Abstract
Transmission of malaria depends on the successful development of the sexual stages of the parasite within the midgut of the mosquito vector. The differentiation process leading to the production of the sexual stages is delineated by several developmental switches. Arresting the progression through this sexual differentiation pathway would effectively block the spread of the disease. The successful development of such transmission-blocking agents is hampered by the lack of a detailed understanding of the program of gene expression that governs sexual differentiation of the parasite. Here we describe the isolation and functional characterization of the Plasmodium falciparum pfs16 and pfs25 promoters, whose activation marks the developmental switches executed during the sexual differentiation process. We have studied the differential activation of the pfs16 and pfs25 promoters during intraerythrocytic development by transfection of P. falciparum and during gametogenesis and early sporogonic development by transfection of the related malarial parasite P. gallinaceum. Our data indicate that the promoter of the pfs16 gene is activated at the onset of gametocytogenesis, while the activity of the pfs25 promoter is induced following the transition to the mosquito vector. Both promoters have unusual DNA compositions and are extremely A/T rich. We have identified the regions in the pfs16 and pfs25 promoters that are essential for high transcriptional activity. Furthermore, we have identified a DNA-binding protein, termed PAF-1, which activates pfs25 transcription in the mosquito midgut. The data presented here shed the first light on the details of processes of gene regulation in the important human pathogen P. falciparum.
Plasmodium falciparum is one of the major debilitating and life-threatening parasitic pathogens of humans. Half of the world’s population lives in areas endemic for malaria, and 2 million to 3 million people are killed annually. Despite years of intensive research, an effective vaccine is still not available and the parasite displays a growing resistance to the currently available drugs. New ways of combatting the disease should be identified, but such efforts require a better understanding of the basic biology of the parasite.
The gene-regulatory processes governing development of the parasite are poorly understood. Classical genetic analysis of the parasite has been hampered by the difficulties encountered in the manipulation of the sexual cycle of the parasite, although the few genetic crosses that have been performed have provided some information on the mechanisms of cell cycle progression (19, 44). Recently, transfection protocols developed for other apicomplexan parasites were successfully adapted to the plasmodia and brought the exciting promise of a functional analysis of genes and their products (47). In addition, a detailed and functional analysis of the gene-regulatory events underlying the development of the parasite may now become feasible. Of particular interest are the molecular mechanisms underlying sexual differentiation of the parasite, as this process leads to the production of parasite stages that are equipped to invade the midgut of the mosquito vector. One approach to control malaria is to prevent sexual development of the parasite and thus transmission to the mosquito (1, 25). Research efforts directed toward such a goal will greatly benefit from a detailed understanding of the gene-regulatory events underlying sexual development.
The process of sexual differentiation is governed by several developmental switches that direct the parasite through a complex series of morphological changes (Fig. 1). First, a subpopulation of asexually reproducing parasites commits to sexual differentiation and undergoes gametocytogenesis, the formation of male and female sex cells. The sexually committed parasites are marked by the expression of the pfs16 gene, which is the earliest event in the sexual differentiation process described to date (13). A second developmental switch defines the sex of the developing gametocyte (39). The next developmental switch is executed following transmission of mature gametocytes to the mosquito midgut. Here, the gametocytes form gametes that fertilize and produce a motile ookinete. This ookinete penetrates the peritrophic membrane surrounding the blood meal and initiates sporogonic development, which leads to the production of new infectious sporozoites. The processes of gametogenesis and fertilization are completed within the 15 to 20 min following the arrival of the gametocytes in the mosquito midgut (1). The onset of gametogenesis is marked by the induction of the pfs25 gene (13, 18), which encodes a membrane protein containing several epidermal growth factor-like domains (27). It has been proposed that Pfs25 is involved in a receptor-ligand interaction required for invasion of the midgut epithelium (40). Antibodies raised against Pfs25 potently block transmission of P. falciparum to the mosquito, and the protein is a leading candidate for a transmission-blocking vaccine (26).
FIG. 1.
Life cycle of the human malaria parasite P. falciparum. Infection in humans begins with the introduction of sporozoites in the bloodstream by a bite of an infected Anopheles mosquito (a). The sporozoites are cleared by the liver (b), where cyclic asexual development initiates (c to g). Merozoites invade erythrocytes and transform into a ring-stage parasite (d). Subsequent trophic growth (e) and mitotic divisions lead to the production of up to 32 merozoites in a schizont (f), which bursts and releases new merozoites in the bloodstream. The merozoites can either reinitiate the erythrocytic asexual multiplication cycle (g) or commit to sexual differentiation (h). Initially, sexually committed parasites adapt the typical postinvasion ring-like shape (i) and cannot be discriminated from asexual parasites on a morphological basis. Sexual differentiation becomes morphologically apparent with the appearance of stage II gametocytes (j), which mature into female and male stage V gametocytes (k). The gametocytes are adapted to infect mosquitoes. Following the blood meal of a mosquito, the female gametocyte transforms in a macrogamete (l). The male gametocyte undergoes three rapid nuclear divisions and produces eight microgametes (m) that fertilize (n) the macrogamete, which then transforms in an invasive ookinete (o). This ookinete traverses the midgut epithelium and forms an oocyst at the side of the basal lamina. Sporogonic development (p) subsequently leads to the production of sporozoites that are released from the oocyst, accumulate in the salivary gland of the mosquito, and are infectious upon a new bite (a). DNA synthesis occurs at the transition from ring-stage parasite to schizont (d to f) (23) and at the transition from sexually committed ring-stage parasite to gametocyte (i and j) (24). Accordingly, both transitions are inhibited by pyrimethamine (9). Transcription of the pfs16 gene is induced in sexually committed ring stages, whereas the pfs25 gene is activated immediately following transmission (13).
The significance of the pfs16 and pfs25 genes as specific markers for two important developmental switches executed by the malaria parasite prompted us to investigate the transcriptional regulation of these genes in greater detail. Therefore, we have isolated the promoters of the P. falciparum pfs16 and pfs25 genes. To study the differential activities of these promoters during intraerythrocytic development of the parasite, we have exploited transient transfections of P. falciparum (10, 47). In addition, we have studied the activities of the pfs16 and pfs25 promoters in the parasite stages that develop within the mosquito midgut, using transfections of the related parasite P. gallinaceum. Our data show that the pfs16 and pfs25 promoters exhibit unusual DNA compositions that are extremely biased toward A and T nucleotides. We have identified the regions in these promoters that are essential for high transcriptional activity. Furthermore, we show that distinct mechanisms control the activities of these promoters during development of the parasite. The pfs16 promoter is induced at the very onset of gametocytogenesis and remains active following transmission of the parasite to the mosquito midgut. The activity of the pfs25 promoter is restricted to the parasite stages that develop within the mosquito midgut. We show that the induction of the pfs25 gene partially relies on a DNA-binding protein, termed PAF-1, which activates pfs25 transcription within the mosquito midgut.
MATERIALS AND METHODS
Transfection vectors.
Transfection vectors pHRPCAT, pA0, and pHLH were kindly provided by Y. Wu (Oxford University, Oxford, England). Plasmids pHLH and pA0 are derivatives of pHRPCAT and PSOCS2, respectively (47), in which cat (chloramphenical acetyltransferase) reporter genes have been replaced by luciferase reporter genes. A general-purpose transfection vector was constructed by substituting the hrp3 (histidine-rich protein 3 gene) promoter from plasmid pHRPCAT with a KpnI/NsiI restriction fragment containing the polylinker of pZERO (InVitrogen). The resulting plasmid was designated pCAT-L.
Isolation of the pfs16 5′ flanking sequences and pfs16 plasmid construction.
