Abstract
Purpose
There is significant variability in the application of positive end-expiratory pressure (PEEP) in patients undergoing invasive mechanical ventilation. There are numerous studies assessing methods of determining optimal PEEP, but many methods, patient populations, and study settings lack high-quality evidence. Guidelines make no recommendations about the use of a specific method because of equipoise and lack of high-quality evidence. We conducted a scoping review to determine which methods of determining optimal PEEP have been studied and what gaps exist in the literature.
Source
We searched five databases for primary research reports studying methods of determining optimal PEEP among adults undergoing invasive mechanical ventilation. Data abstracted consisted of the titration method, setting, study design, population, and outcomes.
Principle findings
Two hundred and seventy-one studies with 17,205 patients met the inclusion criteria, including 73 randomized controlled trials (RCTs) with 10,733 patients. We identified 22 methods. Eleven were studied with an RCT. Studies enrolled participants within an intensive care unit (ICU) (216/271, 80%) or operating room (55/271, 20%). Most ICU studies enrolled patients with acute respiratory distress syndrome (162/216, 75%). The three most studied methods were compliance (73 studies, 29 RCTs), imaging-based methods (65 studies, 11 RCTs), and use of PEEP-FIO2 tables (52 studies, 20 RCTs). Among ICU RCTs, the most common primary outcomes were mortality or oxygenation. Few RCTs assessed feasibility of different methods (n = 3). The strengths and limitations of each method are discussed.
Conclusion
Numerous methods of determining optimal PEEP have been evaluated; however, notable gaps remain in the evidence supporting their use. These include specific populations (normal lungs, patients weaning from mechanical ventilation) and using alternate outcomes (ventilator-free days and feasibility) and they present significant opportunities for future study.
Study registration
Open Science Framework (https://osf.io/atzqc); first posted, 19 July 2022.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12630-024-02871-6.
Keywords: acute respiratory distress syndrome (ARDS), hypoxemic respiratory failure, mechanical ventilation, optimal PEEP, positive end-expiratory pressure (PEEP)
Résumé
Objectif
Il existe une variabilité significative dans l’application de la pression expiratoire positive (PEP) chez les personnes sous ventilation mécanique invasive. De nombreuses études évaluent les méthodes permettant de déterminer la PEP optimale, mais de nombreuses méthodes, populations de patient·es et contextes d’étude manquent de données probantes de haute qualité. Les lignes directrices ne font aucune recommandation concernant l’utilisation d’une méthode spécifique en raison du principe d’équivalence et du manque de données probantes de haute qualité. Nous avons réalisé une étude de portée afin de déterminer quelles méthodes de détermination de la PEP optimale ont été étudiées et quelles lacunes existent dans la littérature.
Sources
Nous avons mené des recherches dans cinq bases de données afin d’en tirer des rapports de recherche primaires étudiant les méthodes de détermination de la PEP optimale chez les adultes sous ventilation mécanique invasive. Les données résumées comprenaient la méthode de titrage, le contexte, la conception de l’étude, la population et les résultats.
Constatations principales
Deux cent soixante et onze études portant sur 17 205 patient·es répondaient aux critères d’inclusion, dont 73 études randomisées contrôlées (ERC) portant sur 10 733 patient·es. Nous avons identifié 22 méthodes. Onze ont été étudiées dans le cadre d’ERC. Les études ont recruté des participant·es dans une unité de soins intensifs (USI) (216/271, 80 %) ou en salle d’opération (55/271, 20 %). La plupart des études réalisées aux soins intensifs ont recruté des patient·es souffrant d’un syndrome de détresse respiratoire aiguë (162/216, 75 %). Les trois méthodes les plus étudiées étaient l’observance (73 études, 29 ERC), les méthodes basées sur l’imagerie (65 études, 11 ERC) et l’utilisation de tables de PEP-FIO2 (52 études, 20 ERC). Parmi les ERC en soins intensifs, les critères d’évaluation principaux les plus courants étaient la mortalité ou l’oxygénation. Peu d’ERC ont évalué la faisabilité de différentes méthodes (n = 3). Les forces et les limites de chaque méthode sont discutées.
