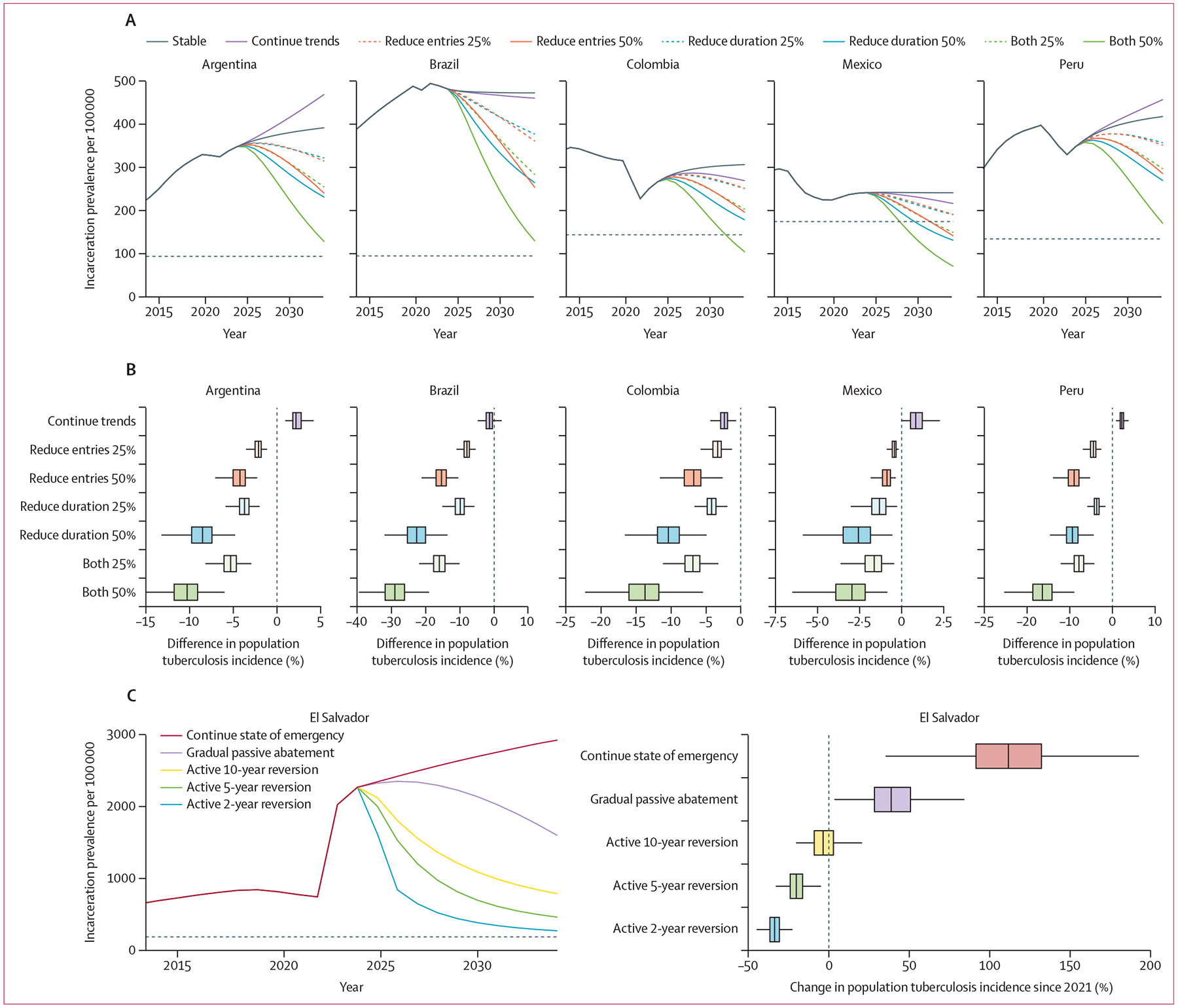
(A) Median incarceration prevalence per 100 000 population aged ≥15 years under incarceration scenarios implemented between 2024 and 2034: stable entry and release rates (reference scenario), continuation of trends from previous 10 years, and 25% or 50% reduction in prison entry rates, duration of incarceration, or both by 2034. The dashed horizontal line represents incarceration prevalence in 1990. (B) Percent difference in population tuberculosis incidence in 2034 under each incarceration scenario, relative to the reference scenario of stable entry and release rates. Outliers are not shown. (C) Left: median incarceration prevalence under each incarceration scenario in El Salvador: continuation of entry and release rates under the state of emergency, passive abatement through gradual reversion of entry and release rates to pre-emergency levels by 2034, active cessation and approximate restoration of pre-emergency incarceration prevalence in 10 years, or restoration of pre-emergency prevalence in 5 years or 2 years with continued decarceration thereafter. The dashed horizontal line represents incarceration prevalence in 1990. Right: percent change in population tuberculosis incidence since 2021 under each scenario.
