Abstract
Background
Progressive systemic right ventricle (sRV) dysfunction is a significant challenge in adult congenital heart disease. Current guidelines do not specify effective heart failure medications for patients with sRV; however, previous studies have relied on semiquantitative assessments. The advancement of cardiac magnetic resonance (CMR) imaging as the gold-standard modality offers quantitatively accurate assessments even for complex cardiac anomalies. Therefore, we aimed to investigate prognostic factors associated with sRV dysfunction in patients on angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACE-I/ARB), using CMR-derived quantitative values.
Methods
We conducted a retrospective cohort study of 17 adult patients with sRV treated with ACE-I/ARB and performed logistic regression analysis, with the primary outcome defined as sRV ejection fraction (sRVEF) deterioration.
Results
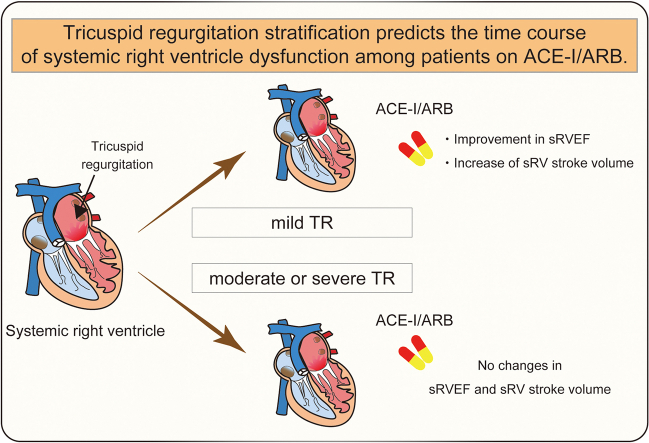
Over an average follow-up period of 68.7 months, sRVEF deterioration occurred in 3 patients (17%). Logistic regression analysis identified tricuspid regurgitation (TR) as a potential independent prognostic factor for the primary outcome (odds ratio = 1.11; 95% confidence interval, 1.00-1.31). Furthermore, patients with mild TR (TR fraction ≤15%; N = 12) experienced improvements in sRVEF between the initial and last CMR assessments (from 49.1% ± 8.4% to 56.7% ± 8.0%, P = 0.0029), with increased stroke volume from 68.2 ± 18.6 to 79.5 ± 17.2 mL (P = 0.0029). In contrast, these changes were not observed in patients with moderate or severe TR (TR fraction >16%) (N = 5).
Conclusions
Our CMR-based evaluation highlights the potential utility of TR stratification in predicting the changes in sRVEF among patients with sRV on ACE-I/ARB. Future randomized controlled trials that consider TR severity are required to elucidate the significance of ACE-I/ARB therapy.
Graphical abstract
Résumé
Contexte
La dysfonction progressive du ventricule droit systémique (VDs) représente un défi important dans la cardiopathie congénitale chez les adultes. Les lignes directrices actuelles n’indiquent aucun médicament efficace contre l’insuffisance cardiaque chez les patients présentant un VDs. Des études antérieures se sont appuyées sur des évaluations semi-quantitatives. La promotion de l’imagerie par résonance magnétique (IRM) cardiaque comme modalité de référence permet des évaluations quantitativement exactes, même en cas d’anomalies cardiaques complexes. C’est pourquoi nous voulions étudier les facteurs pronostiques associés à la dysfonction du VDs chez les patients qui prennent des inhibiteurs de l’enzyme de conversion de l’angiotensine (ECA) ou des antagonistes des récepteurs de l’angiotensine (ARA), en utilisant des valeurs quantitatives dérivées de l’IRM cardiaque.
Méthodologie
Nous avons mené une étude de cohorte rétrospective comptant 17 patients adultes atteints d’un VDs traités par un inhibiteur de l’ECA ou par un ARA et nous avons procédé à une analyse de régression logistique, en utilisant la détérioration de la fraction d’éjection du VDs comme critère d’évaluation principal.
Résultats
Sur une période de suivi moyenne de 68,7 mois, la fraction d’éjection du VDs s’est détériorée chez 3 patients (17 %). L’analyse de régression logistique a permis d’établir que la régurgitation tricuspidienne (RT) était un facteur pronostique indépendant possible pour le critère d’évaluation principal (risque relatif approché = 1,11; intervalle de confiance à 95 % : 1,00-1,31). De plus, la fraction d’éjection du VDs s’est améliorée entre les premiers et les derniers examens d’IRM cardiaque (de 49,1 % ± 8,4 % à 56,7 % ± 8,0 %, p = 0,0029) chez les patients qui présentaient une RT légère (fraction de RT ≤ 15 %; N = 12), le volume systolique passant de 68,2 ± 18,6 à 79,5 ± 17,2 ml (p = 0,0029). Par contre, ces variations n’ont pas été observées chez les patients présentant une RT modérée à sévère (fraction de RT > 16 %) (N = 5).
