ABSTRACT
Case report of a case of acute pancreatitis (AP) at a patient previously known with essential thrombocytosis (ET). The most redoubtable complications of AP in this case were: pancreatic necrosis and splahnic vein thrombosis (SVT). Patient was followed‐up for 3 months with complete resolution of SVT under anticoagulation. As far as we know this is the first case ever published suffering simultaneously from AP and ET, both conditions known for their increased risk of developing thrombi.
Keywords: acute pancreatitis, CT, essential thrombocythemia, necrotizing, splanchnic vein thrombosis
Summary.
We present a case of a 70‐year‐old woman with a previous diagnosis of essential thrombocytosis (ET) and acute necrotizing pancreatitis.
Microvascular disturbances caused by ET might have contributed to the pancreatic necrosis.
First case report presenting co‐existing acute pancreatitis with ET, as far as we know.
1. Introduction
Acute pancreatitis (AP) is an inflammation of the pancreas, with multiple etiologies, having a very heterogeneous aspect regarding both severity and morphology. Almost 20% of the cases are categorized as necrotizing pancreatitis, this pathology being consistent with an increase in mortality and morbidity [1].
Splanchnic vein thrombosis (SVT) represents a rare expression of venous thromboembolism, involving splenic, portal and superior mesenteric vein thrombosis, along with Budd‐Chiari syndrome. Its incidence is 25 times lower than the most common manifestations of venous thromboembolism, such as pulmonary thromboembolism or deep vein thrombosis [2, 3]. Necrotizing pancreatitis can develop venous complications twice as often as the non‐complicated course of the disease, with an increase prevalence of splenic vein thrombosis [3].
Essential thrombocythemia or essential thrombocytosis (ET) represents one of the Philadelphia chromosome‐negative chronic myeloproliferative neoplasms, with a global incidence varying between 0.2 and 2.5 for 100,000 people per year [4]. It is associated with a gain‐of‐function mutation in Janus Kinase 2, with a frequency of 50%–60% [5]. In many cases, the disease is silent, but some patients can develop hemorrhagic or thromboembolic complications, even a transformation of this condition into acute myeloid leukemia or myelofibrosis [5, 6].
Within this context, we present the case of a female patient with essential thrombocythemia, whose clinical journey took a turn as she developed necrotizing acute pancreatitis and double thrombosis, affecting both the splenic and superior mesenteric vein. We were not able to find any previous reports of cases with both ET and AP, both diseases being known to have thrombotic complications.
2. Case History
Our case report displays a 70‐year‐old woman, known hypertensive in treatment with amlodipine 5 mg qd and candesartanum 16 mg qd, former smoker (20‐packs‐year, withdrawn for 30 years), and chronic alcohol consumer (around 5 units of alcohol daily) who presented to the hospital suffering from upper abdominal pain radiating to the back, associated with nausea and vomiting during the past 24 h. Regarding the patient's medical history, she is known to have essential thrombocytosis, arterial hypertension, dyslipidemia, and carotid atheromatosis. She also underwent a hysterectomy and bowel obstruction surgery in the past. The patient did not have any relevant family history.
On admission, her clinical assessment showcased a conscious, overweight, afebrile patient. Her cardiovascular system evaluation revealed a blood pressure of 150/90 mmHg, a heart rate of 100 beats per minute with a regulated rhythm, and normal heart sounds without any heart murmur associated. The pulmonary examination disclosed bilateral vesicular murmur without any rales or crepitus. The abdomen was distended, painful spontaneously and with abdominal guarding, followed in the first 24 h by the formation of a supraumbilical well‐defined solid pseudotumor mass, painful with deep palpation, all of that along with a diminished intestinal transit.
3. Methods
To establish the right diagnosis for our patient, we performed some laboratory and imagistic investigations.
Laboratory tests revealed an inflammatory syndrome with increased levels of C‐reactive protein, leukocytosis with neutrophilia, thrombocytosis, hyperglycemia with a high glycated hemoglobin (HbA1c) level, high lactate dehydrogenase (LDH) levels, a significant increase in serum lipase and amylase, cholestasis with elevated gamma‐glutamyl transferase (GGT) and ALP levels, hyponatremia, hypochloremia, and hyposideremia.
