Graphical abstract
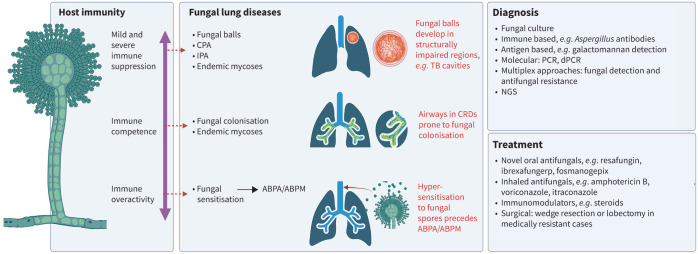
Relationship of fungal lung disease with host immunity, diagnosis and treatment options. CPA: chronic pulmonary aspergillosis; IPA: invasive pulmonary aspergillosis; ABPA: allergic bronchopulmonary aspergillosis; ABPM: allergic bronchopulmonary mycosis; TB: tuberculosis; CRD: chronic respiratory diseases; dPCR: digital PCR; NGS: next-generation sequencing. Figure created with Biorender.com.
Abstract
Fungal lung disease encompasses a wide spectrum of organisms and associated clinical conditions, presenting a significant global health challenge. The type and severity of disease are determined by underlying host immunity and infecting fungal strain. The most common group of diseases are associated with the filamentous fungus Aspergillus species and include allergic bronchopulmonary aspergillosis, sensitisation, aspergilloma and chronic and invasive pulmonary aspergillosis. Fungal lung disease remains epidemiologically heterogenous and is influenced by geography, environment and host comorbidities. Diagnostic modalities continue to evolve and now include novel molecular assays and biomarkers; however, persisting challenges include achieving rapid and accurate diagnosis, particularly in resource-limited settings, and in differentiating fungal infection from other pulmonary conditions. Treatment strategies for fungal lung diseases rely mainly on antifungal agents but the emergence of drug-resistant strains poses a substantial global threat and adds complexity to existing therapeutic challenges. Emerging antifungal agents and increasing insight into the lung mycobiome may offer fresh and personalised approaches to diagnosis and treatment. Innovative methodologies are required to mitigate drug resistance and the adverse effects of treatment. This state-of-the-art review describes the current landscape of fungal lung disease, highlighting key clinical insights, current challenges and emerging approaches for its diagnosis and treatment.
Shareable abstract
Fungal lung disease occurs independently or complicates underlying lung disease. Type and severity are determined by host immunity, geography and environment. Next-generation sequencing is revolutionising diagnostic and treatment strategies. https://bit.ly/3TFwAJ3
Introduction
Fungal lung disease has been increasing in past decades and represents a unique challenge to clinicians worldwide. The spectrum of clinical disease is diverse, ranging from hypersensitivity to colonisation to invasive illness, some with high mortality, with continuing barriers to accurate and prompt diagnostics and limited treatment options. Furthermore, a single patient can “move” between disease states over time, depending on changes to their underlying immunity. With climate change and ageing populations, the array of potential fungal pathogens and associated resistance continues to grow and the increasing use of immunosuppressive or immunomodulating therapies has expanded the “at-risk” patient population.
The intricate relationship between host immune status and fungal infection plays a key role in determining the risk, onset, presentation, course and outcome in all forms of fungal lung disease, exemplified by Aspergillus-associated disease (figure 1). The role of host immunity is important in understanding pathogenesis, achieving diagnosis and selecting treatment for patients, with those immunocompromised at highest risk of invasive disease [1]. Even mild immune alterations, such as those observed in several chronic respiratory disease (CRD) states, predispose to fungal colonisation, allergy and subsequent infection [1–3]. While Aspergillus species are well-recognised causes of fungal lung disease, non-Aspergillus fungi also play a significant role in pulmonary infections. These include a diverse array of pathogens such as Candida, Cryptococcus and Pneumocystis, but include endemic fungi such as Histoplasma, Coccidioides and Blastomyces that can cause severe lung disease, particularly in the immunocompromised patient, presenting unique diagnostic and therapeutic challenges.
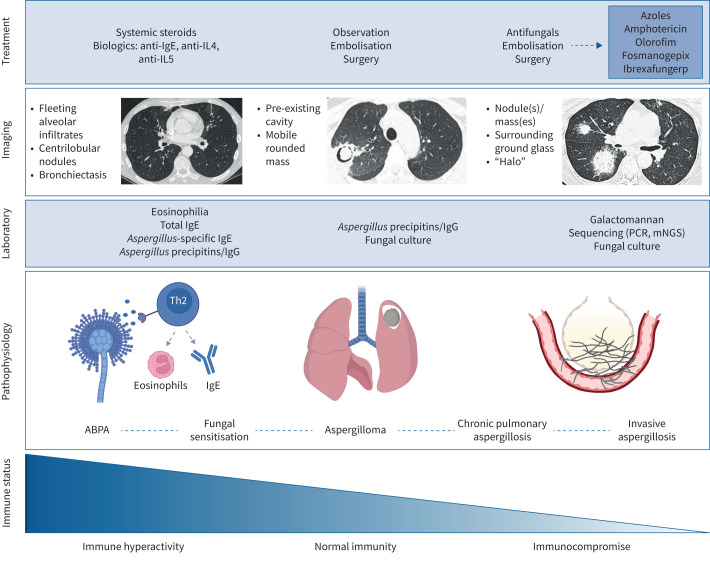
FIGURE 1.
Schematic overview of the clinical spectrum of Aspergillus-associated disease with accompanying relationship to host immune status, radiographic findings, diagnostics and treatment modalities. IL: interleukin; mNGS: metagenomic next-generation sequencing; ABPA: allergic bronchopulmonary aspergillosis; Th2: T-helper type 2. Figure created with Biorender.com.
In this state-of-the-art review, we describe the current landscape of fungal lung disease, highlighting clinical features and the complex relationship to underlying host immunity. We explore current challenges and evaluate emerging, promising approaches for improved diagnosis, including molecular approaches, metagenomic next-generation sequencing (mNGS) and advances in treatment.
Fungal lung disease and immune competence
Individuals with CRDs are prone to fungal colonisation [4–10]. The wide range of reported prevalence (3–57%) is in part due to the varied approaches to detection (culture or PCR) and differing definitions of colonisation, some based on a single sample and others on a series of consecutive samples, demonstrating persistence [4–21]. Aspergillus fumigatus and Candida albicans remain the commonest fungi isolated in CRDs, with key risks for detection including inhaled corticosteroid and antibiotic use, prior exacerbations and isolation of Pseudomonas [5–8, 13, 22, 23]. There is geographic variability in Aspergillus colonisation, with A. fumigatus the most common species identified and variable prevalence reported in India, Spain, Iran and the UK [6, 21, 24–26], while in the Himalayas, increases in A. flavus were noted in patients with CRDs [27]. In cystic fibrosis (CF), A. fumigatus colonisation downregulates the expression of the vitamin D receptor, leading to enhanced T-helper 2 (Th2) inflammation [28]. Treatment with itraconazole reduced Aspergillus burden and Th2 responses, improving symptoms and radiology [28]. Importantly, however, randomised controlled trials evaluating the effect of itraconazole in A. fumigatus-colonised CF have demonstrated unfavourable outcomes, although such trials were limited by small numbers and low detectable therapeutic levels of itraconazole [13, 29]. While the specific role of Aspergillus colonisation is less studied in other CRDs, emerging evidence suggests that an evolution from colonisation to Aspergillus-associated disease exists, including sensitisation, allergic bronchopulmonary mycosis (ABPM), allergic bronchopulmonary aspergillosis (ABPA) and invasive aspergillosis, with implications for lung function, exacerbations and mortality in asthma and COPD [5, 7, 17, 19, 30, 31]. Increased sputum cell counts and neutrophilic inflammation are associated with Aspergillus detection in COPD, while in bronchiectasis, Aspergillus colonisation is prevalent and geographically variable, with A. fumigatus and A. terreus the most common species identified [32]. A. fumigatus is predominant in Asia (i.e. Singapore and Malaysia) while A. terreus is more prevalent in the UK (i.e. Dundee, Scotland) [32]. Enhanced systemic chitinase activity, involved in host defence, is detectable in Aspergillus-colonised bronchiectasis and is associated with exacerbations [33–35]. Taken together, collective evidence supports a potentially deleterious effect of Aspergillus airway colonisation; however, it remains uncertain if such colonisation drives disease progression or represents a marker of severe disease. The lack of standardised protocols for the detection of airway fungi also likely underestimates the true prevalence of fungal colonisation in CRDs.
Aspergillus bronchitis is a lesser described entity characterised by a chronic superficial infection of the trachea and/or bronchial tree by Aspergillus [36]. Proposed diagnostic criteria include chronic respiratory symptoms accompanied by a positive sputum or bronchoalveolar lavage fluid (BALF) result for Aspergillus, determined by culture or PCR, and an elevated Aspergillus IgG serum level in the absence of other underlying fungal-related disease [36]. Characteristic features on bronchoscopy include mucoid impaction and local invasion by Aspergillus hyphae, with COPD and bronchiectasis as the most commonly associated comorbidities [36–38]. Patients should not meet criteria for other established fungal lung disease, particularly ABPA, because these characteristics are also observed in such patients [36]. In CF, Aspergillus bronchitis is reported in 9% of cases, with antifungal treatment showing efficacy in reducing symptoms and improving lung function [37, 39, 40]. Most studies on Aspergillus bronchitis remain limited, small and retrospective, and even if this clinical entity represents a distinct form of fungal lung disease, it warrants further evaluation.
Fungal lung disease and immune overactivity
Fungal sensitisation
Fungal sensitisation represents an important clinical state observed in individuals with chronic respiratory diseases and is characterised by elevated fungal-specific IgE and/or immediate cutaneous hypersensitivity to the respective fungal extract. Aspergillus sensitisation (AS) is most common and defined by an Aspergillus-specific IgE ≥0.35 kUA·L−1 (kilo units of antibody per litre) and/or immediate cutaneous hypersensitivity to Aspergillus extracts [41]. Prevalence of AS in asthma ranges from 5.4% to 16.9% in the community, rising to 25% in tertiary care [42–46]. Patients with severe asthma and AS are referred to as having severe asthma with fungal sensitisation (SAFS), an emerging severe asthma phenotype [41]. Recently, AS and ABPA have been described in COPD with a pooled prevalence of 9.5% [47, 48]. While some studies propose direct associations between AS and increased COPD exacerbation frequency, uncertainty remains about the benefits of antifungal treatment [18, 49]. AS precedes the development of ABPA, and although a quarter of asthma patients in tertiary care are sensitised to A. fumigatus, only a proportion develop ABPA (i.e. 37% of those sensitised) [42]. To prevent progression from AS to ABPA and then subsequent development of bronchiectasis, early evaluation for AS and ABPA is recommended for all asthma patients receiving tertiary care [50].
Allergic bronchopulmonary mycosis/allergic bronchopulmonary aspergillosis
ABPM is a complex group of lung disorders incited by immune overactivity against various fungi colonising the airways of patients with CRD, most commonly asthma or CF [51]. ABPA is a term reserved for disorders caused by Aspergillus species, while ABPM refers to instances of allergic mycoses resulting from fungi other than Aspergillus [50]. Less commonly, ABPA complicates the disease course in other airway diseases including COPD and bronchiectasis, with especially high occurrence in those with concurrent bronchiectasis and COPD [52–55]. The incidence of ABPM is lower than that of ABPA, with most reported as case series [56]. ABPA remains an important cause, consequence and treatable trait in bronchiectasis owing to its treatment responsiveness, although recurrent and sometimes prolonged treatment courses may be required for some [57].
While not predominant in indoor or outdoor air, A. fumigatus is commonly involved in allergic mycoses due to the size of its conidia (2–3.5 µm) and thermotolerant nature facilitating entry and growth into the smallest airways [58]. Two key events in ABPA pathogenesis includes Aspergillus colonisation and a skewed type-2 immune response (figures 2 and 3) [59]. Both events are presumably genetically determined, because not all asthma patients develop ABPA despite comparable exposure to the same environment. The normal human host rapidly eliminates A. fumigatus by innate and adaptive immune mechanisms [60]. Polymorphisms in airway epithelial receptors, innate immune pathways (pattern recognition receptors: toll-like receptors, surfactant proteins, mannose-binding lectin etc.), major histocompatibility complex proteins and adaptive immune pathways (interleukin-4R, interleukin-13 etc.) prevent the elimination of A. fumigatus and promote the development of an aberrant type-2 immune response [61–63]. Eosinophils remain the primary mediators of inflammation in ABPA, with the interaction between eosinophils and A. fumigatus releasing galectin-10 and forming Charcot–Leyden crystals [64]. Subsequently, eosinophils undergo cell death forming histone-rich extracellular traps and increase the viscosity of mucus plugs, which in turn prevents the elimination of A. fumigatus and contributes to ABPA pathogenesis [65]. The prevalence and underlying predisposing factors for the occurrence of ABPA vary between different geographic regions. In a recent systematic review, the prevalence of ABPA in asthma (tertiary care) varied widely (0.8–70%) with a pooled prevalence of 11.3% [42]. In the only community study from North India, the prevalence of ABPA-complicating asthma was 5.7% [45]. Separately, in two large registry studies, ABPA was reported as a cause of bronchiectasis in 2.8% and 8.2% of cases, respectively [66, 67]. The occurrence of ABPA in CF varied widely between 3% and 25%, with a pooled prevalence of 8.9% [68]. Most ABPA patients present with refractory asthma; however, one fifth can have well-controlled asthma, requiring moderate-to-high doses of inhaled steroids.
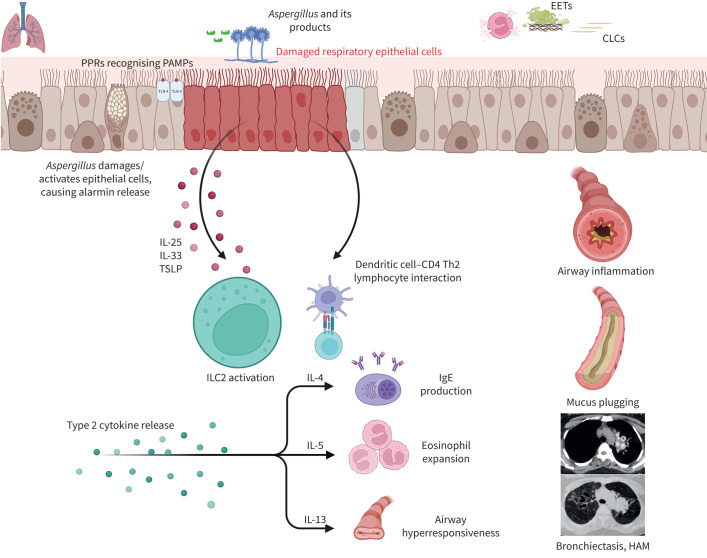
FIGURE 2.