P. falciparum NF54 genomic DNA was digested with EcoRI and additionally sheared by sonication to an average fragment size of 2 kbp. The fragment ends were made blunt ended with T4 polymerase and introduced in a lambda ZAPII vector (Stratagene) with the aid of EcoRI linkers. The library was propagated in Escherichia coli XL-1 Blue cells (Stratagene) and screened with a pfs16-specific cDNA probe (33). Positive plaques were purified to homogeneity, and plasmids were rescued from the phages by an in vivo excision protocol (Stratagene). A plasmid with an insert of 1,461 bp that hybridized to the pfs16 probe was isolated. From this plasmid, the pfs16 upstream region was amplified by PCR using primers PP16.5 (ggctcgagCTACTGTACTTTTTTTGGAC) and PP16.6 (GAACTTTCGAATATgCATGTTGG) (lowercase indicates nucleotides not present in the pfs16 sequence that introduce restriction sites) and introduced into a pGEM-T vector (Promega) to yield plasmid pK16.3.
The pfs16 upstream region was introduced in transfection vector pCAT-L by cloning a XhoI/SphI fragment of pK16.3 in the EcoRV/SphI-digested pCAT-L vector after blunting of the XhoI end with Klenow polymerase. A series of 5′ deletions of the pfs16 upstream region in plasmid pCAT-L16.1 was generated. First, one of the two BamHI sites present in pCAT-L16.1 was eliminated by digesting the plasmid with SmaI/XbaI and blunting of the fragment ends with Klenow polymerase. Religation of the plasmid then resulted in plasmid pCAT-L16.1ΔSmaI/XbaI. Subsequently, this plasmid was digested with KpnI and BamHI. Exonuclease III and S1 nuclease treatment followed by religation of the plasmids then generated a series of incrementing 5′ deletions of the pfs16 upstream region. 3′ deletion mutants of the pfs16 promoter were generated by cloning the products of a partial SspI/EcoRI digest of the pfs16 promoter region of plasmid pCAT-L16.1ΔSmaI/XbaI in an EcoRI/EcoRV-digested pCAT-L vector.
A plasmid containing a luciferase reporter gene under control of the pfs16 promoter was generated by cloning a NsiI/HindIII fragment containing the luciferase gene from plasmid pHLH in the plasmid pExo2.
To visualize the developmental-stage-specific activity of the pfs16 promoter, a green fluorescent protein (GFP) reporter gene was introduced in plasmid pLUC16.1. To this end, a XbaI/PstI fragment of plasmid pKENGFPmut2 (kind gift of B. Cormack, Stanford University, Stanford, Calif.) containing the coding region of GFPmut2 was cloned in pBluescript KS− (Stratagene), and an NsiI restriction site was introduced in the gfp gene by PCR with GFP primer TATACATATGcatAAAGGAGAAG and M13 reverse primer AACAGCTATGACCATG. The PCR product was digested with NsiI and HindIII and inserted in the NsiI and HindIII sites of pLUC16.1
pfs25 plasmid construction.
The characterization of the pfs25 upstream region was based on plasmid pNF4.13, kindly donated by David Kaslow (National Institutes of Health, Bethesda, Md.). The insert of this plasmid is derived from a genomic library of P. falciparum clone 3D7 and contains a 3.5-kb genomic HindIII fragment of the pfs25 gene (27). The plasmid contains 1,006 nucleotides upstream of the ATG start codon, which were amplified by PCR with primer pp25.6 CTGTAAAGTTTATgCATTTTTAAAAG and M13 reverse primer AACAGCTATGACCATG and introduced in vector pGEM5ZF+ (Promega) to yield plasmid pK4. Subsequently, the pfs25 upstream region was introduced in transfection vector pCAT-L by introducing a KpnI/NsiI fragment of plasmid pK4 into the KpnI/NsiI-restricted pCAT-L vector, resulting in plasmid pCAT25.1.
A luciferase gene was placed under control of the pfs25 flanking sequences by cloning a BamHI restriction fragment of pPGS28LUC (16) in a BsaBI-restricted pNF4.13-based plasmid. The latter plasmid, designated pPFS25LUC, contains the luciferase gene inserted in frame with the coding region of the pfs25 gene. A series of 5′ deletion mutants of this plasmid was generated by exonuclease III-S1 nuclease treatment after restriction digestion with KpnI and XhoI. Mutations of a putative transcription factor binding site in plasmid pFS25LUC were generated by using a Quickchange site-directed mutagenesis kit (Stratagene). All plasmids were isolated by a standard alkaline lysis-CsCl gradient centrifugation method, and their integrity was confirmed by restriction mapping and sequence analysis.
Parasites and transfections.
P. falciparum blood-stage parasites were maintained in asynchronous cultures as described elsewhere (36). The asexual and sexual parasitemias were determined as described in reference 7, and blood-stage parasites were transfected as described previously (47). As the mosquito stages of P. falciparum are neither transfectable nor viable in an in vitro culture, we used P. gallinaceum parasites for transfection of the mosquito stages (45). White leghorn chickens were inoculated with P. gallinaceum-infected blood; at a parasitemia of 50 to 80%, blood was withdrawn. Gametogenesis was induced, and gametes and zygotes were isolated and transfected as described elsewhere (16). Following transfection, cells were incubated in ookinete maturation medium (29). Plasmid pCAT-L served as a negative control in all transfections. Plasmid pA0 was used as an internal standard in transfections of blood-stage parasites. Plasmid p49.20, which contains a luciferase gene downstream of a truncated pgs28 promoter (16), served as a control in transfections of the mosquito stages. Cells were harvested 48 h after transfection and reporter assays were as described previously (16, 47). Transfected parasites expressing the GFP reporter were visualized on a Bio-Rad MRC600 confocal laser scanning microscope.
Identification of the transcriptional start sites.
RNA of P. falciparum schizonts, gametocytes, and gametes was isolated as described previously (13). RNase protection assays were performed with an RPAII kit (Ambion). A 312-nucleotide fragment spanning nucleotides −294 to +17 with respect to the translational start site of the pfs16 gene was amplified by PCR using primers CCGTTAAATACTTTTTATACTG and GAACTTTCGAATATTCATGTTGG, cloned in a pGEM-T vector (Promega), and used as a template for the generation of a specific antisense RNA probe by a standard in vitro transcription procedure. Two femtomoles of probe was hybridized to 1 μg of RNA in 25 mM piperazine-N,N′-bis(2-ethanesulfonic acid) PIPES; pH 6.4–1 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA)–1 M NaCl for 100 min at 65°C. The hybrids were then digested with a mixture of RNase A (2.5 U/ml) and RNase T1 (100 U/ml) for 30 min at room temperature. The reaction was terminated, and protected fragments were precipitated as instructed by the manufacturer of the RPAII kit. Protected fragments were run along a sequencing reaction on an 8 M urea–6% acrylamide gel and visualized by autoradiography. A PCR strategy was used to specifically amplify the 5′ end of the pfs25 mRNA, a method often referred to as RACE (rapid amplification of cDNA ends), as specified by the manufacturer of the 5′ Amplifinder RACE kit (Clontech). cDNA was synthesized by reverse transcription from 10 μg of P. falciparum gamete RNA with gene-specific primer GCTAAGTTGAATGAAAAGG. After ligation by T4 RNA ligase of an anchor sequence (GGAGACTTCCAAGGTCTTAGCTATCACTTAAGCAC) to the single-stranded cDNA, the 5′ end of the pfs25 RNA was amplified by PCR using gene-specific nested primer CTATATTGAAGTTTATAAAAACGAC and anchor primer CTGGTTCGGCCCACCTCTGAAGGTTCCAGAATCGATAG. The amplification products were digested with EcoRI, cloned in an EcoRI/SmaI-digested pBluescript SK− vector (Stratagene), and introduced into electrocompetent E. coli SURE cells (Stratagene). Ten individual transformants were selected for sequence analysis.