Conclusion
De nombreuses méthodes ont été évaluées pour déterminer la PEP optimale; cependant, des lacunes notables subsistent dans les données probantes à l’appui de leur utilisation. Il s’agit notamment de populations spécifiques (poumons normaux, patient·es sevré·es de la ventilation mécanique) et de l’utilisation d’autres critères d’évaluation (jours sans ventilateur et faisabilité) et cela représente d’importantes occasions pour des études futures.
Enregistrement de l’étude
Open Science Framework (https://osf.io/atzqc); première publication, 19 juillet 2022.
Positive end-expiratory pressure (PEEP) is an important aspect of invasive mechanical ventilation that is set and titrated by clinicians. Changes in PEEP can influence gas exchange and respiratory mechanics, and can modify the risk of ventilatory-induced lung injury.1,2 By extension, inappropriately high or low PEEP can worsen a patient’s physiology and contribute to poor outcomes.2 Determining the optimal or best PEEP is challenging and complex. Numerous methods to determine optimal PEEP have been tested; however, no single method has consistently been shown to be superior.3 This has resulted in considerable variability in the clinical application of methods of determining optimal PEEP.4–6
There are several studies assessing methods of determining optimal PEEP, but certain methods, patient populations, and study settings lack high-quality evidence. A few large randomized controlled trials (RCTs) in patients with acute respiratory distress syndrome (ARDS) have compared methods such as high and low PEEP-FIO2 tables or titrating to a plateau pressure target but have been unable to show a change in mortality.7–9 Many other methods lack RCTs or data on patient-centred or clinical outcomes.10 Systematic reviews on determining optimal PEEP have generally been limited to methods that have been studied by RCTs, thereby excluding many other potentially viable methods.11–15 Populations outside of ARDS are also underrepresented in high-quality trials of methods of determining optimal PEEP.
Our aim for this study was to describe the methods of determining optimal PEEP in adults undergoing invasive mechanical ventilation in both an intensive care unit (ICU) and operating room (OR) setting that have been reported in the literature. To accomplish the above aim, we used scoping review methodology to systematically identify types of evidence and to delineate gaps in the literature.16 For each PEEP titration method, we sought to synthesize the patient populations, clinical and physiologic outcomes that have been studied, and the study designs that have been used. A better understanding of the gaps in the literature underpinning methods of determining optimal PEEP will guide clinical practice, identify areas of equipoise, and inform opportunities for further clinical trials.
Methods
Framework and registration
We registered this scoping review on 19 July 2022 on Open Science Framework (https://osf.io/atzqc). The protocol was peer-reviewed and published in advance.17 We prepared the review in accordance with the most recent scoping review guidance.18–20 The findings of our research are reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Scoping Review (PRISMA-ScR) statement and checklist21 (see Electronic Supplementary Material [ESM] eAppendix).
Inclusion criteria
We developed our inclusion and exclusion criteria using the “Population, Concept, Context” framework.20 Our population of interest was hospitalized adults receiving invasive mechanical ventilation. We excluded pediatric and neonatal populations, and those undergoing noninvasive or single lung ventilation. The concept of interest was a specific method of setting PEEP along with a measured outcome related to that method, either clinical or physiologic. We excluded studies that set PEEP at an arbitrary level. The context was broad, and we did not limit the searches by gender, geography, language, or duration of mechanical ventilation. We included only primary research studies and excluded case reports, systematic and other reviews, and editorials.
Search strategy
A medical librarian (H. L. R.) created search strategies for MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and Scopus based on the inclusion and exclusion criteria. The search strategy was peer-reviewed by a second medical librarian using the Peer Review of Electronic Search Strategies (PRESS) checklist.22 The search strategy for all databases can be found in ESM eTables 1–5. We completed the search on 22 April 2023 and included all results up to that date. The results were exported to Endnote 20 and screened using the systematic review software Rayyan (Qatar Computing Research Institute, 2016, Doha, Qatar).