Conclusions
Notre évaluation fondée sur l’IRM cardiaque souligne l’utilité possible de la stratification de la RT pour prédire les variations de la fraction d’éjection du VDs chez les patients présentant un VDs traités par un inhibiteur de l’ECA ou un ARA. Il faut d’autres essais contrôlés à répartition aléatoire qui tiendront compte de la gravité de la RT pour élucider la portée du traitement par des inhibiteurs de l’ECA ou des ARA.
Heart failure (HF) therapy in patients with normal heart structure is making remarkable progress;1,2 however, the medical management of patients with congenital heart disease (CHD) remains poorly understood. Surgical advances in CHD over several decades have saved the lives of countless children, and over 90% of them are now able to survive into adulthood.3 This, in turn, has created the new field of adult CHD (ACHD). As these patients with ACHD have not been experienced in clinical settings previously, it is not clear whether the current conventional treatments and strategies for structurally normal patients are also appropriate for patients with ACHD. Notably, there is very limited evidence regarding patients with systemic right ventricle (sRV), who account for approximately 3%-12% of all patients with CHD.4,5 Anatomically, the right ventricle (RV) is distinctively different from the left ventricle (LV) in terms of fibromuscular architecture, shape, and function;6 therefore, novel methodologies that are specifically for the RV are required. Recent advances in cardiac magnetic resonance (CMR) have made it possible to accurately assess the morphologic aspects of any heart with a complex anomaly,7 thus providing reliable data for patients with ACHD.
Representative disorders of sRV in biventricular physiology include congenitally corrected transposition of the great arteries (ccTGA) and dextro-transposition of the great arteries after Mustard/Senning operation (D-TGA (M/S)). Patients with these conditions tend to develop HF in middle age due to progressive sRV dysfunction.8,9 As sRV dysfunction is directly associated with poor prognosis, the outcome for the patients is heart transplantation or death.10 Drug therapy for sRV failure has not been established according to the current guidelines.11,12 A recent report has demonstrated the efficacy of sacubitril/valsartan in these patients;13 however, a previous large retrospective observational study showed no benefits from treatments with angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACE-I/ARB),14 and the most recent meta-analysis also failed to show a beneficial effect of HF medications.15 Our concern is that these studies used echocardiography or nonstandardized assessment of cardiac contractility with various modalities that did not allow an accurate evaluation of sRV ejection fraction (sRVEF) or valve regurgitation. Thus, in the present study, we used the gold-standard modality of CMR to obtain quantitatively accurate data regarding cardiac volume, function, and regurgitation fraction. This study aimed to evaluate the changes in sRV function in patients who were on ACE-I/ARB, using the accurate CMR data.
Methods
Patients and study design
We conducted a retrospective cohort study of all ACHD outpatients with sRV aged 18 years and older who periodically visited the ACHD-specialized clinics at the University of Tokyo Hospital. They included patients with ccTGA and D-TGA (M/S). CMR assessments were performed as part of their routine examinations between April 2012 and December 2022. The patients were consecutively included in the Japanese Network of Cardiovascular Departments for Adult Congenital Heart Disease registry (reference no. 10680).5 The study was performed based on the project “The retrospective analysis associated with clinical indicator, treatment outcome and prognosis about cardiovascular disease” (reference no. 2650-14), both of which were approved by the Research Ethics Committee of the Graduate School of Medicine, the University of Tokyo.
Outcome of interest
The primary outcome was defined as sRVEF deterioration measured using CMR to determine the difference between the oldest (initial) and the latest (last) records in the observational period. After determining the prognostic factors, a single-arm comparison of the initial and last sRVEF on CMR was performed for the poor prognostic factor. In addition, the cutoff value of a continuous variable associated with the prognosis was calculated.
Data collection
Clinical data including cardiac anatomy, age, sex, body surface area, medications, device implantation, and clinical course were retrieved from patients’ electronic records. Regarding the medical information, we recorded “ACE-I/ARB use” when a patient started the medication before or around the time of the initial CMR assessment and continued to take it until the last CMR assessment.
CMR recordings
CMR was performed using Siemens (Bayern, Germany) MAGNETOM Avanto 26063 (1.5T) at the University of Tokyo Hospital, Philips (Amsterdam, Netherlands) Achieva 32775 (1.5T) at the Advanced Imaging Center Yaesu Clinic, Philips Achieva 32230 (1.5T) at the Cardiovascular Imaging Clinic (Iidabashi), Siemens MAGNETOM Vida 175906 (3T) at Seibo Hospital, and in other hospitals. Electrocardiogram-gated CMR recordings were obtained from patients in the supine positions during breath holding and expiration. Collected CMR data included end-diastolic/systolic ventricular volumes (EDV/ESV) calculated from the cine ventriculogram and blood flows measured using the phase contrast method (Supplemental Fig. S1).7 Short-axis ventricular cine images covering the atrioventricular annulus to the apex of the sRV were obtained. Next, the phase-contrast flow was recorded at the bases of the pulmonary artery (PA) and aorta (Ao); at the precardiac levels of the superior and inferior vena cava, respectively; at the main, right, and left PAs; and at major pulmonary veins connecting to the atria, normally consisting of the right superior, right inferior, left superior, and left inferior pulmonary veins.
