Abstract
Pair bonds powerfully modulate health, which becomes particularly important when facing the detrimental effects of aging. To examine the impact of aging on relationship formation and response to loss, we examined behavior in naive 6-, 12-, and 18-month male and female prairie voles, a monogamous species that forms mating-based pair bonds. We found that older males (18-months) bonded quicker than younger voles, while similarly aged female voles increased partner directed affiliative behaviors. Supporting sex differences in bonding behaviors, we found that males were more likely to sample both partner and stranger voles while females were more likely to display partner preference during the initial 20 min of the test. We also found that male voles of all ages show enduring bonding behavior despite four weeks of partner separation while females show an overall decrease in partner-directed affiliation, including an erosion of partner preference in 12-month females. Finally, we found that the number of oxytocin, but not vasopressin, cells in the paraventricular hypothalamus increased at 18 months of age. These results establish prairie voles as a novel model to study the effects of normal and abnormal aging on pair bonding.
Keywords: Prairie voles, Pair-bonding, Loss, Oxytocin, Vasopressin, Aging
1. Introduction
Human social behaviors are complex, evolve over a life course, and become dysfunctional in age-related disorders, such as Alzheimer’s disease (AD). During normal aging, social relationships become more positive and satisfying with age, and social engagement contributes to cognitive resilience, even in the presence of AD pathology (Charles and Piazza, 2007; Luong et al., 2011; Scarmeas et al., 2001; Stern, 2012). Meanwhile, the onset of irritability, depression, heightened anxiety fracture social relationships in prodromal phases of AD and deteriorating social memory in mid to late stages of disease exacerbate relationship stress, negatively impacting the emotional well-being of AD patients and their friends and family (Bediou et al., 2009; Desmarais et al., 2018; Ehrenberg et al., 2018, 2023; Müller-Spahn, 2003; Ren et al., 2023). However, our preclinical understanding of social changes over the course of aging remains largely limited to mice and rats, which do not display many of the complex social phenotypes fundamental to humans.
Monogamous prairie voles (Microtus ochrogaster) provide a promising model for studying the effects of normal and abnormal aging on complex sociocognitive processes relevant to humans. Prairie voles, unlike mice and rats, form life-long pair bonds and have a lifecourse that is similar although not identical to that of laboratory mice and rats. They have an ~21 day gestation and can wean at 20 days. However, they display somewhat faster development with earlier tooth eruption, eye opening, fur growth, accelerated patterns of cortical gene expression for some genes (James et al., 2022; Shapiro and Insel, 1990; Spangenberg et al., 2014), and are reproductively active at an earlier age (females can get pregnant as early as post-natal day 40 if housed with a male; Solomon, 1991). While voles display remarkable longevity in lab settings, with reports of animals living up to 5 years, in the wild they exhibit a much more restricted lifespan and are rarely documented living longer than 12–18 months (Fischer, 1945; Getz et al., 1997; Grippo et al., 2021; Kenkel et al., 2019; Stalling, 1990).
Pair bonding – like other species-typical social behaviors – is mediated by the neuropeptides, oxytocin and vasopressin. These peptide hormones are produced primarily in the paraventricular hypothalamus (PVH) and supraoptic nucleus, with the former contributing to most neural release. Differences in nonapeptide receptor densities in key brain areas such as the nucleus accumbens, prelimbic cortex, and ventral pallidum account, in part, for species differences in pair bonding (Insel and Shapiro, 1992; Olazábal and Young, 2006; Ophir et al., 2012; Young et al., 1996, 2001). Furthermore, direct manipulation of these circuits can facilitate or inhibit pair bonding in prairie voles, and can even produce bonding in typically promiscuous species (Keebaugh et al., 2015; Lim et al., 2004; Lim and Young, 2004; Ross et al., 2009; Winslow et al., 1993; Young et al., 2001). Dynamic changes in oxytocin and vasopressin circuitry occur across postnatal development and in response to pair bonding, pup rearing, and partner separation (Audunsdottir and Quintana, 2022; Bosch et al., 2016; Ebner et al., 2013; Fliers et al., 1985; Fricker et al., 2023; Hiura et al., 2023; Hiura and Ophir, 2018; Ishunina and Swaab, 1999; Kelly et al., 2017, 2018; Kenkel et al., 2019). In addition, upregulated oxytocin receptor and downregulated vasopressin receptor densities have been observed in very old male voles, providing a potential substrate for age-related changes in social behavior (Kenkel et al., 2019).
The dynamics of pair bonding have been well studied in younger animals where female animals form a partner preference more rapidly than male animals (Brusman et al., 2021; DeVries and Carter, 1999; Harbert et al., 2020; Insel and Hulihan, 1995). However, the impacts of age, and its potential interaction with sex, have been studied only in restricted settings. For instance, 2–3 years old male voles show a reduction in the amount of time spent huddling with a stranger compared to younger males (Kenkel et al., 2019), and ~4-year-old male voles display increased immobility during forced swim following separation from their long term partners (Grippo et al., 2021). Yet neither of these studies examined female behavior. Other studies have looked at social behavior outside the context of pair bonds, observing reductions in both prosocial behaviors directed towards a familiar same-sex sibling and aggression towards stranger conspecifics of either sex in non-bonded voles up to ~9 months (Powell et al., 2022). Thus, work to date supports the hypothesis that there are sex- and age-dependent changes in social behavior, bonding, and their underlying hormonal bases, but it has not been thoroughly evaluated.
To comprehensively delineate the age- and sex-dependent changes in social behavior and oxytocin/vasopressin cell number, we tested 6-, 12-, and 18-month-old naive prairie voles in partner preference tests (PPTs) and during unconstrained free interaction. Although prairie voles can live much longer in the laboratory, our goal was to model the lifespan of voles in the wild, reasoning that older ages (e.g. 3+ years) may represent a supraphysiological state for this species. Animals were tested 2 days (short-term) and 2 wks (long-term) following pairing to examine whether age impacts the development of pair bonds (Brusman et al., 2021). We then separated pairs for 4 wks before testing for bond persistence following partner loss via partner preference and free interaction tests (Fricker et al., 2023; Sadino et al., 2021). Finally, we investigated the potential neurochemical mechanisms underlying the evolution of pair bonds dynamics during aging by quantifying the number of oxytocin and vasopressin cells in the PVH. Our results indicate sex-dependent effects of age on pair bonding such that old males bond quicker while females increase partner-directed affiliative behavior and undergo a U-shaped curve of partner preference following long-term partner loss. Within the first 20 min of the PPTs, females were more likely to display partner preference at all ages compared to males. Furthermore, female behavior during PPTs was reflected during unconstrained free interaction, suggesting that female behavior dictates pairwise interactions (Brusman et al., 2021). These changes were met by age-dependent increases in the number of oxytocin, but not vasopressin, neurons within the PVH. Overall, these data provide fundamental knowledge on pair bonding during normal aging in prairie voles which can be used to further develop the species for studying the bidirectional interplay between abnormal aging and socioemotional wellbeing.
2. Materials and methods
2.1. Animals
Prairie voles were bred in-house at the University of Colorado Boulder in a colony originating from wild caught animals from Illinois. Animals were housed in same-sex groups of 2–4 animals in static rodent cages until pairing (see Section 2.2. Experimental timeline), with free access to water, rabbit chow supplemented with sunflower seeds, and cotton nestlets, igloos, and tubes for enrichment. Housing conditions were maintained at 23–26 °C on a 14:10 h light:dark cycle. All procedures were performed during the light cycle and approved by the Institutional Animal Care and Use committee at the University of Colorado Boulder.
2.2. Experimental timeline
Experiments were performed on opposite-sex, non-sibling pairs at 6 (N = 10 pairs; mean age at pairing: 6.2 months, age range at pairing: 5.5–6.4 months), 12 (N = 10 pairs; mean age at pairing: 11.9 months, age range at pairing: 11.3–12.4 months), or 18 months (N = 7 pairs; mean age at pairing: 18.6 months, age range at pairing: 17.7–21.4 months). Animals were paired (Day 0) and PPTs were performed on both members of the pair following 2 days (short-term) and 16 days (long-term/2 wks) of pairing and cohabitation. On days 3 and 17, animals were tested on a free interaction test. After completing the free interaction test on day 17, pairs were separated and single housed for 4 wks in separate vivarium rooms. Animals were subjected to a final PPT on day 44 and free interaction on day 45. Males and females were reproductively intact, and similar birthrates were seen across ages (6 months: 7/10, 12 months: 7/10, 18 months: 4/7). Partner preference and partner huddle time were similar when comparing animals of a specific sex that did and did not have litters within an age group (all p > 0.05; Supplemental Fig. 1). The one exception was 18-month males, where those animals that didn’t have litters huddled less with their partners compared to those that did have litters (F1,5 = 8.553, p = 0.03, η2 = 0.21). Based on these mostly negative findings and to increase statistical power, animals that did and did not have litters were combined for all subsequent analyses. All litters were born and raised by single-housed mothers after separation from their male partners. All pups were born ~1 wk. following separation and remained with mothers through the remainder of the study.
