Abstract
Infected coronary aneurysms are rare in clinical practice and represent a catastrophic complication following percutaneous coronary interventions. A high index of suspicion is imperative in patients presenting with fever a few weeks after percutaneous coronary intervention. Early diagnosis and surgical repair are key to mitigating the morbidity and mortality associated with it.
Key Words: drug-eluting stent, percutaneous coronary intervention, pseudoaneurysm, stent fracture
Graphical Abstract
Infected coronary aneurysms following percutaneous coronary interventions (PCIs) are rare and have only been described in case reports. Despite its low prevalence, its development predicts future catastrophic complications if not promptly detected and treated. They are believed to have multifactorial origins including breach of sterility during stent delivery, improper intervention techniques that lead to vessel wall injury, and possible impairment of host defenses by the antiproliferative agent. We describe a case of giant infected pseudoaneurysms of the left anterior descending (LAD) artery that had a delayed presentation 12 months after PCI.
Take-Home Messages
-
•
Infective complications following a PCI are life-threatening and require a high degree of suspicion for early diagnosis.
-
•
Multimodality imaging should be used in such cases, and timely intervention is essential to reduce the morbidity and mortality associated with it.
History of presentation
A middle-aged man in his 50s presented to the emergency room with complaints of progressively worsening angina (Canadian Cardiovascular Society grade III) and dyspnea (NYHA functional class III) for the past week along with episodes of high-grade fever with chills for 5 days. On physical examination, he was febrile with a body temperature of 102 oF, heart rate of 110 beats/min, and blood pressure of 136/70 mm Hg. Cardiovascular examination was unremarkable and respiratory system examination revealed occasional fine crackles in bilateral basal lung fields.
Past medical history
He was diabetic and hypertensive on oral therapy with a history of PCI to the mid segment of the LAD with a 3.5 × 23 mm Xience Prime (Abbott Vascular) drug-eluting stent (DES) 1 year ago when he had an acute anterior wall myocardial infection.
Investigations
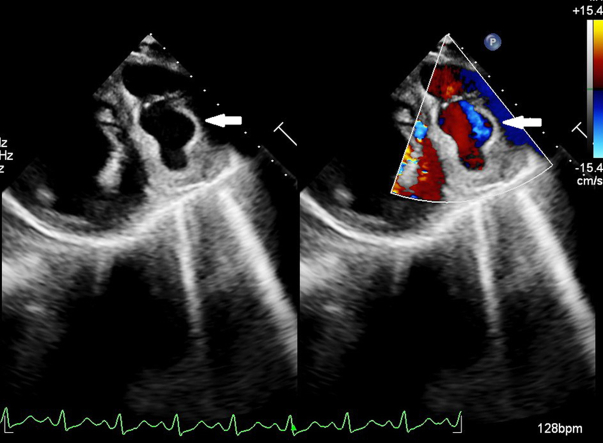
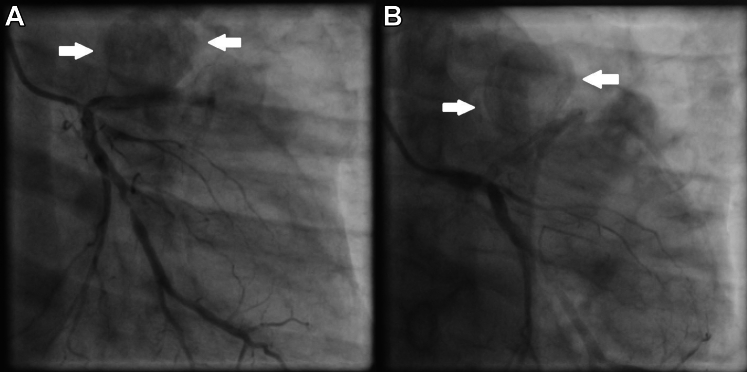
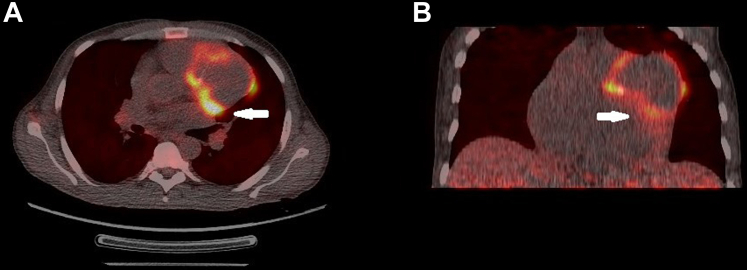
His laboratory investigations were unremarkable apart from leukocytosis with predominant neutrophilia (total leukocyte count: 19,500/mm3, 86% neutrophils) and raised inflammatory markers (CRP 47.1 mg/L, procalcitonin 63.4 ng/mL). All initial blood cultures were negative. Echocardiographic examination revealed a dilated left ventricle with significant hypokinesia of apical, anteroseptal, and anterolateral segments with an ejection fraction of 30% (by modified Simpson’s method) with no evidence of infective endocarditis. Further careful examination revealed a large vascular sac with to-and-fro blood flow (Yin-Yang sign) near the anterior left ventricular wall suggestive of pseudoaneurysm (Figure 1). The pseudoaneurysm was best visualized in parasternal short-axis view with exaggerated anterior tilt. The flow into the pseudoaneurysm could only be appreciated at low aliasing velocities suggestive of possible coronary origin. A coronary angiogram confirmed multiple large pseudoaneurysms arising from the stented LAD segment (Figure 2, Video 1). The LAD stent was patent with a de novo 95% stenosis of the major obtuse marginal (OM) artery. An 18F–fluorodeoxyglucose (FDG) positron emission tomography showed intense FDG uptake in LAD (maximum standard uptake value: 12.2) with the largest outpouching measuring 6.1 × 4.2 cm and hyperdense collection within (Figure 3).