In El Salvador, the projected tuberculosis incidence in 2024 was estimated to be 2·1 (95% UI 1·8–2·4) times as high as expected without the recent state of emergency, corresponding to 2444 (95% UI 1562–3245) excess incident cases in 2024 (appendix p 23). Maintaining the state of emergency for 10 years is projected to increase population tuberculosis incidence in 2034 by 112% (63–176) compared with pre-emergency in 2021 (figure 3C). A gradual, passive abatement of the state of emergency would still increase population tuberculosis incidence in 2034 by a projected 39% (13–72). By contrast, prompt and active cessation of the state of emergency and reversion of incarceration prevalence to approximate pre-emergency levels by 2034 could restore the population tuberculosis incidence in 2034 to its approximate rate in 2021. More decisive actions to revert to pre-emergency incarceration prevalence in 2 years or 5 years and continue further decarceration thereafter could reduce population tuberculosis incidence in 2034 by as much as 34% (25–42) compared with 2021.
Discussion
Across six Latin American countries, more than 34 000 incident cases in 2019 can be attributed to the rise in incarceration since 1990. Collectively in these countries, incarceration accounts for an estimated 27·2% (95% UI 20·9–35·8) of incident cases in 2019 among individuals aged 15 years and older, a far greater fraction than any other determinant.1 Against the backdrop of the region’s alarming increase in tuberculosis incidence over the last decade, we project that policies to reduce incarceration prevalence might considerably reduce future population tuberculosis incidence. Together, the results of this study implicate incarceration as a leading population-level driver of the tuberculosis epidemic in Latin America. In addition to improving prison conditions and implementing biomedical interventions in prisons, criminal legal reforms and development of non-carceral alternatives will be crucial to re-ignite progress towards tuberculosis elimination.
Our results elucidate the harmful impacts of decades of punitive policies on the tuberculosis epidemic in the region. Beginning in the 1990s, amidst rising crime and public support for tough-on-crime initiatives, governments in Latin America expanded police and prosecutorial activity, criminalised new acts, and imposed harsher sentences, including for minor offences.17,18 Consequently, prison populations surged, largely comprised of individuals detained for property-related and drug-related offences.17,19 Meanwhile, inadequate investments in the penitentiary system led to severe overcrowding, inhumane living conditions, deficient health care, corruption among staff, uprisings, and self-governance.17,20 Today, the incarceration rate in Latin America is twice the global rate and higher than all other regions except North America. Criminologists argue that prisons have been ineffective and even counterproductive in curbing crime in the region.17,20 Instead, they have created new challenges, including a worsening crisis of tuberculosis in prisons.
In this study, we show that the scope and magnitude of this crisis is even larger than previously recognised. To date, research and policy guidance has focused on tuberculosis occurring within prisons.3,21-24 However, unlike other tuberculosis risk factors, incarceration is highly dynamic. The constant flow of people who are newly incarcerated and released yields a much larger population ever exposed to the high-risk carceral environment, which we estimate for six countries is over 11 times the size of the population in prison at any given time. By accounting for this phenomenon and its interplay with the variable latent period of tuberculosis, we obtained attributable burden estimates that far exceeded crude, static estimates based on notifications in prisons.2 Of note, most of the difference was due to under-detection in prisons and progression to disease following release, rather than onward transmission. Therefore, although traditional PAF estimates for other tuberculosis determinants might also be underestimated due to not accounting for onward transmission, incarceration is particularly subject to under-recognition by conventional approaches that do not account for its dynamic nature. Policy guidance and future research should recognise incarceration as a tuberculosis driver and social determinant with effects that transcend prison walls.
