Abstract
Background
Cardiovascular impairment has been observed in adults with coronavirus disease 2019 (COVID-19), even in those with mild symptoms. Physical activity can reveal subtle cardiovascular dysfunction that is not apparent at rest. However, there are limited data on cardiovascular function in children and adolescents after the COVID-19 infection. This study aimed to assess cardiovascular function in paediatric and adolescent populations with a history of COVID-19 infection and controls by conducting 2-dimensional transthoracic echocardiography at rest (TTE-R) and exercise stress echocardiography (ESE).
Methods
We conducted TTE-R, including speckle tracking strain analysis of both ventricles, on 100 individuals (median age 12.3 years, 82% male), divided into 2 groups: 73 adolescents with COVID-19 infection and 27 controls. A subset of male participants (40 cases, 15 controls) underwent ESE combined with a cardiopulmonary exercise test (CPET-ESE) to examine the relationship between cardiovascular parameters and contractile reserve. Myocardial contractile reserve was evaluated by measuring the maximum increase in strain values during exercise.
Results
At rest, no signs of myocardial injury or inflammation were observed. Right and left ventricular contractility in the infected group were clinically equivalent to those in the controls. During CPET-ESE, peak oxygen consumption was similar between the infected and control groups. Furthermore, contractile reserve under exercise was similar in both groups.
Conclusions
We found no significant differences in left ventricular systolic and diastolic function and right ventricle systolic function evaluated by TTE-R between participants with a history of mild COVID-19 infection and controls. ESE provided insights for post–COVID-19 young people resuming activities and sports.
Résumé
Contexte
Une atteinte cardiovasculaire a été observée chez des adultes atteints de la maladie à coronavirus 2019 (COVID-19), même en présence de symptômes légers. L’activité physique peut faire apparaître une dysfonction cardiovasculaire subtile non manifeste au repos. Les données sur la fonction cardiovasculaire d’enfants et d’adolescents ayant eu la COVID-19 sont toutefois limitées. Cette étude avait pour but de comparer la fonction cardiovasculaire d’enfants et d’adolescents ayant déjà contracté la COVID-19 à celle de témoins à partir des résultats d’une échocardiographie transthoracique bidimensionnelle au repos (ETT-R) et d’une échocardiographie à l’effort (EE).
Méthodologie
Cent personnes (âge médian : 12,3 ans, 82 % de sexe masculin), réparties dans deux groupes (73 adolescents ayant contracté la COVID-19 et 27 témoins) ont été évaluées par ETT-R, avec analyse de la déformation par un suivi des marqueurs acoustiques naturels (speckle tracking) des deux ventricules. Un sous-groupe de participants de sexe masculin (40 avec antécédents de COVID-19, 15 témoins) s’est prêté à une échocardiographie à l’effort couplée à un test d’effort cardiopulmonaire (TECP-EE) qui avait pour but d’examiner le lien entre les paramètres cardiovasculaires et la réserve contractile. La réserve contractile myocardique a été évaluée en mesurant l’augmentation maximale des valeurs de déformation à l’effort.
Résultats
Au repos, aucun signe d’atteinte ou d’inflammation myocardiques n’a été observé. La contractilité des ventricules gauche et droit des sujets ayant contracté la COVID-19 était cliniquement équivalente à celle des témoins. Durant le TECP-EE, la consommation maximale d’oxygène des sujets ayant contracté la COVID-19 et des témoins était semblable. De plus, la réserve contractile à l’effort était comparable dans les deux groupes.
Conclusion
Nous n’avons observé aucune différence significative en ce qui a trait à la fonction systolique et à la fonction diastolique du ventricule gauche ainsi qu’à la fonction systolique du ventricule droit évaluées par ETT-R entre les participants ayant présenté une forme légère de la COVID-19 et les témoins. L’EE a fourni de l’information concernant les jeunes ayant contracté la COVID-19 qui reprennent leurs activités et se remettent au sport.
Many children and adolescents have experienced mild (42%-54%) or no (15%-36%) upper respiratory tract symptoms due to coronavirus disease 2019 (COVID-19).1 However, COVID-19–related cardiovascular lesions have been observed in some patients.2 One proposed mechanism involves the virus entering cells via the angiotensin-converting enzyme 2 receptor, damaging cardiomyocytes and vascular endothelium. This damage may lead to myocardial inflammation, coronary plaque destabilization, and coagulopathy. In addition, cytokine storms can affect multiple organs and induce myocardial inflammation.3 In children and young adults, a multisystem inflammatory (MIS-C/-A) can occur 2-6 weeks after infection, sometimes accompanied by myocarditis.4 In addition, myocarditis has also been observed in the absence of MIS.4
Early in the pandemic, reports indicated potential COVID-19–related cardiac injury, with 15% of postinfection athletes showing signs of myocardial damage via cardiac magnetic resonance (CMR), even among those asymptomatic or with mild symptoms.5 Although the prevailing perspective is that most young people recover without serious cardiac sequalae,6 the long-term health consequences remain unclear. Although COVID-19 has become common, identifying masked cardiovascular problems related to the infection and preventing cardiovascular events in children and adolescents remains crucial.
Exercise stress echography (ESE) is crucial for assessing cardiovascular function and exercise tolerance and detecting subclinical valvular disease and heart failure. In paediatric cardiology, ESE combined with cardiopulmonary exercise testing (CPET) has been used to evaluate junior athletes7 and cancer survivors.8 This combination allows for quantified high-intensity exercise, heart rate (HR) elevation, and blood pressure (BP) and describes physiological responses.
Myocardial contractile reserve refers to the increase in left ventricular (LV) contractility during exertion compared with pre-exercise contraction. Factors such as myocardial blood flow and degeneration can reduce contractility.9 Speckle-tracking echocardiography strain values can detect potential myocardial dysfunction even in patients with preserved ejection fraction (EF),10 and their use in evaluating contractile reserve has been reported.9 These advantages contribute to risk assessment for individuals resuming daily activity and sports.
The Long-term Impact of COVID-19 on Cardiovascular and Cardiopulmonary Health and Quality of Life in Children and Adolescents (LICO) study aimed to provide subacute and chronic clinical follow-up of the cardiovascular health, physical performance, and quality of life after COVID-19. The main objective of this study was to assess differences in cardiovascular function in children and adolescents with a history of COVID-19 infection and controls, both at rest and during exercise. The study focused on male participants, particularly examining the relationship between cardiovascular parameters and contractile reserve measured by ESE.
Methods
Study design, participant, and inclusion criteria
This study was a prospective, cross-sectional, single-centre, observational study in Bavaria, Germany. From July 2021 to December 2022, participants were recruited through flyers and website advertisements. Study information was disseminated to sports clubs in Bavaria, and face-to-face recruitment was conducted with the permission of these organizations and participants’ guardians.