Evolution during hospitalization is reported in Table 1.
TABLE 1.
Laboratory findings during admission.
| Test | Pre‐admission | 1st day | 3rd day | 6th day | 7th day | 9th day | 11th day | 16th day | 21st day |
|---|---|---|---|---|---|---|---|---|---|
| White blood cells | — | 15.3 ×103/μL | 19.9 ×103/μL | 12.8 ×103/μL | 14.24 ×103/μL | 14.58 ×103/μL | 15.5 ×103/μL | 9.5 ×103/μL | 7.7 ×103/μL |
| Neutrophils (#) | — | 14.1 ×103/μL | 18.3 ×103/μL | 11.4 ×103/μL | 12.3 ×103/μL | 12.9 ×103/μL | 13.6 ×103/μL | 7.7 ×103/μL | 6 ×103/μL |
| Platelets | — | 762 ×103/μL | 526 ×103/μL | 470 ×103/μL | 477.3 ×103/μL | 509.9 ×103/μL | 533 ×103/μL | 600 ×103/μL | 512 ×103/μL |
| C‐reactive protein | — | 14.3 mg/dL | 50.5 mg/dL | — | 53.178 mg/dL | 52.128 mg/dL | — | 18.49 mg/dL | 5.7 mg/dL |
| Amylase | 1692 U/L | — | — | — | 14 U/L | 15 U/L | 12 U/L | 11 U/L | 17 U/L |
| Lipase | 10.049 U/L | 5075 U/L | 975 U/L | — | — | — | — | — | 88 U/L |
| Aspartate transaminase | 30 U/L | 34 U/L | 32 U/L | 22 U/L | 23 U/L | 17 U/L | 23 U/L | 21 U/L | 23 U/L |
| Alanine transaminase | 78 U/L | 65 U/L | 35 U/L | 42 U/L | 28 U/L | 21 U/L | 22 U/L | 14 U/L | 25 U/L |
| Total bilirubin | 0.93 mg/dL | 1.04 mg/dL | 1.47 mg/dL | 0.66 mg/dL | 0.71 mg/dL | 0.53 mg/dL | 0.52 mg/dL | 0.64 mg/dL | 0.7 mg/dL |
| Direct bilirubin | 0.19 mg/dL | 0.34 mg/dL | 0.62 mg/dL | 0.29 mg/dL | 0.28 mg/dL | 0.28 mg/dL | 0.27 mg/dL | — | 0.1 mg/dL |
| Alkaline phosphatase (ALP) | — | 110 U/L | — | — | 148 U/L | 131 U/L | 161 U/L | 157 U/L | — |
| GGT | — | 138 U/L | — | — | 182 U/L | 154 U/L | 216 U/L | 196 U/L | — |
| Glucose | 238 mg/dL | 269 mg/dL | 305 mg/dL | 278 mg/dL | 414 mg/dL | 270 mg/dL | 203 mg/dL | 224 mg/dL | 250 mg/dL |
| HbA1c | — | — | — | — | 7.7% | — | — | — | — |
| LDH | — | — | 679 U/L | — | — | 467 U/L | — | 459 U/L | 310 U/L |
| Na+ | 134 mmol/L | 140 mmol/L | 142 mmol/L | 137 mmol/L | 132 mmol/L | 138 mmol/L | 140 mmol/L | 138 mmol/L | 142 mmol/L |
| K+ | 3.9 mmol/L | 4.8 mmol/L | 4.6 mmol/L | 4.3 mmol/L | 3.8 mmol/L | 4.2 mmol/L | 3.7 mmol/L | 3.6 mmol/L | 3.5 mmol/L |
| Cl− | 95 mmol/L | — | 106 mmol/L | — | — | — | — | — | — |
| Serum iron | — | 14 μg/dL | — | — | — | 19 μg/dL | — | — | — |
| Serum calcium | — | 8.2 mg/dL | 7.1 mg/dL | — | — | 7.54 mg/dL | — | — | 7.6 mg/dL |
| International normalized ratio (INR) | 1.16 | 1.38 | 1.46 | 1.34 | 1.43 | 1.43 | 1.67 | 1.68 | 1.61 |
| Creatinkinase (CK) | 55 U/L | 67 U/L | — | — | — | — | 34 U/L | — | — |
| CK‐MB | 18 U/L | 17 U/L | — | — | — | — | 16 U/L | — | — |
| Reference range |
