Abstract
Staphylococcus aureus is an important bone pathogen, and evidence shows that this organism is internalized by chick osteoblasts. Here we report that S. aureus is internalized by human osteoblasts. Internalization was inhibited by monodansylcadaverine and cytochalasin D and to a lesser extent by ouabain, monensin, colchicine, and nocodazole. We propose that internalization occurs via a receptor-mediated pathway, requiring the participation of cytoskeletal elements, principally actin.
Staphylococcus aureus, a gram-positive facultatively anaerobic bacterium, is an important pathogen in bone disease. It is responsible for about 70% of cases of osteomyelitis (15, 17) and 80% of cases of joint infections in patients with rheumatoid arthritis (15) and is a common factor in several other bone diseases (6, 11, 28). S. aureus infection of bone is associated with rapid, localized destruction of the tissue (11). The mechanism(s) responsible for osteolysis are not established, but exported proteins which associate with the cell surface of this organism are potent stimulators of bone resorption (22). These proteins appear to directly stimulate osteoclast activity (2), although isolates of S. aureus vary in the osteolytic activity of these proteins (23). Additionally, cellular extracts of S. aureus have been demonstrated to inhibit, in a dose-dependent manner, both osteocalcin and type I collagen biosynthesis (19). Localization of S. aureus to bone seems to result from its ability to bind several extracellular matrix proteins, including collagen, fibronectin, and bone sialoprotein (29).
There is growing evidence that bacteria can invade host cells (13, 16, 24–27, 30, 34), with several studies reporting the internalization of S. aureus by both epithelial and endothelial cells (1, 4, 5, 21, 33, 35). Recently, it has also demonstrated that this organism is internalized by embryonic chick osteoblasts in vitro (16), and a preliminary report has suggested that such internalization occurs in vivo (27). It is probable that internalization of S. aureus by bone cells facilitates the progression of disease, by protecting the organism from extracellular host defenses and/or antibiotic therapy. This behavior could help explain the recurrent nature of diseases such as osteomyelitis. The present study was designed to investigate the internalization of S. aureus by human osteoblasts.
Human and bacterial cells.
Normal human osteoblasts, normal human gingival fibroblasts (HGFs), and the human osteoblastic cell line MG-63 were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine and containing 25 mM HEPES, 100 U/ml penicillin (Gibco, Paisley, United Kingdom), streptomycin (100 μg/ml; Gibco), and, for primary osteoblast cultures, amphotericin B (0.25 μg/ml; Sigma, Poole, United Kingdom).
Primary human osteoblasts and HGFs were cultured from patient samples obtained during routine oral surgery. Tissue samples were collected on the day of operation in phosphate-buffered saline (PBS); for bone samples, PBS contained penicillin (100 U/ml; Gibco), streptomycin (100 μg/ml; Gibco). The samples were then either processed the same day or stored overnight at 4°C and then processed. Excess blood was removed by rinsing in PBS several times, and soft tissue was removed from bone samples with a scalpel, aided by rinsing with PBS. Gingival tissue samples were cut into pieces of ∼1 mm3 and cultured in 75-cm3 tissue culture flasks (Sarstedt Ltd., Leicester, United Kingdom) in DMEM supplemented with 10% FBS, 50 μg of l-ascorbic acid per ml, and 2 mM l-glutamine and containing penicillin (100 U/ml; Gibco) and streptomycin (100 μg/ml; Gibco). Bone samples were cut into fragments of ∼1 to 2 mm3 and cultured in 80-cm3 Nunc tissue culture flasks (Gibco) in DMEM, as for routine culture. Once confluent, bone cells were characterized by alkaline phosphatase staining using a leukocyte alkaline phosphatase kit (Sigma) according to the manufacturer’s instructions. The proportion of positively stained cells in each population was estimated by counting a random sample of 100 cells. Cell populations with at least 80% positive cells were further cultured for use in assays. All incubations were carried out at 37°C in a humidified atmosphere containing 5% CO2.