The inclusion criteria were as follows: (1) participants aged 8-17 years, of both sexes in good general health; (2) informed consent obtained from both participants and their guardians; (3) no acute symptoms of infection at the time of examination and a negative COVID-19 screening antigen test before the examination; and (4) for the COVID-19 infected group: participants with confirmed COVID-19 infection via an official polymerase chain reaction test or antibody test for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) during the acute phase, and for the control group: participants with no history of suspected COVID-19 and no positive COVID-19 test.
The resting cardiac assessment was conducted on all participants. It included a series of procedures such as medical history, physical examination, resting BP measurement, electrocardiography (ECG), and resting 2-dimensional transthoracic echocardiography (TTE-R). Information on the health status of the participants (including their current and past medical history, and family history) and physical activity was collected via interviews and questionnaires with the participants and their guardians. COVID-19 vaccination data, including timing, number of doses received, and vaccine name, were collected from all participants at the beginning of Germany’s vaccination programme. The history of COVID-19 infection (timing, acute symptoms, diagnostic method, and late symptoms) was obtained from participants of the infected group. The paediatric cardiologist’s examination results were evaluated before the exercise testing by ESE. In cases of ECG or TTE-R abnormalities suggestive of cardiovascular involvement, a further cardiovascular workup using other modalities was recommended without the exercise test.
Furthermore, the exercise testing by ESE was conducted on a subcohort that met the following inclusion criteria: (1) male participants between 10 and 15 years of age; (2) consent obtained from both participants and their legal guardians; (3) participants with sufficient body size to ride the recumbent cycle ergometer in a semisupine position and the ability to wear a facemask; and (4) safety confirmed by paediatric cardiologists through interviews, physical examination, ECG, and TTE-R during the resting cardiac assessment. Because of low enrolment among female participants and suboptimal imaging quality in the few enrolled females, ESE was restricted to male participants.
Definitions related to COVID-19
According to a review referring to the return to sports after COVID-19 infection, the severity of acute COVID-19 symptoms was categorized into 4 groups based on clinical history11 (Supplemental Fig. S1). Long COVID-19 is usually defined as symptoms persisting beyond 12 weeks after infection.12 This study considered prolonged symptoms when COVID-19–related symptoms persisted for more than 4 weeks in the post-acute phase, as the median interval between COVID-19 diagnosis and cardiovascular evaluation was 75 days [interquartile range: 43-121 days]. The variants of concern were classified into 3 periods based on official information on the infection situation in Germany.13 The first period, from March 2020 to June 2021, was marked by the predominance of the Alpha variant (B.1.1.7). The second period, from July 2021 to December 2021, was dominated by the Delta variant (B.1.617.2). The third period, starting from January 2022, was dominated by the Omicron variant (B.1.1.529). The COVID-19 vaccination for children in Germany began in June 2021 for 12 years and older and in December 2021 for those aged 5-11 years.
Assessment of physical activity
To assess the amount of sports activity in the sports clubs and leisure time, the physical activity score (der Motorik-Modul Aktivitätsfragebogen: MoMo-AFB) was calculated based on the questionnaire and the interview during the examination. Participants were asked about the frequency, duration, and type of their habitual physical activity in sports clubs and during leisure time. Exercise time was calculated based on an algorithm,14 which accounted for the intensity of different sports. Each reported sport was coded with the energy expended as metabolic equivalents of task (METs) per hour. The MET-hours index for sports per week was calculated as the sum of the sports performed in sports clubs and leisure time, indicating the METs expended per week in physical activity.15
Physical measurements and physiological examinations
Peripheral systolic and diastolic BP were measured while participants were lying down after a 5-minute rest using the Mobil-O-Graph on the left arm (Mobil-O-Graph; IEM, Aken, Germany). A 12-lead resting ECG was conducted with participants in a supine position (CARDIOVIT CS-200 Office; SCHILLER AG, Obfelden, Switzerland). Abnormal ECG findings, including supraventricular tachycardia, ventricular arrhythmia, conduction disorder, prolonged QTc interval, ST elevation, abnormal T-wave, wide QRS duration, and low amplitude, were identified by paediatric cardiologists according to the relevant guidelines.16
Echocardiography
Echocardiography (Aplio i900; Canon Medical Systems, Ōtawara, Japan) was performed by paediatric cardiologists at rest and during exercise. Data analysis and evaluation were conducted using the software installed in this echo machine (Aplio i900) by observers blinded to the participants’ information. Basic measurements of wall thickness and ventricle volume were assessed using M-mode. The Z-score for wall thickness was calculated using the Pediatric Heart Network formula.17 This formula has been deemed reliable and used in recent large clinical studies.18 The modified Simpson method determined the left ventricular ejection fraction. The early diastolic velocity of mitral inflow (E), peak systolic velocity (s′), and early diastolic velocity (e′) of mitral annular motion by pulse wave Doppler at the septal wall were measured over several cycles and averaged. The strain was measured under digital loops (average 93 frames/s) with tracing endocardium by an automatic or partially manual process and evaluated at rest, including circumferential strain at the middle ventricle (CS), global longitudinal strain (GLS) from apical 4-, 2-, and 3-chamber views, and longitudinal strain from the 4-chamber view (LS). In the right ventricle (RV), contraction was assessed by tricuspid annular plane systolic excursion (TAPSE), fractional area change, and strain. RV longitudinal strain (RV strain) was evaluated as the average strain values of the 6 segments at the RV-focused 4-chamber view. The LS, CS, and RV strain values were accepted when tracking was satisfactory in 6 segments. For the GLS evaluation, the values were accepted up to one segment with poor monitoring, which was excluded from the calculation.
Cardiopulmonary exercise test and protocol in exercise stress echocardiography
ESE was performed in a semisupine position (approximately 45°) with a slight left-side rotation on a recumbent cycle ergometer (eBike EL; GE Healthcare, Chicago, IL) equipped with a spirometer (MetaMax 3B; Cortex Biophysik GmbH, Leipzig, Germany) with face masks for expiratory gas correction (Fig. 1). BP was measured every 3 minutes. ECG was continuously recorded throughout the examination. The exercise began with a warm-up at 0 watts (W), and the workload was increased in steps every 3 minutes according to a protocol selected from 3 different load types adapted to each participant’s body size, age, and exercise experience. The incremental protocols were as follows: protocol 1 load starts at 25 W and increases 25 W, protocol 2 starts at 50 W and increases 25 W, and protocol 3 starts at 50 W and increases 50 W. Recovery was evaluated at 2 and 6 minutes after the end of exercise. The participants were exercised to the point of maximum exhaustion. The exercise was also terminated if adverse events occurred, including arrhythmia, ischemic electrocardiographic changes, chest pain, severe hypertension (systolic BP >250 mm Hg), a decrease in systolic BP (>10%) during loading, and low oxygen saturation (<90%). Reaching the target HR (85% of maximum predicted HR) or achieving at least 1.05 respiratory exchange ratio was used as the criterion for a valid test.19,20 The Tanaka formula “208 − (0.7 × age) beats/minute (bpm)” was used for maximum predicted HR. This formula was chosen because it provides a more reliable estimate of maximum HR than others, even in children and adolescents.21,22 The peak VO₂ value refers to the highest VO₂ achieved during the test. Peak values were determined to be the maximum values observed over 30 seconds. The anaerobic threshold (AT) was determined using the V-slope method or time trend by a well-trained observer and confirmed by another observer.