We also show the potential effect of alternative incarceration policies on the tuberculosis epidemic in the region. For instance, policies that decrease prison admissions and duration by 50% could reduce future population tuberculosis incidence by more than 10% in Brazil, Peru, Colombia, and Argentina, countries which encompass the majority of Latin America’s tuberculosis burden. In El Salvador, which already had an exorbitant tPAF for incarceration before 2022, the current state of emergency is projected to have catastrophic consequences for tuberculosis. We predict that swift, resolute termination of the state of emergency could enable a return to pre-emergency incidence by 2034, and that further decarceration can recover, at least in part, a decade of lost opportunity for tuberculosis progress. Such measures have precedent in Kazakhstan, where the Royal Netherlands Tuberculosis Foundation and Penal Reform International co-led comprehensive efforts to address tuberculosis in prisons, integrating biomedical interventions with decriminalisation reforms, implementation of alternatives to incarceration, and improvements in prison conditions.25 Following expansion of the programme in 2000, incarceration prevalence decreased by 70% and, with it, the rate of tuberculosis in prisons by 90%.26 Therefore, decarceration interventions, especially if coupled with biomedical interventions and efforts to improve prison conditions, have substantial potential to accelerate progress towards the 2035 End TB Strategy targets.
Our estimates of the tuberculosis burden attributable to incarceration vary greatly across the six countries included, correlating with incarceration prevalence and country-specific disparities in tuberculosis risk between prisons and the general population. Between-country variation in where excess cases arise can also be attributed to distinct carceral dynamics across countries. For instance, in countries with a longer average duration of incarceration, such as El Salvador and Peru, our model predicts that the majority of excess incident cases occur within prisons.4 Conversely, in countries with a shorter average duration of incarceration, such as Brazil and Mexico, a greater proportion of excess incident cases occur in the community after prison release. Therefore, it is crucial to consider incarceration dynamics and changing carceral policies in identifying optimal intervention strategies. These insights might generalise to other countries and regions. Specifically, in most other settings with lower incarceration rates and less disparity in tuberculosis rates between prisons and the general population, incarceration might have a reduced role in the tuberculosis epidemic. Nonetheless, in all settings, the true incarceration-attributable tuberculosis burden probably exceeds crude estimates based on tuberculosis occurring within prisons, especially where prison turnover rates are high.
In response to this public health crisis, bold and decisive investments and actions are needed. First, international health agencies and national tuberculosis programmes must improve reporting of incarceration as a structural determinant of tuberculosis; they must collect information on incarceration history in case notifications databases and current and past incarceration must be included as a key risk factor in WHO’s Global Tuberculosis Report.7 Given the stigma and discrimination faced by individuals with incarceration history, procedures for collecting this information in a sensitive manner should be developed alongside stakeholders with lived experience of incarceration.27 Second, effective strategies to prevent, detect, and treat tuberculosis in individuals who are incarcerated or formerly incarcerated must be identified, incorporated in national guidelines, and implemented at scale.28,29 Although existing research has focused on prison-based interventions, future work should expand to include formerly incarcerated individuals and their community contacts.
