Abstract
Many organisms, including cosmopolitan drosophilids, show circadian plasticity, varying their activity with changing dawn–dusk intervals1. How this behaviour evolves is unclear. Here we compare Drosophila melanogaster with Drosophila sechellia, an equatorial, ecological specialist that experiences minimal photoperiod variation, to investigate the mechanistic basis of circadian plasticity evolution2. D. sechellia has lost the ability to delay its evening activity peak time under long photoperiods. Screening of circadian mutants in D. melanogaster/D. sechellia hybrids identifies a contribution of the neuropeptide pigment-dispersing factor (Pdf) to this loss. Pdf exhibits species-specific temporal expression, due in part to cis-regulatory divergence. RNA interference and rescue experiments in D. melanogaster using species-specific Pdf regulatory sequences demonstrate that modulation of this neuropeptide’s expression affects the degree of behavioural plasticity. The Pdf regulatory region exhibits signals of selection in D. sechellia and across populations of D. melanogaster from different latitudes. We provide evidence that plasticity confers a selective advantage for D. melanogaster at elevated latitude, whereas D. sechellia probably suffers fitness costs through reduced copulation success outside its range. Our findings highlight this neuropeptide gene as a hotspot locus for circadian plasticity evolution that might have contributed to both D. melanogaster’s global distribution and D. sechellia’s specialization.
Subject terms: Evolutionary genetics, Behavioural genetics, Circadian mechanisms
We provide evidence that circadian plasticity has diverged through evolution of the neuropeptide gene Pdf, conferring a selective advantage for Drosophila melanogaster at elevated latitude, whereas Drosophila sechellia probably suffers fitness costs outside its range.
Main
Nervous systems coordinate animals’ behavioural responses to the external world. This task becomes challenging when environments are variable, particularly for broadly distributed species. One way to face changing conditions is with behavioural plasticity—that is, the ability to adjust behavioural phenotypes to match environmental fluctuations. Many examples of plastic behaviours exist: songbirds shift their vocalization frequencies in response to anthropogenic noise3, and lizards change their basking behaviour according to altitude4. How behavioural plasticity is determined and evolves is unknown.
A common example of plastic behaviour is circadian activity, whereby species adjust their activity patterns in response to seasonal variation in day length5. This ability is critical, because circadian activity coordinates specific behaviours with optimal activity throughout the day to maximize food availability and synchronize social behaviours6. Deviations from regular circadian patterns can negatively affect fitness and species persistence7. Drosophilids are a powerful system with which to study circadian behavioural plasticity. These flies show bouts of activity surrounding dawn and dusk (morning and evening activity peaks), separated by a period of relative inactivity8. The best-studied species, the cosmopolitan Drosophila melanogaster, plasticly adjusts its circadian rhythm depending on seasonal variation in photoperiod1. Notably, the degree of photoperiod plasticity of different strains of this species correlates with their latitude of origin9. Moreover, several distantly related, high-latitude species have evolved divergent patterns of activity and extreme plasticity, allowing their daily activity to match long summer days10.
An interesting comparison species to D. melanogaster is the closely related Drosophila sechellia2 (Fig. 1a), which is endemic to the equatorial Seychelles islands, where it experiences little seasonal photoperiod variation (Fig. 1a,b). Here we describe marked differences in the circadian activity and plasticity of D. sechellia and D. melanogaster, and investigate the mechanistic basis of these differences.
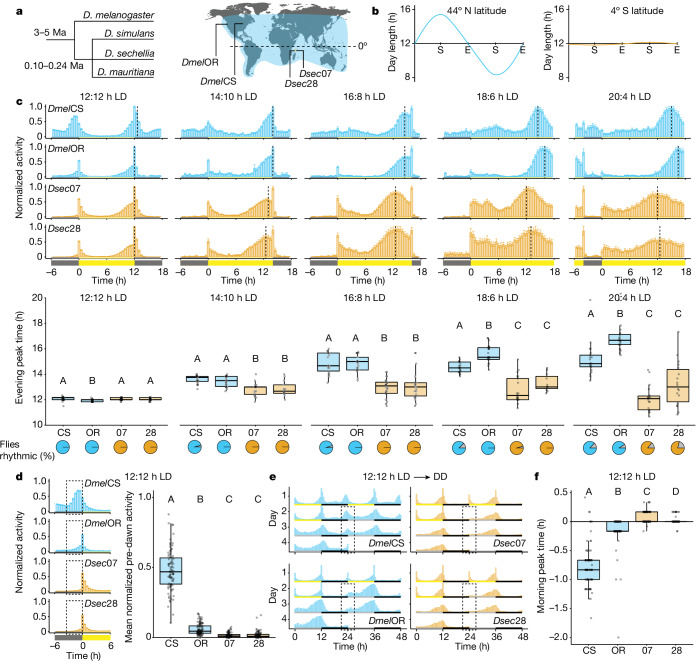
Fig. 1. Interspecific circadian differences in D. sechellia and D. melanogaster.
a, D. melanogaster (Dmel) subgroup phylogeny (left) and ranges (right) of Dmel (blue) and D. sechellia (Dsec) (orange), with approximate collection sites of strains. b, Approximate photoperiod variation (www.srrb.noaa.gov) at collection sites of Dmel (left) and Dsec (right) strains. c, Top, mean normalized activity of Dmel and Dsec under the indicated photoperiods. Yellow and grey bars indicate lights-on and lights-off, respectively; vertical dashed lines indicate average timing of the evening peak; error bars represent s.e.m. Middle, box plots (Methods) depicting evening peak time for individual flies under each photoperiod. Bottom, pie charts representing the proportion of flies that maintained rhythmic behaviour (colour) or no significant periodicity (grey). n, 12:12 h LD: CS (18), OR (21), 07 (24), 28 (19); 14:10 h LD: CS (22), OR (22), 07 (19), 28 (13); 16:8 h LD: CS (18), OR (21), 07 (24), 28 (19); 18:6 h LD: CS (22), OR (23), 07 (21), 28 (11); 20:4 h LD: CS (21), OR (22), 07 (19), 28 (18). d, Left, mean normalized activity of Dmel and Dsec under 12:12 h LD during the morning activity peak (−6 to +6 h data from c). Dashed boxes highlight the predawn period. Right, mean normalized activity of individual flies within the predawn period. n: CS (89), OR (93), 07 (95), 28 (91). e, Double-plotted actograms depicting the transition from the last 2 days of 12:12 h LD to DD for each strain. Dashed boxes highlight morning activity peak period during DD, −3 to +3 h. n: CS (29), OR (32), 07 (29), 28 (22). Grey bars indicate timing of subjective lights-on during DD. f, Morning peak time, from lights-on, for flies from d. c,d,f, Letters A–D indicate significant differences, P < 0.05 (pairwise Wilcoxon test with Bonferroni correction). E, equinox; S, solstice.
Species-specific evening peak plasticity
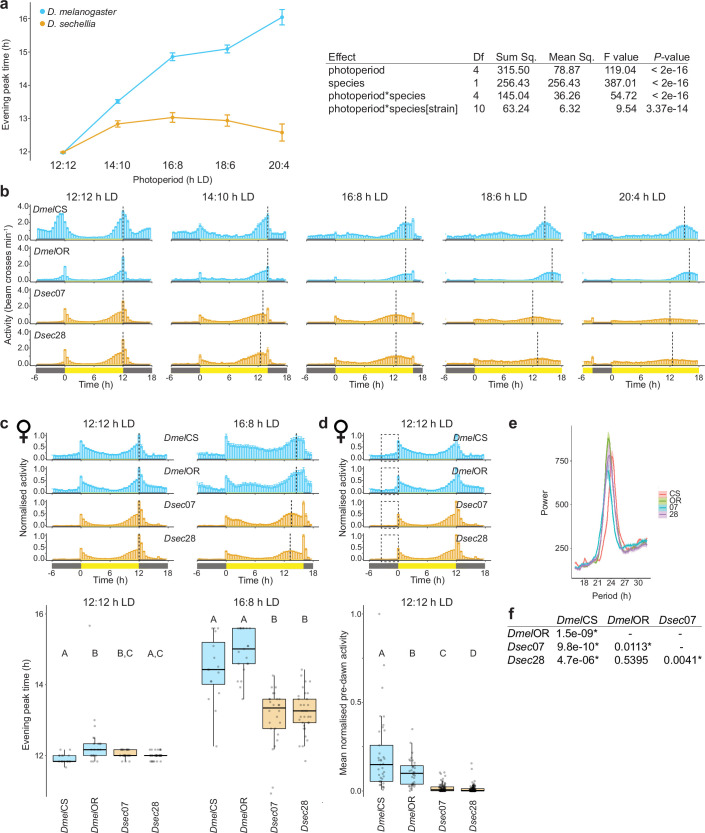
We first measured the circadian behaviour of D. melanogaster and D. sechellia under a 12:12 h light:dark cycle (12:12 h LD) and four extended photoperiods, ranging from mild (14:10 h LD) to extreme (20:4 h LD) (Fig. 1c). We used males of two strains each of D. melanogaster and D. sechellia (Supplementary Table 1), to distinguish inter- from intraspecific differences. The D. melanogaster strains (DmelCS and DmelOR) were collected at around 41° N and 44° N, respectively; the D. sechellia strains (Dsec07 and Dsec28) were from the Seychelles, around 4° S (Fig. 1a). The D. melanogaster and D. sechellia strains thus initially evolved in environments with annual photoperiod variation on the scale of several hours or minutes, respectively (Fig. 1b). Under each photoperiod, all strains showed activity peaks during the morning and evening, although the timing of peak evening activity varied by photoperiod (Fig. 1c). We quantified the average evening peak time of the last 4 of 7 days in a given photoperiod (Methods and Fig. 1c). For D. melanogaster, the timing of the evening activity peak was commensurately delayed as photoperiod increased (Fig. 1c and Extended Data Fig. 1a). By contrast, for D. sechellia we observed notably little evening peak plasticity, with a median delay in evening peak time of maximum around 1 h regardless of photoperiod (Fig. 1c and Extended Data Fig. 1a). Importantly, we detected a significant interaction between photoperiod and species (Extended Data Fig. 1a). Under the most extreme photoperiod, many D. sechellia showed arrhythmicity (Fig. 1c) and decreased activity (Extended Data Fig. 1b), contributing to the increased variability in evening peak time. The absence of evening peak plasticity was also apparent in female D. sechellia (Extended Data Fig. 1c).
Extended Data Fig. 1. Activity of D. melanogaster and D. sechellia males and females under different photoperiods.
a, Left: reaction norm depicting the interaction between species and photoperiod in determining evening peak time. Points represent species means and error bars represent SEM. Right: results of a two-way nested ANOVA detecting a significant interaction between species and photoperiod in determining evening peak time. b, Mean non-normalised activity of flies from Fig. 1c. Vertical dashed lines indicate the average timing of the evening peak for each strain. Error bars represent SEM. Sample sizes as in Fig. 1c. c, Top: mean normalised activity of two D. melanogaster (CS and OR, blue) and two D. sechellia (07 and 28, orange) strains, using female flies, under the indicated photoperiods. Plots depict normalised average activity of the last 4 days of a 7-day photoperiod; for 16:8 h LD, this was preceded by 7 days of 12:12 h LD. Bottom: box plots depict evening peak time quantifications for individual flies under each photoperiod. Here and elsewhere, box plots show the median (bold line), interquartile range (box) and final quartiles (whiskers). Individual data points are overlaid on the box plots; outliers are points that fall beyond the box plot whiskers. Sample sizes (numbers of individual flies) are: 12:12 h LD: CS (37), OR (34), 07 (95), 28 (146); 16:8 h LD: CS (16), OR (21), 07 (30), 28 (33). For b-c, Vertical dashed lines indicate the average timing of the evening peak for each strain. Here and elsewhere, yellow and grey bars indicate timing of lights-on and lights-off, respectively. Error bars represent SEM. d, Mean normalised activity of D. melanogaster and D. sechellia strains under 12:12 h LD during the morning activity peak (same data from c). Top: plots depict average activity of the last 4 days of a 7-day recording period. Dashed boxes highlight the pre-dawn period, 3 h before lights-on. Error bars represent SEM. Bottom: mean normalised activity of individual flies within this pre-dawn period. Sample sizes as in c. For c-d, letters indicate significant differences: P < 0.05 (pairwise Wilcoxon test with Bonferroni correction). e, Periodogram analysis from 5 days of constant darkness (DD) for D. melanogaster (CS and OR) and D. sechellia (07 and 28) strains. Period estimates: CS (24.36 h), OR (23.45 h), 07 (23.16 h), 28 (23.57 h). Sample sizes as in Fig. 1e. f, Table of P-values from all pairwise comparisons of the data in e between strains (Wilcoxon test with Bonferroni correction). No species-specific differences were observed.