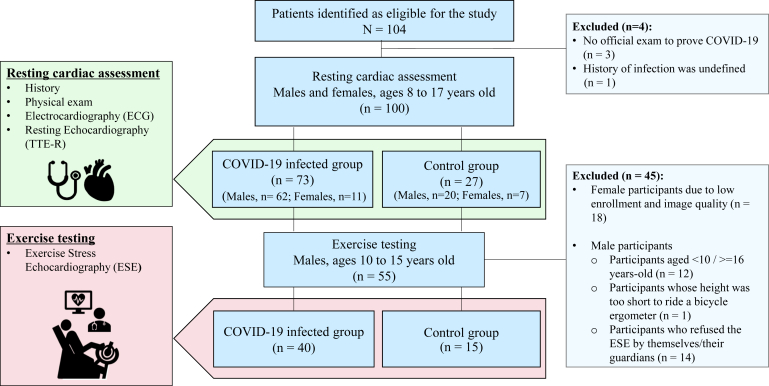
Figure 1.
Study protocol and inclusion criteria.
In the LV, CS, LS, E, septal s′, and e′ were evaluated as much as possible at each workload and recovery. LS from the 4-chamber view and CS at the middle of the LV were specifically evaluated to ensure clinical relevance and minimize missing values, as maintaining adequate image quality throughout the exercise was important in ESE. For the absolute value of LS, the maximum value during exercise divided by the pre-exercise value was expressed as max/base LS. This study defined max/base LS, CS, and s′ as parameters indicative of contractile reserve.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and compared using the t test. Values were expressed as median [interquartile range] for continuous variables without a normal distribution and compared using the Mann-Whitney U test. Categorical variables were reported as frequencies and compared using χ2 tests. The correlation of parameters was assessed using either Pearson’s or Spearman’s rank correlation coefficient. The linear mixed-effect model was used to compare the change in strain values over time during exercise between the infected group and the controls. A P value of <0.05 was used as a threshold for statistical significance. We conducted the analysis using IBM SPSS Statistics version 28.0 (IBM Co SPSS Inc, Chicago, IL) and EZR.23
Intra- and interobserver reliability for strain values
Intra- and interobserver reliability was assessed using randomly selected images taken at rest and during exercise with an HR range of 80-130 bpm. The data sets were reanalysed at least 2 months after the initial assessment to evaluate intraobserver reliability. For interobserver reliability, 2 independent, well-trained observers performed a separate analysis. A reliability threshold of more than 0.7 for intraclass correlation coefficients (ICC) was considered statistically sufficient to assess the reliability.
Results
Baseline characteristics
A total of 104 participants were prospectively enrolled in the study. Four participants were subsequently excluded because of the absence of officially proven COVID-19 or unclear infection history (Fig. 1). The final cohort for the resting cardiac assessment consisted of 100 participants, with 73 in the infected group and 27 in the control group. The exercise testing by ESE was conducted on 55 participants from the original cohort, compromising 40 from the infected group and 15 from the control group. Both groups exhibited similar characteristics, including age, sex, and body size (Table 1). No participants were taking medication for cardiovascular diseases. In the infected group, 85% were asymptomatic or mildly symptomatic during the acute phase of infection. Clinically significant dyspnea, fatigue, and arrhythmias were not observed. Nearly all vaccinated participants (98%) received the Pfizer-BioNTech vaccine, with no reported vaccine-related side effects. Vaccination rates were higher in the control group. Compared with the control group, the infected group showed no apparent reduction in physical activity, as assessed by the MET-hours index in sports per week.
Table 1.
Baseline characteristics in all and in subcohort for exercise stress echocardiography
| Variables | All (resting cardiac assessment) |
Subcohort for ESE (exercise testing) |
||||
|---|---|---|---|---|---|---|
| COVID-19 infected group (n = 73) | Control group (n = 27) | P | COVID-19 infected group (n = 40) | Control group (n = 15) | P | |
| Age (y) | 12.0 [10.0-14.0] | 13.0 [11.5-14.0] | 0.20 | 13.0 [11.0-14.0] | 14.0 [12.5-14.0] | 0.49 |
| Male, n (%) | 62 (84.9) | 20 (74.1) | 0.25 | 40 (100.0) | 15 (100.0) | – |
| Height (cm) | 160.8 ± 15.9 | 163.0 ± 14.5 | 0.52 | 165.5 ± 14.5 | 164.6 ± 15.6 | 0.84 |
| Weight (kg) | 50.8 [37.9-58.3] | 50.2 [40.4-57.9] | 0.89 | 53.9 [41.0-57.8] | 53.2 [38.6-60.1] | 0.90 |
| BSA (m2) | 1.51 [1.27-1.67] | 1.51 [1.35-1.67] | 0.68 | 1.57 [1.32-1.69] | 1.59 [1.28-1.71] | 0.93 |
| SBP (mm Hg) | 113.0 [107.0-116.0] | 111.5 [108.0-121.0] | 0.93 | 114.0 [109.3-119.0] | 116.5 [109.5-126.0] | 0.51 |
| DBP (mm Hg) | 62.0 [58.0-66.0] | 62.0 [59.0-65.8] | 0.89 | 62.0 [58.0-65.3] | 62.0 [60.3-67.3] | 0.40 |
| Heart rate (bpm) | 64.4 ± 8.2 | 65.3 ± 9.9 | 0.66 | 64.0 ± 9.2 | 63.8 ± 10.5 | 0.95 |
| MET-hours index in sports per week |
33.0 [22.5-58.8] |
33.0 [10.7-55.6] |
0.57 |
46.7 [30.3-75.6] |
37.1 [15.1-51.9] |
0.31 |
| COVID-19 |
Infected group |
Control group |
P |
Infected group |
Control group |
P |
| Time interval from diagnosis (d) | 75.0 [43.0-121.0] | 61.5 [39.3-103.5] | ||||
| Infection period, n (%) | ||||||
| First | 7 (9.6) | 2 (5.0) | ||||
| Second | 33 (45.2) | 18 (45.0) | ||||
| Third | 33 (45.2) | 20 (50.0) | ||||
| Acute symptoms, n (%) | ||||||
| None | 3 (4.1) | 2 (5.0) | ||||
| Mild | 59 (80.8) | 32 (80.0) | ||||
| Mod | 10 (13.7) | 6 (15.0) | ||||
| Severe | 1 (1.4) | 0 | ||||
| Prolonged symptoms, n (%), none | 55 (75.3) | 36 (90.0) | ||||
| Vaccination, n (%), yes | 30 (41.1) | 20 (74.1) | <0.01 | 18 (45.0) | 13 (86.7) | 0.01 |
Parameters were expressed as mean ± standard deviation. Continuous variables without a normal distribution were expressed as median [interquartile range]. MET-hours index in sports per week, sports activity time per week in sports club and leisure activity adjusted by metabolic equivalent.14 Acute symptoms are classified with the severity score11 (Supplemental Fig. S1).