2.3. Partner preference tests
Testing was performed as described previously (Brusman et al., 2021; Sadino et al., 2021). Partner and stranger, non-sibling animals were tethered to the ends of a three-chamber plexiglass arena (76.0 cm long, 20.0 cm wide, and 30.0 cm tall) following brief anesthetization with isoflurane. Stranger voles were also reproductively intact and sexually naïve prior to being used in PPTs. Tethers were made of an eyebolt attached to a chain of fishing swivels, with animals being connected via zip ties around the animal’s neck. All animals had access to food and water for the duration of the test (3 h). Experimental animals were placed into the center chamber separated from the other two chambers by opaque dividers. The opaque dividers were removed, and the experimental animal was allowed to move freely around the arena for 3 h. Tracking was performed by overhead Panasonic WVCP304 cameras to record eight chambers simultaneously. Movement from all animals was scored using TopScan High-Throughput software v3.0 (Cleversys Inc) according to (Ahern et al., 2009; Brusman et al., 2021). Both voles in a pair were tested back-to-back at each timepoint, separated by approximately 1 h. Here, timepoint refers to the time since pairing (2 days or 2 wks) or following separation (4 wks separation). Test order (male or female) and partner side within the apparatus was randomized and counterbalanced within an age group. The main dependent variables measured in these tests were total time spent huddling with partner or stranger animal and partner preference (partner huddle time / [partner huddle time + stranger huddle time]). We also examined the time spent in each part of the PPT arena (partner side, stranger side, or center).
To further investigate initial response and habituation to social novelty during PPTs we separately analyzed the first 5 min and the following 15 min of each PPT. We calculated an acute preference index ([partner huddle time − stranger huddle time] / [partner huddle time + stranger huddle time]). We excluded from this analysis any animals that failed to leave the center chamber during the first 20 min of the test (1M from the 12-month and 2F from the 6 month age groups).
2.4. Free interaction
We also recorded unconstrained dyadic partner interactions a plexiglass arena 24 h after the PPT, as previously described (Brusman et al., 2021). Pairs were placed on opposite sides of the two-chamber arena (50.7 cm long, 20.0 cm wide, and 30.0 cm tall) separated by an opaque divider. The divider was removed, and animals were allowed to freely explore the chamber for 3 h. Overhead Logitech C925e webcams were used to record four free interaction tests simultaneously. Social contact between the two animals were scored using TopScan High-Throughput software v3.0 (Cleversys Inc.) using scoring methods optimized within our lab (Brusman et al., 2021). We measured time spent in close social contact (defined by setting the “joint motion” parameter to <5), a metric that is robust to identity swapping.
2.5. PVH oxytocin and vasopressin staining, imaging, and cell counts
After the final free interaction test at the 4 wks separation timepoint, half of the pairs from each age group were injected with a mixture of ketamine/xylazine and perfused with 1× phosphate buffered saline (PBS) followed by 4 % paraformaldehyde. Brains were stored in 4 % paraformaldehyde for 24–48 h and then transferred to 30 % sucrose. Brains were sliced at 40 μm on a Leica SM2010R microtome and slices were stored in 1× PBS with 0.01 % sodium azide at 4 °C until staining. 1–3 sections for each animal encompassing the PVH were atlas matched (approximately − 0.94 to − 1.7 from bregma) to the Mouse Brain in Stereotaxic Coordinates Compact Third Edition. Sections were washed 3 × 5 min in 1× PBS and then incubated for 1 h at room temperature in blocking solution (0.2 % Triton-X, 6 % bovine serum albumin, and 10 % normal goat serum in 1× PBS). Sections were then incubated in 1:500 primary antibodies in blocking solution, mouse anti-NeuN (abcam, ab104224), rabbit anti-oxytocin (Immunostar, 20,068), and guinea pig anti-vasopressin (BMA Biomedicals, T-5048), for 48 h. After 48 h, sections were washed 3 × 5 min in 1× PBS and then incubated in 1:500 Alexa Fluor™ secondary antibodies, goat anti-mouse 405 (ThermoFisher Scientific, A-31553), rabbit 488 (ThermoFisher Scientific, A-11008), and guinea pig 568 (ThermoFisher Scientific, A-11075), diluted in blocking solution for 2 h. Sections were wash 3 × 5 min in 1× PBS and mounted on Diamond White Glass microscope slides (Globe Scientific, Inc.), coverslipped with ProLong™ Diamond Antifade Mountant (ThermoFisher Scientific, P36970), and sealed with nail polish.
Images were acquired on an Olympus iX83 wide field slide scanner with a Chroma Multi LED filter set (Cat: 69401) at 20× for quantification with the following consistent exposures times (DAPI 300 ms, FITC 50 ms, TRITC 100 ms). Representative images for figures were acquired at 10×. Images used for quantification were imported into ImageJ/Fiji and rolling ball subtraction was used to reduce image background (oxytocin = 100, vasopressin = 750). Images were grayscaled and thresholded using the Otsu method (oxytocin = 0–45, vasopressin = 0–65). The watershed function was applied to separate individual cells and analyze particles (size = 50-infinity, circularity = 0.3–1.00) was performed to count the number of oxytocin and vasopressin cells within a manually drawn region of interest (444,655.745 μm2) that was used across all sections. Counts were averaged across all sections for an animal. Male and female histology results were combined due to the smaller sample size; both sexes showed the same overall trends.
2.6. Statistical analysis and data visualization
Data analysis and visualization was performed in GraphPad Prism (version 10.0.0). All behavioral data were analyzed as a two-way repeated measures ANOVA considering age and timepoint as factors. When there were interactions between age and timepoint, we followed up with Tukey’s post-hoc tests. To determine whether groups demonstrated a significant partner preference, we utilized a one-sample t-test to compare group means to the null hypothesis of 0.5 (no preference for partner or stranger). We did not compare time huddling with partner and stranger vole via t-test, as this violates the assumption of independence of measurement. A similar method was used for acute preference indices but compared to the null hypothesis of 0 (no preference for partner or stranger). Histology data was analyzed with a one-way ANOVA considering age as a factor and significant main effects of age were followed up with Tukey’s post-hoc tests. For ANOVAs, η2 was used as a measure of effect size, while Hedge’s g was used for one sample t-tests and significant post-hoc tests. All data used to generate figures are available on Figshare through the following link: https://figshare.com/projects/Aging_leads_to_sex-dependent_effects_on_pair_bonding_and_increased_number_of_oxytocin-producing_neurons_in_monogamous_prairie_voles/220444.
3. Results
3.1. Aging accelerates bond formation in male prairie voles
We first asked whether there were any differences in behavioral metrics during PPTs that depend on age. Because there are sex differences in the development of bonds (Brusman et al., 2021; DeVries and Carter, 1999; Insel and Hulihan, 1995), we analyzed males and females separately. An experimental timeline for PPTs can be found in Fig. 1A. We first examined whether there were any differences in partner preference score following 2 days and 2 wks cohabitation, or after 4 wks separation in male animals (Fig. 1B). There were no effects of age, timepoint or an age × timepoint interaction on partner preference score (all p > 0.05). We also performed a one-sample t-test against the null hypothesis of no preference (50 %), which revealed that 18-month males formed a strong partner preference after only 2 days of cohabitation (t6 = 20.32, p < 0.0001, Hedge’s g = 7.68). In contrast, 6- and 12-month male animals developed a significant partner preference only after 2 wks of cohabitation (6 month: t9 = 3.596, p = 0.0058, Hedge’s g = 1.14; 12 month: t9 = 4.929, p = 0.0008, Hedge’s g = 1.56), which was also maintained by 18-month males (t6 = 14.25, p < 0.0001, Hedge’s g = 5.38). Males across all ages maintained their partner preference after 4 wks separation from their partners (6 month: t9 = 4.555, p = 0.0014, Hedge’s g = 1.44; 12 month: t9 = 3.382, p = 0.0081, Hedge’s g = 1.07; 18 month: t6 = 17.2, p < 0.0001, Hedge’s g = 6.5). There were no differences in the amount of time male animals spent huddling with partner or stranger animals as a result of age, timepoint, or an age × timepoint interaction (all p > 0.05; Fig. 1C and D). Similarly, there was no effect of age, time, or an age × time interaction on proportion of time spent in the partner, stranger, or center chamber of the PPT arena (all p > 0.05; Supplemental Fig. 2A–C).