EMSA.
Nuclear extracts were prepared as described in reference 21. Probes for electrophoretic mobility shift assays (EMSAs) were generated from RsaI/SspI/DraI restriction digests of the pfs25 and pfs16 upstream regions derived from plasmids pK4 and pK16.3, respectively. The individual restriction fragments were supplemented with EcoRI linkers and introduced in an EcoRI-digested pBluescript KS− vector (Stratagene). Fragments were excised from the vector with EcoRI, gel purified, and labeled by filling in the overhanging ends with Klenow polymerase in the presence of [α32P]dATP. Ten femtomoles of end-labeled probe was combined with nuclear extract in 20 mM HEPES (pH 7.9)–100 mM KCl–1 mM EDTA–1 mM dithiothreitol–1 μg of poly(dA-dT) · poly(dA-dT)–4% Ficoll for 15 min at room temperature. Binding reactions were supplemented with specific competitor DNA fragments as indicated in the figure legends and analyzed on a 6% polyacrylamide gel in 0.25× TBE (1× TBE is 0.1 M Tris Base, 0.1 M boric acid, and 2 mM EDTA).
Nucleotide sequence accession numbers.
Sequence data have been submitted to the GenBank database under accession no. AF034389 (pfs16) and AF030628 (pfs25).
RESULTS
Structure of the pfs16 and pfs25 upstream regions.
To assess whether the developmental regulation of Pfs16 and Pfs25 expression is mediated by DNA elements located upstream of the respective genes, and to enable a subsequent characterization of the elements controlling the expression, we set out to isolate the pfs16 and pfs25 upstream regions. For the pfs16 gene, a plasmid with a 1,461-bp insert was obtained. Sequence analysis revealed that this plasmid contains 710 bp upstream of the ATG start codon of the pfs16 gene. The sequence of the pfs25 5′ flanking region was determined from a 3.5-kb genomic HindIII fragment which contains 1,006 nucleotides upstream of the ATG translational start codon. Inspection of the pfs16 and pfs25 upstream regions reveals that both are extremely A/T rich (Fig. 2). The A/T content of the pfs25 upstream region is 85% and reaches 90% in the pfs16 5′ flanking sequence. In both promoters, the A/T richness is characterized by a striking abundance of long homopolymeric (dA:dT) and alternating poly(dA-dT) stretches, a common feature of P. falciparum intergenic regions (12). Further comparison of the two sequences did not reveal any other regions of homology. Comparison of the pfs16 upstream region with sequences in the EPD database of eukaryotic promoter elements (8) revealed two elements with homology to the binding site of the yeast MATα2 transcription factor (Fig. 2A). This homeobox protein binds as a homodimer to two indirectly repeated half sites separated by a 13-nucleotide spacer (46). The elements found in the pfs16 upstream region have an analogous structure with the exception that the repeated sequences are in a direct orientation. The pfs25 upstream region contains numerous TATA boxes, as does the pfs16 upstream region, and a CAAT box (position −571 in Fig. 2B). No additional elements that relate to sequences present in the EPD database were found in the pfs16 and pfs25 upstream regions.
FIG. 2.
(A) Nucleotide sequence of the pfs16 upstream region. Numbering is with respect to the ATG translation initiation codon, which is italicized. The transcription start site as determined by RNase protection is indicated with an arrow. The starting positions of deletion mutants used in transfection studies are indicated in bold and above the sequence. Sequence elements with homology to the binding site of the yeast MATα2 homeobox transcription factor are in bold and overlined. (B) Nucleotide sequence of the pfs25 upstream region. Numbering is with respect to the ATG translation initiation codon (italicized). Arrows indicate positions of the transcription initiation sites. The starting positions of deletion mutants of plasmid pFS25LUC are indicated in bold and above the sequence. The AAGGAATA sequences that serve as the recognition sites for the PAF-1 transcription factor identified in this work are underlined and in bold. A CCAAT box is overlined.
Determination of the transcription initiation sites of the psf16 and pfs25 genes.
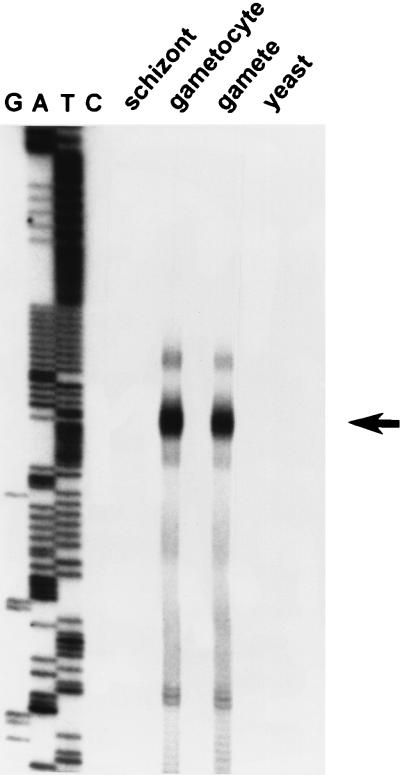
As an initial step in the characterization of the pfs16 and pfs25 promoters, we mapped the transcription initiation sites of the respective genes. The initiation site of the pfs16 gene was analyzed by RNase protection. As shown in Fig. 3, hybridization of a pfs16-specific probe to RNA of gametocytes and gametes results in the protection of fragments that cluster in a discrete region that spans approximately 10 nucleotides and that is located 175 nucleotides upstream of the ATG startcodon. The protected fragments most likely represent a single transcription initiation site, as the observed minor heterogeneity is frequently associated with this type of experimental approach. No protection of the probe was found when an RNA preparation of the schizont stages was used, which is in agreement with the observation that these stages do not express the pfs16 mRNA (13).
FIG. 3.
Analysis of the transcription initiation site of the pfs16 gene. RNase protection was performed on RNA of the different developmental stages of P. falciparum and on yeast RNA as indicated. An arrow indicates the position of the major protected fragment. The letters G, A, T, and C indicate the products of a sequencing reaction that was run along the protected fragments for size determination. Note that the sequence depicted in the figure does not contain a single cytosine base.
To analyze the transcriptional start sites of the pfs25 gene, we used a RACE procedure. The sequences of the amplified products recovered were colinear with the genomic sequence and heterogeneous in their 5′ ends. Figure 2b shows a compilation of the transcription initiation sites on the basis of the 10 independent RACE products analyzed. The initiation sites cluster in an 18-nucleotide region located 267 nucleotides upstream of the ATG translation initiation codon. Of the 10 RACE products, 5 indicated initiation at position −267.
The psf16 and psf25 upstream regions contain differentially regulated promoters.