BSA, body surface area; ESE, exercise stress echocardiography; MET, metabolic equivalents of task; Mod, moderate; S(D)BP, systolic (diastolic) blood pressure.
Echocardiography at rest
There were no signs of global or localized myocardial injury or pericardial effusion among the participants. In the infected group, no abnormalities were observed in the ventricular wall, suggesting the absence of edema or thinning. LV wall thickness and LV volume were not meaningfully different from those of the control group, with similar age and body size (Table 2). The magnitude of the difference in LV septal s′ and the values between the 2 groups was clinically equivalent. Furthermore, both groups’ LV strain values were similar, indicating equivalent LV contractility (Table 2). In the RV, the infected group exhibited a lower TAPSE value than the controls. However, the measured values in both groups were high above the threshold, indicating reduced contraction (<17 mm for adults).24 Considering the fractional area change and RV strain value, the infected group demonstrated no apparent reduction in RV contraction compared with the controls. Consequently, the resting LV and RV contractions observed in the infected group were similar to those in the control group. LV diastolic parameters, including E wave, A wave, and LV septal e′, were also equivalent between the 2 groups25 (Table 2).
Table 2.
Resting echocardiography and exercise expiratory gas analysis and reserve of cardiac parameters
| Variables | All (resting cardiac assessment) |
Subcohort for ESE (exercise testing) |
||||
|---|---|---|---|---|---|---|
| COVID-19 infected group (n = 73) | Control group (n = 27) | P | COVID-19 infected group (n = 40) | Control group (n = 15) | P | |
| Resting echocardiography | ||||||
| Left ventricle | ||||||
| IVSd (mm) | 7.9 [7.2-9.1] | 8.2 [7.3-9.1] | 0.84 | 8.6 [7.6-9.2] | 9.0 [7.6-9.4] | 0.73 |
| IVSd Z-score | 1.36 ± 1.06 | 1.29 ± 0.74 | 0.75 | 1.50 ± 0.99 | 1.51 ± 0.72 | 0.96 |
| LVPWd (mm) | 7.6 [7.0-8.7] | 7.8 [7.0-8.2] | 0.61 | 8.2 [7.5-9.0] | 7.9 [7.4-8.2] | 0.30 |
| LVPWd Z-score | 1.16 ± 0.87 | 0.96 ± 0.89 | 0.30 | 1.37 ± 0.90 | 1.17 ± 0.73 | 0.40 |
| EDVI (mL/m2) | 61.9 ± 8.9 | 62.2 ± 9.5 | 0.92 | 64.4 ± 8.2 | 63.9 ± 7.0 | 0.85 |
| ESVI (mL/m2) | 22.7 ± 4.0 | 22.5 ± 4.3 | 0.80 | 23.7 ± 4.0 | 23.0 ± 3.1 | 0.60 |
| EF (%) | 63.3 ± 3.8 | 63.9 ± 4.0 | 0.57 | 63.3 ± 3.9 | 63.8 ± 3.9 | 0.68 |
| CS (%) | –27.3 ± 2.9 | –28.3 ± 2.9 | 0.14 | –27.8 ±3.0 | –27.5 ± 3.3 | 0.74 |
| GLS (%) | –23.6 ± 2.0 | –24.1 ± 2.3 | 0.40 | –23.6 ± 2.2 | –24.2 ± 2.7 | 0.45 |
| LS (%) | –23.8 ± 2.4 | –24.9 ± 2.8 | 0.08 | –24.2 ± 2.7 | –24.9 ± 2.3 | 0.40 |
| Septal s′ (cm/s) | 7.3 ± 0.9 | 7.8 ± 1.0 | 0.02 | 7.3 ± 1.0 | 7.6 ± 1.1 | 0.35 |
| Septal e′ (cm/s) | 14.0 [12.5–-15.3] | 14.6 [13.6-15.4] | 0.18 | 13.9 [12.1-15.3] | 14.6 [13.7-15.1] | 0.40 |
| E/e′ | 7.0 [6.5-8.1] | 7.3 [6.5-7.9] | 0.88 | 7.3 [6.6-8.1] | 7.71 [7.0-8.7] | 0.37 |
| E (cm/s) | 100.1 [91.3-109.1] | 108.3 [92.6-116.8] | 0.19 | 101.3 [94.0-108.9] | 110.8 [97.5-119.0] | 0.08 |
| A (cm/s) | 45.7 [37.7-52.7] | 43.4 [40.3-59.7] | 0.41 | 44.6 [37.4-52.5] | 54.5 [40.6-60.7] | 0.12 |
| Right ventricle | ||||||
| TAPSE (mm) | 23.9 ± 3.1 | 26.0 ± 3.8 | 0.02 | 24.3 ± 3.0 | 26.3 ± 4.3 | 0.10 |
| FAC (%) | 45.0 [41.0-48.0] | 43.5 [40.8-48.0] | 0.76 | 45.0 [41.0-48.0] | 41.0 [40.0-45.5] | 0.14 |
| Strain (%) |
–26.2 ± 2.9 |
–25.0 ± 3.0 |
0.09 |
–25.4 ± 2.8 |
–24.8 ± 3.1 |
0.52 |
| Respiratory gas analysis (subcohort) |
Infected group |
Control group |
P |
|||
| Rest VE/VCO2 | 24.7 ± 2.7 | 24.1± 2.4 | 0.51 | |||
| Anaerobic threshold | ||||||
| VO2 (mL/min/kg) | 23.4 ± 4.7 | 24.5 ± 4.0 | 0.45 | |||
| Heart rate (bpm) | 121.6 ± 11.5 | 126.4 ± 12.7 | 0.20 | |||
| Work load (W) | 59.2 ± 27.4 | 61.5 ± 24.6 | 0.78 | |||
| Peak | ||||||
| VO2 (mL/min/kg) | 46.6 ± 7.1 | 46.7 ± 4.6 | 0.97 | |||
| peak VO2 <40 mL/min/kg (%) | 6 (15.0) | 0 | 0.17 | |||
| Heart rate (bpm) | 181.6 ± 9.5 | 180.1 ± 15.1 | 0.67 | |||
| peak VO2/HR (mL/beat) | 13.6 ± 3.8 | 13.7 ± 4.5 | 0.89 | |||
| Work load (W) | 160.8 ± 53.8 | 153.2 ± 54.0 | 0.64 | |||
| RER | 1.09 ± 0.06 | 1.07 ± 0.03 | 0.20 | |||
| VE/VCO2 slope |
27.0 ± 3.4 |
27.9 ± 3.7 |
0.44 |
|||
| Echocardiography under exercise |
Infected group |
Control group |
P |
|||
| max/base BP (%) | 160.2 ± 25.6 | 163.6 ± 25.2 | 0.66 | |||
| max/base HR (%) | 254.0 ± 35.3 | 250.2 ± 41.0 | 0.73 | |||
| max/base CS (%) | 121.0 ± 17.3 | 116.5 ± 16.9 | 0.39 | |||
| max/base LS (%) | 125.4 ± 15.1 | 123.3 ± 16.9 | 0.67 | |||
| max/base septal s′ (%) | 210.4 ± 43.6 | 210.1 ± 47.4 | 0.98 | |||
The maximum value during exercise divided by the pre-exercise value was expressed as max/base. Parameters were expressed as mean ± standard deviation. Continuous variables without a normal distribution were expressed as median [interquartile range].