White blood cells: 3.8–11.8 ×103/μL Neutrophils (#): 1.9–8.2 ×103/μL Platelets: 130–408 ×103/μL C‐reactive: 0–0.9 mg/dL Amylase: 15–115 U/L Lipase: 73–393 U/L Aspartate aminotranspherase: 2–40 U/L Alanine aminotranspherase: 3–65 U/L Total bilirubin: 0–1.2 mg/dL Direct bilirubin: 0–0.3 mg/dL Alkaline phosphatase: 40–136 U/L Gamma‐glutamyl transferase: 5–85 U/L Glucose: 70–115 mg/dL HbA1c: 4.3%–6% Na+: 135–150 mmol/L K+: 3.5–5.2 mmol/L Cl−: 98–108 mmol/L Serum iron: 40–160 μg/dL Serum calcium: 8.2–10.7 mg/dL INR: 0.8—1.2 CK: 26–192 U/L CK‐MB: 0–25 U/L |
||||||||
The electrocardiogram depicted a sinus rhythm, normal QRS axis, heart rate of 96 beats per minute, without any ST‐T secondary changes.
The chest X‐ray showed a globally enlarged heart and an accentuation of interstitial and peribronchovascular patterns in the bases bilaterally. The abdominal X‐ray was normal.
The abdominal ultrasound showed an enlarged pancreas with hyperechogenic structure and a visible Wirsung duct. More than that, it depicted non‐dilated common and intrahepatic bile ducts, a normal‐sized gallbladder, with multiple calculi with a maximum diameter of 8 mm, mobile, both kidneys in the normal ecographic range and a thin layer of fluid in Morison's pouch.
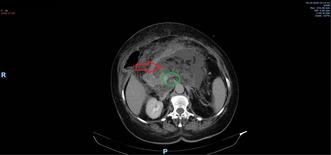
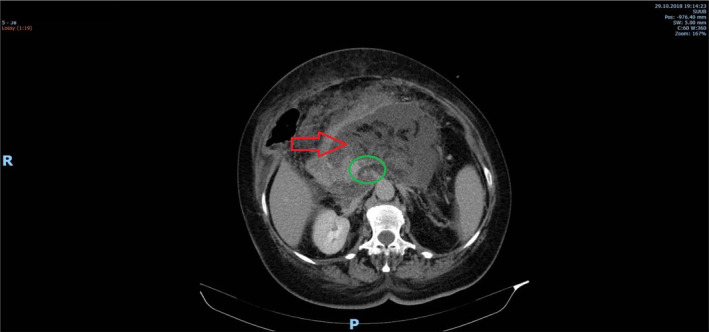
The abdominal computed tomography (CT) scan, both native and post‐contrast, revealed an enlarged pancreas (especially in the cephalic region) with a heterogeneous attenuation of the gland (red arrows on Figure 1). Multiple acute necrosis collection were detected, with a maximum diameter of 14 mm, and areas of cyto‐steatonecrosis. Both small interaortcaval and duodenopancreatic adenopathies were emphasized. The liver was enlarged with a craniocaudal diameter of the right hepatic lobe of 19 cm and an anteroposterior diameter of the left hepatic lobe of 7 cm, showing mild steatosis and perfusion abnormalities in the VIIIth segment (blue arrows in Figure 2), along with subcapsular microcalcifications in segments IV–VIII. There was an identifiable 2 cm‐length heterogeneous aspect in the superior mesenteric vein that occupied 50% of the lumen, and a thrombus in the splenic vein that occupied 80% of the lumen, with the spleen having moderately hypodense triangle‐shaped areas that suggested infarction zones (green circle on Figure 1). The cholecyst was increased in size, with normal wall, its infundibulum was narrowed, and contained an oval macrocalculus with a long axis of 6 mm, without the primary biliary duct and the intrahepatic biliary ducts obstructed nor dilated. The kidneys did not present any abnormalities, except for a cortical cyst on the left kidney, Bosniak type 1, with a diameter of 25 mm. No pleural effusion was noted. A 34‐mm hiatal hernia was observed.