Citation selection
Two reviewers (among S. E., N. K., K. P.) screened each citation to determine eligibility. Disagreements during title and abstract screening were resolved through discussion among the two reviewers and if consensus could not be reached, the third author would arbitrate. We then independently reviewed the full text of all eligible papers (K. P., S. E.) to finalize inclusion. We also reviewed reference lists of included papers and systematic reviews, and conference abstracts were included only if no corresponding manuscript was published. We translated non-English articles with Google Translate (Google LLC, Alphabet Inc., Mountain View, CA, USA) if an embedded PDF was available.23 This approach has been validated for use in systematic reviews.24
Data abstraction
We abstracted relevant study data using a standardized form developed over several iterations with input from all members of the team and pilot testing of ten papers. Abstracted data related to 1) the citation (i.e., author, year and location of publication, journal, study design, funding); 2) population of interest and setting; 3) method of determining optimal PEEP; 4) other ventilator parameters (i.e., tidal volumes); and 5) outcomes (both primary and secondary). Abstracted data were collated in a Microsoft® Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet by one author (S. E.) and the charted data were verified and validated by a second author (K. P.).
Statistical analysis
Given the nature of scoping review methodology, we did not conduct a formal statistical analysis but presented descriptive statistics by summarizing characteristics of the included studies. Nominal data are presented as count (percent) and continuous data are presented as median [interquartile range (IQR)].
Results
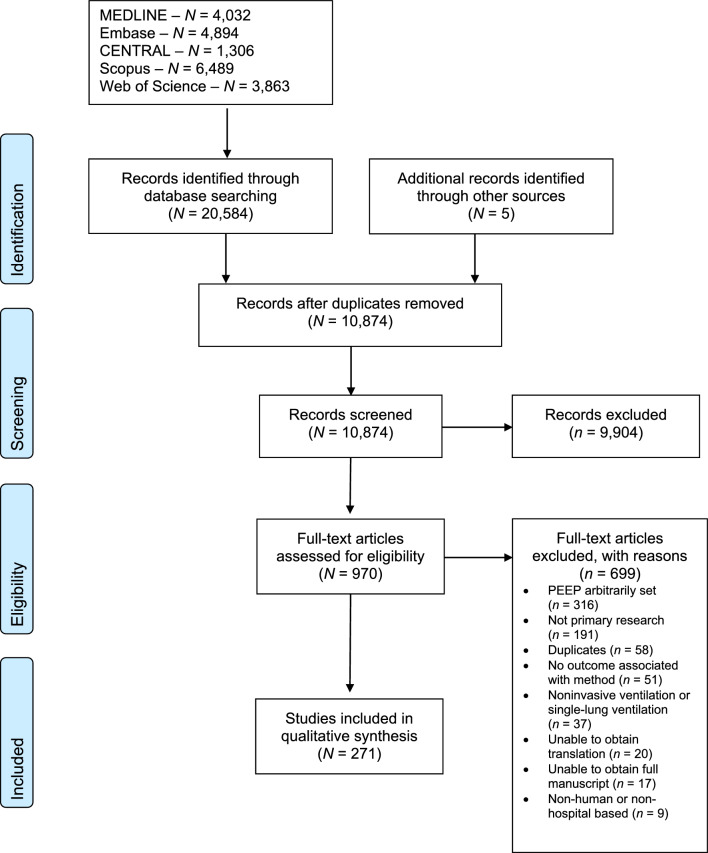
Our search identified 10,874 unique citations, and we included 970 for full-text review. After full-text review, we included 271 articles studying 17,205 patients. Figure 1 shows the PRISMA flow diagram and reasons for exclusion at the full-text screening stage. The complete list of included studies (with study design, population, and method of determining optimal PEEP) can be found in ESM eTable 6.
Fig. 1.
