Abstract
The transcatheter aortic valve replacement (TAVR) with a self-expanding supra-annular valve has shown better hemodynamic outcomes and better valve durability at 5 years when compared with a surgical aortic valve replacement (SAVR). It is possible that the benefits of a self-expanding supra-annular valve can be achieved using a surgical approach. An Evolut (Medtronic) transcatheter aortic valve was surgically implanted in 4 patients undergoing SAVR. Standard surgical methods were used. Three patients had native aortic valve disease, and 1 patient had a degenerated surgical bioprosthesis. The measured valve size was 21 mm in 3 patients and 23 mm in 1 patient; all received a 29-mm Evolut. No valve migration was observed. No patients required a pacemaker. Discharge echocardiography showed low aortic valve gradients (arithmetic mean of 5.3 mm Hg). One patient had a mild paravalvular leak. SAVR using a self-expanding supra-annular valve can be successfully performed in patients not amenable to TAVR.
Key Words: aortic stenosis, self-expanding, supra-annular, transcatheter aortic valves, surgical aortic valve replacement
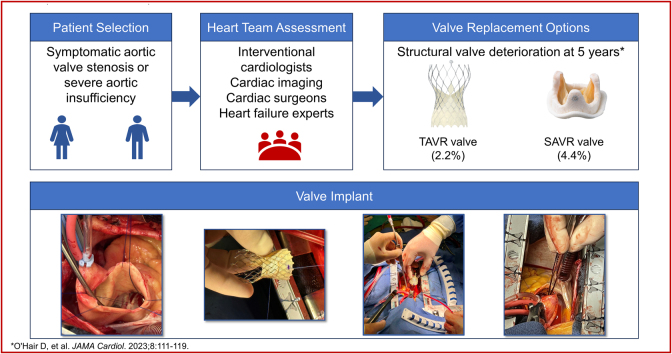
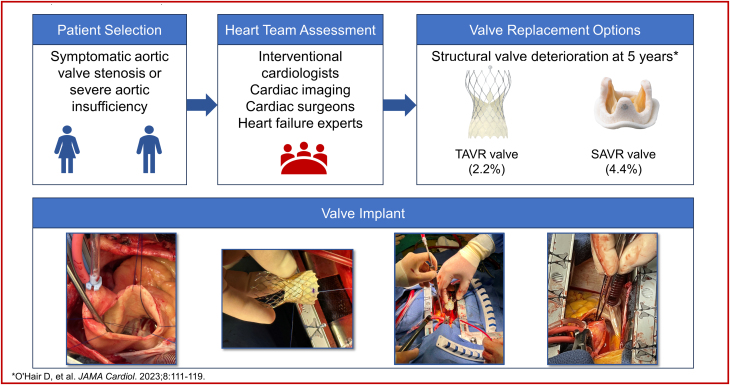
Visual Summary
Visual Summary. Surgical Aortic Valve Replacement Using Supra-Annular Self-Expanding Bioprosthesis
In spite of advances in surgical aortic valve prostheses, prosthesis–patient mismatch (PPM) and valve durability remain concerns for patients requiring aortic valve replacement. Recent reports of 5-year outcomes from large randomized clinical trials comparing transcatheter aortic valve replacement (TAVR) with surgical aortic valve replacement (SAVR) have shown superior hemodynamics and valve durability and less PPM with a self-expanding supra-annular transcatheter valve.1,2 The use of TAVR has now surpassed SAVR in older patients with severe aortic stenosis3 based on favorable outcomes with TAVR, yet not all patients with severe aortic valve disease are able to undergo the TAVR procedure.
Learning Objectives
-
•
To describe the benefits of supra-annular self-expanding transcatheter valves compared with surgery implants for valve durability.
-
•
To define potential issues of prosthesis–patient mismatch.
-
•
To describe the potential benefits of surgical placement of an Evolut transcatheter aortic valve.
Aortic root or annular enlargement has been proposed as a method to mitigate the risk of increased mortality from PPM; however, this procedure itself is not without risk.4 The reduction in the number of SAVR procedures performed has reduced the opportunity for surgeons in training to perform aortic root or annular enlargement. The hemodynamic and durability benefits associated with transcatheter valves may potentially be achieved using a surgical approach in patients for whom TAVR may not be appropriate. We report 4 cases of SAVR using the Evolut PRO+ self-expanding supra-annular porcine bioprosthetic valve (Medtronic).