FIGURE 1.
CT scan. Acute pancreatitis (red arrow) and splenic and superior mesenteric vein thrombosis (green circle).
FIGURE 2.
CT scan. Perfusion abnormalities in liver and spleen.
Differential diagnosis which were taken into consideration were: acute cholecystitis and cholangitis (normal total and both direct and indirect bilirubin, along with no significant ultrasound (US) findings, except some calculi), perforated peptic ulcer disease (CT scan that was performed ruled out this diagnosis), acute hepatitis (normal transaminase levels), bowel obstruction (abdominal X‐ray and CT were normal), basal pneumonia (chest X‐ray was normal, no other specific signs or symptoms) and inferior myocardial infarction electrocardiogram was normal, along with CK and CK‐MB.
The patient was diagnosed with acute necrotizing hemorrhagic pancreatitis—Ranson 3 points, both splenic and superior mesenteric vein thrombosis, arterial hypertension, diabetes mellitus type II, essential thrombocytosis, carotid atheromatosis and hiatal hernia, thus she was admitted to the gastroenterology department of University Emergency Hospital of Bucharest.
Repeated laboratory tests during hospitalization emphasized a drop in the inflammatory syndrome. The lipase and amylase levels decreased to their normal range and her glycemia was suitably managed.
Another computed tomography scan was taken after 7 days of hospitalization and it showed an intensely hypodense edematous pancreas, associated with fluid collections—hard to differentiate from necrosis. No signs of infection were observed. Otherwise, there were no other changes compared to the previous CT scan.
During hospitalization the patient was fluid resuscitated with Ringer lactate 3 mL/kg/h for the first 6 h, afterwards the volume was accordingly to: blood pressure, heart rate, urine output and the volume percentage of red blood cells. As the patient did not tolerate naso‐jejunal tube it had received parenteral nutritional support for 24 h with glucose 10% 500 mL t.i.d. To prevent the extension of the two aforementioned thrombi low molecular weight heparin enoxaparium 150 UI/kg b.i.d. subcutaneously, as recommended by previous studies in this field [7]. In order to control the symptoms of AP we gave the patient: Sodium metamizole 500 mg t.i.d. iv and acetaminophen 500 mg t.i.d. iv to control the pain. For the patients' hyperglycemia, the diabetologist recommended temporary insulin therapy throughout the hospitalization. For arterial hypertension, as recommended by our cardiologist, the patient received metoprolol succinate 50 mg q.d. po, amlodipine 5 mg q.d. po, candesartan 16 mg q.d. po, and for her dyslipidemia simvastatin 20 mg q.d. po. Regarding her underlying hematological disorder, she received hydroxyurea 1000 mg b.i.d po, as recommended prior to hospitalization by her following hematologist.
Our patient's evolution during admission was favorable and she resumed her oral alimentation after 24 h, without any nausea, vomiting, abdominal pain, fever or chills. She was discharged after 21 days.
4. Conclusions and Results
Our patient's outcome was a favorable one, her illnesses being well managed in the hospital. After this episode, she was referred to a diabetologist, a cardiologist, and a hematologist in order to have her diseases under control. The patient was discharged with the following recommendations: to stop alcohol consumption, to quit smoking, and to maintain a diet according to diabetologist indications. In addition, the medical treatment was augmented with apixabanum 5 mg b.i.d. po for 3 months, pantoprazole 40 mg b.i.d po for 7 days and after that 40 mg q.d. po, Domperidone 10 mg t.i.d po for 10 days, allopurinol 100 mg q.d. po, hydroxyurea 1000 mg b.i.d po, candesartan 8 mg q.d. po, metoprolol succinate 50 mg b.i.d. po, amlodipine 10 mg q.d. po, rosuvastatin 10 mg q.d. po, metformin 1000 mg, insulinum glargine 20 UI q.d., pancreatinum 20.00 UI t.i.d. sc, and sodium metamizole 500 mg t.i.d. po. More than that, another recommendation was to repeat after 3 months the patients' CT in order to assess the evolution of the thrombi.