Species-specific morning peak activity
D. sechellia is much less active during the dark phase than D. melanogaster (Fig. 1c). Quantification of predawn activity (3 h preceding lights-on) under 12:12 h LD showed that D. sechellia was generally inactive during this period, whereas D. melanogaster showed ample, albeit strain-specific, activity (Fig. 1d), a difference also seen in females (Extended Data Fig. 1d). We next measured free-running activity by acclimating our strains to 12:12 h LD before submitting them to constant dark conditions (DD). Both D. melanogaster and D. sechellia remained rhythmic under DD (Fig. 1e) showing a period of around 24 h (Extended Data Fig. 1e,f). However, at the subjective dawn, D. melanogaster showed clear activity peaks, whereas D. sechellia exhibited very little activity, even during the first day of DD (Fig. 1e). Low predawn activity therefore reflects a reduced morning activity peak of D. sechellia, and the activity observed after lights-on under 12:12 h LD is probably largely a startle response. Consistently, when we quantified morning peak timing under 12:12 h LD, D. melanogaster reached peak activity before lights-on, as previously described11, whereas D. sechellia peaked only at, or just after, lights-on (Fig. 1f).
D. sechellia-specific loss of plasticity
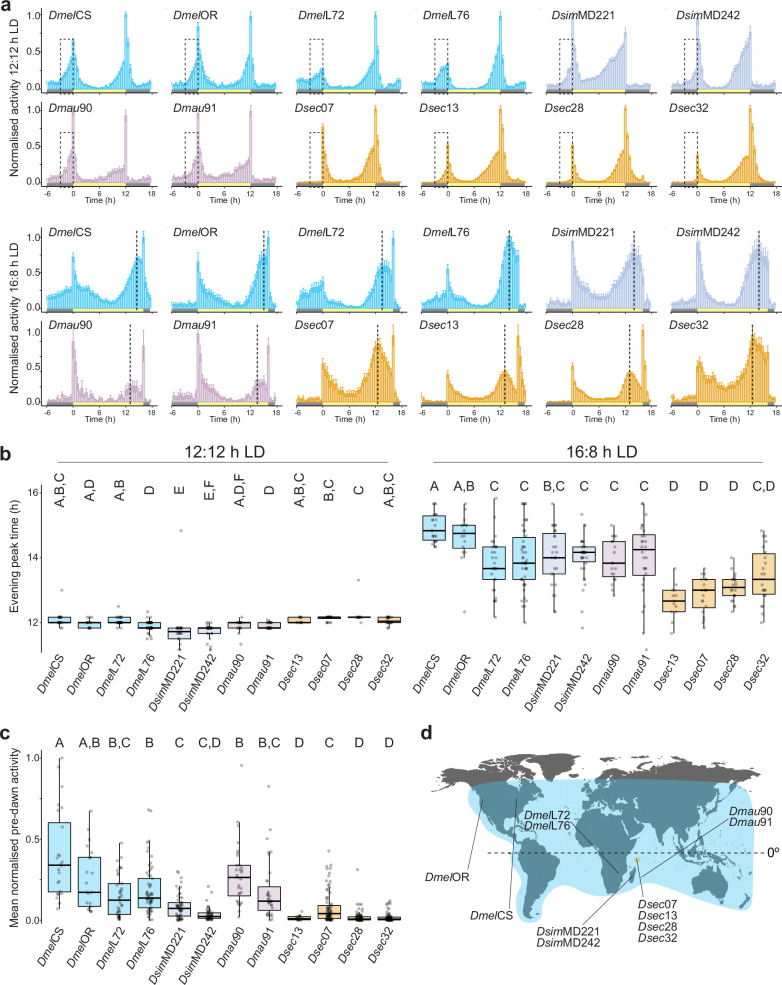
We extended our analyses to two other D. melanogaster strains12 (collected near their ancestral range13 (roughly 16° S)), two additional D. sechellia strains, as well as closer relatives of D. sechellia (Fig. 1a): Drosophila simulans12 (collected from its ancestral range14, around 19° S) and Drosophila mauritiana (endemic to Mauritius, roughly 20° S) (Extended Data Fig. 2a,d). Under 16:8 h LD conditions, all D. melanogaster, D. simulans and D. mauritiana strains exhibited a higher degree of evening peak plasticity compared with D. sechellia (Extended Data Fig. 2b). The degree of evening peak plasticity reflected a latitudinal effect: tropical D. melanogaster strains showed reduced plasticity when compared with high-latitude strains (Extended Data Fig. 2b). The majority of non-D. sechellia strains also exhibited substantial morning activity (Extended Data Fig. 2c). These results indicate that the lack of plasticity and reduction in morning activity observed in D. sechellia probably represent losses in this lineage.
Extended Data Fig. 2. Tropical D. melanogaster, D. simulans and D. mauritiana strains display prominent evening peak plasticity and morning peak activity.
a, Mean normalised activity of laboratory D. melanogaster (DmelCS and DmelOR), two recently-collected strains of D. melanogaster (DmelL72 and DmelL76, from the Lower Zambezi Valley), D. simulans (MD221 and MD242, from Madagascar), D. mauritiana (Dmau90 and Dmau91, from Mauritius) and laboratory D. sechellia (Dsec07, Dsec13, Dsec28 and Dsec32) under 12:12 h LD (top) and 16:8 h LD (bottom). Plots depict average activity of the last 4 days of a 7-day recording period; for 16:8 h LD, this was preceded by 7 days of 12:12 h LD. Dashed boxes highlight the pre-dawn period, 3 h before lights-on. Dashed lines highlight the average evening peak time. Error bars represent SEM. b, Evening peak time for these flies under 12:12 h LD (left) and 16:8 h LD (right). Sample sizes: DmelCS (22), DmelOR (16), DmelL72 (29), DmelL74 (46), DsimMD221 (27), DsimMD242 (34), Dmau90 (19), Dmau91 (28), Dsec07 (19), Dsec13 (20), Dsec28 (24), Dsec32 (22). c, Mean normalised activity of individual flies within this pre-dawn period. Sample sizes: DmelCS (24), DmelOR (21), DmelL72 (41), DmelL74 (61), DsimMD221 (57), DsimMD242 (52), Dmau90 (33), Dmau91 (34), Dsec07 (19), Dsec13 (82), Dsec28 (77), Dsec32 (73). d, The approximate collection sites of the D. melanogaster, D. simulans, D. mauritiana and D. sechellia strains used in a-c. For b-c, letters depict significant differences detected between strains (Wilcoxon tests for all pairwise comparisons with Bonferroni correction, P < 0.05).
Pdf underlies species plasticity loss
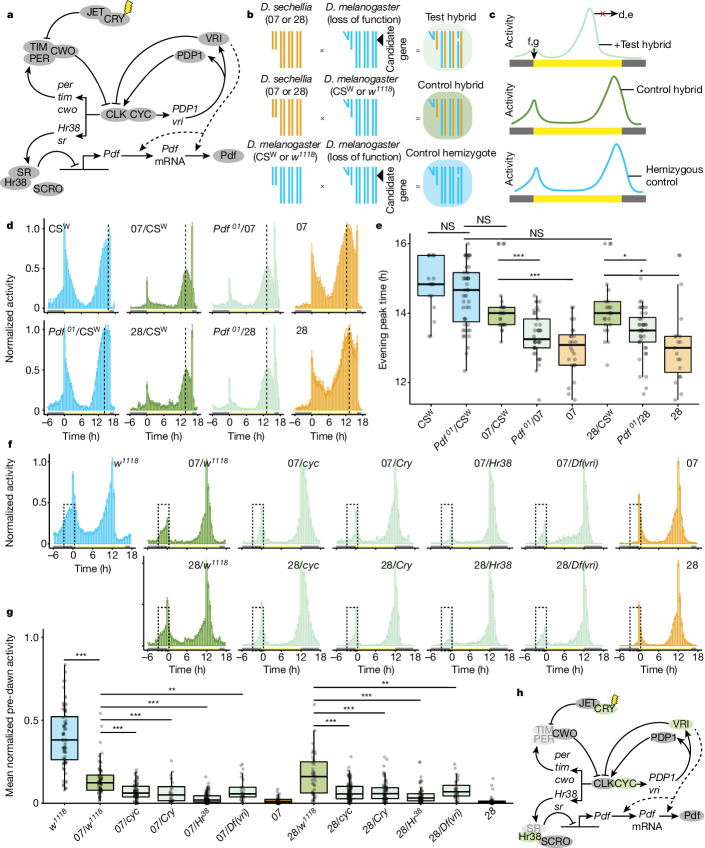
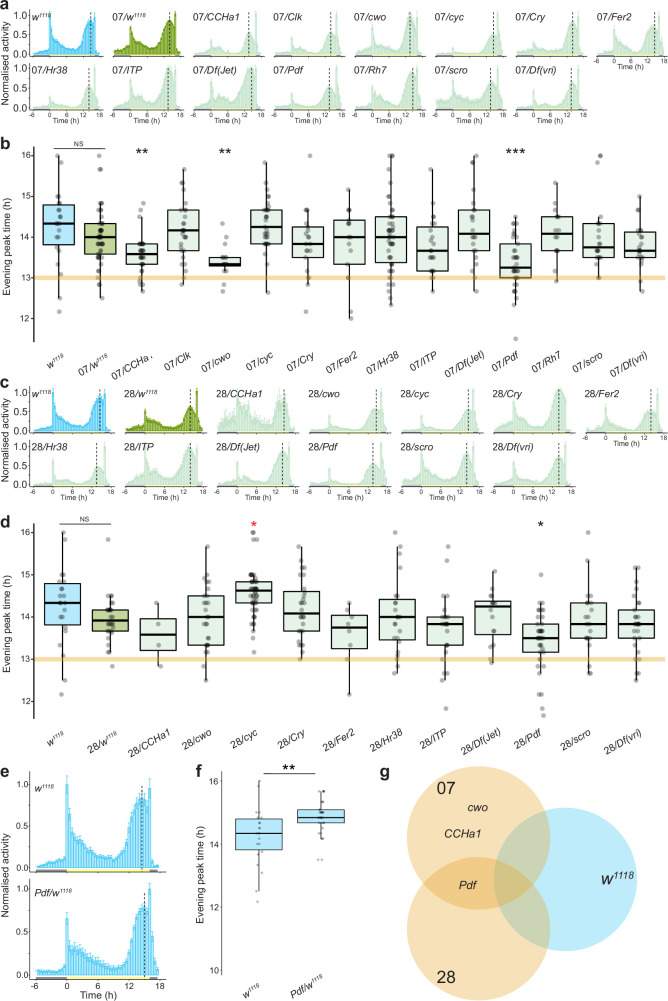
To identify the genetic basis of species differences, we took a candidate approach. In D. melanogaster, 150 circadian neurons contain a molecular feedback loop that tracks a period of approximately 24 h (refs. 15,16) and controls the rhythmic expression of effectors, representing many candidates (Fig. 2a). Because D. sechellia traits reflect evolutionary losses, we reasoned that causal D. sechellia alleles were probably recessive to D. melanogaster and thus designed a screen in D. melanogaster/D. sechellia hybrids (Fig. 2b). We generated hemizygous test hybrids containing D. melanogaster mutations for individual candidates to show any recessive phenotype of the D. sechellia allele at the same locus. We also generated heterozygous control hybrids, using either the D. melanogaster w1118 strain (a common genetic background of mutants) or CSW (pigment-dispersing factor (Pdf); Methods) and each D. sechellia strain. Differences between control and test hybrids are probably due to loss of the D. melanogaster allele in the test hybrid. Gene dosage effects were assessed by testing control D. melanogaster hemizygotes. Genes whose mutations showed a consistent effect in both test hybrid backgrounds compared with control hybrids, but not in hemizygous D. melanogaster, were considered the strongest candidates to explain interspecific differences (Fig. 2c).