Fig. 1.
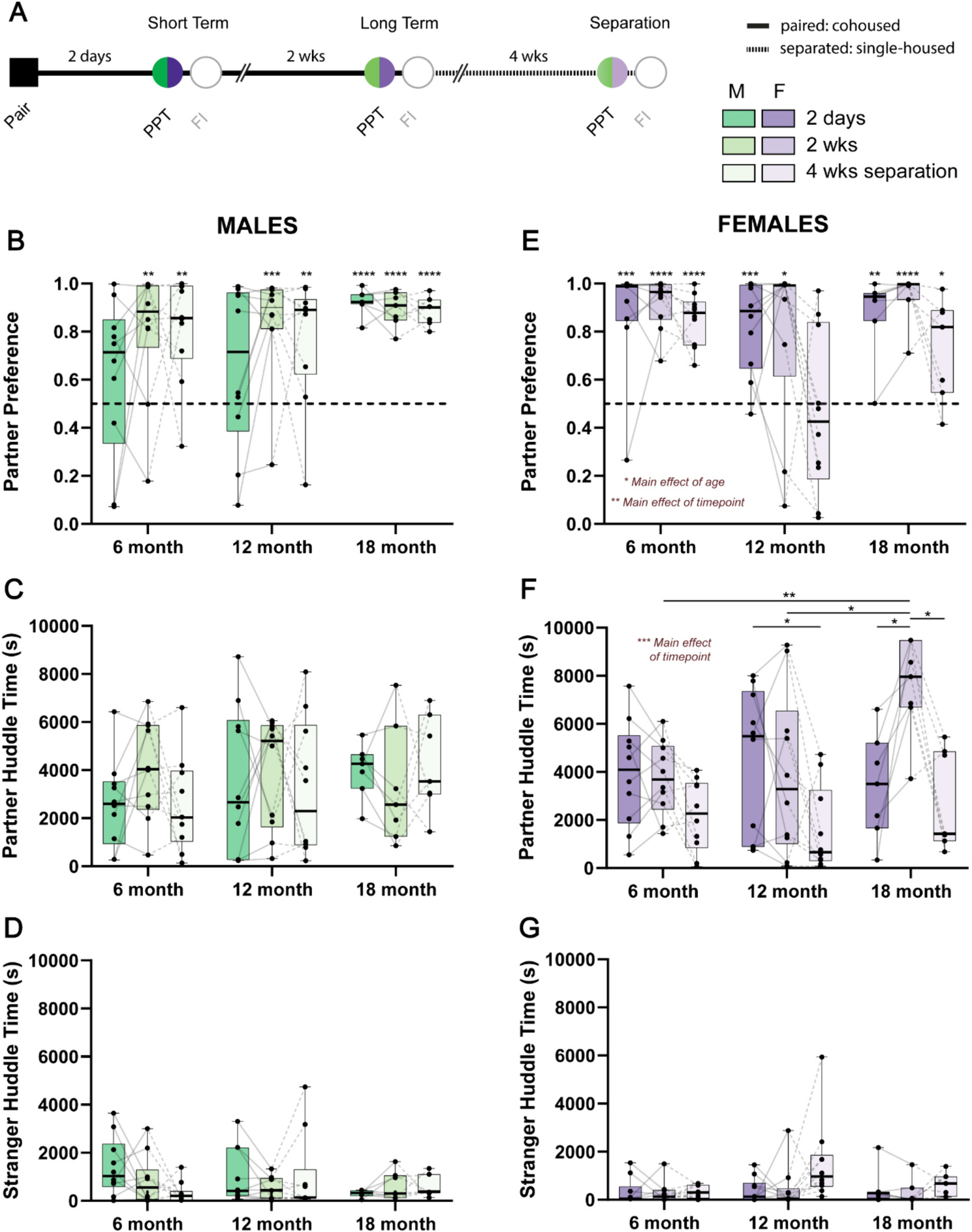
Sex-dependent effects of age on pair bonding. A) Schematic of behavioral timeline where opposite sex voles were paired before undergoing partner preference tests after 2 days (short term) and 2 wks (long term). Pairs were then separated for 4 wks and then tested on another partner preference test. PPT: partner preference test, FI: free interaction. B) Partner preference scores in male 6-, 12-, and 18-month voles. 6- and 12-month male voles only form a partner preference after 2 wks of cohabitation and maintain this preference after 4 wks separation. Meanwhile 18-month male voles form a robust partner preference after 2 days cohabitation which is maintained at other timepoints. Partner preference was tested against the null hypothesis of no preference (0.5, dotted line). C) Partner huddle time is no different in male animals due to age or timepoint. D) Similarly, there are no differences in stranger huddle time due to age or timepoint in male animals. E) Partner preference scores in 6-, 12-, and 18-month female animals. Female animals regardless of age and timepoint displayed a significant partner preference (compared to the null hypothesis of no preference of 0.5, dotted line) F) Unlike males, 18-month females increased their time spent huddling with their partner, specifically after 2 wks of cohabitation, which wasn’t apparent in other age groups. Generally, females in all age groups decreased partner huddle time after 4 wks separation. G) Like males, there were no effects of age or timepoint on time spend huddling with stranger animals. N = 7–10 animals per group; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. Age affects affiliative behavior and partner preference after loss in female prairie voles
We next examined whether there were differences in behavioral phenotypes during PPTs in female animals (Fig. 1E–G). Similar to previous reports, female animals formed a partner preference following 2 days cohabitation regardless of age (6 month: t9 = 5.341, p = 0.0005, Hedge’s g = 1.69; 12 month: t9 = 5.293, p = 0.0005, Hedge’s g = 1.67; 18 month: t6 = 5.794, p = 0.0012, Hedge’s g = 2.19), which was maintained after 2 wks (6 month: t9 = 12.56, p < 0.0001, Hedge’s g = 3.97; 12 month: t9 = 2.645, p = 0.0267, Hedge’s g = 0.84; 18 month: t6 = 11.05, p < 0.0001, Hedge’s g = 4.18). Partner preference was also retained following 4 wks separation, but only in 6- and 18-month animals (6 month: t9 = 10.4, p < 0.0001, Hedge’s g = 3.29; 18 month: t6 = 2.904, p = 0.0272, Hedge’s g = 1.1). Moreover, there was a significant effect of age (F2,24 = 4.686, p = 0.0191, η2 = 0.12) and time (F1.688,40.5 = 7.663, p = 0.0025, η2 = 0.13), but not an age × time interaction (F4,48 = 1.894, p = 0.1268, η2 = 0.06) on partner preference scores. In general, 12-month females displayed lower partner preference scores across each timepoint compared to other age groups, which was most apparent after 4 wks separation. In addition, females showed lower partner preference scores after 4 wks separation compared to the 2 days and 2 wks timepoints.
Unlike males, there were also effects of timepoint (F1.956,46.95 = 13.24, p < 0.0001, η2 = 0.19) and an age × timepoint interaction (F4,48 = 3.419, p = 0.0154, η2 = 0.1) on the amount of time females spent huddling with their partners (Fig. 1F). Females huddled more with their partners after 2 days and 2 wks of cohabitation compared to after 4 wks separation. Post-hoc tests revealed that specifically 18-month female animals displayed increased partner directed huddling at 2 wks compared to both 2 days (q6 = 5.71, p = 0.0161, Hedge’s g = 1.97) and 4 wks separation (q6 = 5.76, p = 0.0154, Hedge’s g = 2.3). 18-month females also displayed a greater amount of partner directed huddling compared to both 6- (q10.71 = 5.93, p = 0.004, Hedge’s g = 2.17) and 12-month (q14.75 = 3.91, p = 0.04, Hedge’s g = 1.24) females after 2 wks. Partner directed huddling time was lower following 4 wks separation compared to 2 days only in the 12-month females (q9 = 3.969, p = 0.0489, Hedge’s g = 1.17).
These phenotypes occurred in the absence of significant differences in stranger huddle time across age and timepoints (all p > 0.05; Fig. 1G). However, partner directed behaviors were associated with differences in which parts of the PPT chambers that female animals spent their time (Supplemental Fig. 2D–F). There was a main effect of timepoint on the proportion of time that females spent in the partner (F1.795,43.08 = 10.25, p = 0.0004, η2 = 0.19) and stranger (F2,24 = 4.528, p = 0.0215, η2 = 0.11) chambers. At each age, females spent more time in their partner’s chamber after 2 wks cohabitation compared to either 2 days or after 4 wks separation. In addition, females spent less time in their partner’s chamber after 4 wks separation. These effects were mirrored in the opposite direction with respect to the chamber that held the stranger animal. Finally, there was an effect of age on the amount of time females spent in the center chamber (F2,24 = 3.844, p = 0.0356, η2 = 0.11), where animals spent gradually less time in the center chamber with increasing age.