Finally, and equally as important, governments must implement structural reforms to reduce the prison population. Although our study focused on tuberculosis, incarceration exposure has been linked to other adverse health outcomes.13,30,31 Therefore, decarceration strategies, especially in conjunction with efforts to transform conditions of confinement, have the potential to both accelerate tuberculosis progress and improve population health at large. Currently, political will and public support for such measures remain low. However, calls are growing for governments to improve prison conditions, decriminalise minor offences, reduce pre-trial detention, and develop restorative justice-based alternatives to incarceration, with several initiatives underway across Latin America.17,19,20,32-35
This study has several limitations. First, for four of six countries (ie, Argentina, El Salvador, Mexico, and Peru) empirical prison-based active case-finding studies were unavailable, so prison incidence estimates for model calibration were based on a regional case detection ratio. For these countries, findings should be viewed as estimates within a plausible range of uncertainty. Second, deterministic compartmental models are unable to capture the full range of complexity in real-world phenomena. The extent to which we were able to incorporate complexity in our model was constrained by inadequate data to inform model parameters and assumptions. For instance, our model did not account for age, gender or sex, socioeconomic status, HIV status, heterogeneity in duration of incarceration, heterogeneity in infectiousness, or multi-drug resistant tuberculosis, which is less common in prisons in the WHO region of the Americas than in other regions.36 Accounting for HIV or multi-drug resistant tuberculosis, which might be exacerbated in prisons, might increase estimates of the incarceration-attributable burden.11 Accounting for other factors such as age or socioeconomic inequities that affect mixing and tuberculosis risk might result in lower estimates for incarceration.23 Moreover, we had little to no data to inform mixing assumptions or stratum-specific parameters for individuals with incarceration history. In these cases of insufficient data, we used wide parameter uncertainty distributions and varied our assumptions in sensitivity analyses, with our findings generally remaining robust. However, the dearth of reliable, publicly accessible data on incarceration and tuberculosis must be urgently addressed. Finally, our future projections are subject to great uncertainty, including uncertainty around how the COVID-19 pandemic has affected and will continue to affect tuberculosis and incarceration. We were unable to model specific policies or reforms (ie, decriminalisation of drug use) due to insufficient data. Our future simulations also do not include changes in any other dimension aside from prison entry and release rates, such as improvements in prison conditions or scale-up of biomedical interventions. Generally, our historical counterfactual and future policy simulations are simplistic, modifying incarceration in isolation from what is inevitably an intricate web of upstream and downstream social, economic, political, and institutional forces that themselves also affect population health and tuberculosis. Nonetheless, our findings underscore the substantial potential for criminal legal reforms to reduce tuberculosis burden in Latin America, impacts which could be enhanced by additional prison-based and community-based interventions.
Mass incarceration policies have undermined tuberculosis control in Latin America to a greater extent than previously recognised. Our estimates of the outsized tuberculosis burden attributable to incarceration eclipse those of other determinants that currently receive far greater attention. However, this exceptional excess burden must not be regarded as inevitable. Health agencies, national tuberculosis programmes, ministries of justice, and other key stakeholders should undertake bold commitments and actions to elevate the prominence of incarceration in national and international strategies for tuberculosis control and elimination, accounting for effects beyond prison walls. These strategies should take an integrated health and human rights approach, combining biomedical interventions and improvements in prison conditions with actions to enable decarceration. Such measures will be crucial to advancing towards regional and global tuberculosis elimination targets.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies on tuberculosis in prisons in Latin America, using the search terms (“tuberculosis”) AND (“prisons” OR “incarceration”) AND (“Latin America” OR “Argentina” OR “Brazil” OR “Colombia” OR “El Salvador” OR “Mexico” OR “Peru”), published in any language. Previous studies have identified a high risk of tuberculosis in prisons in Latin America, finding that notifications in prisons are increasing and account for a growing proportion of all cases in the region. Other national or sub-national studies have found elevated tuberculosis risk among individuals who were formerly incarcerated and identified transmission chains spanning prisons and communities. However, the full contribution of incarceration to the broader tuberculosis epidemic in Latin America—accounting for historical incarceration trends, under-detection in prisons, and spillover effects into communities—has never been quantified. Furthermore, previous studies have focused on biomedical interventions in prisons; the regional effect of alternative incarceration policies on future population-level tuberculosis incidence is unknown.
Added value of this study
In this study, we quantify the full contribution of incarceration to the tuberculosis epidemic in Latin America. Our model captures the dynamic nature of incarceration, incorporating historical and contemporary data sources to account for varying prison turnover rates and mechanisms underlying historical incarceration growth. We estimate the true size of the population ever exposed to incarceration via modelling, which across the six researched countries is over 11 times the size of the population in prison at any one time. We identify the settings in which excess cases occur and compare our results to crude estimates based on notifications in prisons. We show, across six countries with diverse carceral contexts and tuberculosis epidemiology, that incarceration is a leading driver on par with or surpassing other major tuberculosis risk factors. Finally, we demonstrate the potential effect of alternative incarceration policies in reducing future tuberculosis burden in carceral settings and the general population.