Well-trained CMR specialists (K.S., H.T, K.G.) performed volumetric and flow analyses using the CVI42 software (Circle Cardiovascular Imaging, Calgary, Alberta, Canada) following standard methods.16 Briefly, the end-diastolic and end-systolic images at each slice were visually defined, with the endomyocardial border traced and intraventricular muscles removed, automatically providing the EDV and ESV (index) (EDV(I) and ESV(I), respectively; mL [mL/m2]) and systemic heart weight (RV mass, g/m2). Stroke volume (SV, mL) was calculated as the difference between EDV and ESV, and ejection fraction (EF, %) was calculated using the following formula: EF = (EDV – ESV)/EDV. For cardiac output (CO, L/min), SV and heart rate (HR, pulses/min) were measured. The cardiac index (L/min/m2) was determined by dividing CO by body surface area.
For the flow calculations, pulmonary blood flow (index) (Qp(I), L/min [L/min/m2]) was calculated using the following formula: pulmonary SV (pSV, mL) × HR, where pSV was measured from the best one among the flow records of the PA, major pulmonary veins, and atrioventricular valves. The systemic blood flow (index) (Qs(I), L/min [L/min/m2]) was similarly calculated from the best record among the flow records of the Ao, inferior and superior vena cava, and atrioventricular valves. The forward and reverse flows in the tricuspid valve (TV, mL), mitral valve (MV, mL), pulmonary valve (PV, mL), and aortic valve (AoV, mL) were measured, and sRV and pLV in net forward were calculated as (TV forward flow – TV reverse flow) × HR and (MV forward flow – MV reverse flow) × HR, respectively. The regurgitant fractions (%) (pulmonary regurgitation fraction and aortic regurgitation fraction of the PV and AoV, respectively) were directly calculated as reverse flow (mL)/forward flow (mL) in the flow records. On the other hand, tricuspid and mitral regurgitant fractions (TRF and MRF, respectively) were quantified indirectly; for example, TRF was obtained from the following formula: indirect TRF = (sRVSV – Ao forward flow)/sRVSV. The indirect MRF was obtained similarly. The missing values in the CMR parameters were denoted as null values.
Based on a previous CMR guideline,17 we graded tricuspid regurgitation (TR) severity by defining the thresholds of TRF (≤15% for mild, 16%-25% for moderate, 25%-48% for moderate to severe, and >48% for severe), which were borrowed from mitral regurgitation classification based on the description in the “CMR Evaluation of TR Severity” section.
Last, the compatibility of quantitative analysis in CMR was assessed in Supplemental Table S1.
Statistical analysis
Data were presented as counts (%), mean ± standard deviation, or estimates (95% confidence intervals [CIs]) as appropriate. Fisher’s exact test was conducted to compare frequencies because the sample size was <20. A paired t test was used for single-arm analysis. Logistic regression analysis was used to identify the RVEF deterioration factors in CMR parameters. After P values of variables were obtained from univariate analysis, multivariate regression analysis was performed using stepwise selection, with a P value of <0.15 required for entry. In a selection of variables, clinical justification was also considered for variables that have the same meanings not to overlap. To determine the cutoff value for a continuous variable that was a poor prognostic factor, receiver operating characteristic analysis was conducted, and the area under the curve, sensitivity, and specificity were calculated. All statistical tests were 2-sided, and statistical significance was set at P < 0.05. All statistical analyses were performed using JMP 16 (SAS Institute, Cary, NC) and GraphPad Prism 9 (GraphPad Software).
Results
Between April 2012 and December 2022, the electronic records of 24 patients with ccTGA and 54 patients with D-TGA (M/S) were extracted at our facility.5 Patients with sRV who underwent CMR more than once consisted of 10 patients with ccTGA and 10 with D-TGA (M/S) (6 after Mustard operation and 4 after Senning operation). Among these patients, 3 did not use ACE-I/ARB; thus, we focused on 17 patients in our cohort (Supplemental Fig. S2). All patients started taking ACE-I/ARB before or around the time of the initial CMR assessment, and none discontinued these drugs due to side effects. The average duration between the initiation of ACE-I/ARB and the last CMR assessment was 111 ± 82 months (range: 17-317 months).
Patients’ characteristics
This study had male dominance, and the mean age of patients was 44.4 years (range: 18-65 years) at the latest follow-up. Patient characteristics did not differ significantly between the ccTGA and D-TGA (M/S) groups, except for TV replacement (TVR) in CMR intervals and pulmonary hypertension (PH) (Table 1). Two in 4 patients (2 in ccTGA and 0 in D-TGA (M/S)) had TVR before the initial CMR assessment, whereas the other 2 patients (0 in ccTGA and 2 in D-TGA (M/S)) were needed to replace TV for severe TR between the initial and last CMR assessments. Approximately half of the D-TGA (M/S) patients developed PH; in contrast, none of the patients with ccTGA had PH. In our cohort, most patients were asymptomatic or mildly symptomatic, presumably because patients supported with mechanical devices were excluded as this was a contraindication in CMR. Average CMR interval periods were 89.4 ± 20.8 (range: 59-123) and 53.3 ± 17.4 (range: 35-86) months in the ccTGA and D-TGA (M/S) groups, respectively. The follow-up period in the ccTGA group was significantly longer than that in the D-TGA (M/S) group (P = 0.0024) (Supplemental Fig. S3).