A, late diastolic velocity of mitral inflow; BP, blood pressure; CS, circumferential strain; E, early diastolic velocity of mitral inflow; e′, early diastolic velocity of mitral annular motion; ED (S) VI, left ventricular end-diastolic (systolic) volume index; EF, ejection fraction with modified Simpsons method; ESE, exercise stress echocardiography; FAC, fractional area change; GLS, global longitudinal strain; HR, heart rate; IVSd, end-diastolic interventricular septum thickness; LS, longitudinal strain (4-chamber); LVPWd, end-diastolic left ventricle posterior wall thickness; RER, respiratory exchange ratios; s′, peak systolic mitral annular velocity; TAPSE, tricuspid annular plane systolic excursion; VCO2, carbon dioxide production; VE, pulmonary ventilation; VE/VCO2, ventilatory equivalents for carbon dioxide; VO2, oxygen consumption; VO2/HR, oxygen pulse.
Expiratory gas analysis in exercise stress echocardiography
Ninety-five percent of the participants reached the target HR or achieved at least 1.05 of respiratory exchange ratio. The infected group showed no apparent reduction in peak oxygen consumption (VO2) (mL/min/kg). However, 15% of participants in the infected group had a peak VO2 lower than 40 mL/min/kg, the minimum value among the control participants (Table 2). The mean AT value in ESE was approximately 50% of peak VO2.
No parameters strongly correlated with baseline characteristics and echocardiographic parameters related to peak VO2. Under exercise, the rate of HR increase showed only a weak correlation (r = 0.31, P = 0.02) with peak VO2 (Supplemental Table S1). Therefore, evaluating the time trend and relationship of parameters during exercise is necessary for interpreting ESE data.
Physiological reactions to exercise and their relationships evaluated via exercise stress echocardiography
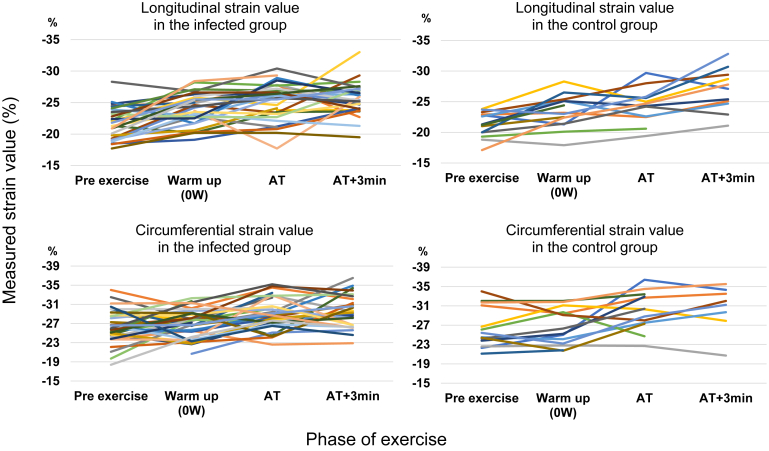
The contractile reserve expressed by the change of CS, LS, and s′ during exercise showed similar results between the 2 groups (Table 2). Focusing on strain values, the trends in CS and LS with changes in time and workload were also not meaningfully different between the 2 groups (Fig. 2).
Figure 2.
Time trends of myocardial strain during exercise. Both groups exhibited similar trends in longitudinal strain (graphs in the upper row, P = 0.08) and circumferential strain (in the lower row, P = 0.86). AT, anaerobic threshold; AT+3min, 3 minutes after AT (the next workload after AT).
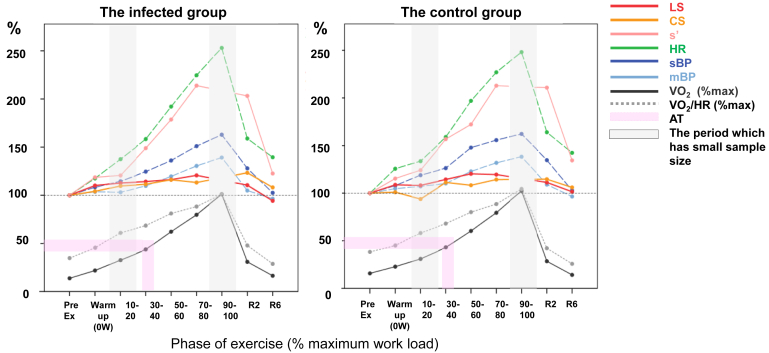
Figure 3 provides parameter changes under exercise in both groups. The increase in HR was notably higher after 30%-40% of the maximum workload, approximately 50% of the peak VO2, and it meant around the AT. BP also increased around this step. In contrast, the increase in strain became smaller around this step. Because of limitations in image acquisition, strain values were occasionally not thoroughly evaluated under the maximum (90%-100%) or submaximum (70%-80%) of the workload; however, both parameters seemed to reach steady states from the submaximum to early recovery. The curves of s′ followed HR increase. The infected group showed reactions and relations in systolic and diastolic parameters similar to the controls. Changes in diastolic parameters are plotted in Supplemental Figure S2. E and e′ elevated, and E/e′ remained constant under exercise.
Figure 3.