Fig. 2. Genetic screens for interspecific circadian differences.
a, The D. melanogaster circadian clock. Arrows denote transcriptional activation, blunt-ended connectors represent repression/degradation and dashed lines indicate hypothesized connections. b, Crossing schemes of the genetic screen. The fourth chromosome is not shown. c, Schematics illustrating the sought-after behavioural phenotypes of hybrid genotypes. d, Mean normalized activity of the indicated genotypes under 16:8 h LD. n: CSW (16), Pdf01/CSW (47), 07/CSW (25), 28/CSW (23), 07/Pdf01 (37), 28/Pdf01(40), 07 (24), 28 (19). Full screen results are given in Extended Data Fig. 3. e, Evening peak time for the flies depicted in d. *P < 0.05, ***P < 0.001 (Wilcoxon tests with Bonferroni correction). Comparisons were made only between control and test hybrids of the same genetic backgrounds. f, Mean normalized activity of the indicated genotypes under 12:12 h LD, illustrating the mutations showing reduced morning activity in test hybrids. Dashed boxes highlight the predawn area used to quantify predawn activity, 3 h before lights-on. Error bars represent s.e.m. Full screen results are shown in Extended Data Fig. 5. g, Mean normalized predawn activity for the genotypes shown in f. **P < 0.01, ***P < 0.001 (Wilcoxon tests comparing each test hybrid with the appropriate control hybrid strain, with Bonferroni correction). h, The circadian molecular network, in which screen hits for morning activity are highlighted in green; genes depicted in light grey could not be tested (Methods). mRNA, messenger RNA; NS, not significantly different.
To assess candidates for an effect on evening peak plasticity, we observed test and control hybrids under 16:8 h LD. Control hybrids of either the w1118 (Extended Data Fig. 3a–d) or CSW background (Fig. 2d,e and Extended Data Fig. 4) showed a higher degree of evening peak plasticity than their D. sechellia parental strain, confirming that the D. melanogaster genotype underlying plasticity is partially dominant to that of D. sechellia, although the degree of plasticity is strain dependent. We screened 14 genes representing the majority of the circadian feedback loop, and many of its modulators and effectors. Mutations in only one reduced evening peak plasticity in both test hybrid backgrounds but not in hemizygous D. melanogaster: Pdf (Fig. 2d,e and Extended Data Fig. 3e–g). This is a promising gene in regard to explaining species differences because, in D. melanogaster, Pdf is essential for delaying the phase of the endogenous clock in circadian neurons under long photoperiods17–19.
Extended Data Fig. 3. Screen results for the genetic basis of interspecific differences in evening peak plasticity.
a, Mean normalised activity of the indicated control and hybrid genotypes under 16:8 h LD. Plots depict average activity of the last 4 days of a 7-day extended photoperiod, following 7 days of 12:12 h LD. Vertical dashed lines indicate the average timing of the evening peak for each genotype. Error bars represent SEM. Sample sizes: w1118 (22), 07/w1118 (53), 07/CCHa1 (34), 07/Clk (29), 07/cwo (16), 07/cyc (33), 07/Cry (21), 07/Fer2 (17), 07/Hr38 (50), 07/ITP (23), 07/Jet (22), 07/Pdf (37), 07/Rh7 (16), 07/scro (22), 07/vri (23), 07 (24). b, Evening peak time for the genotypes in a. Asterisks indicate significant differences: ** P < 0.01 and *** = P < 0.001 (Wilcoxon tests comparing each test hybrid to the control hybrid strain (07/w1118) with Bonferroni correction). NS = not significantly different. The orange line marks the median evening peak delay of the D. sechellia parental strain (07). c, Mean normalised activity of the indicated control and hybrid genotypes under 16:8 h LD. Plots depict average activity of the last 4 days of a 7-day extended photoperiod, following 7 days of 12:12 h LD. Vertical dashed lines indicate the average time of the evening peak for each strain. Error bars represent SEM. Sample sizes: w1118 (22), 28/w1118 (31), 28/CCHa1 (4), 28/cwo (27), 28/cyc (52), 28/Cry (28), 28/Fer (8), 28/Hr38 (23), 28/Itp (25), 28/Jet (16), 28/Pdf (40), 28/scro (31), 28/vri (29), 28 (19). d, Evening peak time for the genotypes in c. Asterisks indicate significant differences: * = P < 0.05 (Wilcoxon tests comparing each test hybrid to the control hybrid strain (28/w1118) with Bonferroni correction). Red asterisk denotes a significant increase in evening peak plasticity. NS = not significantly different. The orange line marks the median evening peak delay of the D. sechellia parental strain (28). e, Mean normalised activity of hemizygous D. melanogaster Pdf mutants (the only mutant that displayed an effect in both hybrid backgrounds) under a 16:8 h LD cycle. Plots depict average activity of the last 4 days of a 7-day extended photoperiod, following 7 days of 12:12 h LD. Vertical dashed lines indicate the average time of the evening peak for each strain. Error bars represent SEM. Sample sizes: w1118 (22), Pdf/w1118 (37). f, Evening peak time for the flies depicted in e. Pdf01 hemizygotes displayed a significant increase in morning peak activity compared to the control strain (w1118). ** = P < 0.01 (Wilcoxon test). g, Summary of the overlapping hits. A priori, we considered the strongest candidates would display a reduction in evening peak plasticity in both Dsec07 and Dsec28 hybrids, but not in w1118 hemizygotes; only Pdf fulfilled these criteria.
Extended Data Fig. 4. Qualitatively similar evening peak plasticity and morning peak activity in two Canton-S strains.
a, Mean normalised activity of two Canton-S (CS and CSW) strains under 12:12 h (left) and 16:8 h LD (right). Plots depict average activity of the last 4 days of a 7-day extended photoperiod, following 7 days of 12:12 h LD. Vertical dashed lines indicate the average timing of the evening peak for each strain. Dashed boxes highlight the pre-dawn period, 3 h before lights-on. Sample sizes: 12:12 h LD CS (29), CSW (42); 16:8 h LD CS (18), CSW (16). b, Evening peak time for the flies in a is shown for each strain under 12:12 h (left) and 16:8 h LD (right). No significant differences were observed between strains (Wilcoxon test). c, Mean normalised pre-dawn activity for flies under 12:12 h LD (from a, within the indicated pre-dawn period). Asterisks indicate significant differences: ** = P < 0.01 (Wilcoxon test).
Many genes affect morning activity
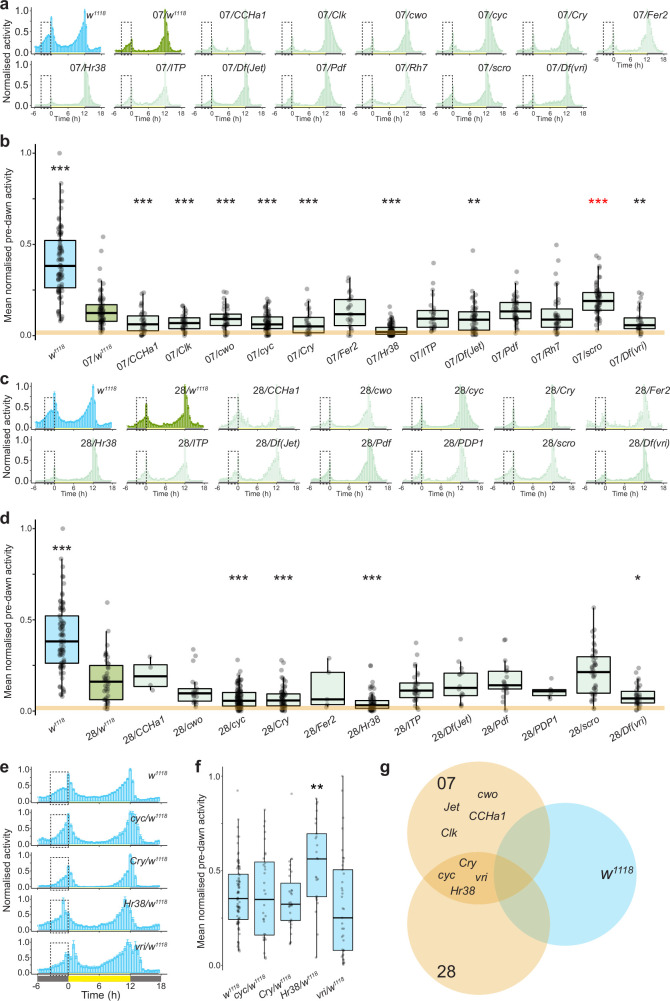
We also screened these genotypes under 12:12 h LD and quantified predawn activity as a measure of morning activity (Extended Data Fig. 5a–d). w1118 control hybrids showed intermediate morning activity relative to parental strains. Consistently, four genes showed an effect in test hybrids of both backgrounds (Fig. 2f,g) but not in hemizygous D. melanogaster (Extended Data Fig. 5e–g). These encode the transcriptional feedback loop proteins CYC and CRY (responsible for light-dependent synchronization of the molecular clock20), and Hr38 and VRI, which are neural activity-dependent transcriptional and post-transcriptional regulators of Pdf, respectively21,22 (Fig. 2h).
Extended Data Fig. 5. Screen results for the genetic basis of interspecific differences in morning activity.
a, Mean normalised activity of the indicated genotypes under 12:12 h LD. Dashed boxes highlight the pre-dawn area used to quantify pre-dawn activity, 3 h before lights-on. Error bars represent SEM. Sample sizes: w1118 (78), 07/w1118 (69), 07/CCHa1 (21), 07/Clk (34), 07/cwo (43), 07/cyc (43), 07/Cry (26), 07/Fer (18), 07/Hr38 (87), 07/ITP (23), 07/Jet (49), 07/Pdf (42), 07/Rh7 (33), 07/scro (58), 07/vri (28), 07 (40). b, Mean normalised pre-dawn activity for the genotypes in a. Asterisks indicate significant differences: ** = P < 0.01 and *** = P < 0.001 (Wilcoxon tests comparing each test hybrid to the control hybrid strain (07/w1118) with Bonferroni correction). Red asterisks denote a significant increase in morning activity relative to control hybrids. The orange line marks the median pre-dawn activity of the D. sechellia parental strain (07). c, Mean normalised activity of the indicated genotypes under 12:12 h LD. Dashed boxes highlight the pre-dawn area used to quantify pre-dawn activity, 3 h before lights-on. Error bars represent SEM. Sample sizes: w1118 (78), 28/w1118 (22), 28/CCHa1 (4), 28/cwo (20), 28/cyc (56), 28/Cry (66), 28/Fer (5), 28/Hr38 (43), 28/ITP (25), 28/Jet (22), 28/Pdf (21), 28/PDP1 (14), 28/scro (38), 28/vri (33), 28 (36). d, Mean normalised pre-dawn activity for the genotypes in c. Asterisks indicate significant differences: * = P < 0.05 and *** = P < 0.001 (Wilcoxon tests comparing each test hybrid to the control hybrid strain (28/w1118) with Bonferroni correction). The orange line marks the median pre-dawn activity of the D. sechellia parental strain (28). e, Mean normalised activity of the indicated hemizygous D. melanogaster genotypes that displayed an effect in a hybrid background under 12:12 h LD. Dashed boxes highlight the pre-dawn area used to quantify pre-dawn activity. Plots depict average activity of the last 4 days of a 7-day recording period. Dashed boxes highlight the pre-dawn period, 3 h before lights-on. Error bars represent SEM. Sample sizes: w1118 (78), cyc/w1118 (31), Cry/w1118 (32), Hr38/w1118 (25), vri/w1118 (46). f, Mean normalised pre-dawn activity for the genotypes in e. Asterisks indicate significant differences: Hr38 hemizygotes displayed a significant increase in morning peak activity compared to the control strain (w1118). ** = P < 0.01 (Wilcoxon tests comparing each test hemizygote to the control strain (w1118) with Bonferroni correction). g, Summary of the overlapping hits from each of the above genotypes. A priori, we considered the strongest candidates to display a reduction in morning peak activity in Dsec07 and Dsec28 hybrids, but not in w1118 hemizygotes. Four genes fulfilled these criteria: cyc, Cry, Hr38 and vri.