Table 1.
Patients’ characteristics
| Characteristic | ccTGA (N = 8) | D-TGA (M/S) (N = 9) | All patients (N = 17) |
|---|---|---|---|
| Gender (male), n (%) | 4 (50) | 5 (55.6) | 9 (52.9) |
| Age (SD, range) (y) | 46.6 (13.4, 18-65) | 42.4 (6.4, 30-51) | 44.4 (10.5, 18-65) |
| BSA (SD) (m2) | 1.64 (0.2) | 1.63 (0.2) | 1.63 (0.20) |
| Surgical repair, n (%) | |||
| Mustard operation | – | 5 (55.6) | – |
| Senning operation | – | 4 (44.4) | – |
| Complex anatomy, n (%) | |||
| Ventricular septal defect | 4 (50) | 3 (33.3) | 7 (41.2) |
| Pulmonary stenosis | 4 (50) | 5 (55.6) | 9 (52.9) |
| Tricuspid regurgitation, n (%) | |||
| Mild | 6 (75) | 6 (66.7) | 12 (70.6) |
| Moderate or severe | 2 (25) | 3 (33.3) | 5 (29.4) |
| Tricuspid valve replacement, n (%) | 2 (25) | 2 (22.2) | 4 (23.5) |
| Tricuspid valve replacement in CMR intervals, n (%) | 0 (0) | 2 (22.2) | 2 (22.2) |
| NYHA class, n (%) | |||
| Ⅰ | 5 (62.5) | 4 (44.4) | 9 (52.9) |
| Ⅱ | 3 (37.5) | 4 (44.4) | 7 (41.2) |
| Ⅲ/Ⅳ | 0 (0) | 1 (11.1) | 1 (5) |
| Pulmonary hypertension, n (%) | 0 (0) | 4 (44.4) | 4 (23.5) |
| Brain natriuretic peptide (pg/mL) | 53.3 (27.8) | 55.1 (39.1) | 54.2 (34.3) |
| ECG, n (%) | |||
| Sinus | 6 (75) | 9 (100) | 15 (88.2) |
| Supraventricular tachycardia | 2 (25) | 0 (0) | 2 (11.8) |
| QRS width (SD) (ms) | 123.4 (22.4) | 124.3 (28.7) | 122.2 (25.7) |
| Medications (at last CMR assessment), n (%) | |||
| β-Blocker | 5 (62.5) | 5 (55.6) | 10 (58.8) |
| SGLT2 inhibitor | 2 (25) | 1 (11.1) | 3 (17.6) |
| MRA | 4 (50) | 5 (55.6) | 9 (52.9) |
| Diuretics | 4 (50) | 5 (55.6) | 9 (52.9) |
BSA, body surface area; ccTGA, congenitally corrected transposition of the great arteries; CMR, cardiac magnetic resonance; D-TGA (M/S), dextro-transposition of the great arteries after Mustard/Senning operations; ECG, electrocardiogram; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SD, standard deviation; SGLT2, sodium-dependent glucose transporter 2 inhibitor.
Logistic regression analysis for the primary endpoint
The event of sRVEF deterioration occurred in 3 patients (1 with ccTGA and 2 with D-TGA (M/S)) (17.6%). First, we performed univariate logistic regression analysis, which showed that among CMR parameters, the odds ratio (OR) of TRF significantly increased (OR = 1.11; 95% CI, 1.02-1.30), whereas other parameters did not show statistically significant differences (Table 2). Through stepwise selection in multivariate logistic regression analysis, only TRF emerged as an independent poor prognostic factor for sRVEF deterioration (OR = 1.11; 95% CI, 1.00-1.31). Notably, there were no significant differences in systolic and diastolic blood pressure between the initial and last assessments in our cohort (Supplemental Fig. S4).
Table 2.
The results of logistic regression analysis for predicting systemic right ventricle ejection fraction
| CMR parameter | sRVEF deterioration |
|||
|---|---|---|---|---|
| Univariate |
Multivariate |
|||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| TRF (%) (per % increase) | 1.11 (1.02-1.30) | 0.0118 | 1.11 (1.00-1.31) | 0.0483 |
| ARF (%) (per % increase) | 0.69 (0.17-1.23) | 0.2998 | – | – |
| MRF (%) (per % increase) | 1.01 (0.86-1.13) | 0.8604 | – | – |
| PRF (%) (per % increase) | 1.03 (0.96-1.11) | 0.3308 | – | – |
| sRVEDV (mL) | 1.00 (0.97-1.02) | 0.8002 | – | – |
| sRVEDVI (mL/m2) | 1.02 (0.96-1.09) | 0.5700 | – | – |
| sRVESV (mL) | 0.97 (0.91-1.01) | 0.2173 | – | – |
| sRVESVI (mL/m2) | 0.97 (0.89-1.05) | 0.4805 | – | – |
| sRVCO (L/min) | 1.61 (0.90-3.81) | 0.1106 | 0.87 (0.18-3.75) | 0.8431 |
| pLVEDV (mL) | 0.99 (0.95-1.02) | 0.4869 | – | – |
| pLVEDVI (mL/m2) | 1.02 (0.96-1.09) | 0.5700 | – | – |
| pLVESV (mL) | 1.00 (0.95-1.05) | 0.8978 | – | – |
| pLVESVI (mL/m2) | 1.02 (0.95-1.09) | 0.6023 | – | – |
| pLVCO (L/min) | 0.84 (0.28-2.31) | 0.7320 | – | – |
| pLVEF (%) | 1.00 (0.93-1.11) | 0.9126 | – | – |
| QsI (L/min/m2) | 0.07 (<0.01-2.26) | 0.1562 | – | – |
| QpI (L/min/m2) | 0.10 (<0.01-3.62) | 0.2333 | – | – |
Bold P values indicate statistical significance.