Figure 1.
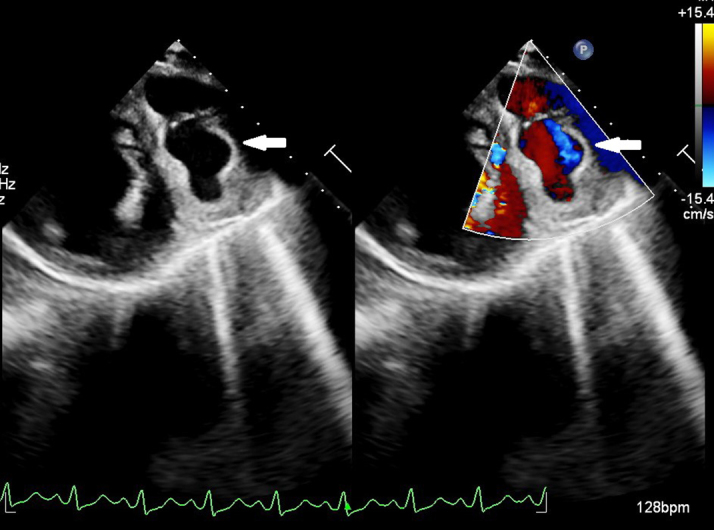
Transthoracic Echocardiogram
Transthoracic echocardiography (short-axis view) in color compare mode showing a vascular sac with to-and-fro blood flow suggestive of a pseudoaneurysm (white arrow).
Figure 2.
Coronary Angiogram
Coronary angiogram of the left coronary system showing pseudoaneurysms (white arrows) from stented left anterior descending artery segment. (A) Right anterior oblique 20o, caudal angulation projection (CAU) 20o. (B) Left anterior oblique 0o, CAU 30o.
Figure 3.
FDG–Positron Emission Tomography
Fluorodeoxyglucose (FDG) positron emission tomography showing intense FDG uptake in the left anterior descending artery with the largest pseudoaneurysm measuring 6.1 × 4.2 cm (white arrow). (A) Axial section. (B) Coronal section.
Diagnosis and management
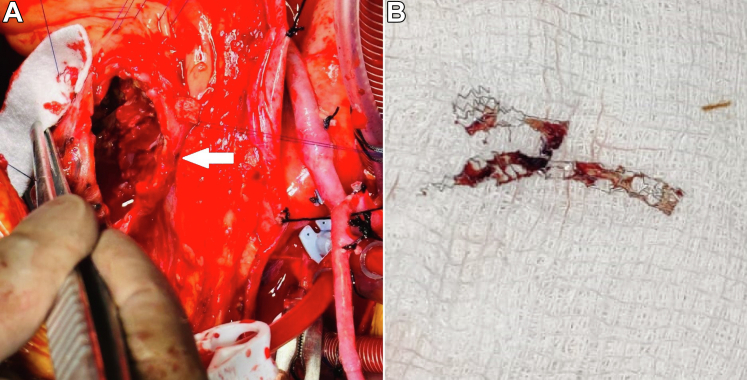
A diagnosis of infected pseudoaneurysms of the LAD was made and the patient received empirical intravenous ceftriaxone and vancomycin. The institute's heart team devised a surgical plan involving bypass grafting of the LAD and major OM and resection of the infected pseudoaneurysms. The mediastinum was accessed through a median sternotomy and the patient was put on cardiopulmonary bypass. Dense adhesions were encountered within the pericardium that warranted extensive adhesiolysis followed by anastomosis of a right saphenous vein graft to the mid-segment of the LAD. The pseudoaneurysms were exposed; dense purulent debris and multiple clots were drained from the sacs (Figure 4A). The DES was extracted only to reveal a fractured scaffold (Figure 4B). This was followed by the plication of the pseudoaneurysms over a Teflon felt. A bypass graft to OM major was attempted but could not be performed because of extensive adhesions and hence was deferred for future percutaneous revascularization.