Species-specific Pdf expression
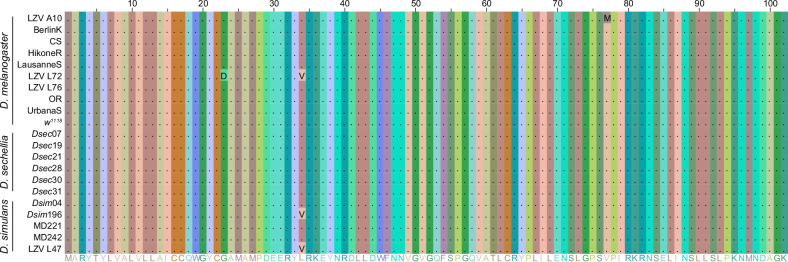
We focused on Pdf, because of its unique effect on evening peak plasticity and evidence that trans-regulation of Pdf expression influences morning peak activity. The Pdf coding sequence is almost perfectly conserved across species (Extended Data Fig. 6), indicating that behavioural divergence must be due to species-specific differences in Pdf expression. In D. melanogaster, Pdf is expressed in eight neurons in each brain hemisphere: four large and four small ventrolateral clock neurons (l-LNvs and s-LNvs, respectively) (Fig. 3a). s-LNvs control the timing of morning activity, whereas l-LNvs can delay the phase of the evening activity peak under long photoperiods23–26, although a functional clock is required in both for photoperiod plasticity24,27. The spatial distribution of this neuropeptide is conserved in D. sechellia (Fig. 3a), consistent with analysis across diverse drosophilids28, suggesting that species-specific differences exist in the temporal pattern and/or levels of Pdf expression.
Extended Data Fig. 6. The predicted Pdf peptide sequence is highly conserved between D. melanogaster, D. sechellia and D. simulans.
Alignment of the predicted Pdf peptide sequence of 10 D. melanogaster, 6 D. sechellia and 5 D. simulans strains. The consensus sequence is displayed at the bottom. Amino acid residues were coloured by chemical similarity using JalView. Periods indicate conserved amino acid residues and letters indicate variable residues. No fixed differences are observed between species.
Fig. 3. Species-specific cis-regulatory elements affect Pdf expression.
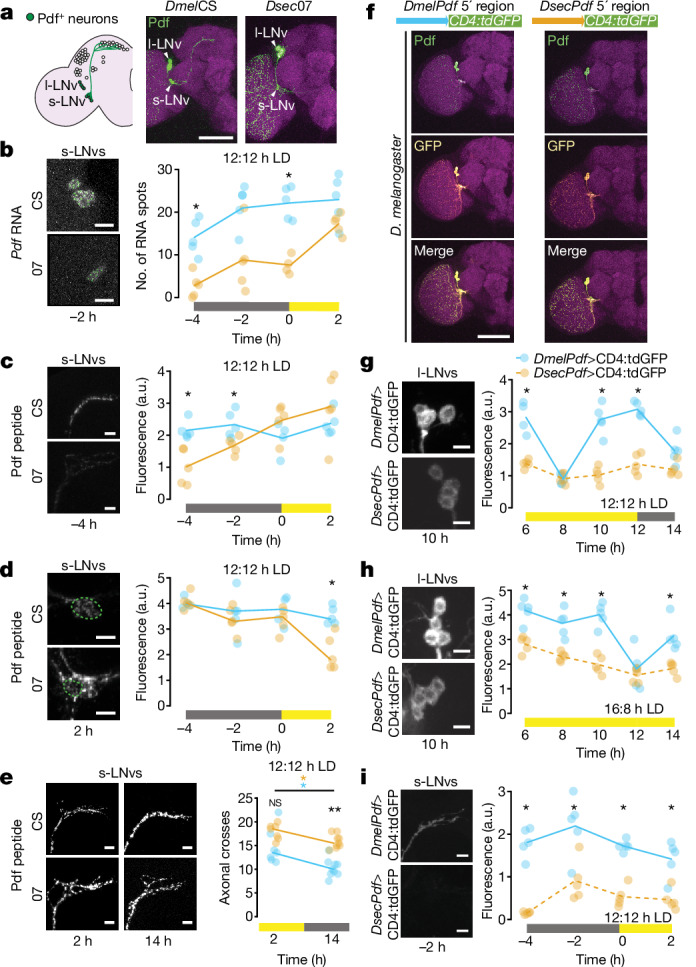
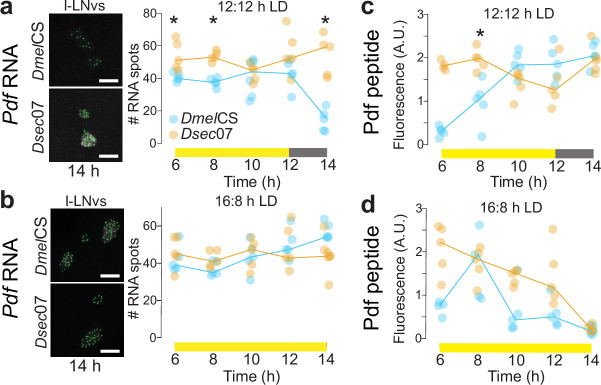
a, Schematic of the D. melanogaster circadian clock neuron network. Pdf-positives-LNvs and l-LNvs are highlighted. Immunofluorescence for Pdf (green) and cadherin-N (magenta) on brains of the indicated strains at 2 h under 12:12 h LD. b–d, Left, representative images of Pdf smFISH (b) and Pdf immunofluorescence (c,d) in s-LNv soma (b,d) and axon termini (c) for strains under 12:12 h LD at the indicated time points. Right, quantifications of each strain at four time points spanning the predawn period. e, Left, Pdf immunofluorescence in s-LNv axon termini for indicated strains during the day (2 h) and night (14 h) under 12:12 h LD. Right, quantifications of axonal branching complexity using Scholl analysis. n: CS 2 h (7), 07 2 h (7), CS 14 h (9), 07 14 h (9). f, Top, schematic illustrating Pdf transcriptional reporters. Bottom, immunofluorescence for Pdf (green), GFP (yellow) and cadherin-N (magenta) in brains of D. melanogaster (at 2 h) expressing species-specific Pdf reporters. g–i, Left, representative images of GFP immunofluorescence in l-LNvs (g,h) and s-LNv axonal projections (i) for Dmel and DsecPdf 5’-regulatory reporter strains under 12:12 h LD (g,i) and 16:8 h LD (h) at the indicated time points. Right, quantifications at time points spanning the evening activity peak period (g,h) and predawn period (i). Although signals are weak in some Dsec images, the structures were readily identified in thresholded images. b–e,g–i, Plotted values are the average of both hemispheres; lines connect medians of time points within genotypes. *P < 0.05, **P < 0.01 (Wilcoxon tests with Bonferroni correction). Scale bars, 100 μm (a,f), 10 μm (b–e, g–i). b–d,g–i, n = 5 brains per strain per time point. a.u., arbitrary units.
Using quantitative single-molecule RNA fluorescence in situ hybridization (smFISH), we compared Pdf transcript levels between D. melanogaster and D. sechellia, focusing primarily on s-LNvs, in which Pdf expression shows a clear temporal pattern that more probably correlates with secretion of this neuropeptide29. Under 12:12 h LD, DmelCS expressed overall more Pdf RNA than Dsec07 throughout the morning activity peak, particularly predawn, with Dsec07 reaching near-similar levels only after lights-on (Fig. 3b). Immunofluorescence quantification of Pdf peptide levels in the axonal projections of s-LNvs for the same time points spanning the morning activity peak (Fig. 3c) showed a consistently high level of Pdf in D. melanogaster, including in the hours preceding lights-on. By contrast, in D. sechellia, Pdf signal was lower in the predawn period and increased to an equivalent amount to D. melanogaster only by lights-on. This pattern corresponds well to that of the relative levels of Pdf transcripts, and to species differences in morning peak activity at these times (Fig. 1c,d). We also analysed Pdf immunofluorescence in the s-LNv soma. Consistent with previous observations29, Pdf signal remained high across the morning peak times in the s-LNv soma of D. melanogaster (Fig. 3d). In D. sechellia, the Pdf signal began high but dropped significantly only after lights-on. These observations suggest that D. sechellia has a weaker and/or shorter pulse of Pdf expression in s-LNvs around the morning peak compared with D. melanogaster, such that once this neuropeptide accumulates to high levels in the axon termini at or after lights-on (Fig. 3c), it becomes depleted from the soma (Fig. 3d).
Pdf expression in the l-LNvs of D. melanogaster has been described as high and constant29. Consistently, using smFISH under both 12:12 and 16:8 h LD, we observed relatively stable expression across time points (Extended Data Fig. 7a,b). However, we noted a D. melanogaster-specific drop in transcript abundance under 12:12 h LD following lights-off that was not apparent under 16:8 h LD, when the lights remained on at the same time point. Pdf immunofluorescence intensity in the l-LNv soma was not significantly different between these species under both photoperiods, but showed substantial variability, particularly for D. sechellia under 16:8 h LD (Extended Data Fig. 7c,d). It remains unclear whether and how Pdf secretion rate in the l-LNv axon termini differs between these species under distinct photoperiods.
Extended Data Fig. 7. Pdf expression in the l-LNvs of D. melanogaster and D. sechellia during the evening peak period.
a,b, Left: representative images of Pdf smFISH in the l-LNv soma in DmelCS and Dsec07 under 12:12 h LD (a) or 16:8 h LD (b) at one timepoint (14 h), with RNA spots (green) identified by RS-FISH. Right: quantifications of RNA spots at 5 timepoints spanning the evening activity peak period. c,d, Quantifications of Pdf signals in the l-LNv soma for DmelCS and Dsec07 at 5 timepoints spanning the evening activity peak period under 12:12 h LD (c) and 16:8 h LD (d). For a-d, lines connect medians of each timepoint within genotypes. * = P < 0.05 (Pairwise Wilcoxon tests with Bonferroni correction).
Pdf neuron structural plasticity
In D. melanogaster, the axonal projections of s-LNvs to the dorsal circadian neurons (Fig. 3a) show circadian structural plasticity, reaching peak branching complexity during the day30. This phenomenon depends, in part, on cyclic expression and release of Pdf from both s-LNvs and l-LNvs and expression of the Pdf receptor31. To test whether the species-specific temporal patterns of Pdf expression are accompanied by differences in neuronal remodelling, we quantified the branching complexity of s-LNv projections during both light (2 h) and dark (14 h) phases under 12:12 h LD (Fig. 3e). During the light phase, we found statistically indistinguishable levels of complexity in the two species and observed in both a decrease in complexity from the light to the dark phase. However, branching complexity in the dark phase was significantly lower in D. melanogaster than in D. sechellia (Fig. 3e). This apparent lower structural plasticity of D. sechellia Pdf neurons corroborates the reduced dynamic changes in Pdf expression in this species.
Cis-regulatory evolution of Pdf
Because our hybrid screen identified an effect of the Pdf locus (and not a trans-regulator) on evening peak delay, we hypothesized that expression differences result from divergence in this gene’s cis-regulatory region. We cloned around 2.4 kb genomic DNA 5’ of Pdf from D. melanogaster (similar to the sequence used in ref. 29) and D. sechellia upstream of a green fluorescent protein (GFP) reporter. These constructs were integrated in an identical location in D. melanogaster, facilitating comparison of their activity in a common genomic and trans environment. Both species’ Pdf reporters exclusively labelled the l-LNvs and s-LNvs (Fig. 3f). We first measured reporter expression in l-LNvs, focusing on behaviourally relevant time points under 12:12 and 16:8 h LD (Fig. 3g,h). In l-LNvs, throughout the evening activity peak under both photoperiods, the D. sechellia 5’-regulatory region consistently drove lower and more constant reporter expression relative to the D. melanogaster sequence (Fig. 3g,h). Notably, the D. melanogaster Pdf reporter showed a sudden drop in expression at either 8 h (under 12:12 h LD) or 12 h (under 16:8 h LD), before returning to a higher level; this expression pattern potentially reflects a new pulse in transcriptional activity that is photoperiod sensitive.