Patients and Methods
Patients at our center requiring aortic valve replacement who were not able to undergo TAVR were included in this report. Boulder Community Hospital waived patient consent for this report.
Valve description
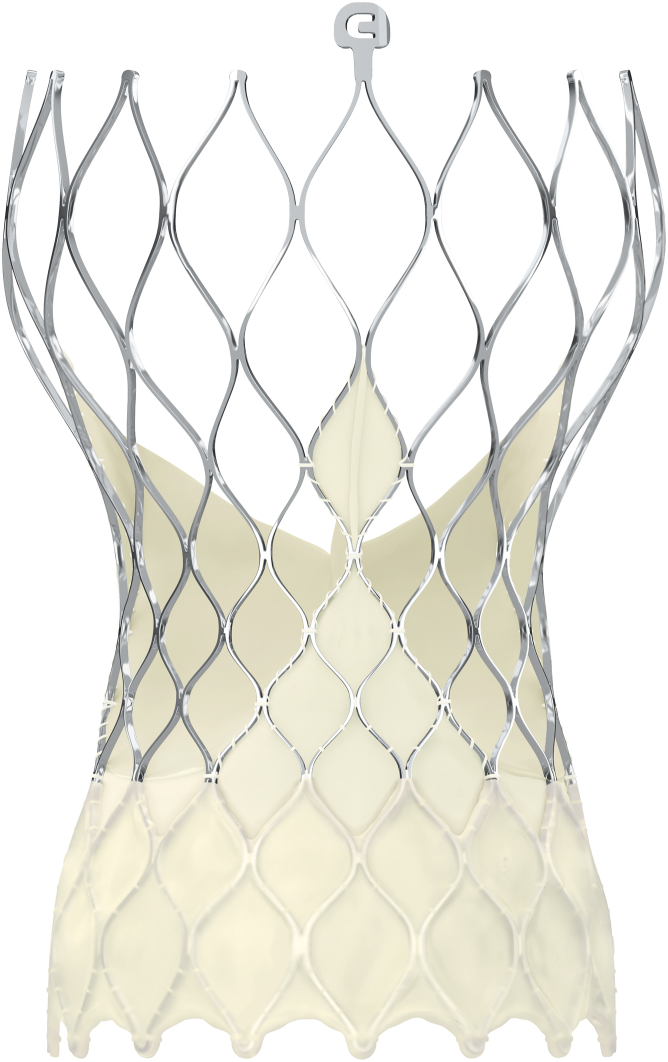
The Evolut transcatheter valve comprises a uniquely designed self-expanding nitinol frame with 3 porcine pericardial leaflets sewn just above a 14-mm pericardial wrap, allowing the new valve to sit above the native annulus (Figure 1).
Figure 1.
The Evolut PRO Valve (© Medtronic 2023)
The Evolut PRO valve comprises 3 porcine pericardial leaflets sewn just above a 14-mm pericardial wrap.