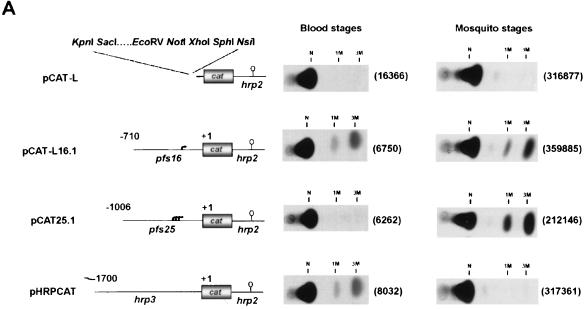
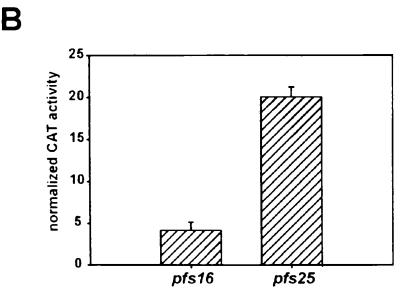
We assessed whether the extremely A/T rich pfs16 and pfs25 upstream regions contain functional promoters. To this end, these sequences were fused to cat reporter genes, the resulting plasmids were introduced into blood- and mosquito-stage parasites by transfection, and CAT activities were determined. As a control, we tested the activity of the hrp3 promoter, which has been described previously (47). For transfection of the blood stages of the parasite, preparations of P. falciparum were used. As the mosquito stages of P. falciparum are neither transfectable nor viable in an in vitro culture, we used P. gallinaceum parasites for the transfection of the mosquito stages (45). The data presented in Figure 4A show that the three promoters show distinct patterns of transcriptional activities. Whereas the pfs16 upstream sequence drives the expression of the cat reporter gene in both the mosquito- and blood-stage preparations, the activity of the pfs25 promoter is restricted to mosquito-stage parasites. Conversely, the activity of the hrp3 promoter peaks in blood-stage parasites but is not detectable in mosquito stages. A quantitative comparison of the activities of the pfs16 and pfs25 promoters in mosquito-stage parasites showed that the pfs25 promoter exhibits an activity approximately fivefold higher than the transcriptional activity of the pfs16 promoter (Fig. 4B). Thus, the transcriptional activities of the pfs16 and pfs25 promoters are quantitatively and qualitatively distinct. The data suggest that activation of the pfs25 promoter marks the transition of the parasite to the mosquito midgut whereas transcription from the pfs16 promoter is activated in the blood stages of the parasite.
FIG. 4.
A. Transfections of mosquito- and blood-stage parasites with pfs16, pfs25, and hrp3 promoter-reporter constructs. CAT signals from P. gallinaceum mosquito- and P. falciparum blood-stage parasites transfected with the plasmids schematically depicted at the left side. Arrows indicate the transcriptional start sites of the pfs16 and pfs25 genes; open circles represent the transcription termination signals provided by the hrp2 sequences. The positions at which the unacetylated (N) and monoacetylated (1M and 3M) forms of [14C]chloramphenicol migrate are indicated. Plasmids p49.20 (P. gallinaceum mosquito-stage transfections) and pA0 (P. falciparum blood-stage transfections) were cotransfected to affirm the success of the transfection, and the numbers in parentheses indicate the relative luciferase activities induced by these plasmids. (B) Comparison of the transcriptional activities of the pfs16 and pfs25 promoters. P. gallinaceum mosquito-stage parasites were transfected with plasmids pCAT-L16.1ΔSX and pCAT25.1. CAT activities were normalized to the luciferase activity induced by cotransfected plasmid p49.20.
The activity of the pfs16 promoter is restricted to the sexual stages.
At the onset of gametocytogenesis, biochemical differentiation precedes morphological differentiation. Initially, sexually committed ring stages are morphologically indistinguishable from asexual ring-stage parasites (Fig. 1) (32). However, they are marked by the induction of the transcriptional activity of the pfs16 gene, which is the earliest event in the sexual differentiation process described to date (13). Following the accumulation of the pfs16 mRNA, the sexually committed ring stages transform into the readily recognizable stage II gametocytes (20). Our data indicate that the pfs16 promoter can drive the expression of a reporter gene in transfection of an asynchronous culture of the blood stages of the parasite (Fig. 4A). Such a culture contains both asexual and sexual parasites, and the experiment depicted in Fig. 4A does not clarify which of the subpopulations is responsible for the pfs16-driven expression of the reporter gene. To corroborate and extent the notion that the activity of the pfs16 gene is restricted to parasites undergoing sexual development, we analyzed the activity of the pfs16 promoter in cultures undergoing sexual differentiation. Given the finding that the transcriptional activity of the hrp3 gene is restricted to asexual parasites (43) and that the hrp3 promoter is silent in the mosquito stages (Fig. 4A), we used the hrp3 promoter as a specific marker for the asexual population of parasites. P. falciparum blood-stage parasite cultures were transfected with hrp3 and pfs16 promoter-reporter constructs, and gametocytogenesis was induced by omitting the supply of fresh erythrocytes (36). Figure 5A shows the parasitemias during the course of the experiment together with the temporal activities of the hrp3 and pfs16 promoters. The asexual parasitemia initially rises and eventually shows a slight decline. The high asexual parasitemia at day 2 triggers gametocytogenesis, as demonstrated by the appearance of stage 2 gametocytes at day 4. The pattern of activity of the hrp3 promoter parallels the asexual parasitemia and persists throughout the course of the experiment. The activity of the pfs16 promoter coincides with the appearance of sexually committed ring-stage parasites in the culture. Thereafter, higher levels of reporter enzyme, driven by the pfs16 promoter, accompany the increase in numbers of gametocytes.
FIG. 5.
Activities of the pfs16 and hrp3 promoters during sexual differentiation of P. falciparum. (A) Asynchronous cultures of P. falciparum were transfected at day 0 with plasmids pHLH and pCAT-L16.1, and reporter activities were monitored over time. The upper graph shows the activities of the pfs16 and hrp3 promoters; the lower graph shows the parasitemia of the culture, discriminating between asexual parasites, gametocytes (gct), and sexually committed ring stages (cr). The number of sexually committed ring stages was inferred from the number of stage II gametocytes appearing 48 h later (7). (B) Cultures were transfected with plasmid pHLH or pLUC16.1 at day 0 and treated with pyrimethamine from days 4 to 7. Error bars indicate standard deviations calculated from three independent experiments.
To assess whether the developing gametocytes are indeed responsible for the observed activity of the pfs16 promoter, transfected cultures were treated with pyrimethamine, which eliminates asexual parasites and sexually committed ring stages but leaves gametocytes that have passed developmental stage I unaffected (36). As a consequence, the activity of the pfs16 promoter is slightly reduced but still persists at a high level (Fig. 5B). By contrast, the pyrimethamine treatment completely abrogates the asexual parasitemia and the activity of the hrp3 promoter. The sharp contrast in the effect of pyrimethamine on the activities of the pfs16 and hrp3 promoters clearly indicates that these reside in different subpopulations of the culture. The pyrimethamine treatment completely abolishes the hrp3-driven expression of the reporter gene, indicating that the activity of the hrp3 promoter is restricted to asexual parasites. The activity of the pfs16 promoter persists at a high level, indicating that this promoter is active in the developing gametocytes.
Transcriptional activity of the pfs16 promoter continues in the parasite stages that invade the mosquito midgut.
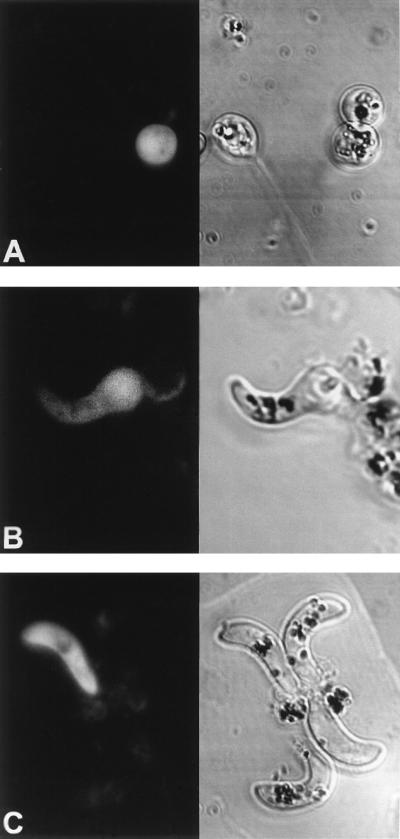
The functional roles of the Pfs16 protein in the sexual differentiation process and the penetration of the mosquito midgut remain elusive. Moreover, data on the expression pattern of Pfs16 are ambiguous. Some studies have reported that Pfs16 is synthesized by gametes and is present on the outer membrane of the gametes (31, 33). Other studies have, however, failed to detect the protein in the gametes and have shown that Pfs16 apparently associated with the gamete membrane is in fact attached to remainders of the parasitophorous vacuole membrane of the gametocytes (2, 6). These remainders eventually are completely shed from the surface of the gamete. Nuclear run-on analysis has shown that the pfs16 gene is transcriptionally active in gametes (13). Furthermore, our data show that the pfs16 promoter is active in the parasite stages that develop within the mosquito midgut (Fig. 4). To specify the pattern of the activity of the pfs16 promoter in the mosquito stages, we visualized this activity by using the gene encoding the GFP of the jellyfish Aequorea victoria as a reporter. Figure 6 shows the GFP expression pattern in the developing midgut stages. The pfs16 promoter drives GFP expression both in unfertilized gametes and in the developing and mature ookinetes. As the morphological differentiation of ookinetes preceded the appearance of the GFP signal, we conclude that the ookinetes were actively synthesizing the GFP protein and did not show fluorescence as a result of an accumulated pool of GFP originating from the gametes. These results lend support to the notion that the pfs16 gene is transcriptionally active in the mosquito stages of the parasite and demonstrate that the pfs16 promoter can drive the expression of foreign genes in the parasite stages that are involved in the invasion of the mosquito midgut epithelium.