3.3. Sex differences in partner preference are evident early in partner preference tests
While the PPT provides an aggregate measure of social choice and partner- and stranger-directed behavior over the course of 3 h, we also analyzed the first 5 min of each test to examine the acute response to a stranger vole across sex and age (Fig. 2). We also examined behavior in minutes 6–20 to examine emergence of preference behavior after the initial 5-min investigatory period, essentially asking whether partner preference emerges within this early period in an age- or sex-dependent manner. In voles, we observed a consistent stranger preference in the first 5 min of the task only for 6-month males after 2 wks and 6-month females after 2 days of cohabitation (males: t9 = 2.4, p = 0.039, Hedge’s g = 1.79; females: t7 = 2.669, p = 0.032 Hedge’s g = 1.94). Of note, this differs from laboratory mice, where a stranger preference is frequently observed within this timeframe (Beery et al., 2018; Crawley et al., 2007; de León Reyes et al., 2023; Moy et al., 2004). In males, it took longer for partner preference to emerge; it was never present in the first 5 min, and it emerged in minutes 6–20 only in 12- and 18-month males that had undergone partner separation (Fig. 2A and B). In contrast, females often displayed a partner preference even within the first 5 min, which remained evident and was strengthened in the succeeding 15 min (Fig. 2C and D). Moreover, there was a significant interaction between age and timepoint (F4,44 = 2.64, p = 0.0463, η2 = 0.12) for partner preference during the initial 5 min of interaction. Post-hoc tests revealed that after 2 days, 12-month females demonstrated a strong partner preference compared to 6-month females (q15.68 = 5.319, p = 0.0047, Hedge’s g = 1.77). There was an additional significant effect of timepoint on the partner preference scores during the initial 5 min (F1.975,45.43 = 4.815, p = 0.013, η2 = 0.1) and subsequent 15 min (F1.872,43.05 = 7.041, p = 0.0027, η2 = 0.12) of the PPTs for male animals only. Males displayed higher partner preference scores at the 4 wks separation timepoint across all ages. Beyond the dynamics noted above, there were no main effects of age, timepoint, or their interaction on partner preference within the first 5 min or the next 15 min of PPTs (all p > 0.05).
Fig. 2.
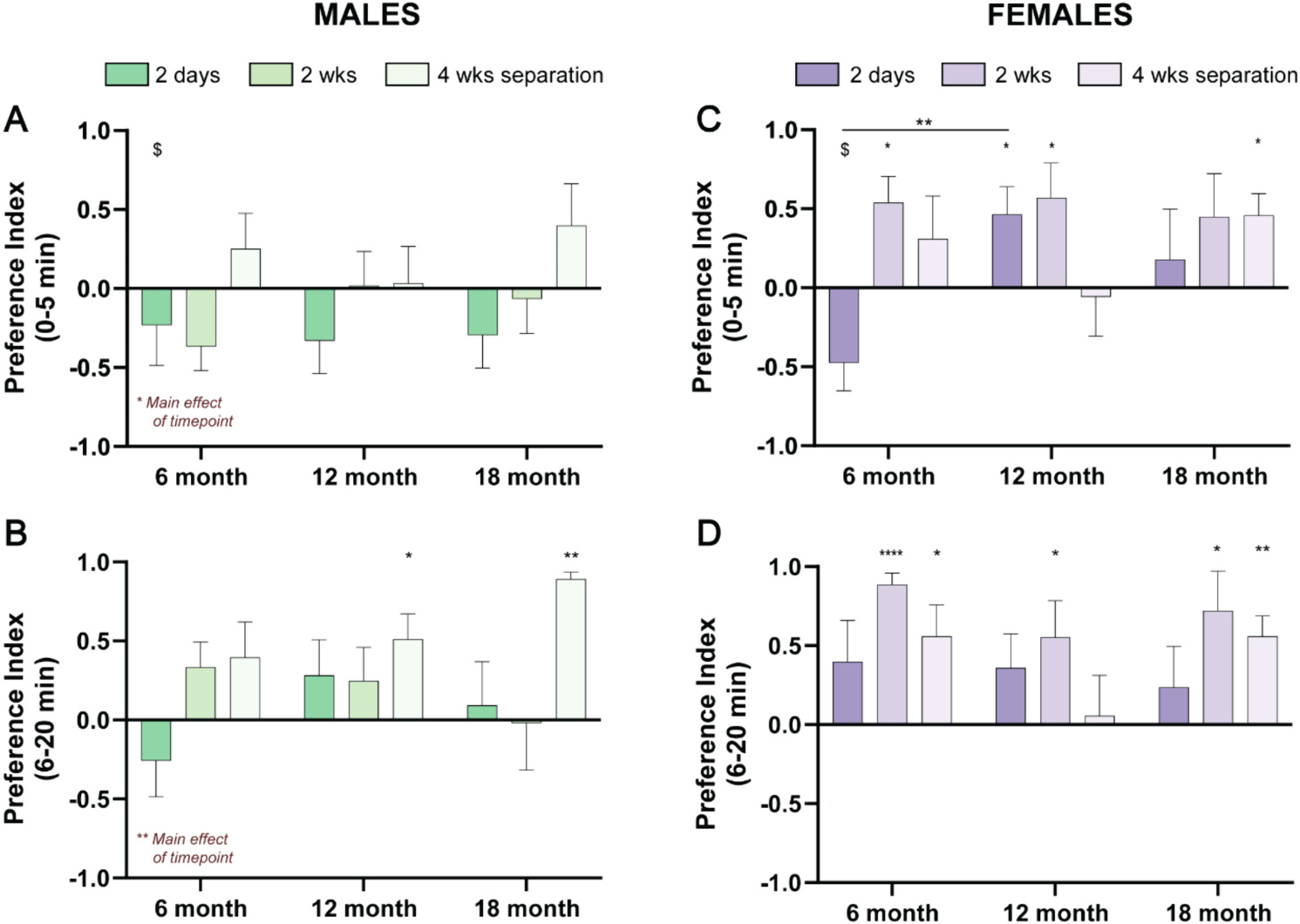
Sex-dependent effects of age on pair bonding are apparent within the initial few minutes of the partner preference test. We used social preference index as a measure of partner or stranger preference during the first 20 min of partner preference tests comparing to a null hypothesis of no preference of 0. A) Male animals displayed a lack of partner or stranger preference during the initial 5 min of partner preference tests, with the exception of 6-month animals after 2 wks of cohabitation, which displayed a stranger preference. B) Similarly, most male animals did not display a partner or stranger preference in the following 15 min of partner preference tests, except for 12- and 18-month after 4 wks separation, which showed a significant partner preference. C) Unlike males, females across ages and timepoints tended to display a significant partner preference within the initial 5 min of partner preference tests, with the exception of 6-month animals after 2 days, which had a stranger preference. D) Female animals of all ages continued to show a significant partner preference in the following 15 min of partner preference tests. N = 7–10 pairs per group; *p < 0.05, **p < 0.01, ****p < 0.0001 for denoting significant main effects and partner preference compared to the null hypothesis of 0 (no preference). $p < 0.05 for denoting significant stranger preference compared to the null hypothesis of 0 (no preference).
3.4. Social contact time during free interaction reflect trends in partner preference tests
PPTs are useful for assessing social choice as the test animal is presented with two tethered voles. However, we have previously shown that behaviors observed during PPTs are only loosely correlated with those observed during unconstrained dyadic interaction (Brusman et al., 2021). We therefore supplemented PPTs with free interaction tests that occurred the following day, recording total interaction time for each pair. The behavioral timeline for free interaction tests can be found in Fig. 3A. In the 18-month cohort, 2 of the 7 pairs were excluded due to technical issues with behavioral recording. We observed a significant effect of age (F2,22 = 15.62, p = 0.0057, η2 = 0.16) and timepoint (F1.526,33.58 = 11.61, p = 0.0048, η2 = 0.12), but no age × timepoint interaction (F4,44 = 2.20, p = 0.08, η2 = 0.07) on the amount of time that pairs spent in close social contact (Fig. 3B). 18-month animals displayed high amounts of social contact compared to 6- and 12-month animals at each timepoint, while social contact generally decreased after 4 wks separation which was most apparent in 12-month pairs.
Fig. 3.