Figure 4.
Intraoperative Findings
(A) Intraoperative image showing exposed pseudoaneurysms (white arrow) with right saphenous vein graft to the left anterior descending artery. (B) Extracted drug-eluting stent showing fractured scaffold.
Outcome
Although the patient was successfully weaned off the cardiopulmonary bypass and shifted out of the operating room in a hemodynamically stable condition on minimal inotropic support, he developed refractory vasoplegia and a complete renal shutdown necessitating renal replacement therapy during the postoperative period. His hemodynamic parameters continued to dwindle despite intra-aortic balloon pump support with rapidly escalating inotropic requirements. A transthoracic echocardiogram revealed markedly reduced cardiac activity and the patient ultimately expired on the second postoperative day.
Discussion
Infected coronary artery pseudoaneurysms are rarely encountered in clinical practice with a reported incidence of 0.3% to 0.6% following PCI and present unique challenges in both diagnosis and management.1 If untreated, they may present with further complications in the form of rupture, thrombosis, embolization, cardiac tamponade, fistula formation, and myocardial ischemia.2 Hence, their prompt identification and early treatment is imperative to prevent catastrophic outcomes. Although the time interval between the primary intervention and presentation ranges from a few weeks to months in most reported cases,3 this case shows a late presentation occurring after a year from PCI.
Restrepo et al2 described a “two-hit” hypothesis for its pathogenesis—the first hit involving compromised vessel wall integrity due to atherosclerosis, connective tissue disease, trauma, or angioplasty techniques involving atherectomy devices, oversized balloons or stents, and high-pressure balloon inflations; the second hit being infiltration of the weakened segment by direct, hematogenous, or embolic spread of microbial pathogens.2 Certain procedure-related risk factors include difficult vascular access, multiple punctures, multiple catheter exchanges through the same arterial sheath, long procedure time, delayed removal of arterial sheaths, presence of heart failure, and unattended access site hematomas.4 DES (particularly, sirolimus-eluting) have a higher degree of association with pseudoaneurysm formation as the antiproliferative agent, apart from preventing re-stenosis, impairs local host defences, and delays endothelisation.5 This creates a nidus for potential microbial sustenance in predisposed people. In our case, no specific risk factors could be identified prima facie; however, the stent extracted intraoperatively was found fractured and it may be hypothesized that high-pressure balloon inflation might have led to stent fracture, vessel wall injury, and subsequent pseudoaneurysm formation.
The diagnosis of this entity demands a high index of suspicion in patients presenting with fever (54.7%) after a coronary intervention; however, some may present with complaints of angina (34%), dyspnea (9.4%), or acute myocardial infarction (7.5%).2 It is mostly detected on coronary angiograms and computed tomography; however, larger aneurysms may also be identified on transthoracic or transesophageal echocardiography.6 Intravascular imaging may further show the mechanism of such complications; however, the introduction of multiple catheters into an infected segment poses a risk of worsening bacteremia.1 Nuclear scans using gallium or FDG have also gained popularity due to the added advantage of detecting infection/inflammation in aneurysms.7 Multimodality imaging is often essential to establish a diagnosis and devise an appropriate management strategy as can be elicited from our case.
Bacterial cultures in such patients, either from blood or resected specimens, have found Staphylococcus aureus (53.3%) as the most common causative agent.2,8 Isolation of an organism enables targeted therapy and hence improves outcomes. In our case, no single causative organism could be isolated, and the patient was treated empirically with intravenous ceftriaxone and vancomycin. The ideal duration of treatment with antibiotics also warrants further research and current evidence advocates at least 4 to 8 weeks of unhindered parenteral antibiotic therapy to prevent recurrence.2,6 Although prophylactic antibiotic therapy in coronary interventions has shown promise in observational studies,9 there is currently no recommendation for its routine use to prevent such complications.