The key events in allergic bronchopulmonary aspergillosis (ABPA) pathogenesis include Aspergillus colonisation followed by skewed type-2 immune responses. Polymorphisms in airway epithelial receptors and innate and adaptive immune pathways prevent elimination of A. fumigatus and promote the development of an aberrant type-2 immune response. Pathogen-associated molecular patterns (PAMPs) from Aspergillus (glucan, galactomannan, galactosaminogalactan, proteases) are recognised by pattern recognition receptors (PRRs), including dectin-1 and toll-like receptors (TLRs), at the lung epithelial cell surface. Fungal proteases can damage the respiratory epithelium and result in release of alarmins (interleukin (IL)-33, IL-25 and thymic stromal lymphopoietin (TSLP)), which in turn stimulate the type 2 innate lymphoid cells (ILC2) and CD4+ type 2 lymphocytes. The dendritic cells also recognise fungal proteins and activate allergen-specific type 2 T-cells (Th2 cells). Eosinophils remain the primary mediators of inflammation in ABPA, with the interaction between eosinophils and A. fumigatus releasing galectin-10 and forming Charcot–Leyden crystals (CLCs). Subsequently, eosinophils undergo cell death forming histone-rich extracellular traps (EETs) and increase the viscosity of mucus plugs, which contribute to ABPA pathogenesis. The skewed type 2 responses lead to secretion of IL-4, IL-5 and IL-13. IL-4 mediates the class switching and production of IgE antibodies, which attach to mast cells and cause mast cell degranulation on allergen exposure. IL-5 is pivotal for eosinophil recruitment, maturation and survival and is central to eosinophilic inflammation. IL-13 from ILC2 and Th2 cells promotes mucus hypersecretion. Finally, immune activation leads to airway inflammation, mucus plugging and bronchiectasis. HAM: high-attenuation mucus. Figure created with Biorender.com.
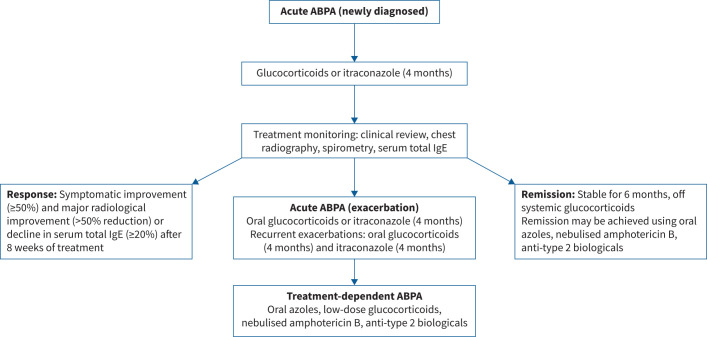
FIGURE 3.
Treatment of various allergic bronchopulmonary aspergillosis (ABPA) categories as suggested by the International Society for Human and Animal Mycology-ABPA working group.
Other important common manifestations of ABPA are fleeting radiological opacities, haemoptysis and mucus plug expectoration. The diagnosis of ABPA is based on a combination of clinical, radiological and immunological findings. The ABPA working group of the International Society for Human and Animal Mycology recently framed new guidelines for diagnosis (table 1) [50]. In an individual with a predisposing condition or compatible clinical presentation, it is essential to demonstrate AS (raised A. fumigatus IgE) and disease activity (raised serum total IgE). The presence of fungal-specific IgG, blood eosinophilia and consistent imaging confirm the diagnosis. ABPA is classified based on chest computed tomography (CT) as serological ABPA (ABPA-S) (ABPA without bronchiectasis), ABPA with bronchiectasis (ABPA-B), ABPA with mucus plugging (ABPA-MP), ABPA with high-attenuation mucus (ABPA-HAM) or ABPA with chronic pleuropulmonary fibrosis (ABPA-CPF). ABPA treatment tenets include anti-inflammatory agents: glucocorticoids and/or biological agents targeting type-2 immune responses to control inflammation; and antifungal agents (oral or nebulised) to decrease airway fungal colonisation. Asymptomatic ABPA is not routinely treated, and systemic therapy is only indicated in those with poor asthma control or recurrent exacerbations. Patients with acute ABPA (newly diagnosed or exacerbations) can be treated with oral glucocorticoids or itraconazole for 4 months [69, 70]. A combination of itraconazole and glucocorticoids is not recommended as first-line therapy for acute ABPA but reserved for treating recurrent (≥2 in the past 1–2 years) ABPA exacerbations [71]. Oral voriconazole, posaconazole and isavuconazole are not first-line agents for acute ABPA [72]. Similarly, high-dose inhaled corticosteroids or biological agents should not be used as primary therapy for acute ABPA. Patients with treatment-dependent ABPA (i.e. ≥2 ABPA exacerbations, each occurring within 3 months of stopping glucocorticoids, or worsening symptoms and imaging, or rise in total IgE by ≥50% within 4 weeks of tapering oral steroids on two separate occasions) may be treated with long-term itraconazole, nebulised amphotericin B or biological agents targeting type-2 inflammation [73–78]. Importantly, patients should be followed up 8–12 weeks post treatment initiation, with treatment response indicated by improvement in symptoms (≥50%) and imaging (significant improvement, ≥50%), along with a ≥20% reduction in total IgE (figure 3). Recently, significant treatment responses were observed with the following chest CT findings: extent and density of mucoid impaction, centrilobular micronodules, consolidation and bronchial wall thickening [79]. Almost 50% of patients experience ABPA exacerbations post treatment cessation [80]. ABPA exacerbations are characterised by a sustained worsening (≥2 weeks) of respiratory symptoms or new opacities on chest imaging and an increase in total IgE by ≥50% from periods of clinical stability [59, 80]. Additional factors contributing to deterioration, notably exacerbations of asthma, COPD and bronchiectasis, all necessitate meticulous exclusion before ABPA-focused interventions are pursued.
TABLE 1.
Revised International Society for Human and Animal Mycology ABPA working group consensus criteria for diagnosing ABPA
| Predisposing conditions | Asthma, cystic fibrosis, COPD and bronchiectasis |
| OR | |
| Compatible clinico-radiological presentation | Expectoration of mucus plugs, finger-in-glove and fleeting opacities on chest radiography, lung collapse and others |
| Essential components | • Aspergillus fumigatus-specific IgE ≥0.35 kUA·L−1a |
| • Serum total IgE ≥500 IU·mL−1b | |
| Other components (any two) | • Positive IgG against A. fumigatusc |
| • Peripheral blood eosinophil count ≥500 cells·µL−1 (could be historical) | |
| • Thin-section chest CT consistent with ABPA (bronchiectasis, mucus plugging and high-attenuation mucusd) or fleeting opacities on chest radiography | |
| Important considerations |
a: a positive type 1 skin test is acceptable when Aspergillus IgE is unavailable b: serum total IgE <500 IU·mL−1 may be acceptable if all other criteria are fulfilled c: A. fumigatus IgG can be detected using lateral flow assays or enzyme immunoassays; cut-offs for A. fumigatus must be developed for specific populations (e.g. ≥27 mgA·L−1, ≥60 mgA·L−1, ≥40 mgA·L−1 for India, Japan and the UK, respectively) and in the absence of population-specific cut-offs, we suggest using manufacturer recommendations d: high-attenuation mucus is pathognomonic of ABPA and confirms ABPA diagnosis even if all other criteria are not fulfilled Elevated IgE against rAsp f1, f2 and f4 can be used as another component |
ABPA: allergic bronchopulmonary aspergillosis; Ig: immunoglobulin; CT: computed tomography.
Fungal lung disease and mild immunosuppression
Chronic pulmonary aspergillosis (CPA) is an indolent, progressive, debilitating disease caused by Aspergillus infection in individuals with pre-existing chronic structural lung disease who may be immunocompetent or have mild immunocompromise interfering with mechanisms of pulmonary fungal clearance [81]. Globally, the most common pulmonary conditions predisposing to CPA are tuberculosis, nontuberculous mycobacterial infection and ABPA, with COPD more common in developed settings [82]. Other prevailing conditions include sarcoidosis, lung cancer and prior thoracic surgery [82–84]. Global epidemiological data are lacking, with an incidence estimated at 22 per 100 000 individuals, which is higher in regions with high tuberculosis prevalence [85]. Presentation is often over months, mimicking deterioration of the underlying chronic lung condition and thus frequently resulting in misdiagnosis. A wide range of radiological phenotypes is seen, from simple aspergillomas (fungal balls) to progressive fibro-cavitary disease, often with overlap [86]. Aspergillomas frequently develop in structurally impaired regions of the lung, such as cavities in individuals with a history of tuberculosis, bronchiectasis and/or sarcoidosis [87–89]. CPA requires a constellation of clinical, radiological, serological and microbiological findings [90]. This is challenging, in part due to resource constraints in some regions, but also due to the lack of sensitivity of current diagnostic tests. Although the possibility of co-infection and lung carcinoma should be carefully considered and excluded where possible, due to underlying comorbidity and the risk of lung biopsy, histological confirmation is rarely obtained in clinical practice. The diagnosis often relies on compatible clinical symptoms and radiology alongside a positive quantitative Aspergillus IgG or precipitin test [91]. The availability of lateral flow-based Aspergillus IgG assays presents a potential low-cost approach for diagnosis in resource-poor settings; however, optimal cut-offs remain unclear, with a significant proportion of CPA demonstrating normal Aspergillus IgG due to non-A. fumigatus infection or an immunocompromised state [92, 93]. Bronchoscopy to detect Aspergillus from lower respiratory tract samples is resource intensive and requires a risk/benefit evaluation, with microbiological culture often showing a poor yield. Use of fungal biomarkers, including galactomannan and Aspergillus PCR, requires significant resources and has relatively poor sensitivity [94]. Microbiological confirmation (when possible) is important to identify aetiological species, enable susceptibility testing and exclude co-infection with other pathogens, especially nontuberculous mycobacteria where fungal co-infection is particularly prevalent [95]. In addition, Aspergillus species referred to as related or cryptic species (e.g. A. lentulus, A. udagawae) have a reported prevalence of ∼25% in individuals with CPA in some settings [96]. These are difficult to identify with conventional methods and demonstrate reduced antifungal sensitivity, further highlighting the importance of microbiological confirmation where possible.
Antifungal therapy with first-line azoles (itraconazole, voriconazole) coupled with meticulous therapeutic drug monitoring is advised in cases with demonstrable clinical or radiological advancement rather than simple aspergillomas [97]. The efficacy of a minimum 12-month treatment duration is superior to shorter regimens in averting disease relapse [98]. Adverse effects, however, are common and often require dose adjustment, therapy change or cessation in up to 30%, with high rates of azole resistance after prolonged therapy [99]. Other therapeutic options include newer azole compounds (posaconazole, isavuconazole), intravenous liposomal amphotericin, echinocandins and adjunctive immunotherapy with interferon-γ [100–102]. Surgical resection should be considered in localised or treatment-refractory disease, with good outcomes reported at specialised centres [83]. When surgical intervention is not feasible, percutaneous or bronchoscopic administration of antifungal agents can be considered. Retrospective case series indicate positive outcomes in terms of cavity size reduction, although potential risks including pneumothorax and bronchopleural fistula necessitate thorough expert evaluation and management [103]. Haemoptysis often presents as a life-threatening complication of CPA, with embolisation recommended in massive haemoptysis, and surgery reserved for medically refractory cases or where a lack of interventional facilities or expertise exists [90]. Despite therapy, mortality remains high, with an estimated 5-year mortality of ∼50%, increasing with underlying comorbidities including COPD, nontuberculous mycobacterial infection, low body mass index, antimicrobial resistance and bilateral disease [104]. The current lack of studies and therapeutic trials on CPA underscores the importance of enhancing clinical outcomes through research efforts, particularly within an evolving antifungal trial landscape. These efforts will be improved by use of the recently published EQUAL score for CPA [105].
Invasive fungal lung disease and severe immunosuppression
Invasive fungal pneumonia is classically thought to be a disease of profound immunocompromise, particularly with prolonged neutropenia and lymphopenia. However, there is increasing recognition that invasive fungal pneumonias can affect a much broader population, including patients with more subtle immunocompromise, and therefore are often under-recognised and undertreated. We outline several categories of fungi frequently encountered in critically ill patients: moulds, Pneumocystis jirovecii and Candida, with a specific focus on invasive pulmonary aspergillosis (IPA), which is associated with particularly high mortality in the severely immunosuppressed.
IPA remains the most common and morbid fungal respiratory infection in immunocompromised patients. Mucorales, Scedosporium and Fusarium are rarer but can have similar clinical features. Most often, invasive mould infections present as fevers with a consolidation, nodule or mass lesion on imaging [106], but they can also present as tracheobronchitis, especially in lung transplant recipients with vulnerable anastomosis sites [107]. The latter can have normal results on chest imaging with abnormalities only visualised on direct inspection of the airway, which can lead to further complications including stenosis and airway dehiscence [108]. Though locally aggressive, these invasive mould infections typically originate from and remain confined to the respiratory tract, but in the extremely immunocompromised, angio-invasive disease can lead to dissemination to other organs such as the brain, eyes, liver and skin, which leads to a very poor prognosis [109]. A notable exception is Fusarium, which disseminates in ∼70% of immunocompromised patients and is frequently associated with positive blood cultures, likely owing to its capacity for in vivo sporulation [110].
Demographic studies show an increasing recognition of IPA in the intensive care unit (ICU), beyond patients with the “classic” severely immunosuppressing risk factors of neutropenia, haematological malignancy and prolonged steroid use [111, 112]. Sepsis, one of the most common reasons for ICU admission, leads to a relative state of immune suppression in its later stages, hampering neutrophil function and predisposing to IPA [113–115]. Steroid use, even short courses, can accelerate fungal growth and depress innate immune function [116, 117]. COPD and cirrhosis, both common comorbidities, have been highlighted as conferring intermediate IPA risk, compelling the European Organisation for Research and Treatment of Cancer (EORTC) to revise definitions of invasive fungal infection to include these groups [111, 118].
Viral infection also predisposes to IPA in both immunocompromised and immunocompetent hosts. In epidemiological studies, the prevalence of IPA tracks with viral pandemics [119, 120].