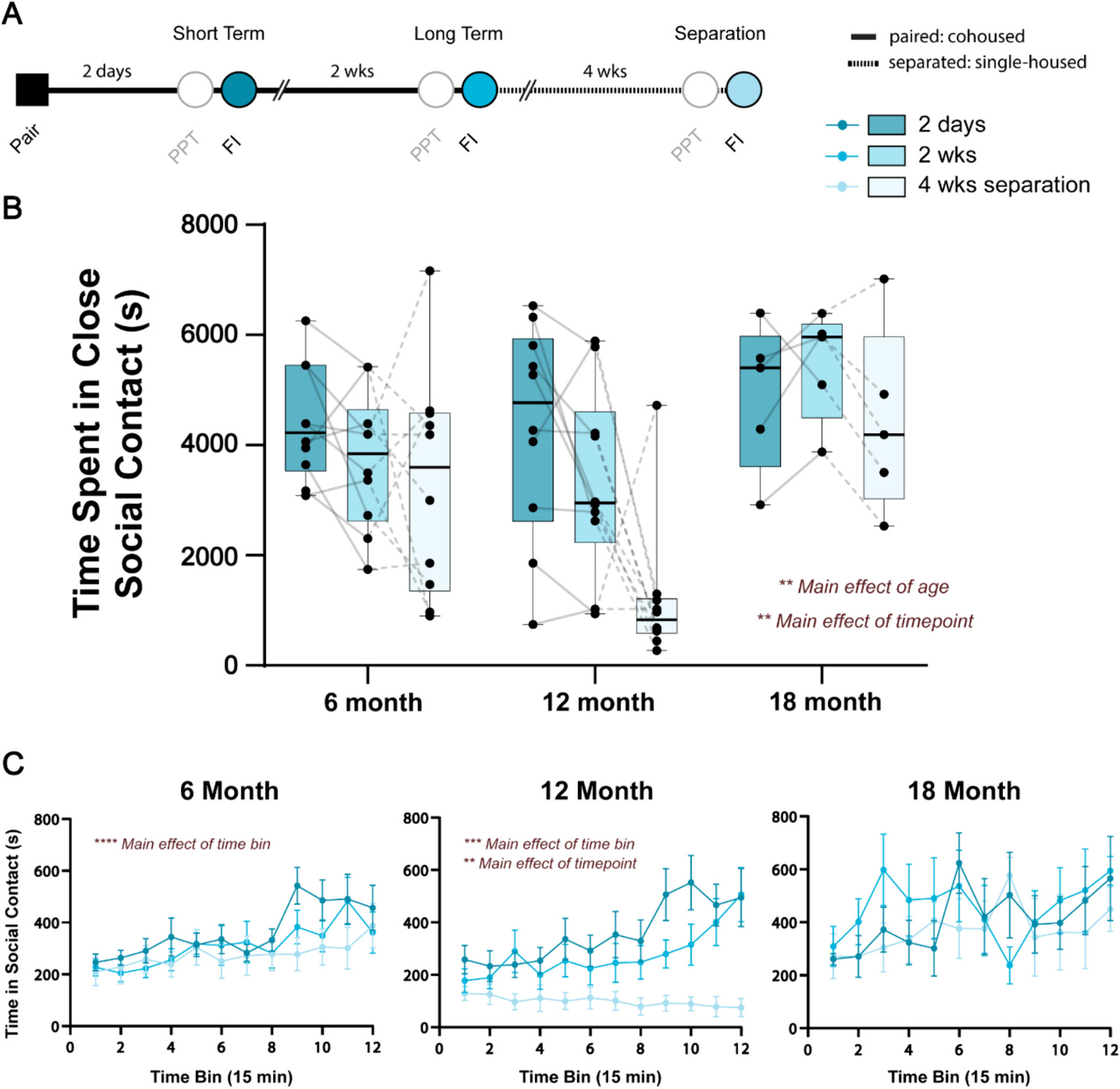
Age and timepoint impact time spent in close social contact during unconstrained free interaction. A) Schematic of behavioral timeline where opposite sex voles were allowed to interact in an open arena the day after each partner preference test at the short term and long term timepoints and following 4 wks separation. PPT: partner preference test, FI: free interaction. B) 18-month animals tended to spend the most time in close social contact, regardless of timepoint. Meanwhile, across ages, pairs spent less time in close social contact after 4 wks separation. C) Time spent in close social contact was then separated into 15-min time bins and analyzed within each age group. There was a main effect of time bin in 6- and 12-month pairs such that time spent in close social contact increased over the course of the 3-h task. There was an additional main effect of timepoint in 12-month pairs, where animals spent less time in close social contact after 4 wks separation. An interaction effect between timepoint and time bin was also apparent for 12-month pairs, and statistics for these comparisons can be found in Supplemental Table 1. There were no effects of time bin, timepoint, or their interaction on the time spent in close social contact during unconstrained free interaction in 18-month pairs. N = 5–10 pairs per group; **p < 0.01, ***p < 0.0001, ****p < 0.0001.
To examine social interaction over time, we used 15-min bins for each age group at each timepoint (Fig. 3C). 6- and 12-month animals showed increases in time spent in close social contact, especially within the last hour of the test. A two-way ANOVA demonstrated a main effect of time bin on time spent in social contact in 6-month pairs (F11,99 = 6.757, p < 0.0001, η2 = 0.1). Meanwhile 12-month animals displayed a significant effect of time bin (F11,99 = 3.571, p = 0.0003, η2 = 0.06), timepoint (F2,18 = 9.22, p = 0.0018, η2 = 0.21), and a time bin × timepoint interaction (F22,198 = 2.864, p < 0.0001, η2 = 0.06) on time spent in close social contact. Notably, time spent in close social contact generally increased over the course of the 3 h test, with the exception of the 4 wks separation timepoint. In addition, huddle time decreased across timepoints. Post-hoc tests for the 12-month pairs revealed many time bins where time in close social contact was lower at the 4 wk separation point compared other timepoints, in addition to a few time bins towards the end of the test where huddling time on day 2 was greater than after 2 wks (statistics available in Supplemental Table 1). Meanwhile, there were no effects of time bin, timepoints, or a time bin × timepoint interaction on time spent in close social contact during free interaction in 18-month animals.
3.5. Number of oxytocin, but not vasopressin, cells increase from 12- to 18-months
The nonapeptides oxytocin and vasopressin synthesized in the PVH have been extensively implicated in pair bonding in voles. We used cell counts to gauge whether these systems are altered during the course of aging and could support behavioral changes observed during PPTs and free interaction (Fig. 4). There was a significant effect of age on the number of oxytocin cells in the PVH (F2,21 = 3.654, p = 0.04, η2 = 0.26). A post-hoc test revealed that the number of oxytocin cells increased from 12- to 18-months (q21 = 3.75, p = 0.04, Hedge’s g = 1.7), while there was a weak trend towards increasing number of cells from 6- to 18-months (q21 = 3.02, p = 0.11, Hedge’s g = 1.01). Meanwhile, there was no effect of age on the number of vasopressin cells in the PVH (F2,17 = 0.43, p = 0.66, η2 = 0.05).
Fig. 4.
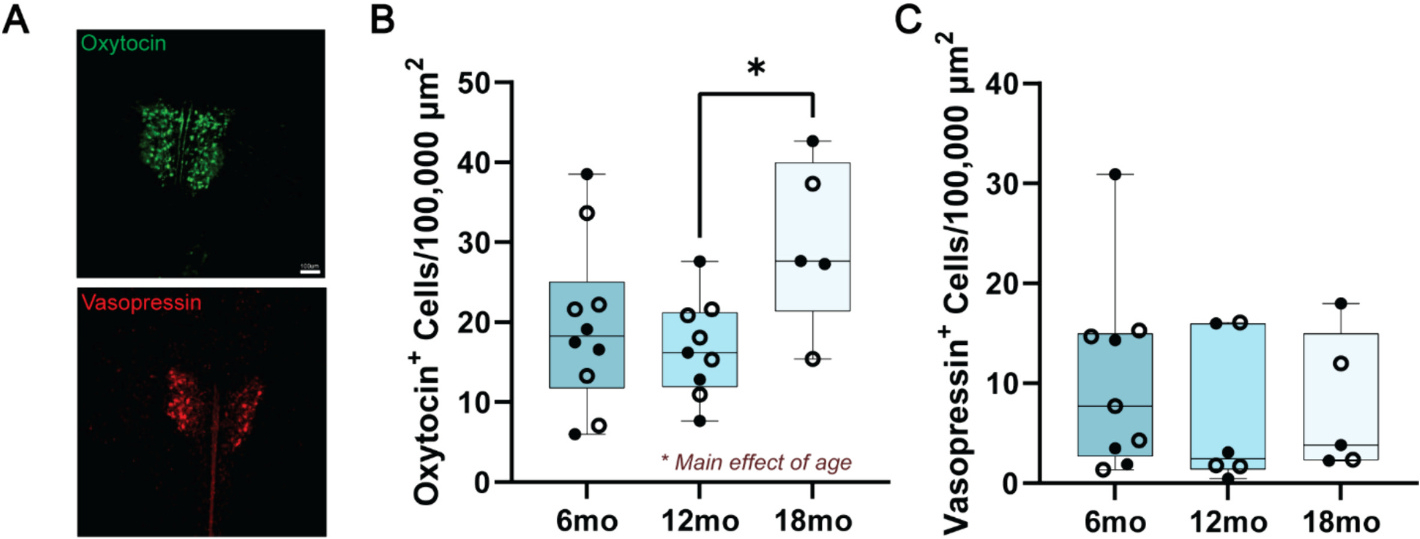
Quantification of oxytocin- and vasopressin-producing cells in the PVH reveals a selective effect of aging on the number of oxytocin, but not vasopressin cells. A) Representative images of oxytocin and vasopressin staining in the PVH from a 6-month animal. Scale bar 100 μm, applicable for both oxytocin and vasopressin. B) There was a main effect of age of the number of oxytocin cells in the PVH. Post-hoc tests revealed a significantly greater number of oxytocin cells in 18-month animals compared to 12-month animals. B) Age did not influence the number of vasopressin cells in the PVH. Empty circles = female, closed circles = male. N = 5–10 animals per group; *p < 0.05.