ARF, aortic regurgitation fraction; CI, confidence interval; MRF, mitral regurgitation fraction; pLVCO, pulmonary left ventricle cardiac output; pLVEDV(I), pulmonary left ventricle end-diastolic volume (index); pLVEF, pulmonary left ventricle ejection fraction; pLVESV(I), pulmonary left ventricle end-systolic volume (index); pLVSV, pulmonary left ventricle stroke volume; PRF, pulmonary regurgitation fraction; sRVCO, systemic right ventricle cardiac output; QpI, pulmonary blood flow index; QsI, systemic blood flow index; sRVEDV(I), systemic right ventricle end-diastolic volume (index); sRVEF, systemic right ventricle ejection fraction; sRVESV(I), systemic right ventricle end-systolic volume index; sRVSV, systemic right ventricle stroke volume; TRF, tricuspid regurgitation fraction.
sRVEF changes based on the extent of tricuspid regurgitation
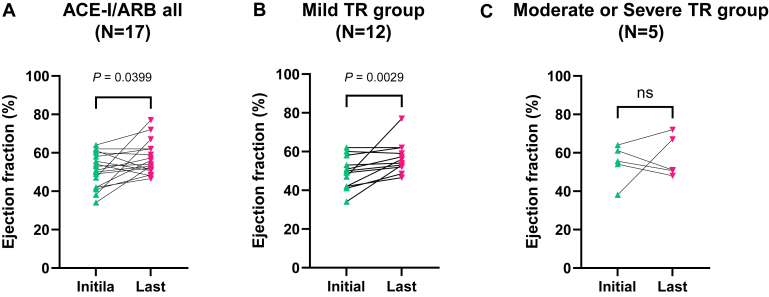
When comparing sRVEF in the 17 patients, there was a significant improvement from 50.7% ± 9.0% at the initial assessment to 57.0% ± 8.6% at the last assessment (P = 0.0399) (Fig. 1A).
Figure 1.
Changes in systemic right ventricle ejection fraction. (A) Systemic right ventricle ejection fraction (sRVEF) improved in all patients treated with ACE-I/ARB from 50.7% ± 9.0% at the initial assessment to 57.0% ± 8.6% at the last assessment (N = 17, Wilcoxon matched-pairs signed-rank test, P = 0.0399). The patients were divided into 2 groups based on the severity of tricuspid regurgitation (TR). (B) The mild TR group showed a significant improvement in sRVEF from 49.1% ± 8.4% to 56.7% ± 8.0% (N = 12, Wilcoxon matched-pairs signed-rank test, P = 0.0029), whereas (C) there was no sRVEF improvement in the moderate or severe TR group (from 54.6% ± 10.0% to 57.7% ± 10.9%; N = 5, Wilcoxon matched-pairs signed-rank test, P > 0.9999). ACE-I/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; ns, not significant; sRVEF, systemic right ventricle ejection fraction; TR, tricuspid regurgitation.
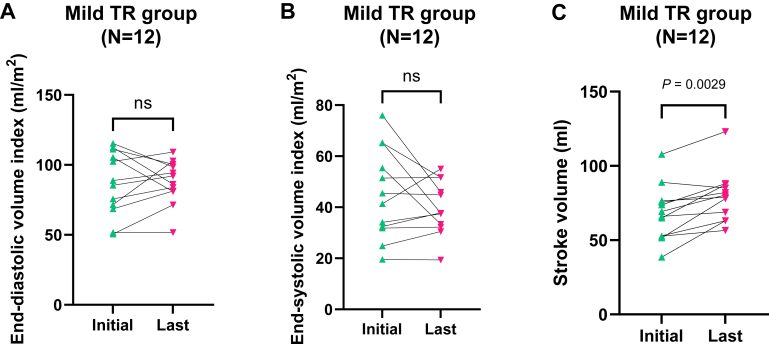
Twelve patients were classified as having mild TR, whereas the remaining patients exhibited moderate or severe TR on the initial CMR assessment. The comparison between the initial and last CMR assessments within the mild TR group revealed a significant sRVEF improvement (from 49.1% ± 8.4% to 56.7% ± 8.0%, P = 0.0029), whereas no significant changes were observed in the moderate or severe TR group (from 54.6% ± 10.0% to 57.7% ± 10.9%, P > 0.9999) (Fig. 1, B and C), although the sample size was very small. In the mild TR group, EDV(I) changed from 86.6 ± 23.2 mL/m2 to 87.3 ± 15.7 mL/m2 (P = 0.8501), and ESV(I) was likely to decrease from 45.2 ± 17.7 mL/m2 to 39.8 ± 10.4 mL/m2, although it did not reach statistical significance (P = 0.6221). On the other hand, sRVSV significantly increased at the last assessment compared with that at the initial one (from 68.2 ± 18.6 mL to 79.5 ± 17.2 mL, P = 0.0029) (Fig. 2).