FIG. 6.
GFP expression driven by the pfs16 promoter in P. gallinaceum mosquito midgut stages. Fluorescence (left) and light transmission (right) images are shown. Gametocytes ingested by a mosquito transform into gametes (A [female gamete]) that fertilize and develop into invasive ookinetes (C) via the intermediate retort stages (B). Parasites that had successfully been transfected with plasmid pGFP16.2 show a bright fluorescence signal (left).
Deletion mapping of the psf16 and psf25 promoters.
To gain insight in the DNA elements involved in the expression of the pfs16 and pfs25 genes, plasmids in which the reporter genes were placed under the control of a series of truncated promoter fragments were transfected to P. gallinaceum mosquito-stage parasites. The data presented in Figure 7 indicate that the transcriptional activity of the pfs16 promoter relies on different components. The −247 to +1 region of the promoter is the shortest region tested here that drives transcription of the reporter gene. Deletion of the sequences between −247 and −209 from this promoter abrogates its activity. The apparent importance of this region for high transcriptional activity and its close proximity to the start site of transcription suggest that this region constitutes the pfs16 core promoter. The region immediately upstream of these sequences, from −247 to −388, is silent with respect to the modulation of transcriptional activity. The sequences upstream of position −388 contribute to the overall efficacy of the promoter but do not contain dominant negative or positive transcriptional control elements. Finally, the results suggest the presence of an activator sequence in the region between nucleotides +1 and −93. However, as this region is located downstream of the transcriptional start site, its deletion affects the transcribed RNA. Hence, the observed lowered CAT activity may be due to a decreased stability or translation efficiency of the mRNA.
FIG. 7.
Deletion mapping of the pfs16 and pfs25 promoters. P. gallinaceum mosquito-stage parasites were transfected with the constructs schematically depicted at the left side. (A) Deletion mapping of the pfs16 promoter. CAT activities were normalized to the luciferase activity induced by cotransfected plasmid p49.20. (B) Deletion mapping of the pfs25 promoter. Luciferase activities were normalized to the CAT activity of cotransfected pCAT-L16.1ΔSX.
The transfection data do not support a functional role for the two elements with homology to the DNA recognition site of the yeast MATα2 repressor that are present in the pfs16 promoter (Fig. 2A). Deletion of either one of these elements does not affect the activity of the promoter (compare the transcriptional activities of exo6 and exo7 and of pCAT-L16.2 and pCAT-L16.3 in Fig. 7). In addition, mutations in the single MAT-like element present in plasmid exo7 do not affect the transcriptional activity of this plasmid in transfections of blood- or mosquito-stage parasites (data not shown).
Mutational analysis of the pfs25 promoter was performed on truncated versions of plasmid pPFS25LUC, which contains a luciferase gene under control of the pfs25 promoter and pfs25 3′ processing signals. The structures of the deletion mutants are schematically depicted in Fig. 7 together with the luciferase activities obtained after transfection of P. gallinaceum mosquito-stage parasites. Deletion from positions −1006 to −959 reduces the activity of the pfs25 promoter to 60% of the activity of the full-length promoter. Further deletions up to position −542 marginally affect the transcriptional activity. The most dramatic effects on the activity of the promoter are seen with next two extended deletions: deletion of the region between positions −542 and −484 results in a 10-fold reduction of the activity of the promoter activity, and further deletion up to position −386 fully abolishes the activity of the promoter. In conclusion, the region between −484 and +1 is the shortest region tested here that drives transcription of the reporter gene. Equipping this region with the region between −484 and −542 potently activates transcription. Finally, the regions upstream of position −542 contribute to an efficient activity of the pfs25 promoter.
The pfs25 upstream region is the target of protein-DNA interactions.
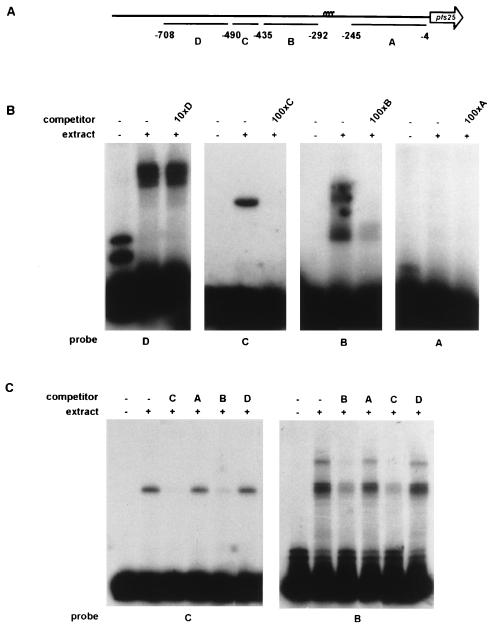
To gain insight in the possible trans-acting factors that play a role in the regulation of transcription of the pfs16 and pfs25 genes, we performed a series of EMSAs. Restriction fragments derived from the pfs16 and pfs25 upstream regions were scanned for the presence of binding sites for proteins present in a nuclear extract of P. gallinaceum gametes. Pilot experiments failed to reveal any specific interactions between DNA-binding proteins and the pfs16 promoter. By contrast, three of the four pfs25-specific probes tested yielded DNA-protein complexes when incubated with a nuclear extract, as shown in Fig. 8A and B. Probe C forms a single complex, indicating a single interaction with a DNA-binding protein. Probes B and D form multiple complexes, revealing a more complex set of interactions on these probes.
FIG. 8.
The pfs25 promoter is a target for DNA-binding proteins. (A) Schematic representation of locations of the probes used in EMSAs. (B) EMSAs with probes A through D and a nuclear extract derived from P. gallinaceum gametes. Competitions included molar excesses of unlabeled probes A through D as indicated. The competition with probe D accidentally was with a 10-fold instead of the intended 100-fold molar excess. (C) Cross-competition assays. Radiolabeled probes B and C were incubated with the nuclear extract. Competitions included 100-fold molar excesses of probes A through D as indicated.
A cross-competition experiment with probe C as the radiolabeled probe reveals that a 100-fold molar excess of probe B disrupts the interaction of probe C with the nuclear factor, whereas probes A and D cannot prevent the interaction between probe C and the nuclear factor (Fig. 8C). Furthermore, a 100-fold molar excess of probe C prevents the formation of a DNA-protein complex on probe B, whereas probes A and D have no effect on complex formation (Fig. 8C). These observations suggest that the nuclear factor that binds to probe C does not have a preference for generally A/T-rich DNA but obeys more specific sequence constraints that are shared by probe B. Close inspection of the sequences of probes B and C reveals that they indeed share a DNA element, AAGGAATA, that is found neither in probes A and D or in other regions of the pfs25 promoter.