Patient was invited to follow‐up at 3 months, and at the contrast‐enhanced computed tomography (CECT) abdominal scan no thrombus was visible in the aforementioned veins (Figure 3). As such, same as the current recommendations in managing non‐cirrhotic SVT [8], at 3 months with no proof of lasting thrombus we have decided to stop the anticoagulation with Apixabanum.
FIGURE 3.
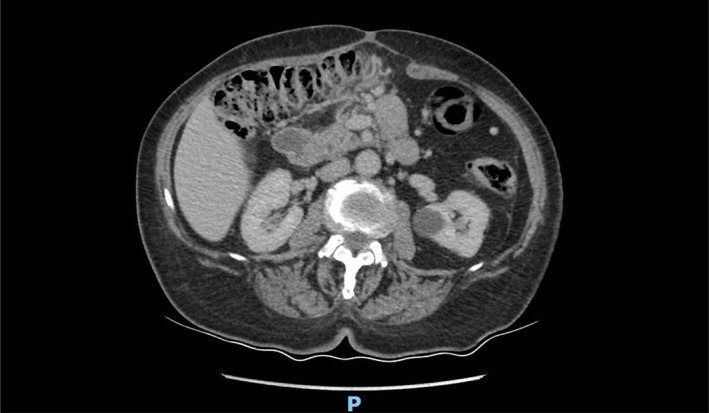
Abdominal CECT scan (venous phase) follow up at 3 months after discharge.
This case report underscores the importance of management and the results of this confluence, where severe acute necrotizing pancreatitis coincides with a pre‐existing diagnosis of essential thrombocythemia.
5. Discussion
Necrotizing pancreatitis can lead to various complications, some of which can be life‐threatening. The most common complications of this type of pancreatitis include infection, organ failure, along with endocrine and exocrine insufficiency and chronic pancreatitis in percentages between 16% and 45% [9]. Besides these, patients can broaden their spectrum of complications with splanchnic vein thrombosis, especially splenic vein, followed by superior mesenteric vein [3, 9]. Cytokine storm and intense inflammatory responses, both local and systemic, lead to a hypercoagulable state, along with endothelial damage due to pancreatic enzymes and stasis caused by compression from the surrounding inflamed tissue [10]. Still, one of the most important factors that increased the chances of setting splanchnic vein thrombosis in our patient was essential thrombocytosis, a diagnosis that the patient had before being admitted.
In essential thrombocythemia, splanchnic vein thrombosis develops with a frequency between 4% and 13%, and it can appear both at the time of myeloproliferative neoplasm (MPN) diagnosis during the follow‐up of these patients [11]. Classical factors linked with the occurrence of thrombosis in case of a MPN are younger age, female gender, prior thrombotic events and the JAK2 V617F mutation [12, 13], our patient having some of them positive. The means by which thrombosis develops in MPN are multiple, but most important are the presence of endothelial oxidative stress, the spontaneous aggregation of thrombocytes, procoagulant, and proinflammatory molecules secreted by leukocytes and erythrocytes aggregation that stimulates leukocyte‐platelet interactions [11, 12]. Also, it is known that the splanchnic venous system is more susceptible to thrombosis [13]. In addition to all, MPN‐SVT showed an increase in mortality and morbidity, essential thrombocytosis (ET)‐SVT presenting a shorter median overall survival compared to ET without SVT [11].
Increased number of platelets was emphasized in our patient laboratory findings, caused by the inflammation from acute pancreatitis augmented by the pathological increase existing in ET. Several studies showed [14, 15, 16] that platelets are directly involved in the inflammatory process of acute pancreatitis, thrombocyte aggregation and adhesiveness leading to a prothrombotic state, with a perturbation in pancreatic microcirculation, accompanied by platelet consumption and bone marrow response. ET and acute pancreatitis at the same patient were not reported previously, as far as we know.