Figure 2.
Comparison of systemic right ventricle volume and stroke volume in patients with mild TR treated with ACE-I/ARB. (A, B) There were no significant differences between the initial and last evaluations of end-diastolic/systolic volume index in the mild TR group treated with ACE-I/ARB (N = 12, Wilcoxon matched-pairs signed-rank test, P = 0.8501 and P = 0.6221, respectively). (C) Stroke volume in the same population significantly increased from 68.2 ± 18.6 mL to 79.5 ± 17.2 mL (N = 12, Wilcoxon matched-pairs signed-rank test, P = 0.0029). ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ns, not significant; TR, tricuspid regurgitation.
To explore the influence of TVR on the changes in sRVEF by TR stratification, sRV function between the initial and last CMR assessments was compared in the population excluding patients who performed TVR. In 13 patients without TV interventions, sRVEF improved from 50.7% ± 9.5% to 56.2% ± 7.8% (P = 0.0493) (Supplemental Fig. S5). Furthermore, patients with mild TR among this population showed a significant sRVEF improvement (from 49.7% ± 8.4% to 54.8% ± 5.6%, P = 0.0156). In contrast, we could not find the significant changes in sRVEF from 52.9% ± 10.8% at the initial CMR assessment to 59.4% ± 11.9% at the last one (P = 0.6250). These findings were consistent with the above results, and our cohort demonstrated that the mild TR group was likely to improve sRVEF between the initial and last CMR assessments, regardless of TVR.
Because β-blockers are also one of the most important HF medications, we divided our cohort into 2 groups based on the use of β-blockers. Ten patients received both ACE-I/ARB and β-blockers, whereas the remaining patients received only ACE-I/ARB. A total of 9 patients (90%) experienced sRVEF improvement in the former group, whereas 5 patients (71.4%) experienced sRVEF improvement in the latter group (P = 0.5368 in Fisher’s exact test) (Supplemental Fig. S6).
Receiver operating curve analysis of tricuspid regurgitation fraction for sRVEF deterioration
For the 17 patients in our cohort, additional analysis regarding TR showed that a cutoff TRF value of 21.6% provided high sensitivity and specificity for predicting sRVEF deterioration (66.7% and 92.9%, respectively). The area under the curve value of 0.86 was considered good, although it did not reach statistical significance (95% CI, 0.6028-1.000, P = 0.0588) (Supplemental Fig. S7).
Discussion
We conducted a retrospective cohort study of 17 adult patients with sRV who are taking ACE-I/ARB in a single centre to evaluate prognostic factors associated with sRV dysfunction. We found that TR stratification could be useful for predicting the changes in the time course of sRVEF among patients on ACE-I/ARB, and patients with mild TR showed improvement of sRVEF and sRVSV over a period of approximately 5.5 years.
Previous reports showed that sRV dysfunction progresses with aging,18 and it is reported that fractional area change in sRV observed using echocardiography decreases yearly by –3%.8 Although the direct comparison of sRVEF by CMR and fractional area change using echocardiography is very difficult, the observational duration over 5 years in our cohort seemed to cause apparent sRVEF deterioration. However, our cohort showed no sRVEF deterioration but rather the improvement of sRVEF and sRVSV among patients with mild TR who were using ACE-I/ARB. Because we did not have a control group in this study, our data could not discuss the efficacy of ACE-I/ARB in patients with sRV. However, the previous randomized trials showed no effective HF medications to improve their prognosis.15 Importantly, the severity of TR was not taken into account in these studies. For example, one of them had the characteristic patient background, in which 45% of the patients had moderate or severe TR at baseline.19 This may influence the results of the lack of efficacy in patients with sRV on ACE-I/ARB. Mitral regurgitation (MR) with normal heart structure may provide useful information on this issue. Over half of patients with severe functional MR fail to experience improved LV function despite optimal medical management,20 and patients with a disproportionately higher degree of MR relative to LV dilatation do not appear to benefit from medical management compared with those who undergo mitral intervention (such as MitraClip).21,22 Therefore, it is understandable that the extent of TR could produce the differences in sRVEF among patients on ACE-I/ARB. Furthermore, semiquantitative assessments of patients with sRV in previous studies may have obscured the changes in sRV function among these patients. According to a report by Ladouceur et al.,14 it was difficult to apply quantitative measurements in patients with sRV because of cardiac complexity. However, recent technological advances in CMR have allowed the use of accurate quantitative evaluations even for these patients, and we demonstrated that patients with mild TR who were taking ACE-I/ARB could experience sRVEF and sRVSV improvement. Our findings suggest that TR stratification is important in predicting the differences in the time course of sRVEF among patients on ACE-I/ARB, and we believe that quantitative and accurate CMR data strengthen the current study.