In D. melanogaster, s-LNvs are essential for resetting the phase of the circadian clock32,33 and morning peak activity23,25. We therefore compared reporter expression in s-LNv axonal projections—in which the highest cyclic Pdf expression was observed over a 24 h period29—for time points spanning the morning activity peak (Fig. 3i). The D. sechellia 5’-regulatory sequence drives lower expression of the reporter but, in contrast to reporter expression in l-LNvs during the evening peak, with a similar temporal pattern.
These results indicate divergence of the Pdf 5’-regulatory region between D. sechellia and D. melanogaster, most probably affecting transcriptional activity. However, because the region encompasses the Pdf 5’-untranslated region, we cannot exclude the possibility that it influences transcript stability or translation34.
Pdf regulatory regions affect plasticity
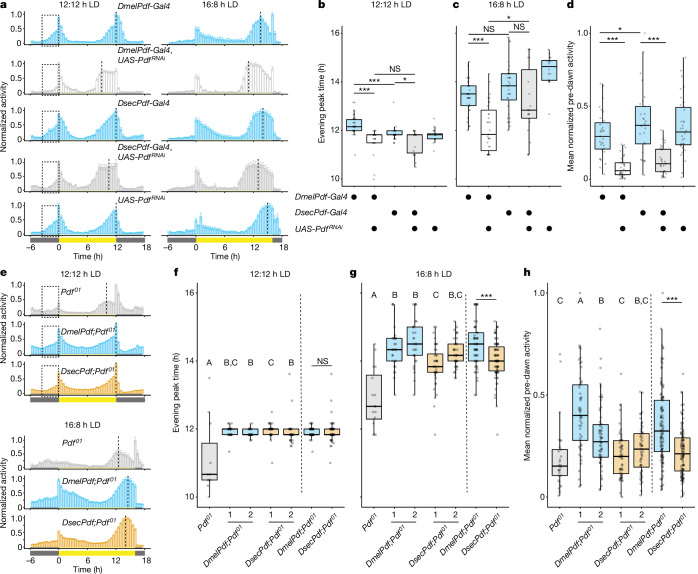
We next asked whether species-specific cis-regulatory activity of Pdf is sufficient to influence behaviour. We initially used the D. melanogaster and D. sechellia Pdf 5’-regulatory regions to generate Pdf neuron drivers to induce ‘strong’ (D. melanogaster Pdf-Gal4) or ‘weak’ (D. sechellia Pdf-Gal4) RNA interference (RNAi) of Pdf31, validating the distinct efficacy of knockdown with smFISH (Extended Data Fig. 8). Under both 12:12 and 16:8 h LD, DmelPdf-Gal4>PdfRNAi flies showed a marked advance in evening peak time relative to controls (Fig. 4a–c). Under 12:12 h LD, there was no difference in the degree of evening peak advance between knockdown treatments. By contrast, under 16:8 h LD, DsecPdf-Gal4>PdfRNAi flies showed a smaller, non-significant, decrease in evening peak time relative to control animals, with a notable increase in variance (Fig. 4a–c). Importantly, under 16:8 h LD, the difference between the PdfRNAi genotypes—of otherwise identical genetic background—was significant. These results indicate that the level (and possibly temporal dynamics) of Pdf expression is sufficient to affect evening peak plasticity. We also quantified the predawn activity (Fig. 4d) of these flies under 12:12 h LD. Both DmelPdf-Gal4>PdfRNAi and DsecPdf-Gal4>PdfRNAi flies exhibited reduced predawn activity relative to controls. However, DmelPdf-Gal4>PdfRNAi and DsecPdf-Gal4>PdfRNAi flies did not show significant differences in predawn activity following post hoc correction for multiple comparisons.
Extended Data Fig. 8. Validation of differential Pdf transcript depletion by RNAi.
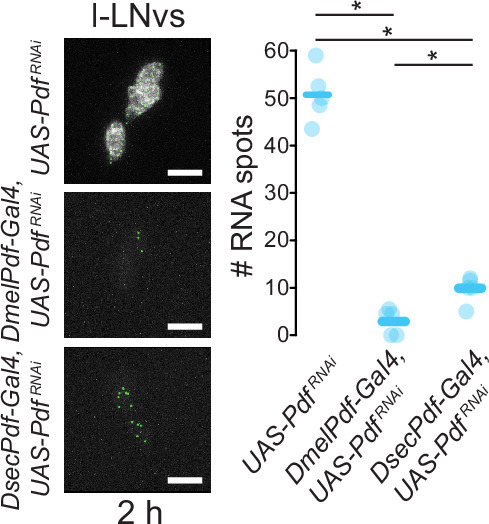
Left: representative images of Pdf smFISH for one genetic control (UAS-PdfRNAi/+), DmelPdf-Gal4/UAS-PdfRNAi and DsecPdf-Gal4/UAS-PdfRNAi strains with RNA spots identified by RS-FISH. Right: quantifications of RNA spots. n = 5 for each genotype. * = P < 0.05 (Pairwise Wilcoxon tests with Bonferroni correction).
Fig. 4. Species-specific Pdf regulatory regions contribute to divergent evening peak plasticity and morning activity.
a, Mean normalized activity of the indicated Dmel genotypes under 12:12 h LD (left) and 16:8 h LD (right). n: 12:12 h LD, DmelPdf-Gal4/+ (31), DsecPdf-Gal4/+ (29), UAS-PdfRNAi/+ (47), DmelPdf-Gal4/UAS-PdfRNAi (34), DsecPdf-Gal4/UAS-PdfRNAi (30); 16:8 h LD, DmelPdf-Gal4/+ (34), DsecPdf-Gal4/+ (32), UAS-PdfRNAi/+ (16), DmelPdf-Gal4/UAS-PdfRNAi (31), DsecPdf-Gal4/UAS-PdfRNAi (24). b,c, Evening peak time for the flies shown in a under 12:12 h LD (b) and 16:8 h LD (c). d, Mean normalized predawn activity for the genotypes shown in a under 12:12 h LD. e, Mean normalized activity of the Pdf mutant and Pdf-rescue genotypes under 12:12 h LD (top) and 16:8 h LD (bottom) (combined data from two independent lines). n: 12:12 h LD, Pdf01 (30); DmelPdf,Pdf01 (89); DsecPdf,Pdf01 (86); 16:8 h LD, Pdf01 (16), DmelPdf,Pdf01 (59); DsecPdf,Pdf01 (79). f,g, Evening peak time for flies under 12:12 h LD (f) and 16:8 h LD (g) shown in e. n: Pdf01 (16); DmelPdf,Pdf01 no. 1 (29); DmelPdf,Pdf01 no. 2 (30); DsecPdf,Pdf01 no. 1 (38); DsecPdf,Pdf01 no. 2 (41). h, Mean normalized predawn activity for flies depicted in e under 12:12 h LD. n: Pdf01 (30); DmelPdf1,Pdf01 (40); DmelPdf1,Pdf01 (49); DsecPdf1,Pdf01 (44); DsecPdf2,Pdf01 (42). b–d,f–h, *P < 0.05, ***P < 0.001 (Wilcoxon tests with Bonferroni correction). f–h, Results for independent species-specific rescue strains and pooled rescue strains are shown in box plots to the left and right of the dashed line, respectively; letters A–C indicate significant differences, P < 0.05 (Wilcoxon tests with Bonferroni correction).
To test explicitly whether divergence of Pdf cis-regulatory regions is sufficient to explain species’ behavioural differences, we rescued Pdf expression in a Pdf-null D. melanogaster background with transgenes containing either the D. melanogaster or D. sechellia Pdf 5’-regulatory region fused to the D. melanogaster Pdf coding sequence. Under 12:12 h LD, rescue strains showed typical patterns of activity, with evening peaks centred around lights-off, whereas Pdf mutants showed a significantly advanced evening peak time (Fig. 4e,f). Under 16:8 h LD, rescue strains showed greater evening peak plasticity than Pdf mutants (Fig. 4g). Importantly, those containing the D. melanogaster Pdf 5’-regulatory region showed slightly, but significantly, greater evening peak delay than those with the D. sechellia 5’-regulatory sequence (Fig. 4g). We also recorded substantial predawn activity in D. melanogaster 5’-regulatory rescue strains compared with Pdf mutants under 12:12 h LD (Fig. 4h), but observed a lower increase in those expressing Pdf under the D. sechellia 5’-regulatory region (Fig. 4h). Importantly, there is again a significant difference between species-specific rescue strains. Together, these results support a contribution of Pdf cis-regulatory divergence to species differences in both evening peak plasticity and morning activity.
Pdf regulatory region sequence evolution
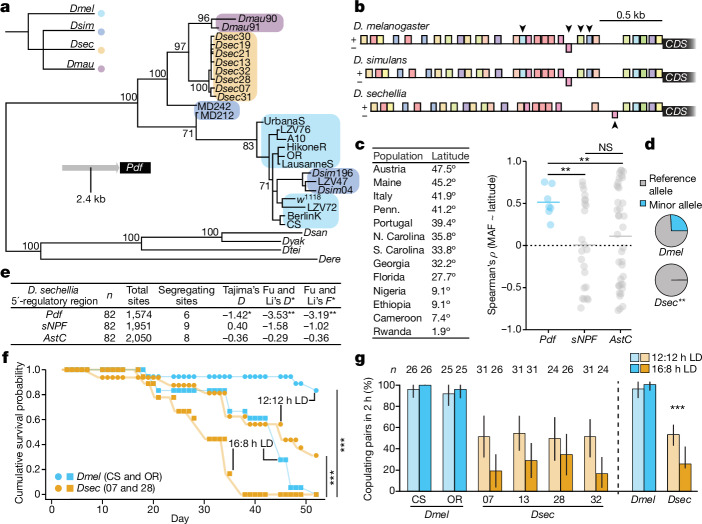
To determine whether the Pdf 5’-regulatory sequence exhibits evolutionary signatures, we constructed a maximum-likelihood phylogeny of sequences of this region from D. melanogaster, D. sechellia, D. simulans and D. mauritiana strains, rooted with sequences from more distant drosophilids (Fig. 5a). Unlike the species tree, D. sechellia Pdf 5’-regulatory sequences formed a monophyletic group, whereas the cosmopolitan D. melanogaster and D. simulans sequences clustered, representing the most derived sequences. D. mauritiana and Madagascan D. simulans strains showed closer similarity to D. sechellia sequences than cosmopolitan D. melanogaster and D. simulans, potentially reflecting inter- and intraspecific variation between populations at different latitudes. Motif enrichment analysis35 identified putative regulatory sequences in these species’ 5’ regions. Although all motifs were shared among D. melanogaster and D. simulans sequences, four were degenerated or absent in D. sechellia and one site was unique to this species (Fig. 5b), potentially affecting its activity through the loss (or gain) of transcription factor-binding sites.
Fig. 5. Evidence for selection on the Pdf 5’-regulatory sequence and fitness effects.
a, Maximum-likelihood phylogeny of Pdf 5’-regulatory sequences from the D. melanogaster subgroup (including D. mauritiana and D. simulans; species tree top left), rooted with sequences from Drosophila santomea, Drosophila yakuba, Drosophila teissieri and Drosophila erecta. Bootstrap support is shown for key internal nodes. b, Predicted motifs in the Pdf 5’-regulatory sequence, shown as coloured boxes on the +/− strands. Dmel/Dsim- and Dsec-specific motifs are marked with downwards and upwards arrowheads, respectively. c, Left, approximate latitude for Dmel populations36; right, significant differences in the distribution of correlation coefficients for MAF of all variable sites in the putative regulatory sequences of Pdf and control genes relative to latitude (t-test). d, Average MAF of variable sites in the Pdf 5’-regulatory sequence (from c) in laboratory Dsec and Dmel strains from a. Variable sites are significantly under-represented in Dsec relative to Dmel (Fisher’s exact test). e, Sequence-based tests of neutrality in the Dsec putative regulatory sequences of Pdf and control genes. Tajima’s D- and Fu and Li’s D*- and F*-statistics were calculated using 82 D. sechellia haplotypes42. f, Cumulative survival probability for species maintained at the indicated photoperiods. No significant differences were observed between strains of the same species by photoperiod (Fisher’s exact test, all P > 0.05), and were thus pooled and compared using a log-rank test. g, Percentage of copulating pairs for Dmel and Dsec after 2 h for flies acclimated to 12:12 h LD and 16:8 h LD; 95% confidence intervals were calculated using 1,000 bootstraps. Because there were no differences between strains, we pooled these by species, finding a significant effect in Dsec (right, Fisher’s exact test). c–g, *P < 0.05, **P < 0.01, ***P < 0.001.