4. Discussion
Most prior studies of pair bond dynamics in prairie voles have been performed in young adult animals. However, pair bonds evolve over a life course and are especially important to maintaining health and well-being in late stages of life. Here, we provide the first comprehensive overview of how age sex-dependently impacts pair bonding in a preclinical rodent model, monogamous prairie voles. Specifically, we found that aging enhances bonding; it accelerates bond formation in males and increases partner-directed affiliative huddling in females. Furthermore, females appear to be more sensitive to long term separation; unlike males, they displayed lower partner preference scores and reduced partner-directed affiliative behavior after 4 wks separation, which was most prevalent at 12-months. Together, these data provide new evidence on the sex-dependent effects of age on the formation and maintenance of pair bonds, a first step towards understanding how bonds and partner loss impact emotional well-being during aging.
4.1. Male and female voles show enhanced bonding at 18 months
We replicated previous reports that male voles require longer cohabitation to display a partner preference (Brusman et al., 2021; DeVries and Carter, 1999; Harbert et al., 2020). Our results show that at the group level, males 12 months and younger do not show a significant partner preference after 2 days of pairing, but 18-months-old males do, indicating that they form bonds faster than their younger counterparts. Males of all ages do eventually form bonds and display a partner preference after 2 wks of cohabitation. Females also display age-dependent increases in bonding behavior, evident in a doubling of partner-directed huddling after 2 wks of cohabitation in 18-month females — greater than at any other timepoint or age. In addition, individual variation is much lower in older voles. Both male and female 18-month voles show consistently strong pair bonds, with nearly all individuals exhibiting a preference score >0.8 and none <0.5.
While our findings in females are new, the age-dependent shift in male behavior is consistent with a prior observation that aged male prairie voles tend to form stronger bonds more quickly than young males regardless of pairing history (Kenkel et al., 2019). This suggests that our finding is likely a reflection of age and not driven solely by prior social history as males in our study were housed with same-sex counterparts until female introduction and those in Kenkel et al. (2019) had diverse prior histories. These findings may reflect increased reproductive urgency in male animals in response to the real or perceived negative impacts of aging on reproductive performance and success (Comizzoli and Ottinger, 2021). Indeed, aged male animals in other species are less selective concerning mate choices despite potential reproductive disadvantages (Baxter et al., 2020; Churchill et al., 2019; Rundus et al., 2015).
Partner preference phenotypes across aging may also be optimized for the varied mating strategies voles employ in the wild (Getz and McGuire, 1993; Shuster et al., 2019). Male prairie voles can either form a territory (residents) or not (wanderers). Wandering in males typically results in longer survival and greater numbers of sired offspring, although this may partly depend on factors such as a population density (Getz and McGuire, 1993; Okhovat et al., 2015; Shuster et al., 2019). However, within male voles that adopt a resident strategy, males sire a higher number of litters when in a monogamous partnership (Shuster et al., 2019), for which quicker bond formation and robust maintenance of partner preference may be advantageous.
4.2. Initial interactions during PPT reflect sex differences in partner preference
We gained more insights into sex differences in partner preference by examining the first 20 min of the PPT. This allowed us to assess novelty response and habituation of interactions with partner and stranger conspecifics at each age. Consistent with the above noted sex difference in overall preference behavior, males typically sampled both partner and stranger animals during the initial portions of the PPT. In contrast, females displayed a greater likelihood for partner preference during both time periods, except for 6-month animals which preferred stranger animals in the very early stages of bonding (2 days cohabitation) in the first 5 min. These trends are in agreement with operant literature showing female but not male animals will preferentially press levers for access to a partner compared to a novel opposite-sex conspecific (Brusman et al., 2021; Vahaba et al., 2022). Both of these results contrast with mice and rats, which display strong social novelty preference in similar tasks (Beery et al., 2018; Crawley et al., 2007; de León Reyes et al., 2023; Moy et al., 2004).
4.3. Male bonds are more resilient than female bonds following long-term partner loss
Metrics of partner preference in response to separation had been well described in male voles but was relatively uncatalogued in females. In accordance with prior reports (Fricker et al., 2023; Sadino et al., 2021), we found that male pair bonds are resilient to separation; males in all age groups show a partner preference and partner-directed affiliation after 4 wks of separation. One study has reported bond deterioration in male voles of unspecified age following 4 wks separation (Sun et al., 2014). Both age and methodological differences (e.g. pairing with a non-ovariectomized versus ovariectomized female; 24 h vs 2 wks total pairing time) may contribute to these discrepancies. While striking, it is not clear why males would maintain bonds for such a long time after separation, but it is worth noting that the males in our study were not provided with an opportunity to form a new bond.
Females display a host of isolation-induced phenotypes, including anhedonia, increased anxiety-like phenotypes and aggression, and neuroendocrine disruption (Grippo et al., 2007, 2008; McNeal et al., 2014; Scotti et al., 2015; Watanasriyakul et al., 2022). One study reported persistence of pair bonds following 4 wks separation, without any effect of sex in ~2–6 month old voles (Fricker et al., 2023). In our experiments, partner preference after 4 wks separation followed a U-curve, where 6- and 18-month females demonstrated a strong partner preference, but 12-month animals did not. Interestingly, about half of the 12-month female voles displayed a partner preference after 4 wks separation while the other half did not, a split that was not observed in any other group. If such trends are seen in future groups of animals, they could provide a way to investigate the neural mechanisms supporting this switch in preference across ages following separation. In addition, females decreased huddling time with a partner after 4 wks of separation, consistent with prior reports that females demonstrate a partial erosion of bond phenotypes (Pierce et al., 2024). These shifts in behavior could promote flexibility to engage with novel animals and potentially form new bonds, but it remains unclear why this would be more evident in females than males.
Similar to males, females can adopt wandering or resident phenotypes to boost reproductive success, which may also be partially reflected in our PPT outcomes (Shuster et al., 2019). Specifically, wandering females tend to produce more offspring when polyandrous versus monandrous (Shuster et al., 2019), which is what might be expected in 12-month females following the loss of a pair bonded partner. The age-dependency for the adoption of either strategy in females is an open question, but does raise the interesting possibility that voles coordinate their mating tactics at a given age in order to maximize reproductive fitness.
4.4. Female pair bond phenotypes dominate intra-pair behaviors during free interaction
Consistent with previous findings from our lab (Brusman et al., 2021), we did not observe strong correlations between behavior during PPT and unconstrained dyadic interaction (data not shown). This likely reflects the inherent differences in these tasks; PPTs include an element of social choice where only one animal has agency to choose, while dyadic interaction is not constrained by choice of a single animal and has no third vole present. However, when we examine overall alignment of PPT and dyadic interaction at a group level, we find that levels of interaction are more consistent with female than male behaviors. This is most evident in the 12-month age group after partner separation where males maintain their pair bonds, but their female partners do not. In the subsequent free interaction test of the 12-month age group after separation, total interaction time is reduced, presumably due to female behavior. Of note, while we show that females in our study drive reduced affiliation following separation, prior work has also shown that females can also increase pairwise interaction as bonds mature (Brusman et al., 2021), so female dominance of pairwise behavior occurs across conditions.
4.5. Neurochemical systems influencing pair bond dynamics across aging
We found that the number of oxytocin, but not vasopressin, cells in the PVH increased with age. This increase was specific when comparing 12- and 18-month animals, while a weak trend was noted between 6- and 18-month animals. While we were underpowered to assess any sex differences in number of oxytocin or vasopressin cells over the course of aging, there were no obvious trends, consistent with previous vole and human literature (Fricker et al., 2023; Ishunina and Swaab, 1999; Wierda et al., 1991). Thus, increased oxytocin cell numbers at 18-months may contribute to the enhanced bonding phenotypes observed at this timepoint.