The ideal mode of treatment is not well-defined and surgical approach has been the most advocated form of management. Although smaller aneurysms <1 cm may be conservatively managed with antibiotic therapy alone, larger aneurysms require isolation of the sac to prevent its enlargement and consequent rupture.6 Ligation and excision of the sac after extraction of the stent scaffold and other infected debris followed by bypass grafting of the involved vessel has been the most favored surgical technique in the literature.2,3,5 Although some investigators have also used covered stents to exclude such aneurysms, it is mostly discouraged due to the risk of covered stent infection as well as aneurysmal rupture.10
The prognosis of infected coronary artery aneurysms hinges on numerous factors, including the extent of coronary artery involvement, the severity of infection, and the promptness of appropriate therapy. Despite advancements in medical and surgical techniques, the condition carries a notable risk for morbidity and mortality, especially in cases complicated by septic embolism, myocardial infarction, or heart failure. In a series of 23 infected coronary pseudoaneurysms, Lim et al11 reported a mortality rate of 43.5%. In our case, it is postulated that the sudden release of endotoxins in the bloodstream might have led to refractory vasoplegia and subsequent hemodynamic instability leading to an unfavorable outcome despite all possible efforts. However, in cases with favorable outcomes, sustained long-term follow-up is indispensable to prevent future recurrence.
Conclusions
Infected coronary aneurysms pose a formidable challenge to clinicians due to their rarity and complex clinical nature. Early recognition, comprehensive evaluation, and timely intervention are essential for improving patient outcomes and mitigating the risk of complications. However, the current experience is limited, and further research is warranted to devise an ideal treatment protocol for its management. Our case shows this potentially devastating outcome of coronary stent infection and highlights the various measures to counter the morbidity and mortality associated with it.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Coronary angiogram showing multiple pseudoaneurysms arising from stented left anterior descending artery segment with a 95% stenosis in major obtuse marginal artery (right anterior oblique 20o caudal angulation projection 20o, left anterior oblique 0o cranial angulation projection 30o).
References
- 1.Aoki J., Kirtane A., Leon M.B., Dangas G. Coronary artery aneurysms after drug-eluting stent implantation. JACC Cardiovasc Interv. 2008;1(1):14–21. doi: 10.1016/j.jcin.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Restrepo C.S., Gonzalez T.V., Baxi A., Rojas C.A. Infected (“mycotic”) coronary artery aneurysm: systematic review. J Cardiovasc Comput Tomogr. 2020;14(6):e99–e104. doi: 10.1016/j.jcct.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Le M.Q., Narins C.R. Mycotic pseudoaneurysm of the left circumflex coronary artery: a fatal complication following drug-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(4):508–512. doi: 10.1002/ccd.21014. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka G., Nakano K., Asano R., Sato A., Kodera K., Tatsuishi W. Purulent pericardial effusion and mycotic pseudoaneurysm following insertion of a bare metal stent. J Card Surg. 2015;30(5):433–435. doi: 10.1111/jocs.12530. [DOI] [PubMed] [Google Scholar]
- 5.Furtado A.D., Bhat S.P.S., Peer S.M., Chikkatur R. Infected pseudoaneurysm involving a drug-eluting stent. Interact Cardiovasc Thorac Surg. 2011;12(4):636–638. doi: 10.1510/icvts.2010.257337. [DOI] [PubMed] [Google Scholar]
- 6.Kukkar V., Kapoor H., Aggarwal A. Mycotic and non-mycotic coronary artery aneurysms — a review of the rarity. J Clin Imaging Sci. 2022;12:13. doi: 10.25259/JCIS_218_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husmann L., Huellner M.W., Gruenig H., et al. Impact of PET/CT among patients with suspected mycotic aortic aneurysms. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0258702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldblatt J., Doi A., Negri J., Nanayakkara S., McGiffin D. Mycotic pseudoaneurysms of the coronary arteries. J Card Surg. 2015;30(7):555–559. doi: 10.1111/jocs.12563. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan A.M., Kundu S., Sacks D., et al. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol. 2010;21(11):1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Crimm H.A., Reoma J.L., Gallagher R.M. Mycotic aneurysm of the proximal LAD successfully excluded with covered stents. Int J Cardiovasc Imaging. 2020;36(11):2299–2300. doi: 10.1007/s10554-020-01930-5. [DOI] [PubMed] [Google Scholar]
- 11.Lim C.P., Ho K.L., Tan T.T., et al. Infected coronary artery pseudoaneurysm after repeated percutaneous coronary intervention. Ann Thorac Surg. 2011;91(2):e17–e19. doi: 10.1016/j.athoracsur.2010.10.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary angiogram showing multiple pseudoaneurysms arising from stented left anterior descending artery segment with a 95% stenosis in major obtuse marginal artery (right anterior oblique 20o caudal angulation projection 20o, left anterior oblique 0o cranial angulation projection 30o).