We next investigated whether sequence divergence between the D. melanogaster and D. sechellia 5’-regulatory sequences results from natural selection. We first determined whether variants within the D. melanogaster Pdf 5’-regulatory region are associated with higher degrees of evening peak plasticity observed with increasing latitudes9. Using single-nucleotide variant frequencies in the genomes of globally distributed D. melanogaster populations36, we chose 13 populations representing a wide range of latitudes (Fig. 5c). We correlated minor allele frequency (MAF) across all variable sites detected within the Pdf 5’-regulatory sequence with the estimated latitude of the population collection site. Because correlations could reflect the underlying population structure as a result of D. melanogaster’s demographic history during emigration from its native African range37, as controls we repeated this analysis for a 2.4 kb 5’ region of two other neuropeptide genes, sNPF and AstC. A positive correlation was consistently observed between population latitude and MAF for each variable site within the Pdf 5’-regulatory region, but not for controls (Fig. 5c). These comparisons indicate that the effect of latitude on MAF of Pdf 5’-regulatory variants is different from that expected due to demography alone, suggesting a role for selection on these sites in D. melanogaster, similar to clinal variation in other circadian genes38–41. Among laboratory D. melanogaster strains, these single-nucleotide variants occur with an approximate MAF of 25%, but none were present in D. sechellia strains (Fig. 5d), consistent with a potential function of these variants in increasing evening peak plasticity.
To test for signs of selection within the D. sechellia population, we used 82 Pdf 5’-regulatory sequences (plus sNPF and AstC control sequences) from individuals recently sampled from the Seychelles42 to calculate Tajima’s D- and Fu and Li’s D*- and F*-statistics40. All three statistics were significantly negative for Pdf, but not for control genes, consistent with recent directional selection acting on this circadian gene (Fig. 5e). Thus, selection might also act to reduce aspects of circadian plasticity at the equator.
Plasticity affects reproductive fitness
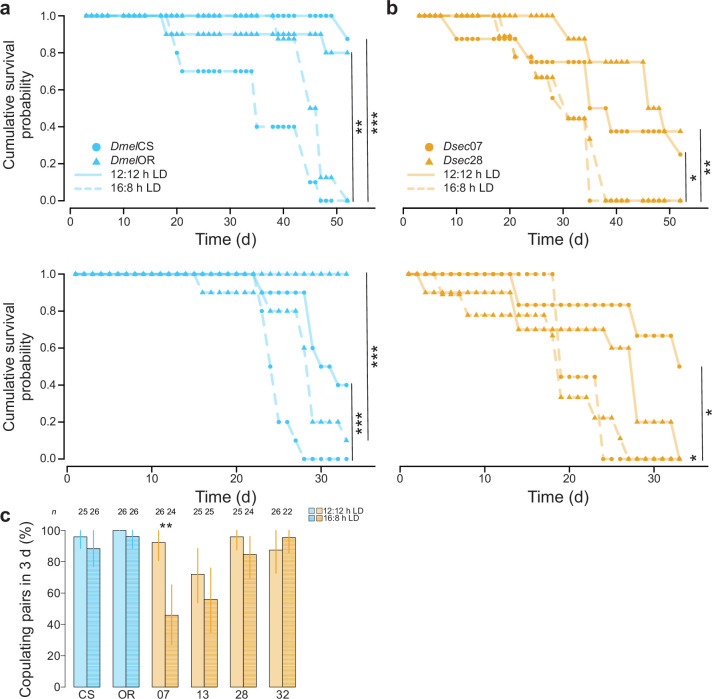
To identify a potential mechanism by which natural selection acts, we asked whether plasticity in circadian activity impacts fitness. We first examined lifespan, which is impacted by photoperiod in other animals43 and probably affects lifetime reproductive output. Both D. melanogaster and D. sechellia maintained under 16:8 h LD showed a significant reduction in lifespan relative to those under 12:12 h LD (Fig. 5f and Extended Data Fig. 9a,b). However, the detrimental effect of longer photoperiod was not observed until several weeks had elapsed, by which time flies could certainly reproduce, making it less likely to impact fitness in nature.
Extended Data Fig. 9. Potential fitness effects of acclimating D. melanogaster and D. sechellia to 16:8 h LD.
a, Two replicates (top and bottom) of the cumulative survival probability for D. melanogaster (DmelCS and DmelOR) maintained at 12:12 h LD or 16:8 h LD. No significant differences were observed between strains of the same species by photoperiod. b, Two replicates (top and bottom) of the cumulative survival probability for D. sechellia (Dsec07 and Dsec28) maintained at 12:12 h LD or 16:8 h LD. No significant differences were observed between strains of the same species by photoperiod. For a-b, asterisks indicate significant differences between photoperiod treatments of the same strain: * = P < 0.05, ** = P < 0.01 and *** = P < 0.001 (log-rank test). The top replicate in both panels is the same data presented in Fig. 5f (where it is pooled by species). c, Percent of copulating pairs observed for D. melanogaster (blue) and D. sechellia (orange) after 3 d for flies acclimated to 12:12 h LD (left) compared to 16:8 h LD (right). Error bars, representing 95% confidence intervals, were calculated using 1000 bootstraps. * = P < 0.05 and ** = P < 0.01 (Wilcoxon tests with Bonferroni correction for multiple comparisons).
Circadian rhythms are important for synchronization of sexual behaviour among conspecifics6,44. We therefore reasoned that if evening peak plasticity (or the lack thereof) influences copulation success, it might affect fitness. We acclimated male and female virgins to 12:12 and 16:8 h LD for 4 days and observed copulation rates among pairs over a 2 h period after lights-on (Fig. 5g). For D. melanogaster, we observed no difference in copulation rates between treatments (Fig. 5g). By contrast, for all D. sechellia strains, there was a consistent reduction in copulation rate for flies acclimated to 16:8 h LD (Fig. 5g, left). Pooling of strains by species showed a significant decrease in overall copulation by D. sechellia acclimated to 16:8 h LD, but not by D. melanogaster (Fig. 5g, right). For some strains, this reduction persisted for several days (Extended Data Fig. 9c). These results demonstrate that D. sechellia’s reproductive success—and thus, probably its fitness—is influenced by its lack of evening peak plasticity when exposed to extended photoperiods that it would never experience in nature. By contrast, D. melanogaster’s behavioural plasticity could allow it to circumvent these negative effects.
Discussion
Identifying the mechanisms of behavioural plasticity is key to understanding how organisms have evolved to inhabit variable environments and to predict how they will persist in increasingly unstable ones45. We have characterized a circadian plasticity difference in drosophilids, providing a rare example of linking changes in gene function, central neuron populations and behavioural divergence.
In D. melanogaster, Pdf is required in the l-LNvs for photoperiod plasticity19, and interspecific spatial differences in Pdf expression exist28, notably in high-latitude species in which Pdf is restricted to the l-LNvs10,46. These observations, combined with our analyses, point to the Pdf locus as a hotspot of evolution. Given Pdf’s terminal placement as an effector gene of the clock network21, its role in broad synchronization of circadian clock neurons47 and its strong influence on circadian behaviours, changes in the cis-regulation of Pdf expression might represent a minimally pleiotropic means of introducing plasticity into the clock neuronal network.
A single locus clearly does not explain the entirety of species differences in plasticity, as is true of most behaviours48. There are almost certainly contributions of additional genes that we have not tested and/or more complex genetic interactions that we cannot identify with our screen design. Beyond the cis-regulatory differences in Pdf characterized here, the translation21, transport and secretion of this neuropeptide49 are all potentially subject to divergent regulation.
D. sechellia also shows greatly reduced morning activity compared with other drosophilids. This phenotype is similar to that observed in D. melanogaster Pdf mutants50, and species-specific Pdf rescue in D. melanogaster indicates a contribution of cis-regulatory divergence of the locus. That our candidate screen did not find an effect of the Pdf locus itself in this difference might reflect coevolution of the Pdf 5’-regulatory region and its trans-acting factors, which could be masked in a hybrid genetic background. Indeed, our screen identified several genes with the ability to regulate Pdf expression in trans, highlighting a different evolutionary trajectory to evening peak plasticity divergence that nevertheless converges on this neuropeptide. The morning and evening oscillators partially overlap in function, sharing synaptic feedback19,51, with both being required for long-photoperiod adaptation27. Potential mechanistic and evolutionary connections between evening peak plasticity and morning activity require further exploration. Moreover, the evolutionary consequences, if any, of reduced morning peak activity in D. sechellia remain unclear. These issues might be illuminated by analyses of the circadian pattern of other behaviours of this species (and its sympatric sister species), such as courtship or feeding, which could constitute further barriers to reproduction52.
Why has D. sechellia lost evening peak plasticity? One hypothesis is that, in a constant photoperiod, selection to maintain plasticity mechanisms is relaxed, leading these to degenerate over time. Alternatively, in stable environments, plasticity might come at a fitness cost, leading selection to favour its loss under constant photoperiods—for example, to enhance the robustness of circadian activity. Regardless, our view of D. sechellia’s specialization should expand beyond evolution of host fruit preference to restriction to an equatorial environment. Indeed, D. sechellia’s circadian phenotype might contribute to its endemism, despite the wider range of M. citrifolia53. Exploration of the impact of differences in circadian plasticity mechanisms on latitudinal constraint of other species seems warranted.
Methods
Drosophila strains and rearing
All flies were reared on a wheat flour/yeast/fruit juice medium in non-overlapping 2 week cycles, and kept in 12:12 h LD at 25 °C. For D. sechellia strains, we added an additional mixture of instant Drosophila medium (Formula 4-24 blue, Carolina bio-supply) mixed with noni juice (Raab Vitalfood).
For comparisons of the circadian behaviour of D. melanogaster, D. sechellia, D. simulans and D. mauritiana, at least two wild-type strains of each species were used (DmelCS, DmelOR, DmelLZV L72, DmelLZV L76, Dsec07, Dsec28, DsimMD221, DsimMD242, Dmau90 and Dmau91). To screen candidate genes for effects on circadian behaviour differences between D. melanogaster and D. sechellia, we used D. melanogaster strains containing loss-of-function mutations for genes previously associated with circadian behaviour phenotypes. A list of fly strains and their hybridization success (when applicable) is provided in Supplementary Table 1. When strains were not available (vrille) or not hybridizable (Jet and timeless), we used D. melanogaster deficiency strains containing engineered chromosomal deletions spanning the region of a candidate gene (in addition to many other loci)54. In the case of timeless, a deficiency strain did not hybridize either. The Pdf strain we used is the Pdf01 allele50 in the Canton-S genetic background (provided by C. Förster, University of Würzburg), because we were unable to hybridize the original Pdf01 strain. To confirm that the effect we observed (Extended Data Fig. 3) was not due to a difference in D. melanogaster genetic background, we compared hybrids made with this Pdf01 strain to the same parental Canton-S (denoted here as DmelCSW, where W represents Würzburg), which showed predawn activity and evening peak plasticity qualitatively similar to our DmelCS strain (Extended Data Fig. 4). To visualize the collection sites of these strains, we generated a world map using the R package ‘maps’.
Hybrid crosses and circadian candidate gene screening
We generated D. melanogaster/D. sechellia hybrids as previously described55. In brief, hours-old virgin females were crossed to males that were collected as virgins and aged at high density (15–20 flies) for 5–7 days. To increase interactions between flies, we pushed a plug into the vial to leave a 2–3 cm space above the food surface. These crosses yielded only sterile, but viable, males. This method does not allow us to test sex-linked candidate genes, including the core transcriptional feedback loop member period56 and the Pdf receptor gene, Pdfr47.
Drosophila activity monitoring
For all activity measurements, we used 1–3-day-old males in the Drosophila activity monitor system57 in small incubators that continuously regulate light and temperature conditions (TriTech Research, no. DT2-CIRC-TK). In brief, this system uses an infrared beam that bisects a glass tube (5 mm inner diameter, 65 mm length) in which the fly is housed, to record activity as the number of beam crosses per minute. Flies were transferred into tubes under light CO2 anaesthesia with a 5% sucrose, 2% agar (w/v) solution at one end and capped with a cotton plug at the other. Each monitor recorded the activity of up to 32 flies simultaneously, and multiple monitors were placed in a single incubator. For each genotype, we tested flies in at least two technical replicates.