Data from human literature show no changes in oxytocin cell number and some changes in composition of vasopressin-positive cells over the course of aging or in AD (Fliers et al., 1985; Ishunina and Swaab, 1999; Wierda et al., 1991). We offer two possibilities for these discrepancies. The first is that there are species differences between voles and humans. The other, non-mutually exclusive explanation is that natural variability in form and function of these socially responsive neural systems lead to different behavioral outputs, even within a specific age range (Audunsdottir and Quintana, 2022; Blumenthal and Young, 2023; Ebner et al., 2013).
4.6. Potential limitations
We did not control for reproduction as all voles were intact. While this may affect behavior, this concern is reduced by the similar fecundity we observed across our age groups, in addition to the lack of major changes in behavior when comparing animals with differing reproductive success (Supplemental Fig. 1). In addition, the animals used as novel conspecifics in the PPT were housed in same-sex cages and were sexually naïve. We therefore cannot rule out the possibility that lack of sexual experience or housing conditions of the stranger vole influenced behavior of the focal animal during PPTs. This is a consideration in any study using PPT in voles, and importantly, these novel animals had the same prior social experience throughout our study.
Oxytocin and vasopressin cell numbers can differ early in life due to social experience (Kelly et al., 2018), making them an ideal first target for examining the effects of age on nonapeptide systems in voles. However, other aspects of oxytocin circuitry (receptor density, innervation patterns, release, and activity) may also be important. Variation in oxytocin receptor density in the nucleus accumbens can predict partner preference and mating strategies (King et al., 2016; Ophir et al., 2012), and vasopressin receptor levels in the retrosplenial cortex reflect mating strategies in the wild (Okhovat et al., 2015). Moreover, ~4 year old male voles do show an increase in oxytocin receptor density in the core of the nucleus accumbens, though with substantial variability (Kenkel et al., 2019). An in-depth examination of this variability across age is thus warranted but was not possible in the current study as tissue fixation for receptor autoradiography and for immunohistochemical detection of the peptides are different.
It is also possible that increased OXT cell number is not attributable to age alone but rather reflects age-dependent response to loss of a bonded partner, given that tissue was taken after 4 wks separation (Fricker et al., 2023; Grippo et al., 2007; Sun et al., 2014). For example, greater numbers of oxytocin cells in the PVH may support the maintenance of partner preference after separation in 18-month males, while lower cell numbers may promote flexibility seen in 12-month females. Future work will employ additional groups of animals to disentangle these factors.
Finally, oxytocin and vasopressin are not the sole neurochemical modulators of pair bonding. Various other molecules like dopamine and corticotropin releasing factor can affect pair bonding (Aragona et al., 2006; Blumenthal and Young, 2023; Devries et al., 2002; Lim et al., 2007; Liu and Wang, 2003; Pierce et al., 2024). These systems are also altered throughout the course of aging and age-related disorders, and may have contributed to some of the phenotypes we observed in our experiments (Curley et al., 2021; Ehrenberg et al., 2023; Kaasinen and Rinne, 2002; Volkow et al., 1998).
5. Conclusions
Our study is the first to show that age has sex-specific effects on the presentation of pair bond behaviors in prairie voles. Specifically, aging facilitates partner preference formation in males and results in enhanced partner-directed affiliation in females. We replicated classic sex differences in partner preference behavior and found that males were less likely to display bond dissolution following long-term separation from their partner. These behaviors are likely influenced by changes in form and function of socially responsive brain regions, such as the observed increase in oxytocin cell number of the PVH during aging. Overall, this work establishes the monogamous prairie vole as a useful preclinical model for studying the effects of normal and abnormal aging on attachment-related behaviors.
Supplementary Material
Acknowledgements
This work was supported by funding from the National Institute of Mental Health (R01 MH125423), National Institute of Aging (U24 AG072701), and National Science Foundation (IOS-2045348) all awarded to ZRD.
Footnotes
Declaration of competing interest
The authors have nothing to disclose.
CRediT authorship contribution statement
Michael A. Kelberman: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Kelly E. Winther: Writing – review & editing, Visualization, Investigation, Data curation. Yana M. Medvedeva: Investigation. Zoe R. Donaldson: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yhbeh.2024.105647.
References
- Ahern TH, Modi ME, Burkett JP, Young LJ, 2009. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods 182, 180–188. 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z, 2006. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139. 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Audunsdottir K, Quintana DS, 2022. Oxytocin’s dynamic role across the lifespan. Aging Brain 2, 100028. 10.1016/j.nbas.2021.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Anderson M, Seelke AM, Kinnally EL, Freeman SM, Bales KL, 2020. Oxytocin receptor binding in the titi monkey hippocampal formation is associated with parental status and partner affiliation. Sci. Rep. 10, 17301. 10.1038/s41598-020-74243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bediou B, Ryff I, Mercier B, Milliery M, Hénaff M-A, D’Amato T, Bonnefoy M, Vighetto A, Krolak-Salmon P, 2009. Impaired social cognition in mild Alzheimer disease. J. Geriatr. Psychiatry Neurol. 22, 130–140. 10.1177/0891988709332939. [DOI] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in sociality: mice and prairie voles exhibit different patterns of peer affiliation. Front. Behav. Neurosci. 12. 10.3389/fnbeh.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal SA, Young LJ, 2023. The neurobiology of love and pair bonding from human and animal perspectives. Biology 12, 844. 10.3390/biology12060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ, 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78. 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusman LE, Protter DSW, Fultz AC, Paulson MU, Chapel GD, Elges IO, Cameron RT, Beery AK, Donaldson ZR, 2021. Emergent intra-pair sex differences and behavioral coordination in pair bonded prairie voles (preprint). Anim. Behav. Cogn. 10.1101/2021.09.03.458892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Piazza JR, 2007. Memories of social interactions: age differences in emotional intensity. Psychol. Aging 22, 300–309. 10.1037/0882-7974.22.2.300. [DOI] [PubMed] [Google Scholar]
- Churchill ER, Dytham C, Thom MDF, 2019. Differing effects of age and starvation on reproductive performance in Drosophila melanogaster. Sci. Rep. 9, 2167. 10.1038/s41598-019-38843-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Ottinger MA, 2021. Understanding reproductive aging in wildlife to improve animal conservation and human reproductive health. Front. Cell Dev. Biol. 9, 680471. 10.3389/fcell.2021.680471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS, 2007. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41, 145–163. 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Curley DE, Webb AE, Sheffler DJ, Haass-Koffler CL, 2021. Corticotropin releasing factor binding protein as a novel target to restore brain homeostasis: lessons learned from alcohol use disorder research. Front. Behav. Neurosci. 15, 786855. 10.3389/fnbeh.2021.786855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais P, Lanctôt KL, Masellis M, Black SE, Herrmann N, 2018. Social inappropriateness in neurodegenerative disorders. Int. Psychogeriatr. 30, 197–207. 10.1017/S1041610217001260. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Carter CS, 1999. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Can. J. Zool. 77, 885–889. 10.1139/z99-054. [DOI] [Google Scholar]
- Devries AC, Guptaa T, Cardillo S, Cho M, Carter CS, 2002. Corticotropin-releasing factor induces social prefrerences in male prairie voles. Psychoneuroendocrinology 27, 705–714. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, MacDonald K, Westberg L, Fischer H, 2013. Oxytocin and socioemotional aging: current knowledge and future trends. Front. Hum. Neurosci. 7, 487. 10.3389/fnhum.2013.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Suemoto CK, França Resende EP, Petersen C, Leite REP, Rodriguez RD, Ferretti-Rebustini REL, You M, Oh J, Nitrini R, Pasqualucci CA, Jacob-Filho W, Kramer JH, Gatchel JR, Grinberg LT, 2018. Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. JAD 66, 115–126. 10.3233/JAD-180688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Kelberman MA, Liu KY, Dahl MJ, Weinshenker D, Falgàs N, Dutt S, Mather M, Ludwig M, Betts MJ, Winer JR, Teipel S, Weigand AJ, Eschenko O, Hämmerer D, Leiman M, Counts SE, Shine JM, Robertson IH, Levey AI, Lancini E, Son G, Schneider C, Egroo MV, Liguori C, Wang Q, Vazey EM, Rodriguez-Porcel F, Haag L, Bondi MW, Vanneste S, Freeze WM, Yi Y-J, Maldinov M, Gatchel J, Satpati A, Babiloni C, Kremen WS, Howard R, Jacobs HIL, Grinberg LT, 2023. Priorities for research on neuromodulatory subcortical systems in Alzheimer’s disease: position paper from the NSS PIA of ISTAART. Alzheimers Dement. 19, 2182–2196. 10.1002/alz.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HJ, 1945. Notes on voles in central Missouri. J. Mammal. 435–437. [Google Scholar]