Influenza-associated pulmonary aspergillosis (IAPA) is similarly well established and estimated to affect 19.2% of patients critically ill with influenza [121]. Other respiratory viral infections show similar associations [122, 123]. More recently, COVID-associated pulmonary aspergillosis (CAPA) has been recognised, with a wide variation in reported incidence of 4–35% of critically ill patients, and perhaps even higher rates in the post-vaccination era [124, 125]. However, caution should be used in conflating CAPA and IAPA; CAPA incidence may be overestimated, with more cases diagnosed as “putative” or “probable”; typically has later onset post-ICU admission; and may not contribute to excess mortality in comparison to IAPA [126, 127]. The pathophysiology of post-viral aspergillosis is incompletely understood but is likely multifactorial, with viral-mediated epithelial damage, dysregulated innate and adaptive immunity, suppression of neutrophil-mediated defences and treatment with high-dose steroids and other immunomodulators [128, 129]. Viral–fungal co-infection is not unique to Aspergillus, and an association between COVID and invasive pulmonary Mucorales infection has also been noted [130]. Revised EORTC guidelines now include severe viral infections in their list of compatible host factors [118].
Despite expanding “at risk” populations for invasive Aspergillus infection, mould growth from respiratory cultures in the ICU is more likely to reflect colonisation than true infection [131], and fungal biomarkers have imperfect sensitivity and specificity, especially in non-neutropenic patients [112, 132]. Radiographic criteria for IPA were developed in neutropenic patients and are often lacking or obscured by other common lung pathology in ICU patients, including acute respiratory distress syndrome, non-fungal pneumonia and/or underlying structural lung disease, making a timely diagnosis of invasive mould infection even more challenging [111].
Pneumocystis jirovecii, a single-celled fungus, nearly exclusively infects immunocompromised hosts and can cause severe, life-threatening pulmonary infection. Pneumocystis pneumonia typically presents with diffuse, geographic bilateral infiltrates, though very rarely can be nodular or cavitating, similar to invasive mould infections [133, 134]. Classic groups at risk for Pneumocystis include those with poorly controlled HIV, recipients of prolonged high-dose corticosteroids and other individuals with severe impairments in T- and B-cell immunity; knowledge of these risk factors has allowed for guideline-based targeted chemoprophylaxis [135].
The incidence and severity of Pneumocystis infections in people without HIV have been rising, and patients previously not considered for chemoprophylaxis are increasingly diagnosed [136]. Increased vigilance for Pneumocystis should be practised in patients on immunotherapy (e.g. checkpoint inhibitors), tyrosine kinase inhibitors, anti-CD20 inhibitors and newer bispecific antibody agents, though prophylaxis is not yet universally recommended for these groups [137–140]. The increased incidence of Pneumocystis in the non-HIV population may be explained in part by the increased use of molecular diagnostics, such as Pneumocystis-targeted PCR, broad-range fungal PCR and plasma mNGS, though these modalities also can identify incident colonisation and lead to false positive diagnoses [141, 142]. Traditional Pneumocystis diagnostics, including microscopy with silver staining and β-D-glucan, have poorer performance in non-HIV immunocompromised patients due to lower organism burden [143, 144]. Generally, prognosis for Pneumocystis is favourable with early appropriate treatment, so a high index of suspicion is critical.
Candida species are perhaps the most isolated fungal organism from critically ill patients. However, their isolation from respiratory tract samples in almost all cases represents colonisation rather than true infection and does not warrant antifungal treatment. Candida species are common commensal flora of the oral, respiratory and upper gastrointestinal tracts, and their detection in culture is promoted by the use of broad-spectrum antibiotics, a common practice in ICU patients [145]. In an autopsy study of 135 ICU patients with pathological evidence of pneumonia, more than half had Candida growth documented from respiratory samples, but none had definitive evidence of pulmonary candidiasis [146]. Importantly, a smaller autopsy study, restricted to immunocompromised oncology patients with pneumonia, demonstrated a 14% prevalence of histologically proven pulmonary candidiasis, so this population may be an exception [147]. Additionally, multifocal Candida colonisation (respiratory tract, urine, skin) is often associated with higher risks of systemic invasive candidiasis by translocation of yeast from the digestive tract [148]. Despite this, Candida isolation from the respiratory tract should not reflexively trigger antifungal treatment.
Endemic mycoses
Endemic mycoses will be briefly discussed outside of this framework, because they infect both immunocompromised and immunocompetent patients with a vast range of severity, from asymptomatic infection to fatal invasive disease. The endemic mycoses (i.e. Coccidioides, Histoplasma, Blastomyces, Paracoccidioides, Sporothrix and Talaromyces) are a diverse group of fungal organisms that have specific ecological niches and are thermally dimorphic with both yeast-like and mould-like states [149]. Depending on the specific organism, infection can be primary or represent reactivation of latent disease, the latter more common in immunocompromised hosts. In both scenarios, dissemination to various sites, including skin, the central nervous system and lymphatics, can occur. Endemic mycoses remain under-recognised causes of community-acquired pneumonia, particularly when they manifest outside of traditional geographic areas [149, 150]. A growing body of work now demonstrates that the historically accepted geographic distributions of individual mycoses is expanding due to several factors, including global climate change, and thus broader clinician awareness is critical in preventing delayed or missed cases that could benefit from antifungal treatment [151, 152]. While still relatively rare compared to the other fungal infections discussed in this review, endemic mycoses can lead to severe and sometimes fatal infections, with a US-based study estimating an in-hospital mortality of 5% in children and 7% in adults, with the majority of infections and deaths in immunocompetent patients [153].
Though technically not an endemic mycosis due to lack of a dimorphic state, Cryptococcus gattii is also worth noting given its similar shifting geography. While previously isolated to tropical regions including Australia and Papua New Guinea, in the last few decades the evolution of a different molecular genotype has led to an ongoing outbreak in western Canada and the USA [154–156]. In contrast to Cryptococcus neoformans, which has a worldwide distribution and infects immunocompromised individuals, C. gattii infections often occur in otherwise healthy hosts, with similar disease manifestations to pneumonia and meningoencephalitis [157, 158].
The pulmonary mycobiome in chronic respiratory disease
mNGS has debunked the dogma of sterile lungs, and we now recognise a diverse resident microbial community, even in health. Like the bacteriome, the mycobiome (the fungal microbiome) in healthy lungs is derived from the oropharynx and upper respiratory tract, largely through micro-aspiration [159, 160]. Owing to the lung architecture and environment, the mycobiome is transient and highly variable, with increasing evidence suggesting key differences between mycobiomes in the healthy state (eubiosis) and those with disease (dysbiosis) (figure 4).
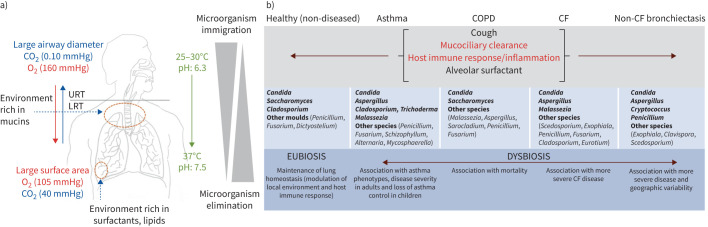
FIGURE 4.
A summary of the homeostatic mechanisms to maintain the lung mycobiome in health and its composition and clinical correlates in chronic respiratory disease. a) Bidirectional flux from the oropharyngeal area, or upper respiratory tract (URT), to the lower respiratory tract (LRT) with biotic (bronchial epithelial cells, respiratory cilia, diameter and cell surface exchanges) and abiotic (temperature, pH, O2/CO2 pressures, airway mucus rich in mucins, a lipid-rich surfactant) factors maintaining a relatively low airway fungal biomass (the mycobiome). The mycobiome maintains a dynamic balance between fungal immigration, driven by inhalation, salivary micro-aspiration and mucosal dispersion, and elimination, driven by cough, mucociliary clearance, innate and adaptive host immune response and antimicrobial activity of alveolar surfactant. This constant dynamic of flux ensures the mycobiome is transient and mobile, with intimate links to the external environment. In health, the lung mycobiome is in a eubiotic state. b) By contrast, dysbiosis of the lung mycobiome occurs across several chronic respiratory diseases including asthma, COPD, cystic fibrosis (CF) and non-CF bronchiectasis. Mechanisms of microbial clearance are differentially impaired based on the pathophysiology of the underlying disease and indicated as normal (black text) to abnormal (red text). In diseased lungs, the mycobiome is more long-lasting and exhibits change, contributing to disease progression.
In healthy lungs, the mycobiome is mainly composed of Ascomycota, characterised by Candida, Saccharomyces and Cladosporium species [159, 161–166]. As the healthy lung evolves to one with disease, lung mycobiomes influence microbial co-habitants and modulate the host immune-inflammatory response, contributing to the onset and progression of lung disease [159, 164]. While the role of the mycobiome has been studied in several CRDs, work focused on mycobiomes specifically in fungal lung disease states is lacking. However, important work in SARS-CoV-2 pneumonia is emerging, illustrating reduced fungal diversity and potentially beneficial effects of antifungal treatment at ICU admission in reducing A. fumigatus-associated mortality in severe COVID-19 [167–170].
The mycobiome in CRDs is predominantly characterised by the genera Aspergillus and Candida, while in asthma Malassezia, a lipophilic basidiomycetous yeast, exhibits a higher abundance during acute exacerbations and in severe asthma [171–175]. Important differences in fungal (and bacterial) microbiota are correlated to asthma endophenotypes [175]. The role of fungi in COPD is emerging, with two distinct mycobiome signatures described: one associated with Saccharomyces and increased symptoms, and another with Aspergillus, Penicillium and Curvularia, frequent exacerbations and higher mortality [3, 18, 165, 176, 177]. Pneumocystis jirovecii is over-represented in the lung mycobiome of HIV-positive COPD patients, highlighting its potential role in COPD pathophysiology [176, 178]. Growing evidence in CF suggests that the fungal community has a key role in disease, with even Candida associated with lung function decline and progressive disease [8]. Malassezia is frequently reported in CF and potentially plays a role in recycling lipids in microbiota, particularly during exacerbations [179–182]. A diversity of moulds is also observed; they are generally considered transient colonisers, except for A. fumigatus, Scedosporium apiospermum and Exophiala dermatitidis, given their multiple and sequential detections [179, 181–184]. Pulmonary exacerbations in CF are associated with an “Attack” community composed of virulent bacteria and viruses [181, 185–189]. Malassezia and Aspergillus form part of this community [182]. According to the “Climax-Attack model”, the “Attack” community is transient, followed by stability characterised by a “Climax” community [185]. The “Climax” community includes bacteria such as Pseudomonas aeruginosa and the fungus Scedosporium, which is associated with poor lung function [182, 188]. Aspergillus, Candida and Penicillium represent the dominant genera in the non-CF bronchiectasis lung, with A. fumigatus, E. dermatitidis and S. apiospermum the most frequently detected species [183]. In association with Cryptococcus and Clavispora, Aspergillus is linked with progressive bronchiectasis, with specific dominant Aspergillus species dependent on the patient's geographic origin [32]. An integrative microbiomics approach incorporating multi-kingdom analysis demonstrated that Cryptococcus is associated with exacerbation risks in bronchiectasis [190].
Fungal signatures as treatable traits in chronic respiratory disease
While fungal colonisation is commonly observed in CRDs, it is unclear when and how such fungi serve as bystanders or pathogens, and to what extent and under what circumstances they contribute to disease onset and progression. Studies evaluating the clinical consequences of fungal colonisation in CRDs reveal contrasting results in relation to symptoms, lung function and exacerbations (table 2) [8, 11–16, 28]. Furthermore, evidence of fungal eradication has proved inconclusive in CF [13, 28, 29]. Nonetheless, emerging evidence demonstrates clear associations between Aspergillus colonisation and the clinical spectrum of aspergillosis, ranging from sensitisation to invasive disease, proposing a role for fungal colonisation as a precursor to the development of fungal lung disease in susceptible individuals [131, 215–220]. Further studies are necessary to elucidate mechanisms related to Aspergillus colonisation in various CRDs. Whether fungal signatures as we currently understand them represent a treatable trait in CRD also remains to be determined.
TABLE 2.
Fungal signatures in chronic lung disease and associated clinical outcomes with potential treatment approaches
| Fungal signature | Disease | Outcomes | Potential treatment |
|---|---|---|---|
| Colonisation | CF | ↑ Radiological abnormalities [11] No difference in lung function [11–13] ↓ Lung function [8, 16] ↑ Exacerbations [8, 14, 15] |
Antifungals [192] |
| Asthma |
Aspergillus sensitisation ↓ Lung function [31] Neutrophilic inflammation [30] ↑ Exacerbations [191] |
||
| COPD | Progression to aspergillosis [19] ↑ Exacerbations [17, 18] Prolonged hospital stay [7] |
||
| Bronchiectasis | ↑ Exacerbations [32, 33] | ||
| Sensitisation | CF | ↓ Lung function [22, 193–195] ↑ Exacerbations [22, 193] |
Corticosteroids [69, 71] Antifungals [192, 201–204] |
| Asthma | ↓ Lung function [30, 191, 196] ↑ Exacerbations [197, 198] |
||
| COPD | ↓ Lung function [5, 49] ↑ Exacerbations [49, 199] |
||
| Bronchiectasis | ↓ Lung function [200] ↑ Disease severity [200] |
||
| ABPA | CF | ↓ Lung function [195] | Corticosteroids [69, 71] Antifungals [201, 203, 208] Biologicals [209–214] |
| Asthma | ↓ Lung function [205] ↑ Exacerbations [205] |
||
| COPD | ↑ Mortality [206] ↑ BCO [55] |
||
| Bronchiectasis | ↓ Lung function [205, 207] ↑ Exacerbations [207] |
CF: cystic fibrosis, ABPA: allergic bronchopulmonary aspergillosis; BCO: bronchiectasis–COPD overlap.
Fungal sensitisation and ABPM/ABPA are associated with adverse outcomes in patients with CRDs (table 2) [30, 191, 196–198, 205–207, 221]. SAFS and ABPA in asthma link to small airway dysfunction, exacerbations, life-threatening asthma and high corticosteroid burden [30, 191, 196–198, 205, 221]. Likewise, in CF, sensitisation to Aspergillus is associated with poorer lung function and exacerbations, detectable using basophil surface biomarkers [22, 193–195]. Reduced serum vitamin D levels are associated with Th2 inflammatory responses in CF-ABPA; however, treatment with vitamin D in clinical trials conferred no demonstrable clinical, radiological or immunological improvement [222, 223]. Case series and small retrospective studies assessing biological therapies in CF-ABPA show favourable results, and while still lacking prospective evaluation in CF, fungal sensitisation and ABPA are clearly important treatable traits in asthma and CF [209–214].
The role of fungi as a treatable trait is less recognised in COPD and bronchiectasis, although the high prevalence of fungal sensitisation is reported in both conditions. Environmental exposure represents a key risk for the development of fungal sensitisation, with measurable responses detectable to outdoor and indoor air fungi and associated allergens in COPD [199]. While ABPA is an established cause and consequence in bronchiectasis but infrequent in COPD, it is associated with increased systemic inflammation and higher mortality compared to non-COPD settings [206]. In contrast to asthma, evidence for biological therapy in COPD and bronchiectasis is only starting to emerge. Considering the vast patient heterogeneity existing in these disease states, personalised endophenotyping will likely be required to identify patient groups that would significantly benefit from leveraging fungal signatures as treatable traits [224, 225].