ET is a well‐known prothrombogenic disease [17, 18, 19, 20] that was found to microvascular circulation disturbances in several other organs like brain, eyes and coronary circulation [21] and further more is it known to provoke a hypercoagulability state by altering the function of von Willebrand factor [22].
In regard to the development and extent of the pancreatic necrosis there is a growing body of evidence in the literature, on both animal [23, 24, 25, 26] and human models [27, 28, 29], that one of the most important pathophysiological events promoting it are the microcirculatory alterations. Also, there is even a study that further more refines the microcirculatory disturbances as endothelial injury in which von Willebrand factor plays a very important role [30].
Bearing in mind all the aforementioned proofs, there is a high probability that ET played a very important role not only in the aforementioned SVT but also on the extent of the pancreatic necrosis. As the extent of pancreatic necrosis is one of the largest mortality drivers [31] in AP we can suggest that any patient that suffers from ET and AP should be better investigated as it is at a higher risk of apparition of pancreatic necrosis.
Author Contributions
Andreea Irina Ghiță: conceptualization, data curation, methodology, project administration, writing – original draft. Arina Ilinca Gheorghe: writing – original draft. Georgiana Elena Beteringhe: writing – original draft. Andreea Ramona Treteanu: data curation. Mihai Radu Pahomeanu: conceptualization, supervision, writing – review and editing.
Ethics Statement
Following COPE recommendation on case study reports (https://publicationethics.org/case/ethical‐approval‐requirements‐case‐study‐reports) this case report is waived from approval from IRB as there were less than 3 patients presented and no intervention was performed.
Consent
The patient signed informed consent regarding using its medical data for research and publication prior to hospitalization.
Conflicts of Interest
Andreea Irina Ghiță, Arina Ilinca Gheorghe, Georgiana Elena Beteringhe, and Andreea Ramona Treteanu none to disclose. Mihai Radu Pahomeanu received lecture fees from Mayoly in the past 12 months.
Acknowledgments
Open access publishing facilitated by Anelis Plus (the official name of “Asociatia Universitatilor, a Institutelor de Cercetare – Dezvoltare si a Bibliotecilor Centrale Universitare din Romania”), as part of the Wiley – Anelis Plus agreement.
Funding: The authors received no specific funding for this work.
Contributor Information
Andreea Irina Ghiță, Email: andreea.irina.ghita@stud.umfcd.ro.
Arina Ilinca Gheorghe, Email: arina-ilinca.gheorghe0721@stud.umfcd.ro.
Georgiana Elena Beteringhe, Email: georgiana-elena.beteringhe0720@stud.umfcd.ro.
Andreea Ramona Treteanu, Email: andreea-ramona.treteanu0720@stud.umfcd.ro.
Mihai Radu Pahomeanu, Email: mihai.pahomeanu@drd.umfcd.ro.
Data Availability Statement
Data available upon reasonable request from the corresponding author.
References
- 1. Leonard‐Murali S., Lezotte J., Kalu R., et al., “Necrotizing Pancreatitis: A Review for the Acute Care Surgeon,” American Journal of Surgery 221, no. 5 (2021): 927–934, 10.1016/j.amjsurg.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wendelboe A. M. and Raskob G. E., “Global Burden of Thrombosis,” Circulation Research 118, no. 9 (2016): 1340–1347, 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 3. Mendelson R. M., Anderson J., Marshall M., and Ramsay D., “Vascular Complications of Pancreatitis,” ANZ Journal of Surgery 75, no. 12 (2005): 1073–1079, 10.1111/j.1445-2197.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 4. Accurso V., Santoro M., Mancuso S., et al., “The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep,” Clinical Medicine Insights: Blood Disorders 13 (2020): 2634853520978210, 10.1177/2634853520978210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tefferi A., Vannucchi A. M., and Barbui T., “Essential Thrombocythemia: 2024 Update on Diagnosis, Risk Stratification, and Management,” American Journal of Hematology 99, no. 4 (2024): 697–718, 10.1002/ajh.27216. [DOI] [PubMed] [Google Scholar]