Bench-side data may be helpful in considering the influence of ACE-I/ARB in sRV. Many basic studies have suggested an essential role of the renin-angiotensin-aldosterone system in the development of cardiac hypertrophy, fibrosis, and LV dysfunction.23, 24, 25 The availability of ACE-I/ARB for patients with sRV remains unclear due to marked differences between the right and left myocardium. However, recent advanced technologies in basic research have revealed gene expression in a single cell level, in which the myocardium in the RV also expresses angiotensin II receptor (ATR) 1, ATR2, and aldosterone receptor (NR3C2),26 suggesting that renin-angiotensin-aldosterone system activation could similarly contribute to sRV dysfunction.
The extent of TRF is another distinguishing issue for patients with sRV. Previous reports have shown that TR promotes sRV dysfunction in the proportion to TR severity.10,18 In current clinical practice, TVR is the treatment of choice for sRV dysfunction. However, when to implant a TV is a challenging question because the extent to which TR could promote sRV dysfunction remains unclear.11 It is considered that TVR before significant sRV dysfunction (sRVEF <40%) is key to preventing sRV failure;27,28 however, there is no clear evidence regarding the definitive value that promotes sRV dysfunction. Using CMR, we identified that a TRF of 21.6% could be the cutoff value as an accelerator of sRVEF deterioration. This value corresponds to “moderate” TR (TRF of 16%-25%) based on the guideline from the American Society of Echocardiography,17 and this result seems to be reasonable considering the Japanese Circulation Society 2022 guideline on the surgical indication for TR in patients with sRV.29 Based on our cohort, a TRF of 21.6% measured using CMR could be a promoting factor for sRVEF deterioration; however, it still remains unknown whether TVR could certainly improve sRV function in these patients. Actually, in our cohort, 1 of 2 patients who received TVR during our observational periods had a decline of sRVEF, whereas the other patient showed no change in sRVEF after TVR. A larger sample size is needed to clarify this issue, and this area should be further investigated in the future.
Last, 10 patients in our cohort were taking ACE-I/ARB and β-blocker, but β-blocker treatment did not seem to affect sRVEF in our cohort. Needless to say, it is important to consider a synergistic effect of ACE-I/ARB and β-blocker,30 and the accumulation of evidence regarding the additive effect of β-blocker in patients with sRV will be required.
Limitations
Our study has several limitations. First, it was a retrospective, single-centre study with a very small sample size, resulting in a broad CI. Therefore, statistical analysis using our small sample size may conceal the truth about changes especially in sRVEF of the moderate or severe TR group. Next, we ignored other HF medications except for β-blocker; thus, the influence of these agents on sRVEF should be considered as a confounding factor. We also defined any decrease in sRVEF as sRVEF deterioration because the current guidelines do not clearly define this. Even in the accurate CMR evaluation, there is a slight measurement error, and the different threshold settings may affect the results. In addition, our research detected the difference in sRVEF between the initial and last CMR assessments based on TR stratification. However, whether this difference could change the prognosis of these patients remained unknown, and much longer observational periods may be required to understand these critical outcomes. Another limitation is that we excluded patients who were using implanted pacemakers, cardiac resynchronization therapy, or implantable cardioverter defibrillators, most of whom were likely to have severe HF. This caused a selection bias. In addition, there was a referral bias, which means that patients with relatively more complex diseases tend to be referred to our centre because it is a certified multiorgan transplant facility. Finally, we only extracted patients’ medical information regarding CMR parameters. Possibly, better predictors of sRVEF deterioration might exist among laboratory data or values obtained from other modalities such as electrocardiogram and echocardiography.
Conclusions
Our study demonstrated that TR stratification could be helpful for predicting the changes in the time course of sRVEF among patients with sRV on ACE-I/ARB. Further randomized control trials considering TR severity may help elucidate the importance of ACE-I/ARB.
Acknowledgements
The authors would like to thank all coauthors for their valuable expertise.
Ethics Statement
The study was approved by the Research Ethics Committee of the Graduate School of Medicine, the University of Tokyo.
Patient Consent
The authors confirm that patient consent forms have been obtained for this article.
Funding Sources
This study was financially supported by the Japan Agency for Medical Research and Development (AMED), the Japanese Association of Congenital Heart Disease with Pulmonary Hypertension Registry Project, and the Japanese Ministry of Health, Labour and Welfare (21FC1014).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2024.07.002.