Molecular diagnostics in fungal lung disease
The inherent limitations of fungal culture and immune-based approaches relying on fungal antigens and/or antibodies necessitates fresh molecular diagnostics to better identify fungal lung disease [226]. Galactomannan detection in serum and BALF remains valuable for the early detection of invasive aspergillosis, particularly in immunocompetent individuals; however, it suffers from poor sensitivity [227–229]. While molecular approaches can confirm culture results, using PCR, digital PCR and mNGS directly on primary clinical specimens, including sputum, BALF and blood, offers significantly improved diagnostic sensitivity, specificity and timeliness against a wide range of fungal pathogens to the species level [230]. Multiplex approaches combine pathogen identification with antifungal resistance testing; for instance, identifying azole (i.e. mutations in CYP51A or ERG11) or echinocandin (mutations in FKS) resistance by PCR leads to earlier initiation of effective antifungals, likely improving clinical outcome [231]. mNGS also offers an added advantage of identifying novel fungal pathogens, especially when clinical presentation and/or epidemiology may be suggestive of a fungal infection but not typical for any particular fungal pathogen precluding PCR diagnostics [231, 232].
Molecular diagnostics offer point of care testing, facilitating rapid diagnosis, particularly in low- and middle-income countries, where access to centralised laboratories is limited [233]. Commercially available and clinically validated PCR assays are widely used to diagnose aspergillosis with BALF or blood [234, 235], candidiasis using blood [236] and Pneumocystis pneumonia using BALF [237, 238], with relatively high accuracy and a rapid turnaround time [239]. The specificity of blood PCR for invasive aspergillosis may be improved with repeat testing, especially in high-risk populations [234]. PCR can also be employed to diagnose some Mucorales species and Cryptococcus neoformans [231].
A diverse range of fungal taxa can be detected by mNGS, including P. jirovecii, Aspergillus and Candida species as well as Cryptococcus, Rhizopus, Fusarium and Alternaria species, Talaromyces marneffei and Histoplasma capsulatum [240]. Compared to traditional diagnostic approaches, NGS is highly sensitive and specific and outperforms traditional culture in detecting fungal pathogens, exemplified by the identification of P. jirovecii, which is not culturable, and other fastidious fungi such as Mucor species [226, 241].
Of note, there are important technical challenges to the use of molecular diagnostics in fungal disease, mostly related to small amounts of fungal DNA often present in clinical specimens [242]. Validation of molecular testing against current gold standards is complicated by the rare nature of some fungal infections. Distinguishing between infection, colonisation and contamination is a challenge with molecular testing [231, 243]. While highly useful in immunocompromised patients with suspected invasive fungal infections, the diagnostic yield of molecular methods is not as high in patients with chronic lung disease who are not necessarily immunocompromised, where a molecular test might be indicative of colonisation and not necessarily invasive infection. This is particularly true in patients with COPD with positive Pneumocystis BALF PCR results or even lung transplant recipients with positive Aspergillus BALF PCR results. Combining Pneumocystis PCR with serum β-D-glucan or Aspergillus PCR with galactomannan will significantly increase the specificity of the test [243, 244]. mNGS at present is more cost and resource intensive than culture and PCR, and may require substantial technical and bioinformatic expertise, currently limiting widespread availability [240]. Additionally, fungal genomic data are not widely available, and inadequately curated databases limit NGS detection accuracy [240]. Moreover, overdiagnosis and erroneous association of pathogens with disease can lead to unnecessary treatment [241]. Despite limitations, molecular diagnostics for fungal infection have become an increasingly common diagnostic approach, with significant potential to replace culture [242]. Its potential to improve patient outcomes makes it an attractive and increasingly important approach for the accurate and timely diagnosis of fungal lung infection.
The antifungal drug development pipeline
With the increasing issue of antifungal resistance, a robust antifungal drug development pipeline is imperative. Aerosolisation presents a promising avenue for drug delivery because it delivers a higher drug concentration to the site of infection while avoiding systemic toxicity, reducing adverse events and administration frequency [245, 246]. Several inhaled antifungals have been examined for the treatment of allergic and invasive pulmonary mycoses with varying degree of success and 50–65% cure rates [247, 248]. Amphotericin B is the most widely studied and used, followed by voriconazole and itraconazole. Inhaled amphotericin B has been also used in combination with systemic azole in highly immunocompromised patients [249]. Newer formulations of azoles (PC945 and PC1244) have been developed specifically for inhaled application with promising results, but lack the clinical and outcome data to support their use [249]. In theory, the advantage of inhaled therapy is in limiting systemic side effects and maximising antimicrobial efficacy at the site of the infection. Combining antifungal agents of different mechanisms of action might be beneficial, and is often used. However, data to support this practice remain scarce. A randomised controlled trial comparing voriconazole and anidulafungin to voriconazole monotherapy showed improved survival in a subgroup of patients but, importantly, not the whole cohort [250].
Rezafungin is an echinocandin, targeting β1,3-D-glucan, like caspofungin, micafungin and anidulafungin. The pharmacological modifications incorporated into rezafungin are designed to provide a longer half-life (133 h), permitting less frequent administration [251]. Equivalent efficacy to other echinocandins was demonstrated by the SENTRY 2015 surveillance programme [252]. Similarly, equivalent efficacy to anidulafungin is reported against Candida species, with potency against fluconazole-resistant isolates but not all anidulafungin-resistant isolates; hence, its value is in its longer half-life rather than increased spectrum of activity over existing echinocandins [253]. Two pivotal preregistration studies for rezafungin established clinical efficacy: the ReSTORE trial [254] and STRIVE [255]. These showed equivalent microbiological cure and mortality outcomes between rezafungin and caspofungin [256].
Ibrexafungerp, a novel synthetic derivative of enfumafungin targeting β1,3-D-glucan synthase, has the added advantage over echinocandins of oral bioavailability [257]. Initial murine studies of candidiasis followed by in vitro data from clinical isolates and other animal models show promise [257–259]. However, like echinocandins, ibrexafungerp remains poorly active against Mucorales and Fusarium [260]. An open-label phase 2 study assessed the efficacy of ibrexafungerp in 27 patients with invasive candidiasis, with comparison to fluconazole or micafungin as standard of care [261]. Clinical success rates were comparable across all groups with no safety concerns. These clinical findings are consistent with in vitro data suggesting ibrexafungerp is fungistatic against A. fumigatus, with demonstrable synergy with azoles and amphotericin B against Aspergillus species [262]. Further trial data for ibrexafungerp are awaited: the SCYNERGIA trial, which investigated its use in combination with voriconazole compared to voriconazole alone in patients with IPA (ClinicalTrials.gov: NCT03672292) has been terminated, while the MARIO study, where an echinocandin followed by either ibrexafungerp or voriconazole was being used to treat patients with invasive candidiasis (ClinicalTrials.gov: NCT05178862), is currently suspended due to manufacturing issues.
Fosmanogepix is a novel first-in-class antifungal targeting Gwt1, an early enzyme in the synthesis of glycosylphosphatidylinositol [263, 264]. Murine neutropenic models suggest it is an effective treatment option for invasive infections with Scedosporium and Fusarium [265] and Candida [266], and promising for Rhizopus arrhizus [267]. A small phase 2 study for invasive candidaemia showed a 30-day survival of 89% [268] with a second phase 2 trial a success rate of 80% [269]. Results of an open-label trial in patients with Aspergillus or rare moulds (ClinicalTrials.gov: NCT04240886) showed a 43% mortality rate (nine out of 21) with early study termination by the sponsor to concentrate on phase 3 studies.
Olorofim (F901318) represents a further novel antifungal inhibiting the enzyme dihydroorotate, a key step in pyrimidine synthesis [270]. In vitro, it is highly active against azole-resistant A. fumigatus, Scedosporium and Fusarium species and endemic mycoses but shows no activity against Mucorales or Candida [271–275]. Provisional results of a phase 2b open-label study in 100 patients with invasive mould infections reported a 44% and 39% success rate at days 42 and 84, respectively [276]. In 2023, the US Food and Drug Administration declined approval, citing a need for more clinical data. A phase 3 study in invasive aspergillosis (ClinicalTrials.gov: NCT05101187) is underway while a phase 2b study in patients with invasive fungal disease lacking other therapeutic options has been recently completed (ClinicalTrials.gov: NCT03583164).
Finally, several other agents remain in the pipeline: tetrazoles (VT-1161, VT-1129 and VT-1598), novel azoles with lower affinity for human cytochrome P450 isoenzymes (CYP2C9, CYP2C19 and CYP3A4) resulting in lower potential for drug–drug interactions, are promising with activity against some fluconazole-resistant isolates such as Candida glabrata, C. krusei and C. auris [277]. T-2307 is an arylamidine similar to pentamidine targeting fungal mitochondria with activity against most Candida, Cryptococcus neoformans, C. gattii and A. fumigatus [266, 277–283]. Further trials are awaited.
In well-selected patients with invasive fungal infections for which medical therapy fails, surgical resection using wedge resection or lobectomy might offer an alternative option with acceptable outcomes [284, 285].
Conclusions
Fungal lung disease remains a rising global health concern with increasing burden. Recognition is difficult and management challenging due to the diverse spectrum of clinical presentations, broad range of fungal pathogens and increasing antifungal drug resistance. Significant progress is being made in understanding its pathophysiology, the spectrum of illness and the key interplay between the host immune system and mycobiome. Novel diagnostics will improve our recognition and the drug development pipeline our treatment options. Harnessing molecular approaches including “omic” technologies and strengthening collaborations between academic, clinical and industry partners is imperative to make progress toward more personalised diagnosis and treatment across the spectrum of fungal lung disease. Standardised guidelines for recognition, diagnosis and management must be implemented and regularly updated in parallel with research advances to maximise the effect at the individual patient level.
Shareable PDF
Acknowledgements
The authors would like to acknowledge the Academic Respiratory Initiative for Pulmonary Health (TARIPH) and the Lee Kong Chian School of Medicine Centre for Microbiome Medicine for collaborative support.
Footnotes
Author contributions: T.K. Jaggi, R. Agarwal, P.Y. Tiew, A. Shah, E.C. Lydon, C.A. Hage, G.W. Waterer, C.R. Langelier and L. Delhaes contributed to study design, data collection, interpretation and writing of the final manuscript. S.H. Chotirmall contributed to study design and conception, data collection, interpretation and analysis, writing of the final manuscript, and obtained study funding.
Conflict of interest: S.H. Chotirmall has served on advisory boards for CSL Behring, Pneumagen Ltd and Boehringer Ingelheim, on data safety monitoring boards for Inovio Pharmaceuticals and Imam Abdulrahman Bin Faisal University, and has received personal fees from AstraZeneca and Chiesi Farmaceutici, all unrelated to this work. The remaining authors have no potential conflicts of interest to disclose.