- 6. Babakhanlou R., Masarova L., and Verstovsek S., “A Review of Essential Thrombocythemia and Its Complications,” Clinical Advances in Hematology & Oncology 21, no. 2 (2023): 76–84. [PubMed] [Google Scholar]
- 7. Valeriani E., Menichelli D., Palumbo I. M., Cammisotto V., Pastori D., and Pignatelli P., “How to Treat Patients With Splanchnic Vein Thrombosis: Recent Advances,” Polskie Archiwum Medycyny Wewnętrznej 133, no. 5 (2023): 16499, 10.20452/pamw.16499. [DOI] [PubMed] [Google Scholar]
- 8. Monaco G., Bucherini L., Stefanini B., Piscaglia F., Foschi F. G., and Ielasi L., “Direct Oral Anticoagulants for the Treatment of Splanchnic Vein Thrombosis: A State of Art,” World Journal of Gastroenterology 29, no. 33 (2023): 4962–4974, 10.3748/wjg.v29.i33.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maatman T. K., Roch A. M., Ceppa E. P., et al., “The Continuum of Complications in Survivors of Necrotizing Pancreatitis,” Surgery 168, no. 6 (2020): 1032–1040, 10.1016/j.surg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borbély R. Z., Szalai E. Á., Philip B. M., et al., “The Risk of Developing Splanchnic Vein Thrombosis in Acute Pancreatitis Increases 3 Days After Symptom Onset: A Systematic Review and Meta‐Analysis,” United European Gastroenterology Journal 12, no. 6 (2024): 678–690, 10.1002/ueg2.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu A., Naymagon L., and Tremblay D., “Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs,” Cancers (Basel) 15, no. 1 (2022): 11, 10.3390/cancers15010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arachchillage D. R. and Laffan M., “Pathogenesis and Management of Thrombotic Disease in Myeloproliferative Neoplasms,” Seminars in Thrombosis and Hemostasis 45, no. 6 (2019): 604–611, 10.1055/s-0039-1693477. [DOI] [PubMed] [Google Scholar]
- 13. How J., Zhou A., and Oh S. T., “Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Pathophysiology and Molecular Mechanisms of Disease,” Therapeutic Advances in Hematology 8, no. 3 (2017): 107–118, 10.1177/2040620716680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mimidis K., Papadopoulos V., Kotsianidis J., et al., “Alterations of Platelet Function, Number and Indexes During Acute Pancreatitis,” Pancreatology 4, no. 1 (2004): 22–27, 10.1159/000077024. [DOI] [PubMed] [Google Scholar]
- 15. Akbal E., Demirci S., Koçak E., Köklü S., Basar Ö., and Tuna Y., “Alterations of Platelet Function and Coagulation Parameters During Acute Pancreatitis,” Blood Coagulation & Fibrinolysis 24, no. 3 (2013): 243–246, 10.1097/MBC.0b013e32835aef51. [DOI] [PubMed] [Google Scholar]
- 16. Osada J., Wereszczynska‐Siemiatkowska U., Dabrowski A., and Dabrowska M. I., “Platelet Activation in Acute Pancreatitis,” Pancreas 41, no. 8 (2012): 1319–1324, 10.1097/MPA.0b013e31824bd89f. [DOI] [PubMed] [Google Scholar]
- 17. Giaccherini C., Marchetti M., and Falanga A., “Thrombocytosis and Risk of Thrombosis in Myeloproliferative Neoplasms,” Biochimica Clinica 41, no. 2 (2017): 128–133, 10.19186/BC_2017.021. [DOI] [Google Scholar]
- 18. Arellano‐Rodrigo E., Alvarez‐Larrán A., Reverter J. C., Villamor N., Colomer D., and Cervantes F., “Increased Platelet and Leukocyte Activation as Contributing Mechanisms for Thrombosis in Essential Thrombocythemia and Correlation With the JAK2 Mutational Status,” Haematologica 91, no. 2 (2006): 169–175. [PubMed] [Google Scholar]