AAGGAATA recruits a mosquito-stage-specific transcription factor.
To test whether the AAGGAATA element constitutes a functional promoter element, a mutant version of this element was tested in transfection studies and EMSAs. The data presented in Fig. 9A show that oligonucleotide TFB25, which contains the AAGGAATA sequence, recruits a DNA-binding protein when incubated with a nuclear extract derived from P. gallinaceum gametes. The interaction can be disrupted by the addition of a 100-fold molar excess of the unlabeled oligonucleotide but not by the addition of an oligonucleotide containing a mutated version of this sequence motif. Accordingly, oligonucleotide TFB25 can compete for the interaction between probe C and the DNA-binding protein, whereas the mutant oligonucleotide cannot (Fig. 9C). These results indicate that AAGGAATA sequence serves as the recognition site for a DNA-binding protein, which we named PAF-1 (pfs25-activating factor 1 [see below]).
FIG. 9.
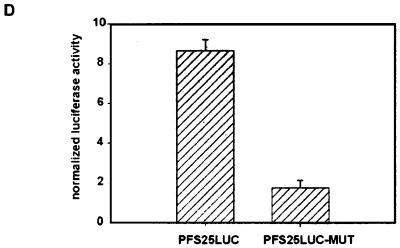
The AAGGAATA elements in the pfs25 promoter recruit a mosquito stage-specific DNA-binding protein that activates transcription. (A) Oligonucleotide TFB25 (AATTCATAAGGAATATAG and its complement AATTCTATATTCCTTATG) was incubated with a nuclear extract derived from P. gallinaceum (g) or P. falciparum (Pfg) gametes or from an asynchronous P. falciparum blood-stage culture that contained both asexual parasites and gametocytes (Pfb). Competitions included either a 100-fold molar excess of the unlabeled oligonucleotide TFB25 or a 100-fold molar excess of an oligonucleotide containing a mutant version of the putative binding site (MUT; AATTCATAAGGCCGCTAG and its complement AATTCTAGCGGCCTTATG). (B) Control on the activity of the P. falciparum blood-stage nuclear extract. Radiolabeled oligonucleotide KAHRP (30) was incubated with a nuclear extract of the blood stages of P. falciparum parasites. The unlabeled oligonucleotide was added as a competitor DNA as indicated. (C) Radiolabeled probe C was incubated with a nuclear extract derived from P. gallinaceum gametes. A batch of nuclear extract different from that used in the experiments depicted in panel A was used, which resulted in the formation of an additional nonspecific complex, indicated by an asterisk. Binding reactions were supplemented with specific competitor DNAs as indicated. (D) Transfection of P. gallinaceum mosquito-stage parasites with plasmid pPFS25LUC and a version of the plasmid in which the two AAGGAATA elements were mutated to AAGGCCGC (pPFS25-LUC-MUT). Plasmids were cotransfected with pCAT-L16.1ΔSX, and luciferase activities were normalized to CAT activities. Values are from three transfections; error bars indicate standard deviations.
We assessed whether PAF-1 is a developmental-stage-specific protein. To this end, the radiolabeled oligonucleotide TFB25 was incubated with nuclear extracts derived from either P. gallinaceum or P. falciparum gametes or from an asynchronous P. falciparum blood-stage parasite culture that contained both asexual parasites and gametocytes. The results shown in Fig. 9A indicate that extracts derived from P. falciparum or P. gallinaceum gametes contain a DNA-binding protein that specifically interacts with the TFB25 oligonucleotide. In contrast, no PAF-1-like activity is present in the nuclear extract derived from P. falciparum blood-stage parasites. As a control on the activity of the blood-stage extract, it was incubated with radiolabeled probe KAHRP, which resulted in the formation of two DNA-protein complexes as described previously (Fig. 9B) (30). We conclude that PAF-1 is a mosquito-stage-specific factor.
Finally, the two copies of the AAGGAATA element within the context of the full-length pfs25 promoter were mutated, and the activity of the mutant promoter was compared to that of the wild-type promoter. Transfections of P. gallinaceum mosquito-stage parasites showed that the activity of the promoter is significantly reduced by the mutations (Fig. 9D). We conclude, therefore, that the AAGGAATA sequence comprises a cis-acting element involved in the activation of the pfs25 promoter. The correlation between a decline in promoter activity and a decreased affinity for PAF-1 upon mutation of the AAGGAATA sequence indicates that PAF-1 is a transcriptional activator that is recruited to the promoter by the AAGGAATA sequence.
DISCUSSION
Sexual differentiation is an obligate part of the life cycle of malaria parasites. It requires a series of developmental decisions to be made during progression from the asexually replicating parasites in the bloodstream of the vertebrate host to the highly specialized cells that invade the mosquito midgut. First, a subpopulation of asexual parasites commits to sexual differentiation; second, the sex of the developing gametocytes is determined; finally, gametogenesis is induced when the arrival of the gametocytes in the mosquito midgut is sensed. A complex network of gene-regulatory events governs the transitions through the sexual differentiation process, as indicated by developmental-stage-specific expression of the pfs16 and pfs25 genes. This paper links the regulatory events operating on the pfs16 and pfs25 promoters to two important developmental switches exhibited by the parasite. Activation of the pfs16 promoter marks commitment to sexual differentiation, whereas the induction of the pfs25 promoter is indicative for the transition to the mosquito midgut. In addition, our data show that the hrp3 gene, which is expressed in asexual parasites, is shut off when parasites commit to sexual differentiation.
A functional analysis of the mechanisms of gene expression underlying sexual development of the malaria parasite has for long been impeded by the lack of a transfection protocol and by the laborious culture of the sexual stages. Recently, transfection methods for the blood stages of P. falciparum have been described (14, 47). However, the in vitro culture and transfection of the mosquito stages of P. falciparum is still not feasible. An alternative is offered by the avian malaria parasite P. gallinaceum, which has been proven to be a versatile model system for the study of sexual differentiation of malaria parasites (16, 45). As P. falciparum and P. gallinaceum are very closely related and are thought to have arisen by lateral transfer between the human and the avian host (41), one might assume that the elements that control gene expression are conserved and interchangeable. Functional conservation has been observed for the P. falciparum hrp3 and the P. berghei dhfr promoters in P. knowlesi (42), for the P. chabaudi dhfr promoter in P. falciparum (10), and for the P. falciparum hsp86 and pcna promoters in P. gallinaceum (11a). P. gallinaceum possesses a close homologue of the Pfs25 protein, termed Pgs25 (28). The regions flanking the pgs25 gene have not been cloned, and a direct comparison of the pfs25 and pgs25 promoters awaits the isolation of the latter sequence. Nonetheless, the results presented here corroborate the notion that the factors required for basal and activated transcription indeed are conserved between the Plasmodium species. Accordingly, the PAF-1 DNA-binding activity was observed in nuclear extracts derived both from P. gallinaceum and from P. falciparum gametes.