S. aureus NCTC 6571, S. carnosus TM300, the clinical isolates strains 15 and 16 (7), and the type strain S. aureus SMH were maintained on Wilkins-Chalgren agar (Oxoid, Basingstoke, United Kingdom) containing 5% horse blood (Oxoid), incubated aerobically at 37°C overnight.
Internalization of S. aureus by human osteoblasts.
Internalization of S. aureus was investigated by using a modification of the method described by Oelschlaeger and Tall (25). Confluent monolayers of normal human osteoblasts, HGFs, or MG-63 cells were washed twice with PBS (containing antibiotics), seeded at 50,000 cells per well onto 24-well tissue culture plates in 1 ml of growth medium, and cultured for 1 to 2 days, until ∼70 to 80% confluent. Two to three hours before the addition of bacteria, the cells were washed twice with 1 ml of PBS and then incubated with 1 ml of assay medium (growth medium without antibiotics).
Prior to internalization assays, bacteria were grown aerobically overnight at 37°C in brain heart infusion broth (Oxoid), adjusted to an A650 of 0.01 with fresh brain heart infusion broth, and further incubated aerobically at 37°C for ∼3 h. Bacterial numbers were estimated spectrophotometrically at 650 nm, and bacteria were collected by centrifugation at 200 × g for 10 min. After centrifugation, bacteria were resuspended in assay medium and added to tissue culture wells at a ratio of ∼30:1 (bacteria:osteoblasts); to minimize clumping of the bacteria, suspensions were mixed thoroughly. Reported studies investigating bacterial uptake by eukaryotic cells have commonly cocultured cells for periods of up to 2 h (4, 5, 16, 24–26, 30, 33, 35).
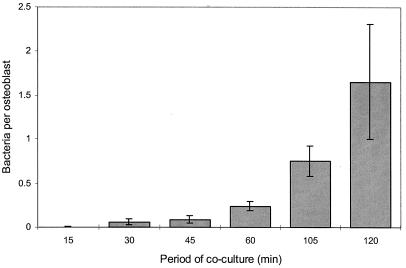
The kinetics of uptake of S. aureus into osteoblasts over this period was investigated, and maximal internalization was observed at 2 h (Fig. 1). Osteoblast-S. aureus cocultures were incubated for 2 h at 37°C in a humidified atmosphere containing 5% CO2. Following internalization, cells were washed twice with 1 ml of PBS and incubated for a further 2 h in 1 ml of fresh medium containing gentamicin (100 μg/ml). Finally, cells were washed twice with 1 ml of PBS and lysed with 0.1% Triton X-100, and intracellular bacteria were enumerated by serial dilution and plate counting.
FIG. 1.
Comparison of the length of the period of coculture on the uptake of S. aureus NCTC 6571 by normal human osteoblasts. Data represent a single experiment performed in triplicate and are shown as the means and standard deviations of the means.
Initially normal human osteoblasts (≤4th passage) were used in internalization assays. However, although internalization could be shown, the number of internalized bacteria per osteoblast was not consistent between experiments. The frequency of internalization was between 0.3 (±0.03) and 3.0 (±0.1) bacteria per osteoblast. The reason for this substantial experimental variation is not known, although it may relate to the stage of differentiation of the osteoblasts or to differences in the source of these cells. To keep the possible effects of cell source consistent, the osteoblast cell line MG-63 was used in subsequent assays. The frequency of internalization into MG-63 was found to be higher than that observed for normal human osteoblasts and more reproducible, with ∼6.8 (±2.1) internalized bacteria per osteoblast. The difference in the number of internalized S. aureus seen in Fig. 1, showing the kinetics of uptake, compared with Fig. 2 was attributed to the use of these different cell types (normal human osteoblasts and MG-63, respectively).
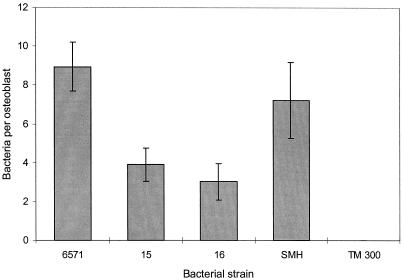
FIG. 2.