All flies were first exposed to 7 days of 12:12 h LD and then shifted to one of four extended photoperiod cycles for an additional 7 days—14:10, 16:8, 18:6 or 20:4 h LD—to allow us to measure 12:12 h LD-associated (that is, predawn activity) and extended photoperiod-associated behaviours (that is, evening peak plasticity) for each animal. For assessment of free-running period, flies were exposed to 7 days of DD following 7 days of 12:12 h LD. For each photoperiod regime, we took the average activity of the final 4 days of each 7 day period. The initial 3 days were considered an acclimation period. All subsequent analyses were performed in R using the Rethomics package58. For activity plots, maximum normalization was performed to standardize all plots to a range of 0–1.
To quantify predawn activity, average normalized activity was calculated for each fly in 30 min bins in the 3 h preceding dawn. To quantify morning and evening peak times, peak activity was identified from the average activity of each fly in 10 min bins during the last 4 days of both the 12:12 h LD and extended photoperiod using custom R scripts (available at github.com/mshahandeh/circ_plasticity). First, a rolling triangular mean was applied to smooth the data, which were then split into two 12 h sections, the first spanning the time around lights-on and the second spanning the time around lights-off (at least 3 h preceding and 3 h after for both). The global peak was identified within each dataset and recorded as the timing of the morning and evening peak, respectively.
For activity plots shown in the figures, average activity is plotted in 30 min bins, with yellow and grey bars at the bottom indicating timing of lights-on and -off, respectively. Error bars indicate the standard error of the mean. For evening peak time, vertical dashed lines indicate the average timing of the evening peak; for predawn activity, dashed boxes highlight the predawn period (3 h before lights-on). For quantifications of both evening peak time and predawn activity, standard box plots were used in which the bold middle line represents the median of the data, the box the interquartile range and the whiskers the remaining quartiles. Outliers are depicted with data points.
smFISH
We used the Pdf probe library described previously59 bound to the Cy5 fluorophore (LubioScience), essentially following a published protocol60. Brains were imaged using an inverted confocal microscope (Zeiss LSM 710 or 880) equipped with a ×40 or ×63 oil-immersion objective, and using fixed settings to maximize the comparability of images within experiments. Images were captured using Microsoft ZEN 2.3 SP1 software. Images were processed in Fiji and RNA spots were counted using the Fiji macro RS-FISH61. No signal was detected outside of the LNv cell bodies. RNA spot counts between strains within photoperiod treatments were compared using a Wilcoxon rank-sum test followed by post hoc correction for multiple tests62. We did not compare between experiments because these flies were dissected, stained and imaged separately. smFISH was repeated for two time points during the morning and evening peaks under both 12:12 and 16:8 h LD to ensure replicability of the overall pattern of expression. We did not pool these data because they are from a separate staining/imaging.
Immunofluorescence
For immunofluorescence of whole-mount Drosophila brains, 1–2-day-old males were collected and acclimated to a specific photoperiod for four additional days. To standardize sampling times, we fixed these flies in 4% paraformaldehyde for 2 h at room temperature with gentle agitation before dissection. Brains were dissected and stained essentially as described63. Primary and secondary antibodies and concentrations used are provided in Supplementary Table 2. Brains were imaged using an inverted confocal microscope (Zeiss LSM 710 or 880) equipped with a ×20 or ×40 objective, and using fixed settings to maximize the comparability of images. Images were captured using Microsoft ZEN 2.3 SP1 software. To quantify fluorescence, images were processed in Fiji by first creating a maximum-intensity projection z-stack, which was thresholded to remove background signal64. Relative fluorescence was measured for each set of neurons by structure (that is, LNv soma or s-LNv dorsal axonal projections) as integrated density of pixel intensity, and the average of both hemispheres was recorded for each brain. We compared Pdf immunofluorescence between strains within photoperiod treatments using a Wilcoxon rank-sum test followed by post hoc correction for multiple tests62. We did not compare between experiments because these flies were dissected, stained and imaged separately. We repeated immunostainings for two time points during the morning and evening peaks under both 12:12 and 16:8 h LD, to ensure replicability of the overall pattern of expression. These data cannot be pooled, however, because they are from a separate staining/imaging and produce different fluorescence measurements (arbitrary units).
For comparison of the structural plasticity of s-LNv axonal projections between D. melanogaster and D. sechellia, we imaged the most dorsal projections during two time points in the light and dark phases (2 and 14 h, respectively) at ×40 with a ×2 digital zoom. We performed Scholl analysis on these images, counting the number of axonal crossings with concentric 10 µm arcs using the Neuroanatomy Fiji plugin65. The number of axonal crossings was averaged per hemisphere for each brain and compared using a Wilcoxon rank-sum test. We performed this experiment in two replicates and pooled replicates for analysis because fluorescence intensity was not measured.
Construction of transgenic lines
Approximately 2.4 kb upstream of the Pdf start codon was PCR amplified from D. melanogaster (DmelCS) or D. sechellia (Dsec28) gDNA and Gateway cloned into the pDONR221 vector, sequenced verified and subcloned into pHemmarG (Addgene, no. 31221) for CD4:tdGFP reporters, pBPGUw (Addgene, no. 17575) for Gal4 drivers or fused to the D. melanogaster (DmelCS) Pdf coding sequence in pattB66 with Gibson assembly, for species-specific rescue constructs. Constructs were injected and integrated into the attP2 landing site (chromosome 3)67 in the D. melanogaster genome by BestGene Inc. Rescue constructs were recombined into the Pdf01 background and genotyped with PCR and Sanger sequencing. For elimination of genetic background effects, for each species-specific rescue construct we collected data for two independent insertions. Oligonucleotides used for cloning and sequence verification are listed in Supplementary Table 3.
Pdf gene region sequence comparisons
We PCR amplified and Sanger sequenced the Pdf gene region using the oligonucleotides listed in Supplementary Table 3. Sequences were assembled and aligned in SnapGene (www.snapgene.com) using MUSCLE v.3.8.1551 (ref. 68) and visually inspected for alignment errors. Pdf coding sequences were translated to an amino acid alignment and visualized using Jalview69 (v.2.11.2). For 5'-regulatory sequences, we used the R package phangorn to generate maximum-likelihood trees70, with the modelTest function to identify the best-fitting substitution model and performing standard bootstrapping to obtain support values. The MEME program was used to discover putative regulatory motifs common across Pdf 5’-regulatory sequences with n > 5 sequences per species35. We restricted this analysis to the top ten most significant motifs identified.
Population genetic analysis of Pdf 5’-regulatory sequences
For detection of genomic patterns of clinal adaptation in Pdf 5’ sequences, we used a dataset of single-nucleotide variants in globally distributed D. melanogaster populations36. We selected populations from this dataset with a read depth greater than five to ensure confidence in variant frequencies. For each population, we calculated MAF across all variable sites in this region, and for the same-sized region upstream of the start codon of two control neuropeptide genes. Spearman’s rho was used to correlate MAF with the latitude of the capital city in each country in which the populations were sampled (precise latitudes were not available). Correlation coefficients for all variable sites within each locus were compared using a t-test.
We obtained 41 D. sechellia genomes sampled in the Seychelles archipelago from the Sequence Read Archive42, aligned them to the D. sechellia reference genome (ASM438219v2), phasing the data by chromosome (Samtools v.1.19.2), and created consensus sequences for the 82 Pdf 5’-regulatory haplotypes. The software DNA sequence polymorphism (v.6) was used to calculate neutrality test statistics, with significance determined by 1,000 coalescence simulations.
Longevity assay
To test for photoperiod-dependent differences in lifespan, we acclimated 1-day-old DmelCS, DmelOR, Dsec07 and Dsec28 males to either 12:12 h LD or 16:8 h LD. We kept ten flies of each genotype individually in vials containing wheat flour/yeast/fruit juice medium, to which we added an additional mixture of instant Drosophila medium (Formula 4-24 blue, Carolina bio-supply), mixed with noni juice for D. sechellia or apple cider vinegar (Denner) for D. melanogaster. Flies were transferred to fresh vials every 3 days to prevent media from drying out. We recorded for each weekday the number of vials in which a fly died until all flies among one treatment per strain were dead. No significant differences were detected between strains within species (Fisher’s exact test, all P > 0.05), which were therefore pooled to represent the species for Fig. 5f. We compared cumulative survival probability using the R package ‘survival’ (ref. 71). We performed a second replicate of these experiments to verify the observed effects (Extended Data Fig. 9).
Copulation assays
To test for photoperiod-dependent differences in copulation rate, we first acclimated 1-day-old virgin DmelCS, DmelOR, Dsec07 and Dsec28 males and females to either 12:12 or 16:8 h LD for 4 days. We aspirated single females into 25-mm-diameter food vials containing wheat flour/yeast/fruit juice medium, returned them to their respective photoperiods and allowed them to recover for 24 h. The following day, 30 min after lights-on, we aspirated a single male of the same genotype into each tube and pushed the plug into the vial so that pairs had a 2 cm space above the food surface, forcing them to interact. We observed courtship for 2 h, recording successfully and unsuccessfully copulating pairs. We reasoned that a consistent reduction in copulation success over 2 h would be sufficient to affect fitness in the field, in which individual flies interact for only much shorter periods of time72,73. We observed no differences between strains within species (Fig. 5g; Fisher’s exact test, all P = 1), so these data were pooled to represent the species for analysis (Fig. 5g).
To determine whether these differences persisted over the long term, following acclimation to either 12:12 h LD or 16:8 h LD for 4 days, single male–virgin female pairs were aspirated into the same food vials and stored at 25 °C for 3 days, after which the males were removed. Vials that produced offspring were counted as successful copulation. Copulation frequencies within species between treatments were compared using Fisher’s exact test.
Data reporting
No statistical methods were used to predetermine sample size. For behavioural experiments, we aimed for a sample size of 25–30 individuals because significant differences are easily detected at these sizes. In the case of our hybrid screen, in which hybrid flies were difficult to produce, we reduced this to 15 individuals per strain but, due to the strong reproductive isolation between species, some genotypes were difficult to cross with D. sechellia, resulting in a lower sample size. For image analyses, we obtained images from five brains from each strain per treatment, because this allowed for parallel processing of multiple genotypes and time points. For both behavioural assays and image analyses, data were collected and analysed blind to treatment (species, genotype, sampling time and so on). We did not randomize within experiments, but instead ran all genotypes in parallel for all experiments, with the exception of the hybrid screen, in which the behaviour of hybrids was measured as offspring were successfully obtained from crosses. In this case, test hybrid behaviour was measured at least once in parallel to control hybrids and parental strains. For all experiments, flies were 3–5 days old at the start of the experiment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-08056-x.
Supplementary information
Supplementary Tables 1–3.
Source data
Source Data Figs. 1–5 and Extended Data Figs. 1–4 and 6–9.
Acknowledgements
We thank E. Bertolini, C. Förster, D. Matute, J. Saltz, the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Developmental Studies Hybridoma Bank (NICHD of the NIH, University of Iowa) for reagents. We thank R. Arguello, E. Bertolini, D. Gatfield and members of the Benton laboratory for discussions and comments on the manuscript. Research in E.N.’s laboratory is supported by the SNSF (no. 310030_189169), and R.B.’s laboratory is supported by the University of Lausanne, an ERC Advanced Grant (no. 833548) and the SNSF (no. 310030B_185377).
Extended data figures and tables
Author contributions
M.P.S. and R.B. conceived the project. M.P.S. designed, performed and analysed most experiments. L.A. performed hybrid screening and sequencing of the Pdf gene region. L.L.D.D. contributed to the experiments shown in Fig. 1c. J.C. contributed to the experiments shown in Extended Data Figs. 3 and 5. R.K. assisted with preliminary behavioural experiments and, together with E.N. and R.B., provided input on experimental design, analysis and interpretation. M.P.S. and R.B. wrote the paper with feedback from all authors.
Peer review
Peer review information
Nature thanks Charlotte Förster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Data supporting the findings of this study are available at Dryad (https://doi.org/10.5061/dryad.vq83bk42z)74. We additionally used D. melanogaster single-nucleotide variation data from ref. 36 (data available at https://doi.org/10.5061/dryad.7440s)75. We used sequences of D. sechellia from ref. 42, available from SRA: SRP113415. To align these sequences, we used the D. sechellia reference genome (ASM438219v2). Source Data are provided with this paper.