Implications of all the available evidence
To date, the true impact of incarceration on the tuberculosis epidemic across the region has been underestimated due to a narrow focus on cases occurring within prisons. Considering the substantial excess tuberculosis burden attributable to incarceration, interventions targeting incarceration can have outsized effects on the broader tuberculosis epidemic in Latin America—much greater than previously appreciated. These interventions should include strategies to reduce tuberculosis risk among currently and formerly incarcerated individuals as well as efforts to end mass incarceration.
Acknowledgments
This study was funded by the National Institutes of Health (grant numbers 5R01AI130058 and 5R01AI149620). We thank Edwin Segura, Hernán Olaeta, Victor Peña Garcia, Noah Bullock, and the National Penitentiary and Prison Institute of Colombia for providing data and useful insights.
Footnotes
Declaration of interests
YEL reports funding from the Stanford Knight Hennessy Scholars Program, the National Science Foundation Graduate Research Fellowship, and the Gerald J Lieberman Fellowship from the Stanford Office of the Vice Provost for Graduate Education; and a previous leadership role in the Stanford Jail and Prison Education Program. MAH reports grants or contracts paid to their institution from the National Institutes of Health (NIH), Gilead Sciences, Insmed, AN2 Therapeutics, and AstraZeneca; and participation on the AIDS Clinical Trials Group Tuberculosis Transformative Science Group Study Monitoring Committee. TC reports grants from the Centers for Disease Control and Prevention and NIH to their institution. JC reports grants or contracts from Valneva–Instituto Butantan, Merck & Co, Sanofi Pasteur, Coalition for Epidemic Preparedness Innovations–Sabin Vaccine Institute, and Takeda; speaking fees from Pfizer; and participation in Advisory Boards for the mRNA-1273 vaccine (for Moderna–Zodiac), RSV maternal vaccine (for Pfizer), Qdenga vaccine (for Takeda), Nirmatrelvir–Ritonavir (for Paxlovid and Pfizer), and the Global Dengue Steering Committee (for Takeda). JRA reports grants from Good Ventures–Open Philanthropy for an ethics evaluation of tuberculosis vaccine trials paid to their institution; payment for expert testimony involving tuberculosis in prisons in the USA; participation on safety monitoring boards and advisory boards for NIH-sponsored clinical studies and trials pertaining to tuberculosis; a leadership role in the TB in Prisons Working Group for the International Union Against Tuberculosis and Lung Disease; and a donation of materials from Cepheid for research use. All other authors declare no competing interests.
For more on incarceration data see https://www.prisonstudies.org/
Contributor Information
Yiran E Liu, Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA; Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University, Stanford, CA, USA.
Yasmine Mabene, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University, Stanford, CA, USA.
Sergio Camelo, Institute for Computational and Mathematical Engineering, Stanford University, Stanford, CA, USA.
Zulma Vanessa Rueda, Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg, MB, Canada; School of Medicine, Universidad Pontificia Bolivariana, Medellin, Colombia.
Daniele Maria Pelissari, National Tuberculosis Program, Ministry of Health, Brasília, Brazil.
Fernanda Dockhorn Costa Johansen, National Tuberculosis Program, Ministry of Health, Brasília, Brazil.
Moises A Huaman, Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Tatiana Avalos-Cruz, Dirección de Prevención y Control de la Tuberculosis, Ministerio de Salud, Lima, Perú.
Valentina A Alarcón, Dirección de Prevención y Control de la Tuberculosis, Ministerio de Salud, Lima, Perú.
Lawrence M Ladutke, New Jersey City University, Jersey City, NJ, USA.
Marcelo Bergman, Center for Latin American Studies on Insecurity and Violence, National University of Tres de Febrero, Buenos Aires, Argentina.
Ted Cohen, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, CT, USA.
