Abstract
A 63-year-old woman with history of mediastinal radiation was admitted for decompensated heart failure due to severe post-radiation aortic and mitral valve disease. To reduce cardiopulmonary bypass time and postoperative pulmonary complications, she underwent a hybrid approach with robotic mitral valve replacement and transcatheter aortic valve replacement.
Key Words: post-radiation valvulitis, robotic mitral valve surgery, TAVR
Graphical Abstract
History of Presentation
A 63-year-old woman was admitted to Massachusetts General Hospital with worsening shortness of breath and dyspnea on exertion. She was diagnosed with hypoxic respiratory failure due to pulmonary edema and bilateral lower limb edema in the setting of decompensated congestive heart failure.
Take-Home Messages
-
•
This case illustrates the benefit of a collaborative heart team approach in high-risk and complex patients with double valve pathology (eg, those with radiation valve disease).
-
•
A hybrid approach to double valve replacement not only avoids sternotomy and reduces cardiopulmonary bypass time, but also allows high-risk patients an earlier recovery and a faster return to an active lifestyle.
Past Medical History
The patient’s medical history includes non-Hodgkin lymphoma treated with mediastinal radiation at 20 years of age, dyslipidemia, hypertension, and hypothyroidism. In 2020, the patient had a node-negative T3a melanoma resected from her back. During hospitalization in 2023, multiple bone lesions in the pelvis and tibia were found. However, the biopsy results were negative for infection or malignancy.
Differential Diagnosis
The initial differential diagnoses included decompensated heart failure from degenerative valve disease, rheumatic heart disease, or valve-related cardiomyopathy. Considering the patient’s radiation history, the most likely diagnosis was acute on chronic decompensated heart failure due to radiation valvulitis and restrictive radiation-related cardiomyopathy.
Investigations
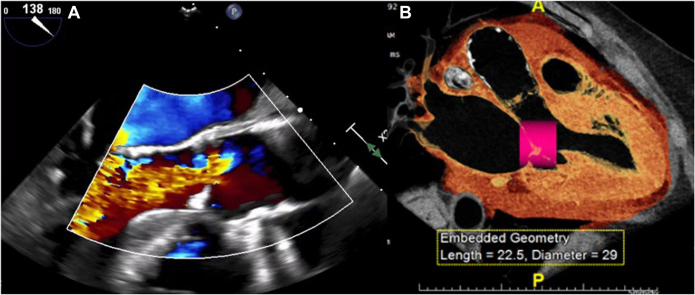
The patient’s transthoracic echocardiography and transesophageal echocardiography (TEE) demonstrated mixed aortic and mitral valve disease. Calcific mitral stenosis had a mean gradient of 10 mm Hg with mild annular calcification, whereas moderate calcific aortic stenosis had a mean gradient of 28 mm Hg with severe regurgitation (Figure 1A). The left ventricular ejection fraction was reduced at 40%.
Figure 1.
Preoperative Multimodal Imaging
(A) Preoperative transesophageal echocardiography demonstrating severe aortic regurgitation. (B) Preoperative computed tomography angiography image of simulated transcatheter mitral valve replacement indicating high risk of left ventricle outflow tract obstruction.
The pulmonary function test revealed reduced forced expiratory volume in one second (FEV1) and FVC (forced vital capacity) with a normal FEV1/FVC ratio, suggesting a restrictive ventilatory deficit. Chest radiograph showed no evidence of pneumonia, pleural effusion, or pulmonary edema. The size and contours of the heart and mediastinum were unremarkable, except for mural calcifications throughout the aortic arch.
Management
The patient’s history of radiation exposure made her a high-risk candidate for extensive open-heart surgery, which would have required prolonged cardiopulmonary bypass (CPB) with a subsequent risk of recalcitrant pleural effusions and slow recovery (Table 1). Therefore, the patient was evaluated by a structural heart team for potential transcatheter mitral valve replacement and transcatheter aortic valve replacement (TAVR) procedures. Computed tomography angiography showed an aortic valve calcium score of 848 with involvement of the aortomitral continuity. The transthoracic echocardiography analysis revealed an acute aorto-mitral angle of 120°, which in the setting of the patient’s upper septal hypertrophy, significantly increased the predicted risk of left ventricle outflow tract obstruction after transcatheter mitral valve replacement (Figure 1B).
Table 1.
Examples of Selection Criteria for Candidates for Hybrid Double Valve Replacement
| Patient with radiation valvulitis |
| High-risk patients requiring double valve replacement |
| Redo sternotomy cases with technically challenging re-entry |
Therefore, the operative plan involved robotic mitral valve replacement via a right mini thoracotomy while addressing the aortic valve with a TAVR procedure, thus reducing CPB time. After the patient’s induction, a right groin incision was made and both the right femoral artery and vein were cannulated using the Seldinger technique. A 6-cm right lateral mini-thoracotomy was performed over the fourth intercostal space, and robotic ports were placed. A cross clamp was applied, and the heart was arrested with antegrade and retrograde cardioplegia. Due to the presence of severe aortic insufficiency, a purse-string suture was placed around the coronary sinus ostium and retrograde cardioplegia catheter was pulled back to optimize biventricular myocardial protection.