Procedure
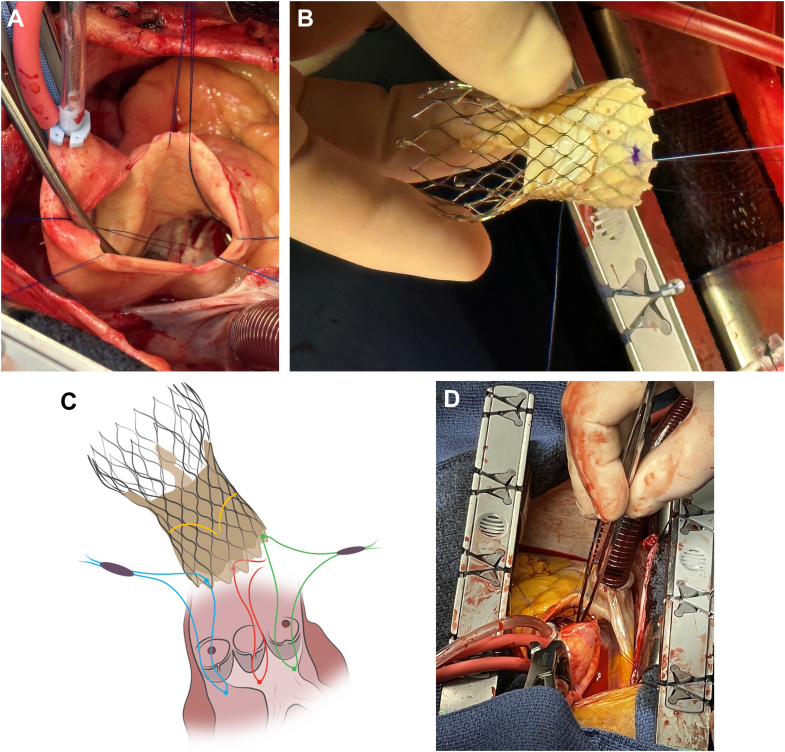
All patients provided informed consent for the surgical procedure, and only anonymized patient health information data are reported. Standard cardiac surgical techniques including cardiopulmonary bypass and cardioplegic arrest were used in all cases. The native leaflets in each case were either debrided (patient 1) or completely removed (patients 2 to 4). During implantation, a prolene suture was passed through the left ventricular outflow tract just below the nadir of each native aortic valve leaflet and then through the corresponding section of the Evolut PRO+ valve wrap (Figure 2) to direct the new valve to the proper depth and to align the commissures for maximal coronary access. This was performed by direct visualization, and annular sealing was confirmed by direct inspection through the valve. Concurrently, the valve was irrigated with iced saline solution to allow sufficient compression of the nitinol to deliver the valve into the aortic root. Once the valve was placed at the proper level (targeted 2- to 4-mm depth) and position, the transcatheter valve was irrigated with warm saline solution to allow full expansion of the nitinol frame. The guide sutures were removed. No valves required postdilation. The ascending aorta was closed using standard techniques.
Figure 2.
Implanting the Transcatheter Valve
(A) Guide sutures are placed in the left ventricular outflow tract. (B) Sutures are then placed through the porcine wrap of the transcatheter valve to facilitate proper depth in deployment and commissural alignment. (C) The transcatheter valve leaflets are located 4 nodes from the bottom of the valve. (D) Inspecting the final valve depth.
Results
Patients
From October 2022 to October 2023, 3 patients with severe aortic stenosis and 1 patient with primary severe aortic insufficiency (AI) underwent surgical implantation of a 29-mm Evolut PRO+ transcatheter valve (Table 1). Patient 1 had a preprocedural computed tomography showing an average annulus diameter of 22.5 mm with an area of 407.84 mm2. Access vessels were adequate for transcatheter access at 6 mm or larger. Because this patients’ payer declined TAVR, and with a body surface area of 1.5 m2, the self-expanding transcatheter valve was implanted surgically to avoid the risk of PPM and the risks of an aortic root enlargement. The annular size for this patient corresponded to a 29-mm Evolut valve with 27% oversizing. Because this patient had a bicuspid valve (Sievers 1, left-right), the fused leaflets were separated, and the edge calcium of each leaflet was reduced before implant. The Evolut valve was positioned so that the bioprosthetic leaflets lie above the native annulus. Symptom relief was immediate and persisted at the 1-year follow-up (1-year gradient of 6 mm Hg). Intraoperative sizing was performed using standard Medtronic surgical valve sizers and valve selection targeted 20% to 30% oversizing. The remaining 3 patients all required a 21-mm valve.
Table 1.