Jeremy D Goldhaber-Fiebert, Department of Health Policy, Stanford University, Stanford, CA, USA; Center for Health Policy, Freeman Spogli Institute, Stanford University, Stanford, CA, USA.
Julio Croda, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, CT, USA; Universidade Federal de Mato Grosso do Sul, Campo Grande, Brazil; Fiocruz Mato Grosso do Sul, Campo Grande, Brazil.
Jason R Andrews, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University, Stanford, CA, USA.
Data sharing
No individual participant data were collected in this study. Data and code used for modelling are available at https://www.github.com/yemloo/tb_incarc_mod.
References
- 1.WHO. Global tuberculosis report 2023. Geneva: World Health Organization, 2023. [Google Scholar]
- 2.Walter KS, Martinez L, Arakaki-Sanchez D, et al. The escalating tuberculosis crisis in central and South American prisons. Lancet 2021; 397: 1591–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cords O, Martinez L, Warren JL, et al. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health 2021; 6: e300–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez L, Warren JL, Harries AD, et al. Global, regional, and national estimates of tuberculosis incidence and case detection among incarcerated individuals from 2000 to 2019: a systematic analysis. Lancet Public Health 2023; 8: e511–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabud TS, de Lourdes Delgado Alves M, Ko AI, et al. Evaluating strategies for control of tuberculosis in prisons and prevention of spillover into communities: an observational and modeling study from Brazil. PLoS Med 2019; 16: e1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequera G, Estigarribia-Sanabria G, Aguirre S, et al. Excess tuberculosis risk during and following incarceration in Paraguay: a retrospective cohort study. Lancet Reg Health Am 2024; 31: 100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sequera G, Aguirre S, Estigarribia G, et al. Incarceration and TB: the epidemic beyond prison walls. BMJ Glob Health 2024; 9: e014722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter KS, Dos Santos PCP, Gonçalves TO, et al. The role of prisons in disseminating tuberculosis in Brazil: a genomic epidemiology study. Lancet Reg Health Am 2022; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JL, Grandjean L, Moore DAJ, et al. Investigating spillover of multidrug-resistant tuberculosis from a prison: a spatial and molecular epidemiological analysis. BMC Med 2018; 16: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanabria GE, Sequera G, Aguirre S, et al. Phylogeography and transmission of Mycobacterium tuberculosis spanning prisons and surrounding communities in Paraguay. Nat Commun 2023; 14: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utpatel C, Zavaleta M, Rojas-Bolivar D, et al. Prison as a driver of recent transmissions of multidrug-resistant tuberculosis in Callao, Peru: a cross-sectional study. Lancet Reg Health Am 2024; 31: 100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevisi L, Brooks MB, Becerra MC, et al. Who transmits tuberculosis to whom: a cross-sectional analysis of a cohort study in Lima, Peru. Am J Respir Crit Care Med 2024; 210: 222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YE, Lemos EF, Gonçalves CCM, et al. All-cause and cause-specific mortality during and following incarceration in Brazil: a retrospective cohort study. PLoS Med 2021; 18: e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra S, Baral SD. Rethinking the population attributable fraction for infectious diseases. Lancet Infect Dis 2020; 20: 155–57. [DOI] [PubMed] [Google Scholar]
- 15.Legislativa Asamblea. Régimen de excepción. 2024. https://www.asamblea.gob.sv/taxonomy/term/1922 (accessed Feb 12, 2024). [Google Scholar]
- 16.Jalal H, Dowd B, Sainfort F, Kuntz KM. Linear regression metamodeling as a tool to summarize and present simulation model results. Med Decis Making 2013; 33: 880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman M, Fondevila G. Prisons and crime in Latin America. Cambridge: Cambridge University Press, 2021. [Google Scholar]
- 18.Hathazy P, Müller M-M. The rebirth of the prison in Latin America: determinants, regimes and social effects. Crime Law Soc Change 2016; 65: 113–35. [Google Scholar]
- 19.Chaparro Hernández SPC. Catalina. Sobredosis carcelaria y política de drogas en América Latina. 2017. https://www.dejusticia.org/publication/sobredosis-carcelaria-y-politica-de-drogas-en-america-latina/ (accessed June 28, 2024). [Google Scholar]
- 20.InSight Crime. The prison dilemma: Latin America’s incubators of organized crime. 2017. https://insightcrime.org/investigations/prison-dilemma-latin-america-incubators-organized-crime/ (accessed June 28, 2024).