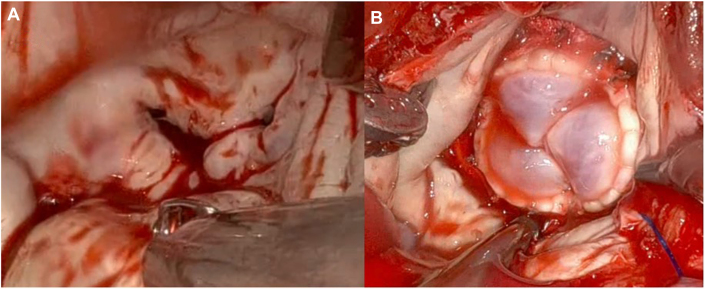
Once the heart had arrested, the left atrium was opened via Sondergaard’s groove. On inspection, mild posterior mitral annular calcification was observed along with diffusely calcified anterior and posterior leaflets, consistent with radiation valve disease (Figure 2A). The anterior leaflet was resected in preparation for valve replacement. A 27-mm Abbott Epic porcine tissue valve was implanted in a standard fashion (Figure 2B).
Figure 2.
Surgical Images of Mitral Valve Replacement
(A) Intraoperative image of the mitral valve consistent with radiation valvulitis. (B) Implanted mitral valve bioprosthesis (27-mm Abbott Epic Porcine Valve).
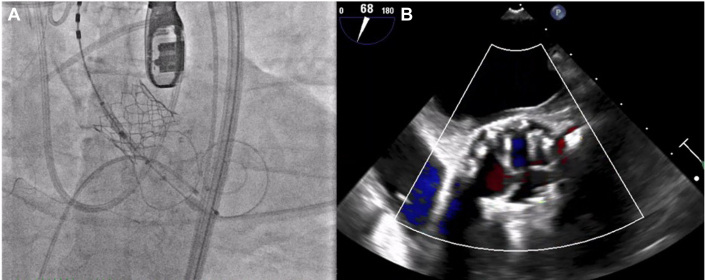
The patient was successfully weaned from CPB, and the valve was found to be competent on the post-bypass TEE. Mini-thoracotomy was closed in a standard fashion, and the patient was decannulated from the peripheral CPB. The total aortic clamp time was 158 minutes, and the CPB time was 362 minutes. Then, a transfemoral TAVR procedure was performed in a standard fashion deploying a 23-mm Edwards SAPIEN RESILIA tissue valve under rapid ventricular pacing (Figure 3A).
Figure 3.
Transcatheter Aortic Valve Replacement Deployment
(A) Deployed transcatheter aortic valve replacement (TAVR) (23-mm Edwards SAPIEN 3 Ultra RESILIA Valve) alongside the implanted bioprosthetic mitral valve replacement. (B) Postoperative transesophageal echocardiography demonstrating no significant paravalvular leak after TAVR deployment.
Final postoperative TEE confirmed well-seated mitral valve bioprosthesis without any paravalvular leaks. Peak and mean gradients were 5 and 3 mm Hg, respectively. TEE also confirmed a good position of the TAVR with no significant paravalvular leaks (Figure 3B). Peak and mean gradients were measured at 7 and 4 mm Hg, respectively. The patient was transferred to the intensive care unit in a stable condition.
Postoperatively, the patient recovered well and was transferred from the intensive care unit to a step-down unit on postoperative day (POD) 4. Prior to transfer, right-sided pleural chest tubes were removed without return of pleural effusions. On POD 7, the patient underwent pacemaker implantation due to a widening left bundle branch block in the setting of the first-degree atrioventricular block. The patient continued to recover well and was discharged home on POD 10.
Early postoperative transthoracic echocardiography showed well-seated valves with trivial posterior paravalvular leak around the implanted TAVR. Peak and mean gradients of the aortic valve bioprosthesis were measured at 19 and 9 mm Hg, whereas peak and mean transmitral gradients were 10 and 5 mm Hg, respectively. The left ventricle outflow tract velocity was initially measured at 1.5 m/s and it reduced itself to 1.1 m/s on a follow-up echocardiography. Left ventricular ejection fraction had improved from 40% to 55%. At 1-month follow-up, both bioprostheses were functioning well. Follow-up chest radiograph showed trace right and mild left pleural effusions, which appeared improved (Figure 4). The patient was asymptomatic, resumed work as an accountant, and had no hospital readmissions since the discharge.
Figure 4.