Patient and Procedure Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Baseline | ||||
| Age, y | 63 | 59 | 83 | 67 |
| Body surface area, m2 | 1.5 | 1.9 | 1.6 | 1.5 |
| Blood pressure, mm Hg | 130/82 | 140/86 | 134/60 | 108/56 |
| Murmur | Systolic | Systolic | Systolic and diastolic | Systolic and diastolic |
| Aortic valve area, cm2 | 0.5 | 0.8 | 2.0 | 1.5 |
| Aortic valve gradient, mm Hg | 50.0 | 49.0 | 11.0 | 20.0a |
| Diagnosis | AS | Surgical AVR/AS | Aortic insufficiency | AS |
| Trancatheter AVR exclusion | Age | Prosthesis–patient mismatch | Primary aortic insufficiency | Age |
| Bicuspid valve | Yes | No | No | Yes |
| Procedure | AVR | Surgical AVR explant/AVR | AVR | AVR |
| Leaflet resection | Partial | Total | Total | Total |
| Measured valve size, mm | 21 | 21 | 21 | 23 |
| Implanted valve size,b mm | 29 | 29 | 29 | 29 |
| Aortic clamp time, min | 46 | 141 | 50 | 69 |
| Perfusion time, min | 57 | 225 | 61 | 87 |
| Postdilation | No | No | No | No |
| Valve migration | No | No | No | No |
| New pacemaker | No | No | No | No |
AS = aortic stenosis; AVR = aortic valve replacement.
Vmax of 3.1 m/s.
All Evolut PRO+ valves.
Patient 2 had a previously implanted 21-mm bovine pericardial valve (Inspiris Resilia, Edwards Lifesciences) but failed to have symptom relief after 3 years because of PPM and early structural valve deterioration (SVD). The surgical valve was explanted and replaced with the 29-mm Evolut PRO+ valve. Patient 3 presented with primary severe aortic valvular AI. After Evolut implantation, the valvular AI was resolved, and a mild paravalvular leak was observed. Patient 4 also had a small body surface area (1.5 m2) and a bicuspid aortic valve (Sievers 1, non-left) with moderate to severe AI.
In the 3 patients with native aortic valve stenosis, all residual native leaflet tissue was removed before placement of the Evolut valve. No patients required a permanent pacemaker. Despite leaflet resection, no patients experienced valve migration. This was assessed intraoperatively using a gentle tug test and postoperatively by confirming valve position by radiography or echocardiography. At discharge echocardiography, all 4 patients had low aortic valve gradients (Table 2), 3 patients had no AI, and 1 patient had mild paravalvular leak.
Table 2.
Postprocedural Echocardiographic Measures
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Gradient, mm Hg | 4.0 | 9.0 | 4.0 | 4.0 |
| Effective orifice area, cm2 | 2.1 | 3.1 | 1.9 | 1.9 |
| Effective orifice area indexed to the body surface area, cm2/m2 | 1.4 | 1.6 | 1.2 | 1.3 |
| Aortic regurgitation | None | None | Mild | None |
Discussion
In spite of advances in SAVR, PPM continues to be reported in up to 30% of patients in carefully adjudicated randomized clinical trials.2 Methods to mitigate the debilitating symptoms and increased mortality associated with PPM include aortic root or annular enlargement. Although this technique may allow the implantation of a larger surgical valve, it requires longer cross-clamp and perfusion times, is associated with numerically higher mortality and morbidity, and is becoming more difficult to teach as the volume of SAVR cases is surpassed by TAVR cases.3 Even the most experienced centers report that fewer than 10% of the patients received a 27- or 29-mm valve after enlargement.5 The Evolut transcatheter aortic bioprosthesis has been reported to have less PPM, larger effective orifice area, and lower gradients than surgical implants in large randomized clinical trials.2,6 The benefits of the self-expanding supra-annular Evolut transcatheter valve can be achieved using a surgical approach that allows accurate and stable placement.
In the cases described, the use of the uniquely designed Evolut valve offered a specific advantage over standard surgical bioprosthetic valves. The Evolut valve has been safely implanted through femoral, axillary, carotid, and direct aortic access vessels. We now report successful direct surgical placement of the Evolut bioprosthesis in patients with small anatomy, avoiding PPM and the need for aortic root enlargement. Excellent hemodynamics were achieved in all patients.
Historically, higher rates of permanent pacemaker implantation have been reported after TAVR as compared with SAVR,6 although these rates have been reduced with the use of new transcatheter aortic valve implantation techniques.7 No patients in our series required a new permanent pacemaker implantation.