Gene regulation is atypical in many parasitic protozoa. Genes of the Kinetoplastida (e.g., Leishmania and Trypanosoma species) seem to be transcribed by a class I RNA polymerase and produce long polycistronic RNA precursors, which are processed into mature RNAs via trans-splicing (35). There is no evidence for polycistronic transcription, trans-splicing, or extensive posttranscriptional regulation of gene expression within the phylum of the Apicomplexa (e.g., Plasmodium spp. and Toxoplasma spp.). Nevertheless, the structure of the gene promoters of organisms of the latter phylum is atypical. Although the deletion mapping of the pfs16 and pfs25 promoters suggests that these promoters resemble typical eukaryotic polymerase II-transcribed promoters, with a core promoter region and more distally located enhancers, the primary structure of the pfs16 and pfs25 promoters is atypical. The extreme A/T richness seems to preclude the assignment of a functional TATA box. Accordingly, analysis of promoters of apicomplexan parasites has revealed that a TATA box is not a conserved promoter element in these organisms (4, 11). Conversely, a repeated (A/T)GAGACG element, which acts as a selector of the transcriptional start site, has been identified as a critical element of many Toxoplasma gondii promoters (4). This element is absent in the pfs16 and pfs25 promoter regions. Our data furthermore indicate that the few elements that do bear homology to known eukaryotic promoter elements, such as the MATα2-like elements in the pfs16 promoter and the CCAAT box in the pfs25 promoter, are not essential for a high transcriptional activity. That P. falciparum promoters are functionally distinct from other eukaryotic promoters is further substantiated by the observation that the pfs16, pfs25, and hrp3 promoters do not function in transfections of mammalian cells (11a). Conversely, viral promoters such as the simian virus 40 promoter do not operate in P. falciparum (22).
Although we failed to identify specific cis- and trans-acting factors operating on the pfs16 promoter, our data show that its mode of action is developmental stage specific. The activity of the pfs16 promoter persists during pyrimethamine treatment, demonstrating that the gametocytes are responsible for the observed activity of the pfs16 promoter in transfections of blood-stage parasites. The pfs16-driven signal of the reporter gene appears at the time at which sexually committed ring-stage parasites appear in the culture, indicating that the pfs16 promoter is induced at the very onset of sexual differentiation. Accordingly, elimination of the sexually committed ring-stage parasites from the culture, a consequence of the pyrimethamine treatment, lowers the level at which the reporter enzyme accumulates. Our results indicate that the hrp3 promoter is silent during gametocytogenesis, as its activity is not observed under pyrimethamine pressure. These results extend the notion that the commitment to sexual differentiation involves the switching to an alternate program of gene expression (1). Asexual genes such as the hrp3 gene are shut off during gametocytogenesis, whereas specific sex genes, such as pfs16, are turned on. The exact nature of the signal that triggers a parasite to commit to sexual development is unknown (1), although it is well documented that the parasite density is an important determinant (5, 7). Our data substantiate the previous finding that the pfs16 gene is activated in sexually committed parasites at the very onset of the sexual differentiation process (13) and indicate that the activation of this gene is immediately downstream of the trigger that activates gametocytogenesis. The early expression of Pfs16 suggests that this protein plays an important role during gametocytogenesis.
Gametocytogenesis ultimately leads to the production of mature gametocytes, which circulate in the bloodstream and remain infective to the mosquito for several weeks (38). Once gametocytes are ingested by a mosquito, their entrance in the midgut induces an array of mutual responses. The drop in temperature following transmission to the poikilothermic mosquito activates gametocytes to produce male and female gametes, a process which is further enhanced by a specific factor in the mosquito midgut (15), recently identified as xanthurenic acid (3). The arrival of the parasites in the mosquito midgut triggers the immune response of the mosquito (37) and induces the expression of mosquito trypsins that help to digest the blood meal (34). The trypsins together with the immune factors provide a hostile environment to the parasite, and ookinetes that do not succeed in penetrating the midgut epithelium are rapidly degraded (17). The key to the escape route through the midgut epithelium is provided by the Pfs25 protein, which appears on the surface of the zygote within 30 min following the arrival of the blood meal in the midgut (18). Antibodies against this protein completely block penetration of the epithelium, suggesting a requirement for this molecule for penetration (40). The pfs25 promoter described here fulfills the need for an immediate expression of the Pfs25 protein. Our data indicate, in agreement with previous data (13), that the pfs25 promoter is silent during asexual growth and gametocytogenesis but activated during gametogenesis. The activation relies in part on the DNA-binding protein PAF-1, which is specific for the mosquito stages of the parasite. Hence, expression of PAF-1 is part of the response of the parasite to the dramatic change in environment following transmission. The recognition site for PAF-1 is novel and is not found in the database of eukaryotic promoter elements (8). Interestingly, the recognition site for PAF-1 is also present in the P. falciparum hsp86 promoter but absent in the hrp3 promoter. Accordingly, the hrp3 promoter is active following transmission to the mosquito (11a), whereas the hrp3 promoter is not (Fig. 4).
The data presented in this paper constitute the first detailed description of cis- and trans-acting elements in Plasmodium. Future analysis of the details of the transcriptional activation of the pfs16 and pfs25 promoters will yield further insight in the mechanisms underlying the sexual differentiation process and might provide new targets for transmission-blocking agents. In addition, the promoters identified and characterized in this study allow the expression of foreign genes in the parasite stages that invade the mosquito midgut and hence provide invaluable tools for the study of parasite-vector interactions.
ACKNOWLEDGMENTS
We gratefully acknowledge Yimin Wu for supplying plasmids pHRPCAT, pHLH, and pA0, Brendan Cormack for providing GFP plasmids, and David Kaslow for the gift of pNF4.13. We thank Jeffrey VanWye for communicating unpublished results on GFP expression in Plasmodium and Patrick van den Boogaard for expert technical assistance.
This investigation received financial support from the Netherlands Ministry for Development Cooperation (grant NL002701), from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and from the Commission of the European Community for Life Sciences and Technologies for Developing Countries. K.J.D. is grateful to the Harald Quintus Bosz Foundation and the Netherlands Organization for the Advancement of Pure Research (NWO) for gifts that covered travel expenses.
Footnotes
We dedicate this paper to the memory of the late Ruud Konings.
REFERENCES
- 1.Alano P, Carter R. Sexual differentiation in malaria parasites. Annu Rev Microbiol. 1990;44:429–449. doi: 10.1146/annurev.mi.44.100190.002241. [DOI] [PubMed] [Google Scholar]
- 2.Baker D A, Daramola O, McCrossan M V, Harmer J, Targett G A T. Subcellular localization of Pfs16, a Plasmodium falciparum gametocyte antigen. Parasitology. 1994;108:129–137. doi: 10.1017/s0031182000068219. [DOI] [PubMed] [Google Scholar]
- 3.Billker O, Lindo V, Panico M, Etienne A E, Paxton T, Dell A, Rogers M, Sinden R E, Morris H R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 4.Bohne W, Wirsing A, Gross U. Bradyzoite-specific gene expression in Toxoplasma gondii requires minimal genomic elements. Mol Biochem Parasitol. 1997;85:89–98. doi: 10.1016/s0166-6851(96)02814-9. [DOI] [PubMed] [Google Scholar]
- 5.Bruce M C, Alano P, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 6.Bruce M C, Carter R N, Nakamura K, Aikawa M, Carter R. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 7.Carter R, Miller L H. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull W H O. 1979;57:37–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Cavin R, Junier T, Bucher P. The eukaryotic promoter database EPD, release 50. Epalinges s/Lausanne, Switzerland: Swiss Institute for Experimental Cancer Research; 1997. [Google Scholar]
- 9.Chutmongkonkul M, Maier W A, Seitz H M. A new model for testing gametocidal effects of some antimalarial drugs on Plasmodium falciparum in vitro. Ann Trop Med Parasitol. 1992;86:207–215. doi: 10.1080/00034983.1992.11812656. [DOI] [PubMed] [Google Scholar]
- 10.Crabb B S, Cowman A F. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dechering K J. Gene regulation during sexual development of the human malaria parasite Plasmodium falciparum. Nijmegen, The Netherlands: University of Nijmegen; 1998. [Google Scholar]
- 11a.Dechering, K. J. Unpublished results.