Support statement: This work is supported by the National Research Foundation Singapore under its Open Fund-Large Collaborative Grant (MOH-001636) and administered by the Singapore Ministry of Health’s National Medical Research Council (S.H. Chotirmall); the Singapore Ministry of Health’s National Medical Research Council under its Clinician-Scientist Individual Research Grant (MOH-001356) (S.H. Chotirmall); Clinician Scientist Award (MOH-000710) (S.H. Chotirmall); Transition Award (MOH-001275-00) (P.Y. Tiew); Open Fund Individual Research Grant (MOH-000955) (S.H. Chotirmall); and the Singapore Ministry of Education under its AcRF Tier 1 Grant (RT1/22) (S.H. Chotirmall). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chotirmall SH, Bogaert D, Chalmers JD, et al. Therapeutic targeting of the respiratory microbiome. Am J Respir Crit Care Med 2022; 206: 535–544. doi: 10.1164/rccm.202112-2704PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mac Aogain M, Jaggi TK, Chotirmall SH. The airway microbiome: present and future applications. Arch Bronconeumol 2022; 58: 8–10. doi: 10.1016/j.arbres.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Tiew PY, Mac Aogain M, Ter SK, et al. Respiratory mycoses in COPD and bronchiectasis. Mycopathologia 2021; 186: 623–638. doi: 10.1007/s11046-021-00539-z [DOI] [PubMed] [Google Scholar]
- 4.Hong G, Miller HB, Allgood S, et al. Use of selective fungal culture media increases rates of detection of fungi in the respiratory tract of cystic fibrosis patients. J Clin Microbiol 2017; 55: 1122–1130. doi: 10.1128/JCM.02182-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J 2014; 43: 64–71. doi: 10.1183/09031936.00162912 [DOI] [PubMed] [Google Scholar]
- 6.Huerta A, Soler N, Esperatti M, et al. Importance of Aspergillus spp. isolation in acute exacerbations of severe COPD: prevalence, factors and follow-up: the FUNGI-COPD study. Respir Res 2014; 15: 17. doi: 10.1186/1465-9921-15-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong X, Cheng A, Xu H, et al. Aspergillus fumigatus during COPD exacerbation: a pair-matched retrospective study. BMC Pulm Med 2018; 18: 55. doi: 10.1186/s12890-018-0611-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chotirmall SH, O'Donoghue E, Bennett K, et al. Sputum Candida albicans presages FEV1 decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010; 138: 1186–1195. doi: 10.1378/chest.09-2996 [DOI] [PubMed] [Google Scholar]
- 9.Nelson LA, Callerame ML, Schwartz RH. Aspergillosis and atopy in cystic fibrosis. Am Rev Respir Dis 1979; 120: 863–873. [DOI] [PubMed] [Google Scholar]
- 10.Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J 2002; 20: 658–664. doi: 10.1183/09031936.02.00248102 [DOI] [PubMed] [Google Scholar]
- 11.McMahon MA, Chotirmall SH, McCullagh B, et al. Radiological abnormalities associated with Aspergillus colonization in a cystic fibrosis population. Eur J Radiol 2012; 81: e197–e202. doi: 10.1016/j.ejrad.2011.01.114 [DOI] [PubMed] [Google Scholar]
- 12.de Vrankrijker AM, van der Ent CK, van Berkhout FT, et al. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin Microbiol Infect 2011; 17: 1381–1386. doi: 10.1111/j.1469-0691.2010.03429.x [DOI] [PubMed] [Google Scholar]
- 13.Blomquist A, Inghammar M, Al Shakirchi M, et al. Persistent Aspergillus fumigatus infection in cystic fibrosis: impact on lung function and role of treatment of asymptomatic colonization - a registry-based case–control study. BMC Pulm Med 2022; 22: 263. doi: 10.1186/s12890-022-02054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin R, Dupuis A, Aaron SD, et al. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010; 137: 171–176. doi: 10.1378/chest.09-1103 [DOI] [PubMed] [Google Scholar]
- 15.Breuer O, Schultz A, Garratt LW, et al. Aspergillus infections and progression of structural lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2020; 201: 688–696. doi: 10.1164/rccm.201908-1585OC [DOI] [PubMed] [Google Scholar]
- 16.Al Shakirchi M, Sorjonen K, Klingspor L, et al. The effects of Aspergillus fumigatus colonization on lung function in patients with cystic fibrosis. J Fungi (Basel) 2021; 7: 944. doi: 10.3390/jof7110944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YX, Zuo YH, Cheng QJ, et al. Respiratory Aspergillus colonization was associated with relapse of acute exacerbation in patients with chronic obstructive pulmonary disease: analysis of data from a retrospective cohort study. Front Med (Lausanne) 2021; 8: 640289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiew PY, Dicker AJ, Keir HR, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J 2021; 57: 2002050. doi: 10.1183/13993003.02050-2020 [DOI] [PubMed] [Google Scholar]
- 19.Barberan J, Garcia-Perez FJ, Villena V, et al. Development of aspergillosis in a cohort of non-neutropenic, non-transplant patients colonised by Aspergillus spp. BMC Infect Dis 2017; 17: 34. doi: 10.1186/s12879-016-2143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borman AM, Palmer MD, Delhaes L, et al. Lack of standardization in the procedures for mycological examination of sputum samples from CF patients: a possible cause for variations in the prevalence of filamentous fungi. Med Mycol 2010; 48: Suppl. 1, S88–S97. doi: 10.3109/13693786.2010.511287 [DOI] [PubMed] [Google Scholar]
- 21.Pashley CH, Fairs A, Morley JP, et al. Routine processing procedures for isolating filamentous fungi from respiratory sputum samples may underestimate fungal prevalence. Med Mycol 2012; 50: 433–438. doi: 10.3109/13693786.2011.615762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baxter CG, Moore CB, Jones AM, et al. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest 2013; 143: 1351–1357. doi: 10.1378/chest.12-1363 [DOI] [PubMed] [Google Scholar]
- 23.Mortensen KL, Jensen RH, Johansen HK, et al. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol 2011; 49: 2243–2251. doi: 10.1128/JCM.00213-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurhade AM, Deshmukh JM, Fule RP, et al. Mycological and serological study of pulmonary aspergillosis in central India. Indian J Med Microbiol 2002; 20: 141–144. doi: 10.1016/S0255-0857(21)03246-1 [DOI] [PubMed] [Google Scholar]
- 25.Shahi M, Ayatollahi Mousavi SA, Nabili M, et al. Aspergillus colonization in patients with chronic obstructive pulmonary disease. Curr Med Mycol 2015; 1: 45–51. doi: 10.18869/acadpub.cmm.1.3.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahid M, Malik A, Bhargava R. Prevalence of aspergillosis in chronic lung diseases. Indian J Med Microbiol 2001; 19: 201–205. [PubMed] [Google Scholar]
- 27.Biswas D, Agarwal S, Sindhwani G, et al. Fungal colonization in patients with chronic respiratory diseases from Himalayan region of India. Ann Clin Microbiol Antimicrob 2010; 9: 28. doi: 10.1186/1476-0711-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlan CA, Chotirmall SH, Renwick J, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 999–1007. doi: 10.1164/rccm.201203-0478OC [DOI] [PubMed] [Google Scholar]
- 29.Aaron SD, Vandemheen KL, Freitag A, et al. Treatment of Aspergillus fumigatus in patients with cystic fibrosis: a randomized, placebo-controlled pilot study. PLoS One 2012; 7: e36077. doi: 10.1371/journal.pone.0036077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fairs A, Agbetile J, Hargadon B, et al. IgE sensitisation to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med 2010; 182: 1362–1368. doi: 10.1164/rccm.201001-0087OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agbetile J, Fairs A, Desai D, et al. Isolation of filamentous fungi from sputum in asthma is associated with reduced post-bronchodilator FEV1. Clin Exp Allergy 2012; 42: 782–791. doi: 10.1111/j.1365-2222.2012.03987.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mac Aogain M, Chandrasekaran R, Lim AYH, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J 2018; 52: 1800766. doi: 10.1183/13993003.00766-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poh TY, Tiew PY, Lim AYH, et al. Increased chitotriosidase is associated with Aspergillus and frequent exacerbations in South-East Asian patients with bronchiectasis. Chest 2020; 158: 512–522. doi: 10.1016/j.chest.2020.02.048 [DOI] [PubMed] [Google Scholar]
- 34.Chang D, Sharma L, Dela Cruz CS. Chitotriosidase: a marker and modulator of lung disease. Eur Respir Rev 2020; 29: 190143. doi: 10.1183/16000617.0143-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohara S, Tazawa Y, Tanai C, et al. Clinical characteristics of patients with Aspergillus species isolation from respiratory samples: comparison of chronic pulmonary aspergillosis and colonization. Respir Investig 2016; 54: 92–97. doi: 10.1016/j.resinv.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 36.Chrdle A, Mustakim S, Bright-Thomas RJ, et al. Aspergillus bronchitis without significant immunocompromise. Ann NY Acad Sci 2012; 1272: 73–85. doi: 10.1111/j.1749-6632.2012.06816.x [DOI] [PubMed] [Google Scholar]
- 37.Ozyigit LP, Monteiro W, Rick EM, et al. Fungal bronchitis is a distinct clinical entity which is responsive to antifungal therapy. Chron Respir Dis 2021; 18: 1479973120964448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barberan J, Sanchez-Haya E, del Castillo D, et al. Report of 38 cases of tracheobronchitis in non-immunocompromised patients with dual isolation of Aspergillus in lower respiratory tract samples. Rev Esp Quimioter 2014; 27: 110–114. [PubMed] [Google Scholar]
- 39.Shoseyov D, Brownlee KG, Conway SP, et al. Aspergillus bronchitis in cystic fibrosis. Chest 2006; 130: 222–226. doi: 10.1378/chest.130.1.222 [DOI] [PubMed] [Google Scholar]
- 40.Brandt C, Roehmel J, Rickerts V, et al. Aspergillus bronchitis in patients with cystic fibrosis. Mycopathologia 2018; 183: 61–69. doi: 10.1007/s11046-017-0190-0 [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R, Muthu V, Sehgal IS. Relationship between Aspergillus and asthma. Allergol Int 2023; 72: 507–520. doi: 10.1016/j.alit.2023.08.004 [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Muthu V, Sehgal IS, et al. Prevalence of Aspergillus sensitisation and allergic bronchopulmonary aspergillosis in adults with bronchial asthma: a systematic review of global data. J Allergy Clin Immunol Pract 2023; 11: 1734–1751. doi: 10.1016/j.jaip.2023.04.009 [DOI] [PubMed] [Google Scholar]
- 43.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: results from the 2005–2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract 2013; 1: 501–508. doi: 10.1016/j.jaip.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 44.Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol 2006; 117: 642–648. doi: 10.1016/j.jaci.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Soundappan K, Muthu V, Dhooria S, et al. Population prevalence of allergic bronchopulmonary aspergillosis in asthma: an epidemiological study of 43,261 participants from North India. Clin Exp Allergy 2023; 53: 777–780. doi: 10.1111/cea.14299 [DOI] [PubMed] [Google Scholar]
- 46.Toppila-Salmi S, Huhtala H, Karjalainen J, et al. Sensitization pattern affects the asthma risk in Finnish adult population. Allergy 2015; 70: 1112–1120. doi: 10.1111/all.12670 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R, Hazarika B, Gupta D, et al. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol 2010; 48: 988–994. doi: 10.3109/13693781003743148 [DOI] [PubMed] [Google Scholar]
- 48.Muthu V, Prasad KT, Sehgal IS, et al. Obstructive lung diseases and allergic bronchopulmonary aspergillosis. Curr Opin Pulm Med 2021; 27: 105–112. doi: 10.1097/MCP.0000000000000755 [DOI] [PubMed] [Google Scholar]
- 49.Tiew PY, Narayana JK, Quek MSL, et al. Sensitisation to recombinant Aspergillus fumigatus allergens and clinical outcomes in COPD. Eur Respir J 2023; 61: 2200507. doi: 10.1183/13993003.00507-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal R, Sehgal IS, Muthu V, et al. Revised ISHAM-ABPA working group clinical practice guidelines for diagnosing, classifying, and treating allergic bronchopulmonary aspergillosis/mycoses. Eur Respir J 2024; 63: 2400061. doi: 10.1183/13993003.00061-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal R, Muthu V, Sehgal IS. Clinical manifestation and treatment of allergic bronchopulmonary aspergillosis. Semin Respir Crit Care Med 2024; 45: 114–127. doi: 10.1055/s-0043-1776912 [DOI] [PubMed] [Google Scholar]
- 52.Hammond EE, McDonald CS, Vestbo J, et al. The global impact of Aspergillus infection on COPD. BMC Pulm Med 2020; 20: 241. doi: 10.1186/s12890-020-01259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chotirmall SH, Martin-Gomez MT. Aspergillus species in bronchiectasis: challenges in the cystic fibrosis and non-cystic fibrosis airways. Mycopathologia 2018; 183: 45–59. doi: 10.1007/s11046-017-0143-7 [DOI] [PubMed] [Google Scholar]
- 54.Yii AC, Koh MS, Lapperre TS, et al. The emergence of Aspergillus species in chronic respiratory disease. Front Biosci (Schol Ed) 2017; 9: 127–138. doi: 10.2741/s477 [DOI] [PubMed] [Google Scholar]
- 55.Tiew PY, Lim AYH, Keir HR, et al. High frequency of allergic bronchopulmonary aspergillosis in bronchiectasis–COPD overlap. Chest 2022; 161: 40–53. doi: 10.1016/j.chest.2021.07.2165 [DOI] [PubMed] [Google Scholar]
- 56.Chowdhary A, Agarwal K, Kathuria S, et al. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol 2014; 40: 30–48. doi: 10.3109/1040841X.2012.754401 [DOI] [PubMed] [Google Scholar]
- 57.Boaventura R, Sibila O, Agusti A, et al. Treatable traits in bronchiectasis. Eur Respir J 2018; 52: 1801269. doi: 10.1183/13993003.01269-2018 [DOI] [PubMed] [Google Scholar]
- 58.Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus – what makes the species a ubiquitous human fungal pathogen? Plos Pathog 2013; 9: e1003743. doi: 10.1371/journal.ppat.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal R, Muthu V, Sehgal IS, et al. Allergic bronchopulmonary aspergillosis. Clin Chest Med 2022; 43: 99–125. doi: 10.1016/j.ccm.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 60.Chotirmall SH, Mirkovic B, Lavelle GM, et al. Immunoevasive Aspergillus virulence factors. Mycopathologia 2014; 178: 363–370. doi: 10.1007/s11046-014-9768-y [DOI] [PubMed] [Google Scholar]
- 61.Overton NL, Denning DW, Bowyer P, et al. Genetic susceptibility to allergic bronchopulmonary aspergillosis in asthma: a genetic association study. Allergy Asthma Clin Immunol 2016; 12: 47. doi: 10.1186/s13223-016-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanaujia R, Arora A, Chakrabarti A, et al. Occurrence of cystic fibrosis transmembrane conductance regulator gene mutations in patients with allergic bronchopulmonary aspergillosis complicating asthma. Mycopathologia 2022; 187: 147–155. doi: 10.1007/s11046-022-00631-y [DOI] [PubMed] [Google Scholar]
- 63.Kanaujia R, Arora A, Chakrabarti A, et al. Polymorphisms in innate and adaptive immune genes in subjects with allergic bronchopulmonary aspergillosis complicating asthma. Mycopathologia 2024; 189: 23. doi: 10.1007/s11046-024-00834-5 [DOI] [PubMed] [Google Scholar]
- 64.Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 2019; 364: eaaw4295. doi: 10.1126/science.aaw4295 [DOI] [PubMed] [Google Scholar]
- 65.Muniz VS, Silva JC, Braga YAV, et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 2018; 141: 571–585. doi: 10.1016/j.jaci.2017.07.048 [DOI] [PubMed] [Google Scholar]
- 66.Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 67.Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019; 7: e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4 [DOI] [PubMed] [Google Scholar]
- 68.Maturu VN, Agarwal R. Prevalence of Aspergillus sensitisation and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy 2015; 45: 1765–1778. doi: 10.1111/cea.12595 [DOI] [PubMed] [Google Scholar]