Avoidance of aortic root or annular enlargement may reduce the longer cross-clamp times, longer cardiopulmonary bypass times, and longer procedure times associated with enlargement. Haunschild et al8 reported in 4,120 patients no significant differences in early mortality in patients undergoing concomitant AVR and aortic root enlargement compared with AVR alone; however, they cautioned surgeons of a higher risk of respiratory failure.
Estimates of bioprosthetic valve durability have often been in the range of 10 to 15 years; however, the largest reports are limited by the lack of systematic follow-up, significant patient attrition, and outdated definitions of SVD.9 More rigorous data from contemporary randomized clinical trials are now available. We recently reported the superior durability of self-expanding supra-annular valves (CoreValve and Evolut valves; Medtronic) compared with surgery out to 5 years.1 Limited data are available beyond 5 years, but the NOTION trial, which evaluated the first-generation CoreValve self-expanding valve, reported similar rates of more than moderate SVD (15.4% TAVR vs 20.8% SAVR, P = 0.20) and significantly less severe SVD in the TAVR group compared with SAVR at 10 years (1.5% vs 10.0%, P = 0.004).10
This report describes a single-center experience, and longer-term echocardiographic measures are pending. Use of the Evolut TAV as an alternative to a surgical aortic valve bioprosthesis is considered off-label. For the patients presented here, we believe it was the best option for them. The technique described is fast, simple, and broadly applicable using a device with superior performance and durability.
Funding Support and Author Disclosures
Dr O’Hair has a relationship with Medtronic that includes consulting or advisory. Dr Iyengar is a proctor for Medtronic. Dr Ware has reported that she has no relationships relevant to the contents of this article to disclose.
Acknowledgments
Jane Moore, MS, ELS, MSRM, LLC, drafted this report under the direction of Dr O’Hair, funded by Boulder Community Health. No generative artificial intelligence or artificial intelligence–assisted technologies were used in the development of this manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.O'Hair D., Yakubov S.J., Grubb K.J., et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. 2023;8:111–119. doi: 10.1001/jamacardio.2022.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Mieghem N.M., Deeb G.M., Søndergaard L., et al. Self-expanding transcatheter vs surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol. 2022;7:1000–1008. doi: 10.1001/jamacardio.2022.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma T., Krishnan A.M., Lahoud R., Polomsky M., Dauerman H.L. National trends in TAVR and SAVR for patients with severe isolated aortic stenosis. J Am Coll Cardiol. 2022;80:2054–2056. doi: 10.1016/j.jacc.2022.08.787. [DOI] [PubMed] [Google Scholar]
- 4.Sá M.P.B.O., Carvalho M.M.B., Sobral Filho D.C., et al. Impact of surgical aortic root enlargement on the outcomes of aortic valve replacement: a meta-analysis of 13 174 patients. Interact Cardiovasc Thorac Surg. 2019;29:74–82. doi: 10.1093/icvts/ivy364. [DOI] [PubMed] [Google Scholar]
- 5.Peterson M.D., Borger M.A., Feindel C.M., David T.E. Aortic annular enlargement during aortic valve replacement: improving results with time. Ann Thorac Surg. 2007;83:2044–2049. doi: 10.1016/j.athoracsur.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Forrest J.K., Deeb G.M., Yakubov S.J., et al. 3-Year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. 2023;81:1663–1674. doi: 10.1016/j.jacc.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Grubb K.J., Gada H., Mittal S., Nazif T., et al. Clinical impact of standardized TAVR technique and care pathway: insights from the Optimize PRO Study. JACC Cardiovasc Interv. 2023;16:558–570. doi: 10.1016/j.jcin.2023.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Haunschild J., Scharnowski S., Mende M., et al. Aortic root enlargement to mitigate patient-prosthesis mismatch: do early adverse events justify reluctance? Eur J Cardiothorac Surg. 2019;56:335–342. doi: 10.1093/ejcts/ezz016. [DOI] [PubMed] [Google Scholar]
- 9.Johnston D.R., Soltesz E.G., Vakil N., et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–1247. doi: 10.1016/j.athoracsur.2014.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thyregod H.G.H., Jørgensen T.H., Ihlemann N., et al. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J. 2024;45:1116–1124. doi: 10.1093/eurheartj/ehae043. [DOI] [PMC free article] [PubMed] [Google Scholar]