- Fliers E, Swaab DF, Pool W, Verwer RWH, 1985. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 342, 45–53. 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- Fricker BA, Roshko VC, Jiang J, Kelly AM, 2023. Partner separation rescues pair bond-induced decreases in hypothalamic oxytocin neural densities. Sci. Rep. 13, 4835. 10.1038/s41598-023-32076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL, McGuire B, 1993. A comparison of living singly and in male-female pairs in the prairie vole, Microtus ochrogaster. Ethology 94, 265–278. 10.1111/j.1439-0310.1993.tb00444.x. [DOI] [Google Scholar]
- Getz LL, Simms LE, McGuire B, Snarski ME, 1997. Factors affecting life expectancy of the prairie vole, Microtus ochrogaster. Oikos 80, 362. 10.2307/3546604. [DOI] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Sue Carter C, 2007. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS, 2008. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety 25, E17–E26. 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, McNeal N, Normann MC, Colburn W, Dagner A, Woodbury M, 2021. Behavioral and neuroendocrine consequences of disrupting a long-term monogamous social bond in aging prairie voles. Stress 24, 239–250. 10.1080/10253890.2020.1812058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbert KJ, Pellegrini M, Gordon KM, Donaldson ZR, 2020. How prior pair-bonding experience affects future bonding behavior in monogamous prairie voles. Horm. Behav. 126, 104847. 10.1016/j.yhbeh.2020.104847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura LC, Ophir AG, 2018. Interactions of sex and early life social experiences at two developmental stages shape nonapeptide receptor profiles. Integr. Zool. 13, 745–760. 10.1111/1749-4877.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura LC, Lazaro VA, Ophir AG, 2023. Plasticity in parental behavior and vasopressin: responses to co-parenting, pup age, and an acute stressor are experience-dependent. Front. Behav. Neurosci. 17. 10.3389/fnbeh.2023.1172845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ, 1995. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 109, 782–789. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE, 1992. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. 89, 5981–5985. 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF, 1999. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. J. Clin. Endocrinol. Metab. 84, 4637–4644. 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- James SS, Englund M, Bottom R, Perez R, Conner KE, Huffman KJ, Wilson SP, Krubitzer LA, 2022. Comparing the development of cortex-wide gene expression patterns between two species in a common reference frame. Proc. Natl. Acad. Sci. 119, e2113896119. 10.1073/pnas.2113896119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Rinne JO, 2002. Functional imaging studies of dopamine system and cognition in normal aging and Parkinson’s disease. Neurosci. Biobehav. Rev. 26, 785–793. 10.1016/S0149-7634(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ, 2015. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 10, 561–570. 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, Ophir AG, 2017. Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integr. Comp. Biol. 57, 603–618. 10.1093/icb/icx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Ophir AG, 2018. Rapid nonapeptide synthesis during a critical period of development in the prairie vole: plasticity of the paraventricular nucleus of the hypothalamus. Brain Struct. Funct. 223, 2547–2560. 10.1007/s00429-018-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, Yee JR, Carter CS, 2019. Rewritable fidelity: how repeated pairings and age influence subsequent pair-bond formation in male prairie voles. Horm. Behav. 113, 47–54. 10.1016/j.yhbeh.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ, 2016. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry Autism Soc. Brain 80, 160–169. 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de León Reyes NS, Sierra Díaz P, Nogueira R, Ruiz-Pino A, Nomura Y, de Solis CA, Schulkin J, Asok A, Leroy F, 2023. Corticotropin-releasing hormone signaling from prefrontal cortex to lateral septum suppresses interaction with familiar mice. Cell 186, 4152–4171.e31. 10.1016/j.cell.2023.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ, 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757. 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ, 2007. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm. Behav. 51, 508–515. 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX, 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. [DOI] [PubMed] [Google Scholar]
- Luong G, Charles ST, Fingerman KL, 2011. Better with age: social relationships across adulthood. J. Soc. Pers. Relat. 28, 9–23. 10.1177/0265407510391362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, Trahanas DM, Grippo AJ, 2014. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci. 180, 9–16. 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN, 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302. 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Müller-Spahn F, 2003. Behavioral disturbances in dementia. Dialogues Clin. Neurosci. 5, 49–59. 10.31887/DCNS.2003.5.1/fmuellerspahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM, 2015. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350, 1371–1374. 10.1126/science.aac5791. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ, 2006. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm. Behav. 49, 681–687. 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng D-J, Phelps SM, 2012. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav. 61, 445–453. 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AF, Protter DSW, Watanabe YL, Chapel GD, Cameron RT, Donaldson ZR, 2024. Nucleus accumbens dopamine release reflects the selective nature of pair bonds. Curr. Biol. 34, 519–530.e5. 10.1016/j.cub.2023.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JM, Garvin MM, Lee NS, Kelly AM, 2022. Behavioral trajectories of aging prairie voles (Microtus ochrogaster): adapting behavior to social context wanes with advanced age. PLoS One 17, e0276897. 10.1371/journal.pone.0276897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Savadlou A, Park S, Siska P, Epp JR, Sargin D, 2023. The impact of loneliness and social isolation on the development of cognitive decline and Alzheimer’s disease. Front. Neuroendocrinol. 69, 101061. 10.1016/j.yfrne.2023.101061. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ, 2009. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312. 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundus AS, Biemuller R, DeLong K, Fitzgerald T, Nyandwi S, 2015. Age-related plasticity in male mate choice decisions by Schizocosa retrorsa wolf spiders. Anim. Behav. 107, 233–238. 10.1016/j.anbehav.2015.06.020. [DOI] [Google Scholar]
- Sadino JM, Bradeen XG, Kelly CJ, Walker DM, Donaldson ZR, 2021. Prolonged partner separation erodes nucleus accumbens transcriptional signatures of pair bonding in male prairie voles (preprint). BioRxiv Neurosci. 10.1101/2021.07.14.452355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y, 2001. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57, 2236–2242. 10.1212/WNL.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti M-AL, Carlton ED, Demas GE, Grippo AJ, 2015. Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster). Horm. Behav. 70, 7–13. 10.1016/j.yhbeh.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR, 1990. Infant’s response to social separation reflects adult differences in affiliative behavior: a comparative developmetal study in prairie and montane voles. Dev. Psychobiol. 23, 375–393. 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- Shuster SM, Willen RM, Keane B, Solomon NG, 2019. Alternative mating tactics in socially monogamous prairie voles, Microtus ochrogaster. Front. Ecol. Evol. 7. 10.3389/fevo.2019.00007. [DOI] [Google Scholar]
- Solomon NG, 1991. Age of pairing affects reproduction in prairie voles. Lab. Anim. 25, 232–235. 10.1258/002367791780808428. [DOI] [PubMed] [Google Scholar]
- Spangenberg E, Wallenbeck A, Eklöf A-C, Carlstedt-Duke J, Tjäder S, 2014. Housing breeding mice in three different IVC systems: maternal performance and pup development. Lab. Anim. 48, 193–206. 10.1177/0023677214531569. [DOI] [PubMed] [Google Scholar]
- Stalling DT, 1990. Microtus ochrogaster. Mammal. Spec. 355, 1–9. [Google Scholar]
- Stern Y, 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11, 1006–1012. 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z, 2014. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res. 265, 22–31. 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahaba DM, Halstead ER, Donaldson ZR, Ahern TH, Beery AK, 2022. Sex differences in the reward value of familiar mates in prairie voles. Genes Brain Behav. 21, e12790. 10.1111/gbb.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S, Hitzemann R, Smith G, Logan J, 1998. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. AJP 155, 344–349. 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Watanasriyakul WT, Scotti M-AL, Carter CS, McNeal N, Colburn W, Wardwell J, Grippo AJ, 2022. Social isolation and oxytocin antagonism increase emotion-related behaviors and heart rate in female prairie voles. Auton. Neurosci. 239, 102967. 10.1016/j.autneu.2022.102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda M, Goudsmit E, Van Der Woude PF, Purba JS, Hofman MA, Bogte H, Swaab DF, 1991. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol. Aging 12, 511–516. 10.1016/0197-4580(91)90081-T. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Huot B, Nilsen R, Wang Z, Insel TR, 1996. Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J. Neuroendocrinol. 8, 777–783. 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR, 2001. Cellular mechanisms of social attachment. Horm. Behav. 40, 133–138. 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