Comparison of internalization of S. aureus NCTC 6571, 15, 16, and SMH and S. carnosus TM300 by MG-63. Data are from a representative experiment performed at least three times in triplicate and are given as the means and standard deviations of the means.
It was considered that clinical isolates of S. aureus might have a greater propensity to be internalized by osteoblasts than NCTC 6571. This organism is a laboratory reference strain originally isolated more than half a century ago, and in consequence it may have lost some of its virulence. To address this question, MG-63 cells were cocultured with a nonpathogenic organism of the same genus, namely, S. carnosus TM300 (an organism used in the meat industry for fermenting meat products) (Fig. 2), two clinical isolates of S. aureus, strains 15 and 16 (7), and the prototypic bone pathogen SMH. S. carnosus was not internalized, but S. aureus strains 15, 16, and SMH, along with 6571, were (Fig. 2). This finding suggests that the simple interaction of human and bacterial cells is not sufficient for bacterial internalization. However, although it was anticipated that strains 15, 16, and SMH would show greater internalization than NCTC 6571, it was observed that strains 15 and 16 were actually internalized two- to threefold less than NCTC 6571. There was no difference in internalization between strains SMH and NCTC 6571 (Fig. 2).
Internalization of S. aureus by HGFs was also investigated. S. aureus exhibited a lower propensity for internalization by HGFs than MG-63, with 1.8 (±1.0) internalized bacteria per cell. It is of interest, however, that Hudson et al. (16) reported no significant difference between S. aureus internalization of osteoblasts and fibroblasts isolated from 16- to 18-day-old chick embryos.
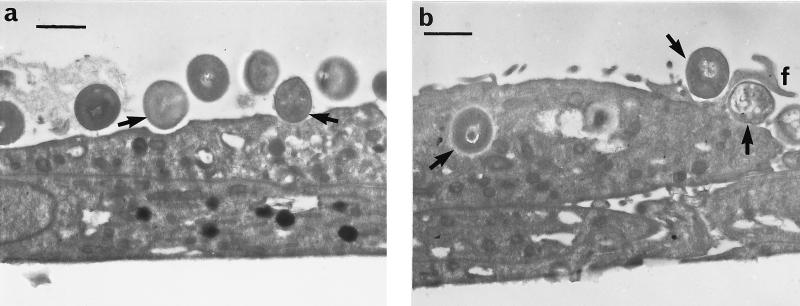
Internalization was confirmed by transmission electron microscopy (Fig. 3). Normal human osteoblasts were seeded at 50,000 cells per well onto Nunc eight-well chamber slides (Gibco) in a 500-μl volume of growth medium and cultured overnight. Internalization was tested by the standard assay except that all washes and changes of media were in 500-μl volumes. Following internalization, cells were fixed in situ with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C for 3 h and postfixed in 1% osmium tetroxide at 4°C for 2 h. Slides were then fractured for further processing. Finally, samples were dehydrated in a graded series of alcohol (20 to 90%) washes, infiltrated with L.R. White resin, and then embedded in L.R. White at 0°C. Prior to viewing, slides were removed from resin blocks and sections were taken at 90 to 100 nm. The sections were stained with uranyl acetate and lead citrate and viewed in a JEOL 100CX transmission electron microscope. Figure 3a shows S. aureus interacting with the osteoblast cell surface, and Fig. 3b demonstrates the internalization of S. aureus by osteoblasts.
FIG. 3.
Transmission electron micrographs of normal human osteoblasts infected with S. aureus NCTC 6571. Bars represent 1 μm. (a) S. aureus interacting with the osteoblast cell surface are arrowed. (b) Internalized S. aureus and S. aureus associated with filopodia (f) are arrowed.
Internalization in the presence of inhibitors.