- 12.Dechering K J, Cuelenaere K, Konings R N H, Leunissen J A M. Distinct frequency-distributions of homopolymeric DNA tracts in different genomes. Nucleic Acids Res. 1998;26:4056–4062. doi: 10.1093/nar/26.17.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechering K J, Thompson J, Dodemont H J, Eling W, Konings R N H. Developmentally regulated expression of pfs16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 1997;89:235–244. doi: 10.1016/s0166-6851(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 14.Fidock D A, Wellems T E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia G E, Wirtz R A, Rosenberg R. Isolation of a substance from the mosquito that activates Plasmodium fertilization. Mol Biochem Parasitol. 1997;88:127–135. doi: 10.1016/s0166-6851(97)00086-8. [DOI] [PubMed] [Google Scholar]
- 16.Goonewardene R, Daily J, Kaslow D, Sullivan T J, Duffy P, Carter R, Mendis K, Wirth D. Transfection of the malaria parasite and expression of firefly luciferase. Proc Natl Acad Sci USA. 1993;90:5234–5236. doi: 10.1073/pnas.90.11.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouagna L C, Mulder B, Noubissi E, Tchuinkam T, Verhave J P, Boudin C. The early sporogonic cycle of Plasmodium falciparum in laboratory-infected Anopheles gambiae: an estimation of parasite efficacy. Trop Med Int Health. 1998;3:21–28. doi: 10.1046/j.1365-3156.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 18.Gouagna L C, Sinden M, Kieboom J, Kroneman R, Verhave J P. Kinetics and efficiency of Plasmodium falciparum development in the midguts of Anopheles gambiae, An. funestus, and An. nili. Ann Trop Med Parasitol. 1998;92:115–118. doi: 10.1080/00034989860247. [DOI] [PubMed] [Google Scholar]
- 19.Guinet F, Dvorak J A, Fujioka H, Keister D B, Muratova O, Kaslow D C, Aikawa M, Vaidya A B, Wellems T E. A developmental defect in Plasmodium falciparum male gametogenesis. J Cell Biol. 1996;135:269–278. doi: 10.1083/jcb.135.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawking F, Wilson M E, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe-Seyler F, Butz K, Rittmueller C, von Kneber Doeberitz M. A rapid microscale procedure for the simultaneous preparation of cytoplasmic RNA, nuclear DNA binding proteins and enzymatically active luciferase extracts. Nucleic Acids Res. 1991;19:5080. doi: 10.1093/nar/19.18.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horrocks P, Dechering K J, Lanzer M. Control of gene expression in P. falciparum. Mol Biochem Parasitol. 1998;95:171–181. doi: 10.1016/s0166-6851(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 23.Jacobberger J W, Horan P K, Hare J D. Cell cycle analysis of asexual stages of erythrocytic malaria parasites. Cell Prolif. 1992;25:431–45. doi: 10.1111/j.1365-2184.1992.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 24.Janse C J, Ponnudurai T, Lensen A H W, Meuwissen J H E T, Ramesar J, Van Der Ploeg M, Overdulve J P. DNA synthesis in gametocytes of Plasmodium falciparum. Parasitology. 1988;96:1–7. doi: 10.1017/s0031182000081609. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow D C, Bathurst I C, Barr P J. Malaria transmission-blocking vaccines. Trends Biotechnol. 1992;10:388–391. doi: 10.1016/0167-7799(92)90280-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaslow D C, Isaacs S N, Quakyi I A, Gwadz R W, Moss B, Keister D B. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991;252:1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 27.Kaslow D C, Quakyi I A, Syin C, Raum M G, Keister D B, Coligan J E, McCutchan T F, Miller L H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;335:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 28.Kaslow D C, Syin C, McCutchan T F, Miller L H. Comparison of the primary structure of the 25 kDa ookinete surface antigens of Plasmodium falciparum and Plasmodium gallinaceum reveal six conserved regions. Mol Biochem Parasitol. 1989;33:283–288. doi: 10.1016/0166-6851(89)90090-x. [DOI] [PubMed] [Google Scholar]
- 29.Kaushal D C, Carter R, Howard R J, McAuliffe F M. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. I. Zygote surface antigens. Mol Biochem Parasitol. 1983;8:53–69. doi: 10.1016/0166-6851(83)90034-8. [DOI] [PubMed] [Google Scholar]
- 30.Lanzer M, de Bruin D, Ravetch J V. A sequence element associated with the Plasmodium falciparum KAHRP gene is the site of developmentally regulated protein-DNA interactions. Nucleic Acids Res. 1992;20:3051–3056. doi: 10.1093/nar/20.12.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo C A, Konings R N, Kumar N. Expression of early gametocyte-stage antigens Pfg27 and Pfs16 in synchronized gametocytes and non-gametocyte and non-gametocyte producing clones of Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:151–154. doi: 10.1016/0166-6851(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 32.Lobo C A, Kumar N. Sexual differentiation and development in the malaria parasite. Parasitol Today. 1998;14:146–150. doi: 10.1016/s0169-4758(97)01210-6. [DOI] [PubMed] [Google Scholar]
- 33.Moelans I I, Meis J F, Kocken C, Konings R N, Schoenmakers J G. A novel protein antigen of the malaria parasite Plasmodium falciparum, located on the surface of gametes and sporozoites. Mol Biochem Parasitol. 1991;45:193–204. doi: 10.1016/0166-6851(91)90086-l. [DOI] [PubMed] [Google Scholar]
- 34.Mueller H M, Crampton J M, della Torre A, Sinden R, Crisanti A. Members of a trypsin gene family in Anopheles gambiae are induced in the gut by blood meal. EMBO J. 1993;12:2891–2900. doi: 10.1002/j.1460-2075.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pays E, Vanhamme L, Berberof M. Genetic controls for the expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. doi: 10.1146/annurev.mi.48.100194.000325. [DOI] [PubMed] [Google Scholar]
- 36.Ponnudurai T, Lensen A H W, Meis J F G M, Meuwissen J H E T. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology. 1986;93:263–227. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- 37.Richman A M, Dimopoulos G, Seeley D, Kafatos F C. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J. 1997;16:6114–6119. doi: 10.1093/emboj/16.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smalley M E, Sinden R E. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74:1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- 39.Trager W, Tershakovec M, Lyandvert L, Stanley H, Lanners N, Gubert E. Clones of the malaria parasite Plasmodium falciparum obtained by microscopic selection: their characterization with regard to knobs, chloroquine sensitivity, and formation of gametocytes. Proc Natl Acad Sci USA. 1981;78:6527–6530. doi: 10.1073/pnas.78.10.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermeulen A N, Ponnudurai T, Beckers P J A, Verhave J P, Smits M A, Meuwissen J H E T. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters A P, Higgins D G, McCutchan T F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci USA. 1991;88:3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Well A M, Tomas A M, Kocken C H M, Malhotra P, Janse C J, Waters A P, Thomas A W. Transfection of the primate malaria Plasmodium knowlesi using entirely heterologous constructs. J Exp Med. 1997;185:1499–1503. doi: 10.1084/jem.185.8.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellems T E, Howard R J. Homologues genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci USA. 1986;83:6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellems T E, Panton L J, Gluzman U Y, do Rosario V E, Gwadz R W, Walker-Jonah A, Krogstad D J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990;345:253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- 45.Wirth D F. Drug resistance and transfection in Plasmodium. In: Boothroyd J C, Komuniecki R, editors. Molecular approaches to parasitology. New York, N.Y: Wiley-Liss; 1995. pp. 227–241. [Google Scholar]
- 46.Wolberger C, Vershon A K, Liu B, Johnson A D, Pabo C O. Crystal structure of a MATa2 homeodomain-operator complex suggests a general model for homeodomain-DNA interactions. Cell. 1991;67:517–528. doi: 10.1016/0092-8674(91)90526-5. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Sifri C D, Lei H H, Su X Z, Wellems T E. Transfection of Plasmodium falciparum within human red blood cells. Proc Natl Acad Sci USA. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]