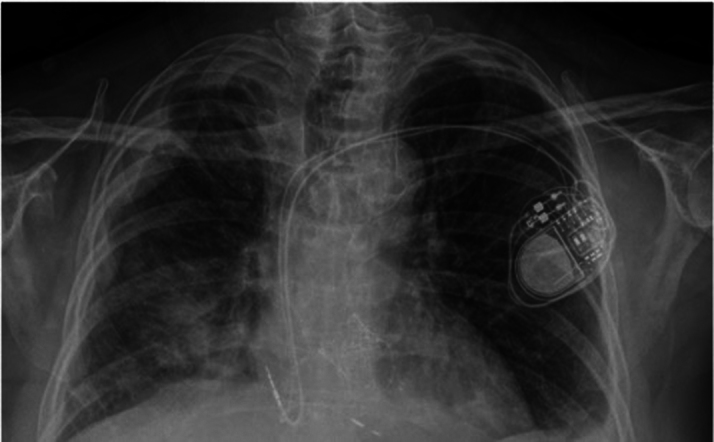
Postoperative Radiograph
Discussion
Extensive mediastinal radiation therapy used in treating Hodgkin and non-Hodgkin lymphomas significantly increases the risk of cardiovascular disease. This can result in delayed onset of coronary artery disease, cardiomyopathy, conduction abnormalities, pericardial constriction, and mixed valvular heart dysfunctions, collectively known as radiation valvulitis.1 Radiation also affects aortomitral curtain and aorta, leading to its significant calcification. Similar effects, although to a lesser extent, may occur with radiation therapy for breast and other thoracic malignancies.
Radiation exposure to the heart triggers fibroblast stimulation leading to increased collagen deposition, osteoblast-like differentiation, and calcium deposition. Despite the heart’s anterior position, studies indicate a higher incidence of left-sided valvular disease, implicating factors like flow dynamics and pressure alongside radiation dose.2 Fibrotic changes in the mediastinum contribute to restrictive lung disease, complicating surgical management and prognosis. Radiation-induced pleural effusion, driven by pulmonary fibrosis, vascular changes, and lymphatic obstruction, is a serious complication. Extensive calcification, especially in critical areas like the aorto-mitral curtain, raises operative complexity and mortality risk. Patients undergoing double valve replacement, possibly with aortomitral curtain reconstruction, often face prolonged recovery and recurrent pleural effusions due to compromised lymphatic drainage.3
After radiation therapy, guidelines recommend undergoing functional noninvasive stress tests and echocardiograms every 5 to 10 years, with more frequent assessments if symptoms or concerning indications arise sooner.
In terms of surgical management, formal guidelines are lacking due to limited data for robust analysis. However, recent studies have directly compared outcomes after TAVR and surgical aortic valve replacement in the post-radiation patient population. The findings commonly demonstrate lower mortality, postoperative complications, and length of stay with TAVR. This is predominantly attributed to poor wound healing in surgical aortic valve replacement and the necessity for concomitant procedures afterward (eg, coronary artery bypass grafting).4,5
According to the 2020 American Heart Association/American College of Cardiology guidelines6 on valvular heart disease, expanded eligibility criteria for TAVR have been presented, making TAVR a feasible alternative for high-, intermediate-, and low-risk patients as opposed to surgical aortic valve replacement.
Conclusions
Double valve replacement in the setting of post-radiation valvulitis is associated with a high surgical risk and can lead to a complicated and prolonged postoperative recovery. Post-radiation patients tend to have recalcitrant pulmonary effusions after a long CPB run.7,8 Therefore, staging such a lengthy operation into a mitral valve replacement via a right mini-thoracotomy approach followed by a TAVR can significantly reduce CPB time. It should then decrease the risk of intractable pleural effusions postoperatively and allow an earlier discharge and return to an active lifestyle. In summary, patients with a high surgical risk and unsuitable anatomy for a complete interventional approach can be treated using the presented hybrid approach, while decreasing surgical trauma and enabling quick recovery.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Brown J.A., Aranda-Michel E., Kilic A., et al. Impact of thoracic radiation on patients undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. 2022;34(1):136–143. doi: 10.1053/j.semtcvs.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Lee C., Hahn R T. Valvular heart disease associated with radiation therapy: a contemporary review. Struct Heart. 2022;7(2) doi: 10.1016/j.shj.2022.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salz T., Zabor E.C., De Nully Brown P., et al. Cardiovascular risk factors, radiation therapy, and myocardial infarction among lymphoma survivors. Acta Oncologica. 2022;61(9):1064–1068. doi: 10.1080/0284186X.2022.2107402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belzile-Dugas E., Eisenberg M.J. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdchi F., Hirji S.A., Nohria A., et al. Transcatheter compared with surgical aortic valve replacement in patients with previous chest-directed radiation therapy. JACC CardioOncol. 2021;3(3):397–407. doi: 10.1016/j.jaccao.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto C.M., Rick Nishimura C.C.A., Robert Bonow C.C.O., et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Belzile-Dugas E., Fremes S.E., Eisenberg M.J. Radiation-induced aortic stenosis: an update on treatment modalities. JACC Adv. 2023;2(1) doi: 10.1016/j.jacadv.2022.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauffal V., Bay C., Shah P.B., et al. Short-term outcomes of transcatheter versus isolated surgical aortic valve replacement for mediastinal radiation-associated severe aortic stenosis. Circ Cardiovasc Interv. 2021;14(2) doi: 10.1161/CIRCINTERVENTIONS.120.010009. [DOI] [PubMed] [Google Scholar]