Rate of systolic parameters in LV and cardiovascular reaction during exercise (value under exercise/baseline [%]). The percentage of the whole workload is presented on the horizontal line. The mean values of the increase from the pre-exercise baseline are shown on the vertical line. Oxygen consumption (VO2) and oxygen pulse (VO2/HR) are presented as the rate per the maximum value during the exercise. The 10%-20% of the workload has a small number of samples due to the protocol, and the 90%-100% of the workload has a low analysis rate due to the low feasibility of echo evaluation under higher heart rate and respiration. 10-20, 10%-20% of the maximum workload; AT, anaerobic threshold; CS, circumferential strain; HR, heart rate; LS, longitudinal strain (4-chamber); LV, left ventricular; mBP, mean blood pressure ; R2, recovery 2 minutes; s′, peak systolic mitral annular velocity; sBP, systolic blood pressure.
Intra- and interobserver reliability in resting and exercise stress echocardiography
Supplemental Table S2 provides the intra- and interobserver reliability data. For intraobserver, the ICC surpassed 0.7 for all strain values at rest and during ESE. Regarding interobserver reliability, the ICC for CS under exercise was slightly below 0.7. This could be attributed to the short-axis view being influenced by frequent respiration and motion artefacts under high-load exercise, requiring partially manual tracing for CS.
Discussion
The objective of the present study was to assess the impact of COVID-19 infection on cardiovascular function at rest and during exercise. From the resting cardiac assessment, most baseline characteristics and TTE-R results did not reveal specific abnormalities related to COVID-19 infection. In the exercise testing, post–COVID-19 participants showed cardiovascular responses to exercise similar to those of controls in ESE with CPET.
Background: infection and the physical activity levels
The amount of physical activity assessed by the MET-hours index per week was similar between the 2 groups. However, the physical activity levels observed in this study appear lower than those reported in previous studies conducted before the pandemic (MET-hours index per week: average 47 in 11-13 years old and 52 in 14-17 years old).14 This discrepancy could be related to the pandemic, which may have influenced the amount of physical activity recorded during the study visit.
The influence of COVID-19 on cardiovascular function and exercise tolerance
Several studies have reported on exercise tolerance after COVID-19 infection using CPET with/without echocardiography. In adults, peak VO2 and an increase of EF under exercise were reduced in post–COVID-19 patients, particularly those who experienced more than moderate symptoms in the acute phase.26 A cohort study among adults with moderate or severe infection showed that half of the patients had percent-predicted peak VO2 <80%, whereas LV function was preserved.27 In young athletes with COVID-19–related cardiac involvement, abnormalities during stress, including ventricular ectopy, wall motion abnormalities, and/or elevated ventilatory equivalents for carbon dioxide (VE/VCO2), were observed. However, VO2 max and GLS were not significantly different from cases without myocardial involvement, although the study had a small sample size.28 A meta-analysis indicated a mild reduction in exercise tolerance among individuals with Long-COVID-19, with factors such as deconditioning, dysfunctional breathing, chronotropic incompetence, and abnormal peripheral oxygen extraction potentially contributing to reduced exercise tolerance.29
Resting echocardiographic parameters to evaluate cardiac contraction and dilatation
Although intervendor differences should be accounted for when measuring strain values, the LV and RV strain values in the infected group in this study were clinically equivalent to those of controls and within the reference values for the age group.30, 31, 32 Regarding LV septal s′, our data were deemed to be within the normal range, given that the standard threshold for s′ on the LV lateral wall in adults is >8 cm/s.33 The reference values for LV lateral s′ in children and LV septal s′ are 7 and 6 cm/s, respectively.34 TAPSE is a simple method to evaluate RV function, with a standard threshold of 17 mm or more for adults.24 However, TAPSE is an angle-dependent value and can occasionally overestimate or underestimate RV function. RV strain is a more sensitive indicator of potential RV dysfunction in patients with cardiac disease than TAPSE.35 In our study, a slight difference in height between the groups may account for the variation in LV septal s′ and TAPSE, as these values depend on body size.
We also evaluated LV diastolic function using parameters reflecting elevated LV filling pressure, including E, A, and e′. Our findings indicated that these parameters were within normal ranges, suggesting that LV diastolic function was not significantly impaired in the infected group compared with the controls. Furthermore, peak tricuspid regurgitation velocity, left atrial volume index, and reservoir strain may be assessed to provide comprehensive insights into LV diastolic function for adults;36 however, these were not evaluated in the present study. These parameters may be affected by age,37 and reference values in children and adolescents are not well defined and remain difficult to evaluate thoroughly.38
Contractile reserve and parameters assessed by exercise stress echocardiography
Several parameters evaluate contractile reserve, including the difference of left ventricular ejection fraction, wall motion score index, the ratio between systolic arterial pressure and LV end-systolic volume, LV strain, and s′.9 Because some parameters, such as ventricular volume, cannot be precisely measured in children under exercise due to small heart size and high HR, strain value and s′ under incremental load were better for evaluation in the present research. Evaluating intrinsic cardiac contractility is complex as preload and afterload dynamically change under exercise. Under high heart stimulation, contraction usually increases with an up-sloping curve (force-frequency relationship) to the upper limitation, typically around 125 bpm39 or baseline HR +50 bpm.40 However, in patients with heart failure, contraction peaks at a lower HR, for example, 100 bpm31 or baseline HR +10 bpm,40 with a biphasic or flat curve.40 Although the upper limit HR for contraction in children remains unclear, the increase in strain, especially LS, showed up-sloping curves and reached a steady state in the present study (Fig. 3). Beyond the upper limit of contraction, the significant increase in HR more contributes to maintaining cardiac output.
Strong cardiac contraction does not simply mean good function because cardiac efficiency should be considered in the context of optimal exercise oxygen demand.41 Adequate contractile reserve is required for increased HR, BP, and oxygen demand. The diagnostic steps for cases with low peak VO2 and AT include confirming resting strain values compared with normal thresholds and determining pathophysiology during exercise by referring to the HR and contractile reserve.
Concerning diastolic function, exercise tests can reveal an elevation of LV filling pressure masked at rest in adults, such as those with heart failure with preserved EF42 or hypertrophic cardiomyopathy.43 Noninvasive diastolic stress tests indicate 3 parameters: septal e′ <7 cm/s at baseline, septal E/e′ >15 with exercise (or average E/e′ >14), and tricuspid regurgitation velocity >2.8 m/s with exercise.44 E and e′ typically elevate with incremental workload, and E/e′ remains constant. However, the role of E/e′ in assessing LV filling pressure under exercise is still controversial.45 Factors such as LV end-diastolic stiffness, increased venous return as preload, and incomplete relaxation due to shortening of the diastolic phase at high HR may affect this assessment.