- 21.World Health Organization. WHO consolidated guidelines on tuberculosis. Module 2: screening—systematic screening for tuberculosis disease, 2021. Geneva: World Health Organization, 2021. [PubMed] [Google Scholar]
- 22.World Health Organization. Tuberculosis in prisons. 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/featured-topics/tb-in-prisons (accessed Feb 2, 2024).
- 23.Pelissari DM, Diaz-Quijano FA. Impact of incarceration on tuberculosis incidence and its interaction with income distribution inequality in Brazil. Trans R Soc Trop Med Hyg 2020; 114: 23–30. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, de Macedo Couto R, Pelissari DM, et al. Excess tuberculosis cases and deaths following an economic recession in Brazil: an analysis of nationally representative disease registry data. Lancet Glob Health 2022; 10: e1463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atabay T, Laticevschi V, Vasil’eva TF. Human rights and health in prisons: a review of strategy and practice. The Hague: Penal Reform International and Royal Netherlands Tuberculosis Foundation, 2006. [Google Scholar]
- 26.Pak S. Reforming the prison system: focusing on pre-trial detainees. EUSAT-RCS Prison TB Alliance Meeting; March 19–20, 2024. [Google Scholar]
- 27.Sue K. How to talk with patients about incarceration and health. AMA J Ethics 2017; 19: 885–93. [DOI] [PubMed] [Google Scholar]
- 28.Charalambous S, Velen K, Rueda Z, et al. Scaling up evidence-based approaches to tuberculosis screening in prisons. Lancet Public Health 2023; 8: e305–10. [DOI] [PubMed] [Google Scholar]
- 29.Narayan A, Salindri AD, Keshavjee S, et al. Prioritizing persons deprived of liberty in global guidelines for tuberculosis preventive treatment. PLoS Med 2023; 20: e1004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman-Retana O, Servan-Mori E, Bertozzi SM, Orozco-Nuñez E, Bautista-Arredondo S, Lopez-Ridaura R. Prison environment and non-communicable chronic disease modifiable risk factors: length of incarceration trend analysis in Mexico City. J Epidemiol Community Health 2018; 72: 342–48. [DOI] [PubMed] [Google Scholar]
- 31.Marmolejo L, Barberi D, Bergman M, Espinoza O, Fondevila G. Responding to COVID-19 in Latin American prisons: the cases of Argentina, Chile, Colombia, and Mexico. Vict Offenders 2020; 15: 1062–85. [Google Scholar]
- 32.COPLAD. Latin American jurists propose a regional pact to humanize penitentiary policies. 2024. https://copolad.eu/en/alternative-penalties-workshop-barcelona/ (accessed July 2, 2024).
- 33.Brazil National Council of Justice. Training guide on alternatives to imprisonment I: postulates, principles and guidelines for the policy of alternatives to imprisonment in Brazil, 2023. 2023. https://www.cnj.jus.br/wp-content/uploads/2023/09/guide-on-alternatives-to-imprisonment-i-postulates-and-principles-digital.pdf (accessed July 2, 2024).
- 34.Mahtani N. El modelo ‘antiBukele’ funciona en Costa Rica. 2023. https://elpais.com/america-futura/2023-11-13/el-modelo-antibukele-funciona-en-costa-rica.html#?rel=mas (accessed July 2, 2024). [Google Scholar]
- 35.Youngers C. Colombia to implement law on alternatives to incarceration for women heads of household. 2022. https://www.wola.org/analysis/colombia-law-alternatives-incarceration-women-heads-household/ (accessed July 2, 2024).
- 36.Gygli SM, Loiseau C, Jugheli L, et al. Prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis. Nat Med 2021; 27: 1171–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No individual participant data were collected in this study. Data and code used for modelling are available at https://www.github.com/yemloo/tb_incarc_mod.