- 69.Agarwal R, Aggarwal AN, Dhooria S, et al. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2016; 47: 490–498. doi: 10.1183/13993003.01475-2015 [DOI] [PubMed] [Google Scholar]
- 70.Agarwal R, Dhooria S, Singh Sehgal I, et al. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest 2018; 153: 656–664. doi: 10.1016/j.chest.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 71.Agarwal R, Muthu V, Sehgal IS, et al. A randomised trial of prednisolone versus prednisolone and itraconazole in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2022; 59: 2101787. doi: 10.1183/13993003.01787-2021 [DOI] [PubMed] [Google Scholar]
- 72.Agarwal R, Dhooria S, Sehgal IS, et al. A randomised trial of voriconazole and prednisolone monotherapy in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2018; 52: 1801159. doi: 10.1183/13993003.01159-2018 [DOI] [PubMed] [Google Scholar]
- 73.Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342: 756–762. doi: 10.1056/NEJM200003163421102 [DOI] [PubMed] [Google Scholar]
- 74.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol 2003; 111: 952–957. doi: 10.1067/mai.2003.1388 [DOI] [PubMed] [Google Scholar]
- 75.Ram B, Aggarwal AN, Dhooria S, et al. A pilot randomized trial of nebulized amphotericin in patients with allergic bronchopulmonary aspergillosis. J Asthma 2016; 53: 517–524. doi: 10.3109/02770903.2015.1127935 [DOI] [PubMed] [Google Scholar]
- 76.Albogami S. Use of biologic drugs for treatment of allergic bronchopulmonary aspergillosis. Int J Pulm Respir Sci 2021; 5: 555663. doi: 10.19080/IJOPRS.2021.05.555663 [DOI] [Google Scholar]
- 77.Godet C, Couturaud F, Marchand-Adam S, et al. Nebulised liposomal amphotericin-B as maintenance therapy in allergic bronchopulmonary aspergillosis: a randomised, multicentre trial. Eur Respir J 2022; 59: 2102218. doi: 10.1183/13993003.02218-2021 [DOI] [PubMed] [Google Scholar]
- 78.Muthu V, Dhooria S, Sehgal IS, et al. Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: a systematic review and meta-analysis. Pulm Pharmacol Ther 2023; 81: 102226. doi: 10.1016/j.pupt.2023.102226 [DOI] [PubMed] [Google Scholar]
- 79.Godet C, Brun A-L, Couturaud F, et al. CT imaging assessment of response to treatment in allergic bronchopulmonary aspergillosis in adults with bronchial asthma. Chest 2024; 165: 1307–1318. doi: 10.1016/j.chest.2024.02.026 [DOI] [PubMed] [Google Scholar]
- 80.Agarwal R, Sehgal IS, Muthu V, et al. Long-term follow-up of allergic bronchopulmonary aspergillosis treated with glucocorticoids: a study of 182 subjects. Mycoses 2023; 66: 953–959. doi: 10.1111/myc.13640 [DOI] [PubMed] [Google Scholar]
- 81.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016; 47: 45–68. doi: 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 82.Maitre T, Cottenet J, Godet C, et al. Chronic pulmonary aspergillosis: prevalence, favouring pulmonary diseases and prognosis. Eur Respir J 2021; 58: 2003345. doi: 10.1183/13993003.03345-2020 [DOI] [PubMed] [Google Scholar]
- 83.Bongomin F, Olum R, Kwizera R, et al. Surgical management of chronic pulmonary aspergillosis in Africa: a systematic review of 891 cases. Mycoses 2021; 64: 1151–1158. doi: 10.1111/myc.13359 [DOI] [PubMed] [Google Scholar]
- 84.Uzunhan Y, Nunes H, Jeny F, et al. Chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J 2017; 49: 1602396. doi: 10.1183/13993003.02396-2016 [DOI] [PubMed] [Google Scholar]
- 85.Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis 2024; 24: e428–e438. doi: 10.1016/S1473-3099(23)00692-8 [DOI] [PubMed] [Google Scholar]
- 86.Tamkeviciute L, Tumenas A, Zaveckiene J, et al. Different forms of pulmonary aspergillosis: a pictorial essay. Eur J Radiol 2024; 171: 111290. doi: 10.1016/j.ejrad.2024.111290 [DOI] [PubMed] [Google Scholar]
- 87.Jaggi TK, Ter SK, Mac Aogain M, et al. Aspergillus-associated endophenotypes in bronchiectasis. Semin Respir Crit Care Med 2021; 42: 556–566. doi: 10.1055/s-0041-1730947 [DOI] [PubMed] [Google Scholar]
- 88.Denning DW, Riniotis K, Dobrashian R, et al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003; 37: Suppl. 3, S265–S280. doi: 10.1086/376526 [DOI] [PubMed] [Google Scholar]
- 89.Tunnicliffe G, Schomberg L, Walsh S, et al. Airway and parenchymal manifestations of pulmonary aspergillosis. Respir Med 2013; 107: 1113–1123. doi: 10.1016/j.rmed.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 90.Evans TJ, Lawal A, Kosmidis C, et al. Chronic pulmonary aspergillosis: clinical presentation and management. Semin Respir Crit Care Med 2024; 45: 88–101. doi: 10.1055/s-0043-1776914 [DOI] [PubMed] [Google Scholar]
- 91.Mei ZX, Han JF, Yu HW, et al. Detection of serum Aspergillus-specific IgM and IgG antibody levels for the diagnosis of chronic pulmonary aspergillosis developed in patients with tuberculosis. Eur J Clin Microbiol Infect Dis 2023; 42: 1081–1089. doi: 10.1007/s10096-023-04637-2 [DOI] [PubMed] [Google Scholar]
- 92.Ray A, Chowdhury M, Sachdev J, et al. Efficacy of LD Bio Aspergillus ICT lateral flow assay for serodiagnosis of chronic pulmonary aspergillosis. J Fungi (Basel) 2022; 8: 400. doi: 10.3390/jof8040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hunter ES, Wilopo B, Richardson MD, et al. Effect of patient immunodeficiencies on the diagnostic performance of serological assays to detect Aspergillus-specific antibodies in chronic pulmonary aspergillosis. Respir Med 2021; 178: 106290. doi: 10.1016/j.rmed.2020.106290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chowdhury M, Singh G, Pandey M, et al. The utility of galactomannan and polymerase chain reaction assays in bronchoalveolar lavage for diagnosis of chronic pulmonary aspergillosis. Mycopathologia 2023; 188: 1041–1053. doi: 10.1007/s11046-023-00797-z [DOI] [PubMed] [Google Scholar]
- 95.Geurts K, Zweijpfenning SMH, Pennings LJ, et al. Nontuberculous mycobacterial pulmonary disease and Aspergillus co-infection: Bonnie and Clyde? Eur Respir J 2019; 54: 1900117. doi: 10.1183/13993003.00117-2019 [DOI] [PubMed] [Google Scholar]
- 96.Rozaliyani A, Abdullah A, Setianingrum F, et al. Unravelling the molecular identification and antifungal susceptibility profiles of Aspergillus spp. isolated from chronic pulmonary aspergillosis patients in Jakarta, Indonesia: the emergence of cryptic species. J Fungi (Basel) 2022; 8: 411. doi: 10.3390/jof8040411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sprute R, Salzer HJF, Seidel D. CPAnet: the challenges of gaining evidence-based knowledge in chronic pulmonary aspergillosis. Eur Respir J 2022; 59: 2102879. doi: 10.1183/13993003.02879-2021 [DOI] [PubMed] [Google Scholar]
- 98.Sehgal IS, Dhooria S, Muthu V, et al. Efficacy of 12 months oral itraconazole versus 6 months oral itraconazole to prevent relapses of chronic pulmonary aspergillosis: an open-label, randomised controlled trial in India. Lancet Infect Dis 2022; 22: 1052–1061. doi: 10.1016/S1473-3099(22)00057-3 [DOI] [PubMed] [Google Scholar]
- 99.Bongomin F, Harris C, Hayes G, et al. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS One 2018; 13: e0193732. doi: 10.1371/journal.pone.0193732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monk EJM, Harris C, Doffinger R, et al. Interferon-γ replacement as salvage therapy in chronic pulmonary aspergillosis: effects on frequency of acute exacerbation and all-cause hospital admission. Thorax 2020; 75: 513–516. doi: 10.1136/thoraxjnl-2019-213606 [DOI] [PubMed] [Google Scholar]
- 101.Nwankwo L, Gilmartin D, Matharu S, et al. Experience of isavuconazole as a salvage therapy in chronic pulmonary fungal disease. J Fungi (Basel) 2022; 8: 362. doi: 10.3390/jof8040362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osborne W, Fernandes M, Brooks S, et al. Pulsed echinocandin therapy in azole intolerant or multiresistant chronic pulmonary aspergillosis: a retrospective review at a UK tertiary centre. Clin Respir J 2020; 14: 571–577. doi: 10.1111/crj.13171 [DOI] [PubMed] [Google Scholar]
- 103.Yang L, Yang C, Wan N, et al. Bronchoscopic instillation of amphotericin B is a safe and effective measure to treat pulmonary mycosis. Front Pharmacol 2023; 14: 1167475. doi: 10.3389/fphar.2023.1167475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 2017; 49: 1601062. doi: 10.1183/13993003.01062-2016 [DOI] [PubMed] [Google Scholar]
- 105.Sprute R, Van Braeckel E, Flick H, et al. EQUAL CPA Score 2022: a tool to measure guideline adherence for chronic pulmonary aspergillosis. J Antimicrob Chemother 2022; 78: 225–231. doi: 10.1093/jac/dkac378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horger M, Hebart H, Einsele H, et al. Initial CT manifestations of invasive pulmonary aspergillosis in 45 non-HIV immunocompromised patients: association with patient outcome? Eur J Radiol 2005; 55: 437–444. doi: 10.1016/j.ejrad.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 107.Mehrad B, Paciocco G, Martinez FJ, et al. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest 2001; 119: 169–175. doi: 10.1378/chest.119.1.169 [DOI] [PubMed] [Google Scholar]
- 108.Samanta P, Clancy CJ, Nguyen MH. Fungal infections in lung transplantation. J Thorac Dis 2021; 13: 6695–6707. doi: 10.21037/jtd-2021-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Upton A, Kirby KA, Carpenter P, et al. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 2007; 44: 531–540. doi: 10.1086/510592 [DOI] [PubMed] [Google Scholar]
- 110.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 2007; 20: 695–704. doi: 10.1128/CMR.00014-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meersseman W, Lagrou K, Maertens J, et al. Invasive aspergillosis in the intensive care unit. Clin Infect Dis 2007; 45: 205–216. doi: 10.1086/518852 [DOI] [PubMed] [Google Scholar]
- 112.Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 2004; 170: 621–625. doi: 10.1164/rccm.200401-093OC [DOI] [PubMed] [Google Scholar]
- 113.Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis 2019; 10: 782. doi: 10.1038/s41419-019-2015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hartemink KJ, Paul MA, Spijkstra JJ, et al. Immunoparalysis as a cause for invasive aspergillosis? Intensive Care Med 2003; 29: 2068–2071. doi: 10.1007/s00134-003-1778-z [DOI] [PubMed] [Google Scholar]
- 115.Neyton LPA, Sinha P, Sarma A, et al. Host and microbe blood metagenomics reveals key pathways characterizing critical illness phenotypes. Am J Respir Crit Care Med 2024; 209: 805–815. doi: 10.1164/rccm.202308-1328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet 2003; 362: 1828–1838. doi: 10.1016/S0140-6736(03)14904-5 [DOI] [PubMed] [Google Scholar]
- 117.Tejerina EE, Abril E, Padilla R, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses 2019; 62: 673–679. doi: 10.1111/myc.12927 [DOI] [PubMed] [Google Scholar]
- 118.Bassetti M, Azoulay E, Kullberg BJ, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the Intensive Care Unit Working Group. Clin Infect Dis 2021; 72: Suppl. 2, S121–S127. doi: 10.1093/cid/ciaa1751 [DOI] [PubMed] [Google Scholar]
- 119.Gold JAW, Adjei S, Gundlapalli AV, et al. Increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021. Emerg Infect Dis 2023; 29: 1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lat A, Bhadelia N, Miko B, et al. Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis 2010; 16: 971–973. doi: 10.3201/eid1606.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu LY, Lee HM, Burke A, et al. Prevalence, risk factors, clinical features, and outcome of influenza-associated pulmonary aspergillosis in critically ill patients: a systematic review and meta-analysis. Chest 2024; 165: 540–558. doi: 10.1016/j.chest.2023.09.019 [DOI] [PubMed] [Google Scholar]
- 122.Apostolopoulou A, Clancy CJ, Skeel A, et al. Invasive pulmonary aspergillosis complicating noninfluenza respiratory viral infections in solid organ transplant recipients. Open Forum Infect Dis 2021; 8: ofab478. doi: 10.1093/ofid/ofab478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Magira EE, Chemaly RF, Jiang Y, et al. Outcomes in invasive pulmonary aspergillosis infections complicated by respiratory viral infections in patients with hematologic malignancies: a case–control study. Open Forum Infect Dis 2019; 6: ofz247. doi: 10.1093/ofid/ofz247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feys S, Lagrou K, Lauwers HM, et al. High burden of COVID-19-associated pulmonary aspergillosis in severely immunocompromised patients requiring mechanical ventilation. Clin Infect Dis 2024; 78: 361–370. doi: 10.1093/cid/ciad546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21: e149–e162. doi: 10.1016/S1473-3099(20)30847-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janssen NAF, Nyga R, Vanderbeke L, et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis. Emerg Infect Dis 2021; 27: 2892–2898. doi: 10.3201/eid2711.211174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lamoth F, Lewis RE, Walsh TJ, et al. Navigating the uncertainties of COVID-19-associated aspergillosis: a comparison with influenza-associated aspergillosis. J Infect Dis 2021; 224: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dewi IM, Janssen NA, Rosati D, et al. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr Opin Microbiol 2021; 62: 21–27. doi: 10.1016/j.mib.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 129.Salazar F, Bignell E, Brown GD, et al. Pathogenesis of respiratory viral and fungal coinfections. Clin Microbiol Rev 2022; 35: e0009421. doi: 10.1128/CMR.00094-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe 2022; 3: e543–e552. doi: 10.1016/S2666-5247(21)00237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vandewoude KH, Blot SI, Depuydt P, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care 2006; 10: R31. doi: 10.1186/cc4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006; 42: 1417–1427. doi: 10.1086/503427 [DOI] [PubMed] [Google Scholar]
- 133.Crans CA Jr, Boiselle PM. Imaging features of Pneumocystis carinii pneumonia. Crit Rev Diagn Imaging 1999: 40: 251–284. doi: 10.3109/10408379991249194 [DOI] [PubMed] [Google Scholar]
- 134.Ferré C, Báguena F, Podzamczer D, et al. Lung cavitation associated with Pneumocystis carinii infection in the acquired immunodeficiency syndrome: a report of six cases and review of the literature. Eur Respir J 1994; 7: 134–139. doi: 10.1183/09031936.94.07010134 [DOI] [PubMed] [Google Scholar]
- 135.Zhou S, Aitken SL. Prophylaxis against Pneumocystis jirovecii pneumonia in adults. JAMA 2023; 330: 182–183. doi: 10.1001/jama.2023.9844 [DOI] [PubMed] [Google Scholar]
- 136.Kolbrink B, Scheikholeslami-Sabzewari J, Borzikowsky C, et al. Evolving epidemiology of Pneumocystis pneumonia: findings from a longitudinal population-based study and a retrospective multi-center study in Germany. Lancet Reg Health Eur 2022; 18: 100400. doi: 10.1016/j.lanepe.2022.100400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin-Garrido I, Carmona EM, Specks U, et al. Pneumocystis pneumonia in patients treated with rituximab. Chest 2013; 144: 258–265. doi: 10.1378/chest.12-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raje N, Anderson K, Einsele H, et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: consensus recommendations from an expert panel. Blood Cancer J 2023; 13: 116. doi: 10.1038/s41408-023-00879-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryan CE, Cheng MP, Issa NC, et al. Pneumocystis jirovecii pneumonia and institutional prophylaxis practices in CLL patients treated with BTK inhibitors. Blood Adv 2020; 4: 1458–1463. doi: 10.1182/bloodadvances.2020001678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xia S, Gong H, Wang YK, et al. Pneumocystis jirovecii pneumonia associated with immune checkpoint inhibitors: a systematic literature review of published case reports and disproportionality analysis based on the FAERS database. Front Pharmacol 2023; 14: 1129730. doi: 10.3389/fphar.2023.1129730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Azoulay É, Bergeron A, Chevret S, et al. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2009; 135: 655–661. doi: 10.1378/chest.08-1309 [DOI] [PubMed] [Google Scholar]