- 19. Barbui T., Finazzi G., and Falanga A., “Cancer‐Associated Thrombotic Disease Myeloproliferative Neoplasms and Thrombosis,” Blood 122, no. 13 (2013): 2176–2184, 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 20. Passamonti F., Rumi E., Arcaini L., et al., “Prognostic Factors for Thrombosis, Myelofibrosis, and Leukemia in Essential Thrombocythemia: A Study of 605 Patients,” Haematologica 93, no. 11 (2008): 1645–1651, 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 21. Michiels J. J., Berneman Z., Schroyens W., et al., “Platelet‐Mediated Erythromelalgic, Cerebral, Ocular and Coronary Microvascular Ischemic and Thrombotic Manifestations in Patients With Essential Thrombocythemia and Polycythemia Vera: A Distinct Aspirin‐Responsive and Coumadin‐Resistant Arterial Thrombophilia,” Platelets 17, no. 8 (2006): 528–544, 10.1080/09537100600758677. [DOI] [PubMed] [Google Scholar]
- 22. Fabris F., Casonato A., Delben M., Demarco L., and Girolami A., “Abnormalities of Vonwillebrand‐Factor in Myeloproliferative Disease—A Relationship With Bleeding Diathesis,” British Journal of Haematology 63, no. 1 (1986): 75–83, 10.1111/j.1365-2141.1986.tb07497.x. [DOI] [PubMed] [Google Scholar]
- 23. Keck T., Werner J., Banafsche R., et al., “Oxygen Radicals Promote ICAM‐1 Expression and Microcirculatory Disturbances in Experimental Acute Pancreatitis,” Pancreatology 3, no. 2 (2003): 156–163, 10.1159/000070085. [DOI] [PubMed] [Google Scholar]
- 24. Foitzik T., Eibl G., Hotz H. G., Faulhaber J., Kirchengast M., and Buhr H. J., “Endothelin Receptor Blockade in Severe Acute Pancreatitis Leads to Systemic Enhancement of Microcirculation, Stabilization of Capillary Permeability, and Improved Survival Rates,” Surgery 128, no. 3 (2000): 399–407, 10.1067/msy.2000.107104. [DOI] [PubMed] [Google Scholar]
- 25. Du B.‐Q., Yang Y.‐M., Chen Y.‐H., Liu X.‐B., and Mai G., “N‐Acetylcysteine Improves Pancreatic Microcirculation and Alleviates the Severity of Acute Necrotizing Pancreatitis,” Gut Liver 7, no. 3 (2013): 357–362, 10.5009/gnl.2013.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bostanci H., Sahin T. T., Dikmen K., et al., “Candesartan Mediates Microcirculation in Acute Necrotizing Pancreatitis,” Bratislava Medical Journal 116, no. 4 (2015): 270–275, 10.4149/BLL_2015_052. [DOI] [PubMed] [Google Scholar]
- 27. Antkowiak R., Bialecki J., Chabowski M., and Domoslawski P., “Treatment of Microcirculatory Disturbances in Acute Pancreatitis Where Are We Now?,” Pancreas 51, no. 5 (2022): 415–421, 10.1097/MPA.0000000000002044. [DOI] [PubMed] [Google Scholar]
- 28. Smeets X. J. N. M., Litjens G., Gijsbers K., et al., “The Accuracy of Pancreatic Perfusion Computed Tomography and Angiography in Predicting Necrotizing Pancreatitis: A Systematic Review,” Pancreas 47, no. 6 (2018): 667–674, 10.1097/MPA.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 29. Menger M. D., Plusczyk T., and Vollmar B., “Microcirculatory Derangements in Acute Pancreatitis,” Journal of Hepato‐Biliary‐Pancreatic Surgery 8, no. 3 (2001): 187–194, 10.1007/s005340170015. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y., Ke L., Meng L., et al., “Endothelial Markers Are Associated With Pancreatic Necrosis and Overall Prognosis in Acute Pancreatitis: A Preliminary Cohort Study,” Pancreatology 17, no. 1 (2017): 45–50, 10.1016/j.pan.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 31. Jha A. K., Goenka M. K., Kumar R., and Suchismita A., “Endotherapy for Pancreatic Necrosis: An Update,” JGH Open 3, no. 1 (2019): 80–88, 10.1002/jgh3.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon reasonable request from the corresponding author.