The role of exercise stress echocardiography—comparison with CMR
CMR has been a noninvasive tool in diagnosing myocarditis in children.46 The original Lake Louise criteria, including T1 and T2 mapping in 2018, were more sensitive for diagnosing myocarditis.47 CMR’s efficacy was reported in diagnosing and monitoring COVID-19–related or vaccine-associated myocarditis lesions.47 CMR has a superior ability to detect inflammation and fibrosis, can evaluate localized lesions, and is recommended for patients with chest symptoms or abnormalities in other examinations. However, it is not always practical to perform CMR on all post–COVID-19 patients, given health care economics and resource limitations.48 Regarding the duration with which myocardial injury is detected, there have been reports of fulminant COVID-19–related myocarditis in which myocardial injury findings via CMR persisted for 50-150 days.49 In contrast to other forms of viral myocarditis, patients with MIS-C-related myocarditis tend to recover more rapidly.50 At the follow-up CMR examination conducted 3-5 months later, only a few patients exhibited findings that met the criteria for myocarditis.51
The advantages of ESE are the ability to assess cardiac contraction in combination with other cardiovascular parameters and monitor arrhythmias during exercise. It can provide helpful information for children and adolescents, especially competitive athletes, concerned about when and how to return to daily activities and sports in the subacute or late stages of relatively mild COVID-19 infection or the absence of abnormal cardiovascular findings at rest.
Limitations
Our study has limitations. As a single-centre investigation, it may not fully capture the diversity of youth populations across different regions or demographics. Despite our efforts to reach out to female participants, the ESE assessment was limited to male youth participants due to low female enrolment. This restricts the generalizability of our ESE findings. However, similar challenges regarding sex differences in interest and willingness to participate have been reported in the literature.52,53 Future studies should explore the potential causes for low female representation and implement strategies to increase diverse participation.
Regarding the severity of cases, our infection group consisted primarily of asymptomatic and mildly symptomatic patients during the acute phase of infection. Consequently, our findings may not reflect outcomes in moderate and severe cases, which could potentially yield different results. From the perspective of COVID-19 spread and vaccine development at the time of study design, we selected the control group based on the self-reported absence of clear infection history. Given the subsequent widespread transmission, antibody testing would have been ideal. However, because of limited resources, we did not perform such testing. This introduces a potential misclassification bias, as some asymptomatic infected individuals could have been inadvertently included among the controls. Nevertheless, this scenario is not very likely because most controls were enrolled before the large-scale pandemic wave associated with the Omicron variant and subsequent stay-at-home periods. Lastly, adjustments for factors such as vaccination history and infection duration should be considered when estimating the impact of viral load on cardiovascular function. However, our study lacked sufficient participants for robust adjustment and matching.
Conclusions
Among participants with a history of COVID-19 infection and controls, we could not show any meaningful difference in LV systolic and diastolic function, as well as in RV systolic function, evaluated by TTE-R. Noninvasive evaluation by ESE showed no apparent reduction in exercise tolerance or cardiovascular response focusing on contractile reserve in the infected group. This finding provides valuable insight for children and adolescents concerned about resuming daily activities and sports during the subacute and chronic stages of COVID-19 infection.
Acknowledgements
The authors would like to express their sincere gratitude to Dr Patricia C. da Rosa (Institute of Social Determinants of Health and Preventive Pediatrics, Department Health and Sport Sciences, TUM School of Medicine and Health, Technical University of Munich) for her invaluable guidance throughout writing the article.
Ethics Statement
The LICO study received approval from the Medical Ethics Committee of the Technical University of Munich (140/21 S-EB).
Patient Consent
Written informed consent was given to the participants and their guardians before enrolment.
Funding Sources
The Canon Medical Systems Europe B.V. and the Japan Heart Foundation, Japan funded this work.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2024.08.001
Supplementary Material
References
- 1.Viner R.M., Ward J.L., Hudson L.D., et al. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child. 2020;106:802–807. doi: 10.1136/archdischild-2020-320972. [DOI] [PubMed] [Google Scholar]
- 2.Vasichkina E., Alekseeva D., Kudryavtsev I., et al. COVID-19 heart lesions in children: clinical, diagnostic and immunological changes. Int J Mol Sci. 2023;24:1147. doi: 10.3390/ijms24021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naeem A., Tabassum S., Gill S., et al. COVID-19 and cardiovascular diseases: a literature review from pathogenesis to diagnosis. Cureus. 2023;15 doi: 10.7759/cureus.35658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D.L., Davogustto G., Soslow J.H., et al. Characteristics of COVID-19 myocarditis with and without multisystem inflammatory syndrome. Am J Cardiol. 2022;168:135–141. doi: 10.1016/j.amjcard.2021.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jone P.N., John A., Oster M.E., et al. SARS-CoV-2 infection and associated cardiovascular manifestations and complications in children and young adults: a scientific statement from the American Heart Association. Circulation. 2022;145:e1037–e1052. doi: 10.1161/CIR.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 7.Pieles G.E., Gowing L., Forsey J., et al. The relationship between biventricular myocardial performance and metabolic parameters during incremental exercise and recovery in healthy adolescents. Am J Physiol Heart Circ Physiol. 2015;309:H2067–H2076. doi: 10.1152/ajpheart.00627.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifra B., Chen C.K., Fan C.S., et al. Dynamic myocardial response to exercise in childhood cancer survivors treated with anthracyclines. J Am Soc Echocardiogr. 2018;31:933–942. doi: 10.1016/j.echo.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Thein P.M., Mirzaee S., Cameron J.D., Nasis A. Left ventricular contractile reserve as a determinant of adverse clinical outcomes: a systematic review. Intern Med J. 2022;52:186–197. doi: 10.1111/imj.14995. [DOI] [PubMed] [Google Scholar]
- 10.Holland D.J., Marwick T.H., Haluska B.A., et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–1066. doi: 10.1136/heartjnl-2014-307391. [DOI] [PubMed] [Google Scholar]
- 11.Kriemler S., Siaplouras J., Förster H., Joisten C. COVID-19 bei jugendlichen Athleten: diagnose und return to sports. Pädiatrie Schweiz. 2022;33:42–47. [Google Scholar]
- 12.Parhizgar P., Yazdankhah N., Rzepka A.M., et al. Beyond acute COVID-19: a review of long-term cardiovascular outcomes. Can J Cardiol. 2023;39:726–740. doi: 10.1016/j.cjca.2023.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert Koch Institute SARS-CoV-2 Varianten in Deutschland. https://public.data.rki.de/t/public/views/IGS_Dashboard/DashboardVOC?%3Aembed=y&%3AisGuestRedirectFromVizportal=y Available at:
- 14.Karlsruher Instituts für Technologie Der Motorik-Modul Aktivitätsfragebogen MoMo-AFB: Leitfaden zur Anwendung und Auswertung. https://publikationen.bibliothek.kit.edu/1000062199 Available at:
- 15.Spengler S., Mess F., Schmocker E., Woll A. Longitudinal associations of health-related behavior patterns in adolescence with change of weight status and self-rated health over a period of 6 years: results of the MoMo longitudinal study. BMC Pediatr. 2014;14:242. doi: 10.1186/1471-2431-14-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritsch P., Ehringer-Schetitska D., Dalla Pozza R., et al. Cardiovascular pre-participation screening in young athletes: recommendations of the Association of European Paediatric Cardiology. Cardiol Young. 2017;27:1655–1660. doi: 10.1017/S1047951117001305. [DOI] [PubMed] [Google Scholar]
- 17.Lopez L., Colan S., Stylianou M., et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network normal echocardiogram database. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forså M.I., Smedsrud M.K., Haugaa K.H., et al. Distinguishing left ventricular hypertrophy from hypertrophic cardiomyopathy in adolescents: a longitudinal observation study. Eur J Prev Cardiol. 2024;31:591–598. doi: 10.1093/eurjpc/zwad361. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes J., Ubeda Tikkanen A., Jenkins K.J. Exercise testing and training in children with congenital heart disease. Circulation. 2010;122:1957–1967. doi: 10.1161/CIRCULATIONAHA.110.958025. [DOI] [PubMed] [Google Scholar]
- 20.Amedro P., Matecki S., Pereira Dos Santos T., et al. Reference values of cardiopulmonary exercise test parameters in the contemporary paediatric population. Sports Med Open. 2023;9:68. doi: 10.1186/s40798-023-00622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 22.Carli M.E.C., Moraes Junior F.B., Menezes-Junior F.J., Tadiotto M.C., Mota J., Leite N. Prediction equations for maximal heart rate in obese and nonobese children and adolescents: a systematic review and meta-analysis. Rev Paul Pediatr. 2023;41 doi: 10.1590/1984-0462/2023/41/2021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Szekely Y., Lichter Y., Sadon S., et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. 2021;34:1273–1284.e9. doi: 10.1016/j.echo.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vannini L., Quijada-Fumero A., Martín M.P.R., Pina N.C., Afonso J.S.H. Cardiopulmonary exercise test with stress echocardiography in COVID-19 survivors at 6 months follow-up. Eur J Intern Med. 2021;94:101–104. doi: 10.1016/j.ejim.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitrani R.D., Alfadhli J., Lowery M.H., et al. Utility of exercise testing to assess athletes for post COVID-19 myocarditis. Am Heart J Plus. 2022;14 doi: 10.1016/j.ahjo.2022.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durstenfeld M.S., Sun K., Tahir P., et al. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farsalinos K.E., Daraban A.M., Ünlü S., et al. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr. 2015;28:1171–1181.e2. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Levy P.T., Machefsky A., Sanchez A.A., et al. Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2016;29:209–225.e6. doi: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy P.T., Sanchez Mejia A.A., Machefsky A., et al. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–560.e3. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho C.Y., Solomon S.D. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396–e398. doi: 10.1161/CIRCULATIONAHA.105.579268. [DOI] [PubMed] [Google Scholar]
- 34.Cifra B., Mertens L., Mirkhani M., et al. Systolic and diastolic myocardial response to exercise in a healthy pediatric cohort. J Am Soc Echocardiogr. 2016;29:648–654. doi: 10.1016/j.echo.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Tadic M., Nita N., Schneider L., et al. The predictive value of right ventricular longitudinal strain in pulmonary hypertension, heart failure, and valvular diseases. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.698158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smiseth O.A., Donal E., Boe E., et al. Phenotyping heart failure by echocardiography: imaging of ventricular function and haemodynamics at rest and exercise. Eur Heart J Cardiovasc Imaging. 2023;24:1329–1342. doi: 10.1093/ehjci/jead196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyoshi T., Addetia K., Citro R., et al. Left ventricular diastolic function in healthy adult individuals: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr. 2020;33:1223–1233. doi: 10.1016/j.echo.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Lopez L., Saurers D.L., Barker P.C.A., et al. Guidelines for performing a comprehensive pediatric transthoracic echocardiogram: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;37:119–170. doi: 10.1016/j.echo.2023.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Gierula J., Paton M.F., Lowry J.E., et al. Rate-response programming tailored to the force-frequency relationship improves exercise tolerance in chronic heart failure. JACC Heart Fail. 2018;6:105–113. doi: 10.1016/j.jchf.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Bombardini T., Zoppè M., Ciampi Q., et al. Myocardial contractility in the stress echo lab: from pathophysiological toy to clinical tool. Cardiovasc Ultrasound. 2013;11:41. doi: 10.1186/1476-7120-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand V., Kane G.C., Scott C.G., et al. Prognostic value of peak stress cardiac power in patients with normal ejection fraction undergoing exercise stress echocardiography. Eur Heart J. 2021;42:776–785. doi: 10.1093/eurheartj/ehaa941. [DOI] [PubMed] [Google Scholar]
- 42.Obokata M., Kane G.C., Reddy Y.N., et al. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izawa H., Yokota M., Takeichi Y., et al. Adrenergic control of the force-frequency and relaxation-frequency relations in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2959–2968. doi: 10.1161/01.cir.96.9.2959. [DOI] [PubMed] [Google Scholar]
- 44.Ha J.W., Andersen O.S., Smiseth O.A. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging. 2020;13:272–282. doi: 10.1016/j.jcmg.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 45.Sunderji I., Singh V., Fraser A.G. When does the E/e' index not work? The pitfalls of oversimplifying diastolic function. Echocardiography. 2020;37:1897–1907. doi: 10.1111/echo.14697. [DOI] [PubMed] [Google Scholar]
- 46.Law Y.M., Lal A.K., Chen S., et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez Tijmes F., Thavendiranathan P., Udell J.A., Seidman M.A., Hanneman K. Cardiac MRI assessment of nonischemic myocardial inflammation: state of the art review and update on myocarditis associated with COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3 doi: 10.1148/ryct.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira V.M., Plein S., Wong T.C., et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: recommendations by the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson. 2023;25:21. doi: 10.1186/s12968-023-00933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hara S., Miwa N., Hachiya H., Sasano T. Inflammatory process of the COVID-19 fulminant myocarditis in the multimodality imaging: a case report. Eur Heart J Case Rep. 2023;7 doi: 10.1093/ehjcr/ytad125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel T., Kelleman M., West Z., et al. Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dove M.L., Oster M.E., Hashemi S., Slesnick T.C. Cardiac magnetic resonance findings after multisystem inflammatory syndrome in children. J Pediatr. 2022;245:95–101. doi: 10.1016/j.jpeds.2022.02.049. [DOI] [PubMed] [Google Scholar]
- 52.Costello J.T., Bieuzen F., Bleakley C.M. Where are all the female participants in Sports and Exercise Medicine research? Eur J Sport Sci. 2014;14:847–851. doi: 10.1080/17461391.2014.911354. [DOI] [PubMed] [Google Scholar]
- 53.Nuzzo J.L., Deaner R.O. Men and women differ in their interest and willingness to participate in exercise and sports science research. Scand J Med Sci Sports. 2023;33:1850–1865. doi: 10.1111/sms.14404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.