Internalization assays were performed as described above in the presence of either 10 μM colchicine, 20 μM nocodazole, 250 μM monodansylcadaverine, 250 μM ouabain, 71 μM cycloheximide, 2 μM cytochalasin D, 40 μM monensin (each added 1 h prior to the addition of bacteria), or 31 or 310 μM chloramphenicol (added together with bacteria). All inhibitors were obtained from Sigma, and the concentrations used were based on previous reports of similar studies investigating bacterial internalization by cultured epithelial cells (24–26). Osteoblast viability following incubation with these inhibitors, for the maximum period used in internalization assays, was confirmed by staining with 0.2% (wt/vol) trypan blue. Viability was estimated by counting approximately 100 cells and calculating the percentage of cells excluding the stain. In both control (no inhibitor) and test samples, ∼95% osteoblast viability was observed.
The effect of inhibitors on S. aureus uptake was tested for significance by the two-tailed t test. Data from three separate experiments were collated (n = 18, df = 16), and control and test samples compared in SPSS for Windows version 7.5.1 (SPSS Inc., Chicago, Ill.). A P value of <0.05 was taken as significant.
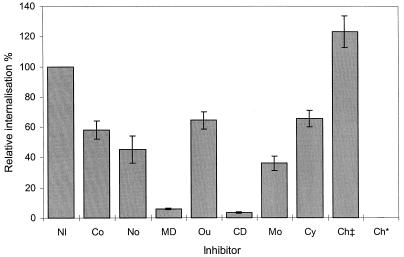
Inhibition of protein synthesis suggested a requirement for eukaryotic, but not bacterial, de novo protein synthesis for efficient staphylococcal internalization. Preincubation of osteoblasts with 71 μM cycloheximide reduced S. aureus internalization by ∼35% (Fig. 4), an effect greater than that seen with uptake of Proteus mirabilis strains by different epithelial cell lines (25), although this did not prove statistically significant (P = 0.081). In contrast, internalization of S. aureus appeared to be inhibited by >99% upon the addition of 310 μM chloramphenicol to the medium (Fig. 4), suggesting that de novo bacterial protein synthesis is critical for efficient internalization by osteoblasts. However, in separate experiments S. aureus was incubated at ∼3 × 106 cells per ml in assay medium with and without 31, 155, or 310 μM chloramphenicol. At 31 μM, >80% of the initial inoculum was recovered, but at 155 and 310 μM, viability was reduced by >90 and >95%, respectively. Chloramphenicol had no inhibitory effect on internalization at 31 μM (Fig. 4). In previous studies, internalization of P. mirabilis and Klebsiella pneumoniae was greatly reduced in the presence of chloramphenicol (25, 26). These data suggest that the S. aureus internalization pathway mainly exploits preexisting cellular components. However, it is possible that at 31 μM, chloramphenicol had no apparent effect on protein synthesis in S. aureus. Indeed, reported studies of bacterial internalization used 310 μM chloramphenicol (25, 26), a concentration that was not found to be toxic to the organisms.
FIG. 4.
Relative internalization of S. aureus NCTC 6571 into the human osteoblast cell line MG-63 either in the absence of any inhibitor (NI; defined as 100%) or in the presence of 10 μM colchicine (Co), 20 μM nocodazole (No), 250 μM monodansylcadaverine (MD), 250 μM ouabain (Ou), 2 μM cytochalasin D (CD), 40 μM monensin (Mo), 71 μM cycloheximide (Cy), or 31 or 310 μM chloramphenicol (Ch‡ or Ch*, respectively). Data are representative of experiments repeated at least three times in triplicate and are given as the means and standard deviations of the means. Note that inhibitors were assayed in various combinations, not all simultaneously.
Coated pit formation was inhibited with the addition of either 250 μM monodansylcadaverine or 250 μM ouabain to S. aureus–MG-63 cocultures, resulting in ∼95 and ∼35% (P = 0.023) reductions in uptake, respectively (Fig. 4). The former compound inhibits transglutaminase, which interferes with receptor recycling (8, 32), while the latter blocks Na+/K+-ATPase, causing the arrest of coated pit formation by inhibition of the interaction of clathrin and adapter proteins (14, 18). If coated pits per se were critical for internalization, one might expect to see similar inhibitory effects with both of these compounds. The observed difference in the ability of monodansylcadaverine and ouabain to inhibit internalization suggests that transglutaminase inhibition might have some other effect on osteoblast function which is responsible for S. aureus internalization.