- 142.Wang D, Fang S, Hu X, et al. Metagenomic next-generation sequencing is highly efficient in diagnosing Pneumocystis jirovecii pneumonia in the immunocompromised patients. Front Microbiol 2022; 13: 913405. doi: 10.3389/fmicb.2022.913405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Limper AH, Offord KP, Smith TF, et al. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 1989; 140: 1204–1209. doi: 10.1164/ajrccm/140.5.1204 [DOI] [PubMed] [Google Scholar]
- 144.Thomas CF Jr, Limper AH. Pneumocystis pneumonia: clinical presentation and diagnosis in patients with and without acquired immune deficiency syndrome. Semin Respir Infect 1998: 13: 289–295. [PubMed] [Google Scholar]
- 145.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med 2013; 5: 63. doi: 10.1186/gm467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009; 35: 1526–1531. doi: 10.1007/s00134-009-1482-8 [DOI] [PubMed] [Google Scholar]
- 147.Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002; 34: 400–403. doi: 10.1086/338404 [DOI] [PubMed] [Google Scholar]
- 148.Leon C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006; 34: 730–737. doi: 10.1097/01.CCM.0000202208.37364.7D [DOI] [PubMed] [Google Scholar]
- 149.Thompson GR 3rd, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis 2021: 21: e364–e374. doi: 10.1016/S1473-3099(21)00191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir Med 2012; 106: 769–776. doi: 10.1016/j.rmed.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 151.Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia 2020; 185: 843–865. doi: 10.1007/s11046-020-00431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mazi PB, Sahrmann JM, Olsen MA, et al. The geographic distribution of dimorphic mycoses in the United States for the modern era. Clin Infect Dis 2023; 76: 1295–1301. doi: 10.1093/cid/ciac882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chu JH, Feudtner C, Heydon K, et al. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 2006; 42: 822–825. doi: 10.1086/500405 [DOI] [PubMed] [Google Scholar]
- 154.Byrnes EJ 3rd, Bildfell RJ, Frank SA, et al. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis 2009: 199: 1081–1086. doi: 10.1086/597306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hagen F, Khayhan K, Theelen B, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 2015; 78: 16–48. doi: 10.1016/j.fgb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 156.Harris JR, Lockhart SR, Debess E, et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis 2011; 53: 1188–1195. doi: 10.1093/cid/cir723 [DOI] [PubMed] [Google Scholar]
- 157.Baddley JW, Chen SC, Huisingh C, et al. MSG07: An international cohort study comparing epidemiology and outcomes of patients with Cryptococcus neoformans or Cryptococcus gattii infections. Clin Infect Dis 2021; 73: 1133–1141. doi: 10.1093/cid/ciab268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mitchell DH, Sorrell TC, Allworth AM, et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 1995; 20: 611–616. doi: 10.1093/clinids/20.3.611 [DOI] [PubMed] [Google Scholar]
- 159.Li R, Li J, Zhou X. Lung microbiome: new insights into the pathogenesis of respiratory diseases. Signal Transduct Target Ther 2024; 9: 19. doi: 10.1038/s41392-023-01722-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Natalini JG, Singh S, Segal LN. The dynamic lung microbiome in health and disease. Nat Rev Microbiol 2023; 21: 222–235. doi: 10.1038/s41579-022-00821-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012; 186: 536–545. doi: 10.1164/rccm.201204-0693OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Enaud R, Vandenborght LE, Coron N, et al. The mycobiome: a neglected component in the microbiota-gut-brain axis. Microorganisms 2018; 6: 22. doi: 10.3390/microorganisms6010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu H, Liang Z, Cao N, et al. Airway bacterial and fungal microbiome in chronic obstructive pulmonary disease. Med Microecol 2021; 7: 100035. doi: 10.1016/j.medmic.2021.100035 [DOI] [Google Scholar]
- 164.Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol 2014; 14: 827–835. doi: 10.1038/nri3769 [DOI] [PubMed] [Google Scholar]
- 165.Martinsen EMH, Eagan TML, Leiten EO, et al. The pulmonary mycobiome: a study of subjects with and without chronic obstructive pulmonary disease. PLoS One 2021; 16: e0248967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nguyen LD, Viscogliosi E, Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol 2015; 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Viciani E, Gaibani P, Castagnetti A, et al. Critically ill patients with COVID-19 show lung fungal dysbiosis with reduced microbial diversity in patients colonized with Candida spp. Int J Infect Dis 2022; 117: 233–240. doi: 10.1016/j.ijid.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang L, Cao JB, Xia BB, et al. Metatranscriptome of human lung microbial communities in a cohort of mechanically ventilated COVID-19 Omicron patients. Signal Transduct Target Ther 2023; 8: 432. doi: 10.1038/s41392-023-01684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xie L, Chen L, Li X, et al. Analysis of lung microbiome in COVID-19 patients during time of hospitalization. Pathogens 2023; 12: 944. doi: 10.3390/pathogens12070944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weaver D, Gago S, Bassetti M, et al. Mycobiome analyses of critically ill COVID-19 patients. bioRxiv 2023; preprint [ 10.1101/2023.06.13.543274]. [DOI] [Google Scholar]
- 171.Fraczek MG, Chishimba L, Niven RM, et al. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J Allergy Clin Immunol 2018; 142: 407–414. doi: 10.1016/j.jaci.2017.09.039 [DOI] [PubMed] [Google Scholar]
- 172.van Woerden HC, Gregory C, Brown R, et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community-based case control study. BMC Infect Dis 2013; 13: 69. doi: 10.1186/1471-2334-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vandenborght LE, Enaud R, Urien C, et al. Type 2-high asthma is associated with a specific indoor mycobiome and microbiome. J Allergy Clin Immunol 2021; 147: 1296–1305. doi: 10.1016/j.jaci.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rick EM, Woolnough KF, Seear PJ, et al. The airway fungal microbiome in asthma. Clin Exp Allergy 2020; 50: 1325–1341. doi: 10.1111/cea.13722 [DOI] [PubMed] [Google Scholar]
- 175.Sharma A, Laxman B, Naureckas ET, et al. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J Allergy Clin Immunol 2019; 144: 1214–1227. doi: 10.1016/j.jaci.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cui L, Lucht L, Tipton L, et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am J Respir Crit Care Med 2015; 191: 932–942. doi: 10.1164/rccm.201409-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Su J, Liu HY, Tan XL, et al. Sputum bacterial and fungal dynamics during exacerbations of severe COPD. PLoS One 2015; 10: e0130736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xue T, Chun-Li A. Role of Pneumocystis jirovecii infection in chronic obstructive pulmonary disease progression in an immunosuppressed rat Pneumocystis pneumonia model. Exp Ther Med 2020; 19: 3133–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Delhaes L, Monchy S, Frealle E, et al. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community – implications for therapeutic management. PLoS One 2012; 7: e36313. doi: 10.1371/journal.pone.0036313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Enaud R, Lussac-Sorton F, Charpentier E, et al. Effects of lumacaftor-ivacaftor on airway microbiota-mycobiota and inflammation in patients with cystic fibrosis appear to be linked to Pseudomonas aeruginosa chronic colonization. Microbiol Spectr 2023; 11: e0225122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nguyen LD, Deschaght P, Merlin S, et al. Effects of propidium monoazide (PMA) treatment on mycobiome and bacteriome analysis of cystic fibrosis airways during exacerbation. PLoS One 2016; 11: e0168860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soret P, Vandenborght LE, Francis F, et al. Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci Rep 2020; 10: 3589. doi: 10.1038/s41598-020-60015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cuthbertson L, Felton I, James P, et al. The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis. J Cyst Fibros 2021; 20: 295–302. doi: 10.1016/j.jcf.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kramer R, Sauer-Heilborn A, Welte T, et al. Cohort study of airway mycobiome in adult cystic fibrosis patients: differences in community structure between fungi and bacteria reveal predominance of transient fungal elements. J Clin Microbiol 2015; 53: 2900–2907. doi: 10.1128/JCM.01094-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Conrad D, Haynes M, Salamon P, et al. Cystic fibrosis therapy: a community ecology perspective. Am J Respir Cell Mol Biol 2013; 48: 150–156. doi: 10.1165/rcmb.2012-0059PS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hampton TH, Thomas D, van der Gast C, et al. Mild cystic fibrosis lung disease is associated with bacterial community stability. Microbiol Spectr 2021; 9: e0002921. doi: 10.1128/Spectrum.00029-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lim YW, Evangelista JS 3rd, Schmieder R, et al. Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J Clin Microbiol 2014: 52: 425–437. doi: 10.1128/JCM.02204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Quinn RA, Lim YW, Maughan H, et al. Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung. mBio 2014; 5: e00956–e00913. doi: 10.1128/mBio.00956-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Quinn RA, Whiteson K, Lim YW, et al. A Winogradsky-based culture system shows an association between microbial fermentation and cystic fibrosis exacerbation. ISME J 2015; 9: 1024–1038. doi: 10.1038/ismej.2014.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mac Aogáin M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021; 27: 688–699. doi: 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 191.Welsh KG, Holden KA, Wardlaw AJ, et al. Fungal sensitisation and positive fungal culture from sputum in children with asthma are associated with reduced lung function and acute asthma attacks respectively. Clin Exp Allergy 2021; 51: 790–800. doi: 10.1111/cea.1379 [DOI] [PubMed] [Google Scholar]
- 192.Lin CY, Huang YC, Huang HY, et al. Effectiveness of low-dose itraconazole in fungal-associated severe asthma: a retrospective study. J Asthma Allergy 2020; 13: 453–461. doi: 10.2147/JAA.S276289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Peetermans M, Goeminne P, De Boeck C, et al. IgE sensitisation to Aspergillus fumigatus is not a bystander phenomenon in cystic fibrosis lung disease. Chest 2014; 146: e99–e100. doi: 10.1378/chest.14-0635 [DOI] [PubMed] [Google Scholar]
- 194.Mirkovic B, Lavelle GM, Azim AA, et al. The basophil surface marker CD203c identifies Aspergillus species sensitisation in patients with cystic fibrosis. J Allergy Clin Immunol 2016; 137: 436–443. doi: 10.1016/j.jaci.2015.07.045 [DOI] [PubMed] [Google Scholar]
- 195.Fillaux J, Bremont F, Murris M, et al. Assessment of Aspergillus sensitisation or persistent carriage as a factor in lung function impairment in cystic fibrosis patients. Scand J Infect Dis 2012; 44: 842–847. doi: 10.3109/00365548.2012.695454 [DOI] [PubMed] [Google Scholar]
- 196.Chan R, Lipworth B. Severe asthma with fungal sensitisation is associated with worse small airway dysfunction but similar symptom control. Ann Allergy Asthma Immunol 2023; 130: 518–520. doi: 10.1016/j.anai.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 197.Goh KJ, Yii ACA, Lapperre TS, et al. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy 2017; 10: 131–140. doi: 10.2147/JAA.S130459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000; 55: 501–504. doi: 10.1034/j.1398-9995.2000.00293.x [DOI] [PubMed] [Google Scholar]
- 199.Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J 2020; 56: 2000418. doi: 10.1183/13993003.00418-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mac Aogáin M, Tiew PY, Lim AYH, et al. Distinct “immunoallertypes” of disease and high frequencies of sensitisation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2019; 199: 842–853. doi: 10.1164/rccm.201807-1355OC [DOI] [PubMed] [Google Scholar]
- 201.Pasqualotto AC, Powell G, Niven R, et al. The effects of antifungal therapy on severe asthma with fungal sensitisation and allergic bronchopulmonary aspergillosis. Respirology 2009; 14: 1121–1127. doi: 10.1111/j.1440-1843.2009.01640.x [DOI] [PubMed] [Google Scholar]
- 202.Denning DW, O'Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitisation: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 2009; 179: 11–18. doi: 10.1164/rccm.200805-737OC [DOI] [PubMed] [Google Scholar]
- 203.Chishimba L, Niven RM, Cooley J, et al. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitisation. J Asthma 2012; 49: 423–433. doi: 10.3109/02770903.2012.662568 [DOI] [PubMed] [Google Scholar]
- 204.Risum M, Hare RK, Gertsen JB, et al. Azole-resistant Aspergillus fumigatus among Danish cystic fibrosis patients: increasing prevalence and dominance of TR34/L98H. Front Microbiol 2020; 11: 1850. doi: 10.3389/fmicb.2020.01850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zeng Y, Xue X, Cai H, et al. Clinical characteristics and prognosis of allergic bronchopulmonary aspergillosis: a retrospective cohort study. J Asthma Allergy 2022; 15: 53–62. doi: 10.2147/JAA.S345427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang P, Ma Y, Chen X, et al. The difference in all-cause mortality between allergic bronchopulmonary aspergillosis with and without chronic obstructive pulmonary disease. J Asthma Allergy 2022; 15: 1861–1875. doi: 10.2147/JAA.S389985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kumar R, Chopra D. Evaluation of allergic bronchopulmonary aspergillosis in patients with and without central bronchiectasis. J Asthma 2002; 39: 473–477. doi: 10.1081/JAS-120004905 [DOI] [PubMed] [Google Scholar]
- 208.Francis NZ, Southern KW. Antifungal therapies for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev 2022; 9: CD002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wark P, Hussaini S, Holder C, et al. Omalizumab is an effective intervention in severe asthma with fungal sensitisation. J Allergy Clin Immunol Pract 2020; 8: 3428–3433. doi: 10.1016/j.jaip.2020.05.055 [DOI] [PubMed] [Google Scholar]
- 210.Dhariwal J, Hearn AP, Kavanagh JE, et al. Real-world effectiveness of anti-IL-5/5R therapy in severe atopic eosinophilic asthma with fungal sensitisation. J Allergy Clin Immunol Pract 2021; 9: 2315–2320. doi: 10.1016/j.jaip.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 211.Aydin O, Sozener ZC, Soyyigit S, et al. Omalizumab in the treatment of allergic bronchopulmonary aspergillosis: one center's experience with 14 cases. Allergy Asthma Proc 2015; 36: 493–500. doi: 10.2500/aap.2015.36.3909 [DOI] [PubMed] [Google Scholar]
- 212.Moss RB. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur Respir J 2014; 43: 1487–1500. doi: 10.1183/09031936.00139513 [DOI] [PubMed] [Google Scholar]