Supplementary Material
References
- 1.Abdin A., Bauersachs J., Soltani S., et al. A practical approach to the guideline-directed pharmacological treatment of heart failure with reduced ejection fraction. ESC Heart Fail. 2023;10:24–31. doi: 10.1002/ehf2.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42:681–683. doi: 10.1093/eurheartj/ehaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moons P., Bovijn L., Budts W., Belmans A., Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122:2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343. [DOI] [PubMed] [Google Scholar]
- 4.Brida M., Diller G.P., Gatzoulis M.A. Systemic right ventricle in adults with congenital heart disease: anatomic and phenotypic spectrum and current approach to management. Circulation. 2018;137:508–518. doi: 10.1161/CIRCULATIONAHA.117.031544. [DOI] [PubMed] [Google Scholar]
- 5.Yao A., Inuzuka R., Mizuno A., et al. Status of adult outpatients with congenital heart disease in Japan: the Japanese Network of Cardiovascular Departments for Adult Congenital Heart Disease Registry. J Cardiol. 2022;80:525–531. doi: 10.1016/j.jjcc.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Haddad F., Hunt S.A., Rosenthal D.N., Murphy D.J. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa Y. Cardiac magnetic resonance imaging. Pediatr Cardiol Cardiac Surg. 2016;32:291–306. [Google Scholar]
- 8.Egbe A.C., Miranda W.R., Jain C.C., Connolly H.M. Prognostic implications of progressive systemic ventricular dysfunction in congenitally corrected transposition of great arteries. JACC Cardiovasc Imaging. 2022;15:566–574. doi: 10.1016/j.jcmg.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirjavainen M., Happonen J.M., Louhimo I. Late results of Senning operation. J Thorac Cardiovasc Surg. 1999;117:488–495. doi: 10.1016/s0022-5223(99)70329-6. [DOI] [PubMed] [Google Scholar]
- 10.van Dissel A.C., Opotowsky A.R., Burchill L.J., et al. End-stage heart failure in congenitally corrected transposition of the great arteries: a multicentre study. Eur Heart J. 2023;44:3278–3291. doi: 10.1093/eurheartj/ehad511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgartner H., De Backer J., Babu-Narayan S.V., et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 12.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 13.Fusco F., Scognamiglio G., Merola A., et al. Safety and efficacy of sacubitril/valsartan in patients with a failing systemic right ventricle: a prospective single-center study. Circ Heart Fail. 2023;16 doi: 10.1161/CIRCHEARTFAILURE.122.009848. [DOI] [PubMed] [Google Scholar]
- 14.Ladouceur M., Segura de la Cal T., Gaye B., et al. Effect of medical treatment on heart failure incidence in patients with a systemic right ventricle. Heart. 2021;107:1384–1389. doi: 10.1136/heartjnl-2020-318787. [DOI] [PubMed] [Google Scholar]
- 15.Zaragoza-Macias E., Zaidi A.N., Dendukuri N., Marelli A. Medical therapy for systemic right ventricles: a systematic review (part 1) for the 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e801–e813. doi: 10.1161/CIR.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 16.Fratz S., Chung T., Greil G.F., et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15:51. doi: 10.1186/1532-429X-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Prieto L.R., Hordof A.J., Secic M., Rosenbaum M.S., Gersony W.M. Progressive tricuspid valve disease in patients with congenitally corrected transposition of the great arteries. Circulation. 1998;98:997–1005. doi: 10.1161/01.cir.98.10.997. [DOI] [PubMed] [Google Scholar]
- 19.van der Bom T., Winter M.M., Bouma B.J., et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127:322–330. doi: 10.1161/CIRCULATIONAHA.112.135392. [DOI] [PubMed] [Google Scholar]
- 20.Nasser R., Van Assche L., Vorlat A., et al. Evolution of functional mitral regurgitation and prognosis in medically managed heart failure patients with reduced ejection fraction. JACC Heart Fail. 2017;5:652–659. doi: 10.1016/j.jchf.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 22.Obadia J.F., Messika-Zeitoun D., Leurent G., et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 23.Brilla C.G., Zhou G., Matsubara L., Weber K.T. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 24.Hale T.M. Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition. J Mol Cell Cardiol. 2016;93:125–132. doi: 10.1016/j.yjmcc.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Wollert K.C., Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovasc Res. 1999;43:838–849. doi: 10.1016/s0008-6363(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 26.Tucker N.R., Chaffin M., Fleming S.J., et al. Transcriptional and cellular diversity of the human heart. Circulation. 2020;142:466–482. doi: 10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Son J.A., Danielson G.K., Huhta J.C., et al. Late results of systemic atrioventricular valve replacement in corrected transposition. J Thorac Cardiovasc Surg. 1995;109:642–652. doi: 10.1016/S0022-5223(95)70345-4. [discussion: 652-43] [DOI] [PubMed] [Google Scholar]
- 28.Mongeon F.P., Connolly H.M., Dearani J.A., Li Z., Warnes C.A. Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. J Am Coll Cardiol. 2011;57:2008–2017. doi: 10.1016/j.jacc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi H., Kawata M., Uemura H., et al. JCS 2022 Guideline on management and re-interventional therapy in patients with congenital heart disease long-term after initial repair. Circ J. 2022;86:1591–1690. doi: 10.1253/circj.CJ-22-0134. [DOI] [PubMed] [Google Scholar]
- 30.Miller R.J.H., Howlett J.G., Fine N.M. A novel approach to medical management of heart failure with reduced ejection fraction. Can J Cardiol. 2021;37:632–643. doi: 10.1016/j.cjca.2020.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.