Primary amines and ionophores inhibit endosome acidification (9, 20) and can affect receptor recycling (3, 31). Inclusion of monensin, which has an inhibitory effect on low-density lipoprotein receptor recycling (3), at a concentration of 40 μM in the assay medium reduced S. aureus internalization by ∼65% (Fig. 4; P < 0.001). It can be inferred from the above data that receptor recycling is of major import for efficient staphylococcal internalization by osteoblasts and that the process is not totally dependent on clathrin-coated vesicles. That internalization of osteoblasts by S. aureus likely involves receptor-mediated endocytosis is also supported by the observation of filopodia under electron microscopic examination (Fig. 3b).
Microfilament dependence is a characteristic of many systems of bacterial internalization (1, 4, 5, 21, 25, 26) but not all (24). To assess whether microfilaments are required for osteoblasts internalization of S. aureus, internalization assays were performed in the presence of the microfilament-depolymerizing agent cytochalasin D (2 μM), which reproducibly inhibited the internalization of S. aureus by ∼95% (Fig. 4). Similar results have been reported for S. aureus uptake by epithelial and endothelial cells (1, 4, 21). It is interesting that while depolymerization of microfilaments drastically reduces staphylococcal internalization, there appears to be less dependence on the formation of clathrin-coated vesicles in the light of recent reports which suggest a role for microfilaments in clathrin-dependent endocytosis (10, 12), a process which can be inhibited by cytochalasin D.
Depolymerization of microtubules by 10 μM colchicine reduced uptake of S. aureus by ∼40% (Fig. 4; P = 0.002). Nocodazole (20 μM) was marginally more effective at blocking internalization, reducing uptake by ∼55% (Fig. 4; P = 0.001). Although a significant reduction in internalization, the mechanism of uptake of S. aureus would appear to be more dependent on intact microfilaments.
We do not understand how S. aureus causes the destruction of the skeleton when it infects bone. However, it has been shown that this organism is internalized by chick osteoblasts both in vitro (16) and, apparently, in vivo (27), which suggests a possible pathogenic mechanism. The data presented here support these findings and indicate that internalization of S. aureus by human osteoblasts occurs via a receptor-mediated pathway. This process appears to be heavily dependent on the presence of intact actin filaments but less dependent on either clathrin-coated vesicles or microtubules. There also seems to be some requirement for osteoblast protein synthesis in this process. Further characterization of the mechanisms involved in this process will be useful in understanding staphylococcal bone disease and should enable the development of novel therapeutic strategies. It is possible that internalization of S. aureus by osteoblasts protects the organism from antibiotic therapy. Additionally, intracellular S. aureus induce apoptosis in epithelial and endothelial cells (4, 21, 33), and there is the possibility that intracellular S. aureus can also lead to osteoblast cell death. Investigations are now under way to determine the fate of osteoblasts following internalization of S. aureus. Induction of osteoblast death in vivo might lead to a reduction in the formation of bone matrix and a disruption of the homeostatic balance between osteoblasts and osteoclasts. Such a phenomenon could help explain the bone loss observed during S. aureus infection.
Acknowledgments
This work was supported by the Wishbone Trust.
We gratefully acknowledge Gary Best (Medical College of Georgia, Augusta) for providing the clinical isolates of S. aureus strains 15 and 16 and the type strain SMH.