Code availability
Code used for analyses is available from GitHub (github.com/mshahandeh/circ_plasticity).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael P. Shahandeh, Email: Michael.P.Shahandeh@hofstra.edu
Richard Benton, Email: Richard.Benton@unil.ch.
Extended data
is available for this paper at 10.1038/s41586-024-08056-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-08056-x.
References
- 1.Rieger, D., Stanewsky, R. & Helfrich-Forster, C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms18, 377–391 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Auer, T. O., Shahandeh, M. P. & Benton, R. Drosophila sechellia: a genetic model for behavioral evolution and neuroecology. Annu. Rev. Genet.55, 527–554 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Roca, I. T. et al. Shifting song frequencies in response to anthropogenic noise: a meta-analysis on birds and anurans. Behav. Ecol.27, 1269–1274 (2016). [Google Scholar]
- 4.Caldwell, A. J., While, G. M. & Wapstra, E. Plasticity of thermoregulatory behaviour in response to the thermal environment by widespread and alpine reptile species. Anim. Behav.132, 217–227 (2017). [Google Scholar]
- 5.Muraro, N. I., Pirez, N. & Ceriani, M. F. The circadian system: plasticity at many levels. Neuroscience247, 280–293 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Wang, G. et al. Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science371, 411–415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn, M. et al. The circadian clock improves fitness in the fruit fly, Drosophila melanogaster. Front. Physiol.10, 1374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardeland, R. & Stange, G. Comparative studies on the circadian rhythms of locomotor activity of 40 Drosophila species. J. Interdiscipl. Cycle Res.4, 353–359 (1973). [Google Scholar]
- 9.Rieger, D., Peschel, N., Dusik, V., Glotz, S. & Helfrich-Forster, C. The ability to entrain to long photoperiods differs between 3 Drosophila melanogaster wild-type strains and is modified by twilight simulation. J. Biol. Rhythms27, 37–47 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Beauchamp, M. et al. Closely related fruit fly species living at different latitudes diverge in their circadian clock anatomy and rhythmic behavior. J. Biol. Rhythms33, 602–613 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Bywalez, W. et al. The dual-oscillator system of Drosophila melanogaster under natural-like temperature cycles. Chronobiol. Int.29, 395–407 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Matute, D. R., Gavin-Smyth, J. & Liu, G. Variable post-zygotic isolation in Drosophila melanogaster/D. simulans hybrids. J. Evol. Biol.27, 1691–1705 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Lachaise, D. et al. in Historical Biogeography of the Drosophila melanogaster Species Subgroup (eds. Hecht, M. K., Wallace, B. & Prance, G. T.) 159–225 (Plenum, 1988).
- 14.Dean, M. D. & Ballard, J. W. Linking phylogenetics with population genetics to reconstruct the geographic origin of a species. Mol. Phylogenet. Evol.32, 998–1009 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Hardin, P. E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet.74, 141–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermann-Luibl, C. & Helfrich-Forster, C. Clock network in Drosophila. Curr. Opin. Insect Sci.7, 65–70 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Vaze, K. M. & Helfrich-Forster, C. The neuropeptide PDF is crucial for delaying the phase of Drosophila’s evening neurons under long Zeitgeber periods. J. Biol. Rhythms36, 442–460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshii, T. et al. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. J. Neurosci.29, 2597–2610 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlichting, M. et al. A neural network underlying circadian entrainment and photoperiodic adjustment of sleep and activity in Drosophila. J. Neurosci.36, 9084–9096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peschel, N., Chen, K. F., Szabo, G. & Stanewsky, R. Light-dependent interactions between the Drosophila circadian clock factors Cryptochrome, Jetlag, and Timeless. Curr. Biol.19, 241–247 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Gunawardhana, K. L. & Hardin, P. E. VRILLE controls PDF neuropeptide accumulation and arborization rhythms in small ventrolateral neurons to drive rhythmic behavior in Drosophila. Curr. Biol.27, 3442–3453 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Mezan, S., Feuz, J. D., Deplancke, B. & Kadener, S. PDF signaling is an integral part of the Drosophila circadian molecular oscillator. Cell. Rep.17, 708–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grima, B., Chelot, E., Xia, R. & Rouyer, F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature431, 869–873 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Delventhal, R. et al. Dissection of central clock function in Drosophila through cell-specific CRISPR-mediated clock gene disruption. eLife8, e48308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoleru, D., Peng, Y., Agosto, J. & Rosbash, M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature431, 862–868 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Sheeba, V., Gu, H., Sharma, V. K., O’Dowd, D. K. & Holmes, T. C. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J. Neurophysiol.99, 976–988 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menegazzi, P. et al. A functional clock within the main morning and evening neurons of D. melanogaster is not sufficient for wild-type locomotor activity under changing day length. Front. Physiol.11, 229 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann, C. et al. The circadian clock network in the brain of different Drosophila species. J. Comp. Neurol.521, 367–388 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Park, J. H. et al. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc. Natl Acad. Sci. USA97, 3608–3613 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez, M. P., Berni, J. & Ceriani, M. F. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol.6, e69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrero, A. et al. Coupling neuropeptide levels to structural plasticity in Drosophila clock neurons. Curr. Biol.30, 3154–3166 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Zhang, L. et al. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr. Biol.20, 591–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang, X., Holy, T. E. & Taghert, P. H. A series of suppressive signals within the Drosophila circadian neural circuit generates sequential daily outputs. Neuron94, 1173–1189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryczek, N., Lys, A. & Makalowska, I. The functional meaning of 5’UTR in protein-coding genes. Int. J. Mol. Sci.24, 2976 (2023). [DOI] [PMC free article] [PubMed]
- 35.Bailey, T. L. & Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol.2, 28–36 (1994). [PubMed] [Google Scholar]
- 36.Bergland, A. O., Tobler, R., Gonzalez, J., Schmidt, P. & Petrov, D. Secondary contact and local adaptation contribute to genome-wide patterns of clinal variation in Drosophila melanogaster. Mol. Ecol.25, 1157–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arguello, J. R., Laurent, S. & Clark, A. G. Demographic history of the human commensal Drosophila melanogaster. Genome Biol. Evol.11, 844–854 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatib, L., Subasi, B. S., Fishman, B., Kapun, M. & Tauber, E. Unveiling subtle geographical clines: phenotypic effects and dynamics of circadian clock gene polymorphisms. Biology12, 858 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamaze, A. et al. A natural timeless polymorphism allowing circadian clock synchronization in “white nights”. Nat. Commun.13, 1724 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauber, E. et al. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science316, 1895–1898 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Deppisch, P. et al. Adaptation of Drosophila melanogaster to long photoperiods of high-latitude summers is facilitated by the ls-timeless allele. J. Biol. Rhythms37, 185–201 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrider, D. R., Ayroles, J., Matute, D. R. & Kern, A. D. Supervised machine learning reveals introgressed loci in the genomes of Drosophila simulans and D. sechellia. PLoS Genet.14, e1007341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, J. C. & Xu, F. Influences of light-dark shifting on the immune system, tumor growth and life span of rats, mice and fruit flies as well as on the counteraction of melatonin. Biol. Signals6, 77–89 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Emerson, K. J., Bradshaw, W. E. & Holzapfel, C. M. Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution62, 979–983 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducatez, S., Sol, D., Sayol, F. & Lefebvre, L. Behavioural plasticity is associated with reduced extinction risk in birds. Nat. Ecol. Evol.4, 788–793 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Menegazzi, P. et al. Adaptation of circadian neuronal network to photoperiod in high-latitude European drosophilids. Curr. Biol.27, 833–839 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Shafer, O. T. et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron58, 223–237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.York, R. A. Assessing the genetic landscape of animal behavior. Genetics209, 223–232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding, K. et al. Imaging neuropeptide release at synapses with a genetically engineered reporter. eLife8, e46421 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renn, S. C., Park, J. H., Rosbash, M., Hall, J. C. & Taghert, P. H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell99, 791–802 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Duhart, J. M. et al. Circadian structural plasticity drives remodeling of E cell output. Curr. Biol.30, 5040–5048 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Shahandeh, M. P., Pischedda, A. & Turner, T. L. Male mate choice via cuticular hydrocarbon pheromones drives reproductive isolation between Drosophila species. Evolution72, 123–135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razafimandimbison, S. G., McDowell, T. D., Halford, D. A. & Bremer, B. Origin of the pantropical and nutriceutical Morinda citrifolia L. (Rubiaceae): comments on its distribution range and circumscription. J. Biogeogr.37, 520–529 (2010). [Google Scholar]
- 54.Cook, R. K. et al. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol.13, R21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahandeh, M. P. & Turner, T. L. The complex genetic architecture of male mate choice evolution between Drosophila species. Heredity (Edinb.)124, 737–750 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, J., Yu, W. & Hardin, P. E. CLOCKWORK ORANGE enhances PERIOD mediated rhythms in transcriptional repression by antagonizing E-box binding by CLOCK-CYCLE. PLoS Genet.12, e1006430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu, J. C., Low, K. H., Pike, D. H., Yildirim, E. & Edery, I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Vis. Exp.43, 2157 (2010). [DOI] [PMC free article] [PubMed]
- 58.Geissmann, Q., Garcia Rodriguez, L., Beckwith, E. J. & Gilestro, G. F. Rethomics: an R framework to analyse high-throughput behavioural data. PLoS ONE14, e0209331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long, X., Colonell, J., Wong, A. M., Singer, R. H. & Lionnet, T. Quantitative mRNA imaging throughout the entire Drosophila brain. Nat. Methods14, 703–706 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Yuan, Y., Padilla, M. A., Clark, D. & Yadlapalli, S. Streamlined single-molecule RNA-FISH of core clock mRNAs in clock neurons in whole mount Drosophila brains. Front. Physiol.13, 1051544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahry, E. et al. RS-FISH: precise, interactive, fast, and scalable FISH spot detection. Nat. Methods19, 1563–1567 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat.6, 65–70 (1979). [Google Scholar]
- 63.Ostrovsky, A., Cachero, S. & Jefferis, G. Clonal analysis of olfaction in Drosophila: immunochemistry and imaging of fly brains. Cold Spring Harb. Protoc.2013, 342–346 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira, T. A. et al. Neuronal morphometry directly from bitmap images. Nat. Methods11, 982–984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA104, 3312–3317 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markstein, M., Pitsouli, C., Villalta, C., Celniker, S. E. & Perrimon, N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet.40, 476–483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res.32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Procter, J. B. et al. Alignment of biological sequences with Jalview. Methods Mol. Biol.2231, 203–224 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schliep, K. P. phangorn: Phylogenetic analysis in R. Bioinformatics27, 592–593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Therneau, T. M. & Grambsch, P. M. Modelling Survival Data: Extending the Cox Model (Springer, 2000).
- 72.Jezovit, J. A., Alwash, N. & Levine, J. D. Using flies to understand social networks. Front. Neural Circuits15, 755093 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dukas, R. Natural history of social and sexual behavior in fruit flies. Sci. Rep.10, 21932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahandeh, M. Data from: circadian plasticity evolves through regulatory changes in a neuropeptide gene. Dryad10.5061/dryad.vq83bk42z (2024). [DOI] [PMC free article] [PubMed]
- 75.Bergland, A. O. et al. Data from: secondary contact and local adaptation contribute to genome-wide patterns of clinal variation in Drosophila melanogaster. Dryad10.5061/dryad.7440s (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–3.
Source Data Figs. 1–5 and Extended Data Figs. 1–4 and 6–9.
Data Availability Statement
Data supporting the findings of this study are available at Dryad (https://doi.org/10.5061/dryad.vq83bk42z)74. We additionally used D. melanogaster single-nucleotide variation data from ref. 36 (data available at https://doi.org/10.5061/dryad.7440s)75. We used sequences of D. sechellia from ref. 42, available from SRA: SRP113415. To align these sequences, we used the D. sechellia reference genome (ASM438219v2). Source Data are provided with this paper.
Code used for analyses is available from GitHub (github.com/mshahandeh/circ_plasticity).