- 213.Manti S, Giallongo A, Parisi GF, et al. Biologic drugs in treating allergic bronchopulmonary aspergillosis in patients with cystic fibrosis: a systematic review. Eur Respir Rev 2022; 31: 220011. doi: 10.1183/16000617.0011-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ramonell RP, Lee FE, Swenson C, et al. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract 2020; 8: 742–743. doi: 10.1016/j.jaip.2019.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bouza E, Guinea J, Pelaez T, et al. Workload due to Aspergillus fumigatus and significance of the organism in the microbiology laboratory of a general hospital. J Clin Microbiol 2005; 43: 2075–2079. doi: 10.1128/JCM.43.5.2075-2079.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gago S, Denning DW, Bowyer P. Pathophysiological aspects of Aspergillus colonization in disease. Med Mycol 2019; 57: Suppl. 2, S219–S227. doi: 10.1093/mmy/myy076 [DOI] [PubMed] [Google Scholar]
- 217.Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 2001; 33: 1824–1833. doi: 10.1086/323900 [DOI] [PubMed] [Google Scholar]
- 218.Alsalman J, Zaid T, Makhlooq M, et al. A retrospective study of the epidemiology and clinical manifestation of invasive aspergillosis in a major tertiary care hospital in Bahrain. J Infect Public Health 2017; 10: 49–58. doi: 10.1016/j.jiph.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 219.Farrant J, Brice H, Fowler S, et al. Fungal sensitisation in severe asthma is associated with the identification of Aspergillus fumigatus in sputum. J Asthma 2016; 53: 732–735. doi: 10.3109/02770903.2016.1154073 [DOI] [PubMed] [Google Scholar]
- 220.Chakrabarti A, Sethi S, Raman DS, et al. Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses 2002; 45: 295–299. doi: 10.1046/j.1439-0507.2002.00738.x [DOI] [PubMed] [Google Scholar]
- 221.O'Hollaren MT, Yunginger JW, Offord KP, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med 1991; 324: 359–363. doi: 10.1056/NEJM199102073240602 [DOI] [PubMed] [Google Scholar]
- 222.Kreindler JL, Steele C, Nguyen N, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest 2010; 120: 3242–3254. doi: 10.1172/JCI42388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dodamani MH, Muthu V, Thakur R, et al. A randomised trial of vitamin D in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Mycoses 2019; 62: 320–327. doi: 10.1111/myc.12879 [DOI] [PubMed] [Google Scholar]
- 224.Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205–214. doi: 10.1056/NEJMoa2303951 [DOI] [PubMed] [Google Scholar]
- 225.Kudlaty E, Patel GB, Prickett ML, et al. Efficacy of type 2-targeted biologics in patients with asthma and bronchiectasis. Ann Allergy Asthma Immunol 2021; 126: 302–304. doi: 10.1016/j.anai.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen P, Sun W, He Y. Comparison of the next-generation sequencing (NGS) technology with culture methods in the diagnosis of bacterial and fungal infections. J Thorac Dis 2020; 12: 4924–4929. doi: 10.21037/jtd-20-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Máiz L, Nieto R, Cantón R, et al. Fungi in bronchiectasis: a concise review. Int J Mol Sci 2018; 19: 142. doi: 10.3390/ijms19010142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Morrissey CO, Chen SC, Sorrell TC, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 2013; 13: 519–528. doi: 10.1016/S1473-3099(13)70076-8 [DOI] [PubMed] [Google Scholar]
- 229.Zhou W, Li H, Zhang Y, et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J Clin Microbiol 2017; 55: 2153–2161. doi: 10.1128/JCM.00345-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jenks JD, White PL, Kidd SE, et al. An update on current and novel molecular diagnostics for the diagnosis of invasive fungal infections. Expert Rev Mol Diagn 2023; 23: 1135–1152. doi: 10.1080/14737159.2023.2267977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kidd SE, Chen SC, Meyer W, et al. A new age in molecular diagnostics for invasive fungal disease: are we ready? Front Microbiol 2019; 10: 2903. doi: 10.3389/fmicb.2019.02903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jiang S, Chen Y, Han S, et al. Next-generation sequencing applications for the study of fungal pathogens. Microorganisms 2022; 10: 1882. doi: 10.3390/microorganisms10101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Trubin P, Azar MM. A fast-track to fungal diagnosis: the potential of molecular diagnostics for fungi at the point of care. Expert Rev Mol Diagn 2024; 24: 143–146. doi: 10.1080/14737159.2023.2287504 [DOI] [PubMed] [Google Scholar]
- 234.Cruciani M, Mengoli C, Loeffler J, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev 2015; 9: CD009551. doi: 10.1002/14651858.CD009551.pub2 [DOI] [PubMed] [Google Scholar]
- 235.Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200: 535–550. doi: 10.1164/rccm.201906-1185ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol 2011; 49: 665–670. doi: 10.1128/JCM.01602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alanio A, Hauser PM, Lagrou K, et al. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71: 2386–2396. doi: 10.1093/jac/dkw156 [DOI] [PubMed] [Google Scholar]
- 238.Sandhu GS, Kline BC, Espy MJ, et al. Laboratory diagnosis of Pneumocystis carinii infections by PCR directed to genes encoding for mitochondrial 5S and 28S ribosomal RNA. Diagn Microbiol Infect Dis 1999; 33: 157–162. doi: 10.1016/S0732-8893(98)00137-0 [DOI] [PubMed] [Google Scholar]
- 239.Fang W, Wu J, Cheng M, et al. Diagnosis of invasive fungal infections: challenges and recent developments. J Biomed Sci 2023; 30: 42. doi: 10.1186/s12929-023-00926-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tsang CC, Teng JLL, Lau SKP, et al. Rapid genomic diagnosis of fungal infections in the age of next-generation sequencing. J Fungi (Basel) 2021; 7: 636. doi: 10.3390/jof7080636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Damerum A, Malka S, Lofgren N, et al. Next-generation DNA sequencing offers diagnostic advantages over traditional culture testing. Am J Vet Res 2023; 84: ajvr.23.03.0054. doi: 10.2460/ajvr.23.03.0054 [DOI] [PubMed] [Google Scholar]
- 242.Wickes BL, Wiederhold NP. Molecular diagnostics in medical mycology. Nat Commun 2018; 9: 5135. doi: 10.1038/s41467-018-07556-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Luong ML, Clancy CJ, Vadnerkar A, et al. Comparison of an Aspergillus real-time polymerase chain reaction assay with galactomannan testing of bronchoalvelolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in lung transplant recipients. Clin Infect Dis 2011; 52: 1218–1226. doi: 10.1093/cid/cir185 [DOI] [PubMed] [Google Scholar]
- 244.Morjaria S, Frame J, Franco-Garcia A, et al. Clinical performance of (1,3) β-D glucan for the diagnosis of Pneumocystis pneumonia (PCP) in cancer patients tested with PCP polymerase chain reaction. Clin Infect Dis 2019; 69: 1303–1309. doi: 10.1093/cid/ciy1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ito K. Inhaled antifungal therapy: benefits, challenges, and clinical applications. Expert Opin Drug Deliv 2022; 19: 755–769. doi: 10.1080/17425247.2022.2084530 [DOI] [PubMed] [Google Scholar]
- 246.Colley T, Alanio A, Kelly SL, et al. In vitro and in vivo antifungal profile of a novel and long-acting inhaled azole, PC945, on Aspergillus fumigatus infection. Antimicrob Agents Chemother 2017; 61: e02280-16. doi: 10.1128/aac.02280-02216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Safdar A, O'Brien S, Kouri IF. Efficacy and feasibility of aerosolized amphotericin B lipid complex therapy in caspofungin breakthrough pulmonary zygomycosis. Bone Marrow Transplant 2004; 34: 467–468. doi: 10.1038/sj.bmt.1704552 [DOI] [PubMed] [Google Scholar]
- 248.Holle J, Leichsenring M, Meissner PE. Nebulized voriconazole in infections with Scedosporium apiospermum – case report and review of the literature. J Cyst Fibros 2014; 13: 400–402. doi: 10.1016/j.jcf.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 249.Brunet K, Martellosio JP, Tewes F, et al. Inhaled antifungal agents for treatment and prophylaxis of bronchopulmonary invasive mold infections. Pharmaceutics 2022; 14: 641. doi: 10.3390/pharmaceutics14030641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162: 81–89. doi: 10.7326/M13-2508 [DOI] [PubMed] [Google Scholar]
- 251.Sofjan AK, Mitchell A, Shah DN, et al. Rezafungin (CD101), a next-generation echinocandin: a systematic literature review and assessment of possible place in therapy. J Glob Antimicrob Resist 2018; 14: 58–64. doi: 10.1016/j.jgar.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 252.Pfaller MA, Messer SA, Rhomberg PR, et al. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents 2017; 50: 352–358. doi: 10.1016/j.ijantimicag.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 253.Hall D, Bonifas R, Stapert L, et al. In vitro potency and fungicidal activity of CD101, a novel echinocandin, against recent clinical isolates of Candida spp. Diagn Microbiol Infect Dis 2017; 89: 205–211. doi: 10.1016/j.diagmicrobio.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 254.Thompson GR 3rd, Soriano A, Cornely OA, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023; 401: 49–59. doi: 10.1016/S0140-6736(22)02324-8 [DOI] [PubMed] [Google Scholar]
- 255.Thompson GR, Soriano A, Skoutelis A, et al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis 2021; 73: e3647–e3655. doi: 10.1093/cid/ciaa1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Thompson GR 3rd, Soriano A, Honore PM, et al. Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials. Lancet Infect Dis 2024; 24: 319–328. doi: 10.1016/S1473-3099(23)00551-0 [DOI] [PubMed] [Google Scholar]
- 257.Lepak AJ, Marchillo K, Andes DR. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 2015; 59: 1265–1272. doi: 10.1128/AAC.04445-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Berkow EL, Angulo D, Lockhart SR. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother 2017: 61: e00435-17. doi: 10.1128/AAC.00435-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ghannoum M, Isham N, Angulo D, et al. Efficacy of ibrexafungerp (SCY-078) against Candida auris in an in vivo guinea pig cutaneous infection model. Antimicrob Agents Chemother 2020; 64: e00854-20. doi: 10.1128/AAC.00854-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lamoth F, Alexander BD. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 2015; 59: 4308–4311. doi: 10.1128/AAC.00234-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Spec A, Pullman J, Thompson GR, et al. MSG-10: a phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother 2019; 74: 3056–3062. doi: 10.1093/jac/dkz277 [DOI] [PubMed] [Google Scholar]
- 262.Ghannoum M, Long L, Larkin EL, et al. Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrob Agents Chemother 2018; 62: e00244-18. doi: 10.1128/AAC.00244-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Trzoss M, Covel JA, Kapoor M, et al. Synthesis of analogs of the Gwt1 inhibitor manogepix (APX001A) and in vitro evaluation against Cryptococcus spp. Bioorg Med Chem Lett 2019; 29: 126713. doi: 10.1016/j.bmcl.2019.126713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel) 2020; 6: 239. doi: 10.3390/jof6040239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Alkhazraji S, Gebremariam T, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Ch 2020; 64: e01735-19. doi: 10.1128/AAC.01735-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of delayed therapy with fosmanogepix (apx001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 2019; 63: e01120-19. doi: 10.1128/AAC.01120-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gebremariam T, Alkhazraji S, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother 2020; 64: e00178-20. doi: 10.1128/AAC.00178-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vazquez JA, Pappas PG, Boffard K, et al. Clinical efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by Candida auris: results from a phase 2 trial. Antimicrob Agents Chemother 2023; 67: e0141922. doi: 10.1128/aac.01419-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pappas PG, Vazquez JA, Oren I, et al. Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidaemia: results from a phase 2 trial. J Antimicrob Chemother 2023; 78: 2471–2480. doi: 10.1093/jac/dkad256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Oliver JD, Sibley GEM, Beckmann N, et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA 2016; 113: 12809–12814. doi: 10.1073/pnas.1608304113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jorgensen KM, Astvad KMT, Hare RK, et al. EUCAST determination of olorofim (F901318) susceptibility of mold species, method validation, and MICs. Antimicrob Agents Chemother 2018; 62: e00487-18. doi: 10.1128/AAC.00487-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother 2019; 74: 1586–1590. doi: 10.1093/jac/dkz078 [DOI] [PubMed] [Google Scholar]
- 273.Biswas C, Law D, Birch M, et al. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 2018; 56: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 274.Buil JB, Rijs A, Meis JF, et al. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 2017; 72: 2548–2552. doi: 10.1093/jac/dkx177 [DOI] [PubMed] [Google Scholar]
- 275.Wiederhold NP, Law D, Birch M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 2017; 72: 1977–1980. doi: 10.1093/jac/dkx065 [DOI] [PubMed] [Google Scholar]
- 276.Maertens J, Thompson G, Spec A, et al. Olorofim for treatment of invasive fungal infections (IFI) due to moulds in patients with limited or no treatment options: interim results from a phase 2b open-label study (NCT03583164). Open Forum Infect Dis 2022: 9: Suppl. 2, ofac492.039. doi: 10.1093/ofid/ofac1492.1039 [DOI] [Google Scholar]
- 277.Nishimoto AT, Wiederhold NP, Flowers SA, et al. In vitro activities of the novel investigational tetrazoles VT-1161 and VT-1598 compared to the triazole antifungals against azole-resistant strains and clinical isolates of Candida albicans. Antimicrob Agents Chemother 2019; 63: e00341-19. doi: 10.1128/AAC.00341-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yamashita K, Miyazaki T, Fukuda Y, et al. The novel arylamidine T-2307 selectively disrupts yeast mitochondrial function by inhibiting respiratory chain complexes. Antimicrob Agents Chemother 2019; 63: e00374-19. doi: 10.1128/AAC.00374-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Abe M, Nakamura S, Kinjo Y, et al. Efficacy of T-2307, a novel arylamidine, against ocular complications of disseminated candidiasis in mice. J Antimicrob Chemother 2019; 74: 1327–1332. doi: 10.1093/jac/dkz020 [DOI] [PubMed] [Google Scholar]
- 280.Mitsuyama J, Nomura N, Hashimoto K, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 2008; 52: 1318–1324. doi: 10.1128/AAC.01159-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nishikawa H, Fukuda Y, Mitsuyama J, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. J Antimicrob Chemother 2017; 72: 1709–1713. doi: 10.1093/jac/dkx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nishikawa H, Sakagami T, Yamada E, et al. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J Antimicrob Chemother 2016; 71: 1845–1855. doi: 10.1093/jac/dkw095 [DOI] [PubMed] [Google Scholar]
- 283.Wiederhold NP, Najvar LK, Fothergill AW, et al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J Antimicrob Chemother 2016; 71: 692–695. doi: 10.1093/jac/dkv398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Habicht JM, Reichenberger F, Gratwohl A, et al. Surgical aspects of resection for suspected invasive pulmonary fungal infection in neutropenic patients. Ann Thorac Surg 1999; 68: 321–325. doi: 10.1016/S0003-4975(99)00513-5 [DOI] [PubMed] [Google Scholar]
- 285.Theodore S, Liava'a M, Antippa P, et al. Surgical management of invasive pulmonary fungal infection in hematology patients. Ann Thorac Surg 2009; 87: 1532–1538. doi: 10.1016/j.athoracsur.2009.02.069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This PDF extract can be shared freely online.
Shareable PDF ERJ-00803-2024.Shareable (808.6KB, pdf)