REFERENCES
- 1.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Arora M, Shah N, Meghji S, Henderson B, Harris M, Nair S, Wilson M, Gray C M, Jones S J, Boyde A. Effect of Staphylococcus aureus extracellular proteinaceous fraction in an isolated osteoclastic resorption assay. J Bone Miner Metab. 1998;16:158–161. [Google Scholar]
- 3.Basu S K, Goldstein J L, Anderson R G, Brown M S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981;24:493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- 4.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekhuizen H, Van De Gevel J S, Olsson B, Van Benten I J, Van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 6.Cimmino M A. Recognition and management of bacterial arthritis. Drugs. 1997;54:50–60. doi: 10.2165/00003495-199754010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Clark B A, Rissing J P, Buxton T B, Best N H, Best G K. The effect of growth temperature on Staphylococcus aureus binding to type I collagen. Microb Pathog. 1994;17:239–251. doi: 10.1006/mpat.1994.1069. [DOI] [PubMed] [Google Scholar]
- 8.Davies P J A, Davies D R, Levitzki A, Maxfield F R, Milhaud P, Willingham M C, Pastan I H. Transglutaminase is essential in receptor mediated endocytosis of α2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- 9.Dean R T, Jessup W, Roberts C R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984;217:27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrbach A, Louvard D, Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996;109:457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg D L, Reed J I. Bacterial arthritis. N Engl J Med. 1985;312:764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassmé H U C, Ireland R M, Van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen S H, Sandvig K, Van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho G. Bacterial arthritis. In: McCarty D J, Koopman W P, editors. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1993. pp. 2003–2024. [Google Scholar]
- 16.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe H L. Skeletal lesions caused by certain other infectious agents. In: Jaffe H L, editor. Metabolic, degenerative and inflammatory diseases of bone and joints. Philadelphia, Pa: Lea & Febiger; 1972. pp. 1015–1031. [Google Scholar]
- 18.Larkin J M, Brown M S, Goldstein J L, Anderson R G W. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 19.Lerner U H, Sundqvist G, Ohlin A, Rosenquist J B. Bacteria inhibit biosynthesis of bone matrix proteins in human osteoblasts. Clin Orthop Relat Res. 1998;346:244–254. [PubMed] [Google Scholar]
- 20.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 21.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair S, Song Y, Meghji S, Reddi K, Harris M, Ross A, Poole S, Wilson M, Henderson B. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Miner Res. 1995;10:726–734. doi: 10.1002/jbmr.5650100509. [DOI] [PubMed] [Google Scholar]
- 23.Nair S P, Meghji S, Wilson M, Nugent I, Ross A, Ismael A, Bhudia K, Harris M, Henderson B. Clinical isolates of Staphylococcus aureus have osteolytic surface proteins and a proportion of the population have antibodies that block this activity: is this of prognostic significance? Br J Rheumatol. 1997;36:328–332. doi: 10.1093/rheumatology/36.3.328. [DOI] [PubMed] [Google Scholar]
- 24.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oelschlaeger T A, Tall B A. Uptake pathways of clinical isolates of Proteus mirabilis into human epithelial cell lines. Microb Pathog. 1996;21:1–16. doi: 10.1006/mpat.1996.0037. [DOI] [PubMed] [Google Scholar]
- 26.Oelschlaeger T A, Tall B A. Internalization of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reilly S S, Ramp W K, Zane S F, Hudson M C. Internalization of Staphylococcus aureus by embryonic chicken osteoblasts in vivo. J Bone Miner Res. 1997;12:S231. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 28.Ross A C. Infected arthroplasties. Curr Opin Rheumatol. 1991;3:628–633. doi: 10.1097/00002281-199108000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Rydén C, Tung H S, Nikolaev V, Engström Å, Oldberg Å. Staphylococcus aureus causing osteomyelitis binds to a nonapeptide sequence in bone sialoprotein. Biochem J. 1997;327:825–829. doi: 10.1042/bj3270825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schramm N, Wyrick P B. Cytoskeletal requirements in Chlamydia trachomatis infection of host cells. Infect Immun. 1995;63:324–332. doi: 10.1128/iai.63.1.324-332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz A L, Bolognesi A, Fridovich S E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984;98:732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Leuven F, Cassiman J J, Van Den Berghe H. Primary amines inhibit recycling of α2M receptors in fibroblasts. Cell. 1980;20:37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- 33.Wesson C A, Liou L E, Todd K M, Bohach G A, Trumble W R, Bayles K W. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect Immun. 1998;66:5238–5243. doi: 10.1128/iai.66.11.5238-5243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiest P M, Johnson J H, Flanigan T P. Microtubule inhibitors block Cryptosporidium parvum infection of a human enterocyte cell line. Infect Immun. 1993;61:4888–4890. doi: 10.1128/iai.61.11.4888-4890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao L, Bengualid V, Lowy F D, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]