PRISMA flow diagram
Overall studies
Table 1 highlights characteristics of included studies. Of the 271 studies, 216 (80%) were set in the ICU including 13,157 patients, and 55 (20%) were set in the OR including 4,048 patients. The most common study designs were observational studies (163/271 studies, 60%, 5,479 patients), followed by RCTs (73/271 studies, 27%, 10,733 patients). The first ICU studies were published in the 1970s but the first OR study did not appear until 2006 (ESM eFig. 1). Half of the studies were among patients from Europe (139/271, 51%), but individuals from all continents were represented in the included studies (Table 1). Among the 216 studies performed in the ICU, most involved individuals with ARDS (n = 162, 75%). Among the 55 studies done in the OR, most patients had normal lungs (n = 46, 84%).
Table 1.
Characteristics of included studies
| Study characteristic | Number of studies* | ||
|---|---|---|---|
| Overall | ICU | OR | |
| Number of studies, N | 271 | 216 | 55 |
| Study design, n/total N (%) | |||
| Observational | 163/271 (60%) | 147/216 (68%) | 16/55 (29%) |
| RCT | 73/271 (27%) | 39/216 (18%) | 34/55 (62%) |
| Nonrandomized trial | 14/271 (5%) | 13/216 (6%) | 1/55 (2%) |
| Randomized crossover | 13/271 (5%) | 12/216 (6%) | 1/55 (2%) |
| Reanalysis | 8/271 (3%) | 5/216 (2%) | 3/55 (6%) |
| Continent of origin,† n/total N (%) | |||
| Europe | 139/271 (51%) | 114/216 (53%) | 25/55 (46%) |
| Asia | 67/271 (25%) | 46/216 (21%) | 21/55 (38%) |
| North America | 38/271 (14%) | 36/216 (17%) | 2/55 (4%) |
| South America | 19/271 (7%) | 15/216 (7%) | 4/55 (7%) |
| Africa | 10/271 (4%) | 8/216 (4%) | 2/55 (4%) |
| Australia/New Zealand | 9/271 (3%) | 8/216 (4%) | 1/55 (2%) |
| Time period, n/total N (%) | |||
| 1970s | 4/271 (2%) | 4/216 (2%) | 0/55 (0%) |
| 1980s | 12/271 (4%) | 12/216 (6%) | 0/55 (0%) |
| 1990s | 10/271 (4%) | 10/216 (5%) | 0/55 (0%) |
| 2000s | 34/271 (13%) | 32/216 (15%) | 2/55 (4%) |
| 2010s | 100/271 (37%) | 85/216 (39%) | 15/55 (27%) |
| 2020s | 111/271 (41%) | 73/216 (34%) | 38/55 (69%) |
| Patient population,† n/total N (%) | |||
| ARDS | 162/271 (60%) | 162/216 (75%) | 0/55 (0%) |
| Normal | 46/271 (17%) | 0/216 (0%) | 46/55 (84%) |
| Laparoscopic | 30/271 (11%) | 0/216 (0%) | 30/55 (55%) |
| COVID-19 | 27/271 (10%) | 27/216 (13%) | 0/55 (0%) |
| Obese | 25/271 (9%) | 16/216 (7%) | 9/55 (16%) |
| Mixed | 22/271 (8%) | 22/216 (10%) | 0/55 (0%) |
| AHRF | 12/271 (4%) | 12/216 (6%) | 0/55 (0%) |
| COPD | 10/271 (4%) | 10/216 (5%) | 0/55 (0%) |
| Postoperative | 10/271 (4%) | 10/216 (5%) | 0/55 (0%) |
| ECMO | 10/271 (4%) | 10/216 (5%) | 0/55 (0%) |
| Proned | 6/271 (2%) | 4/216 (2%) | 2/55 (4%) |
Included studies are stratified by overall and study setting
*Percentages are for total studies within given column
†Sum of columns is greater than total as some articles fit more than one category
AHRF = acute hypoxemic respiratory failure; ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; OR = operating room; RCT = randomized controlled trial
Methods of determining optimal positive end-expiratory pressure
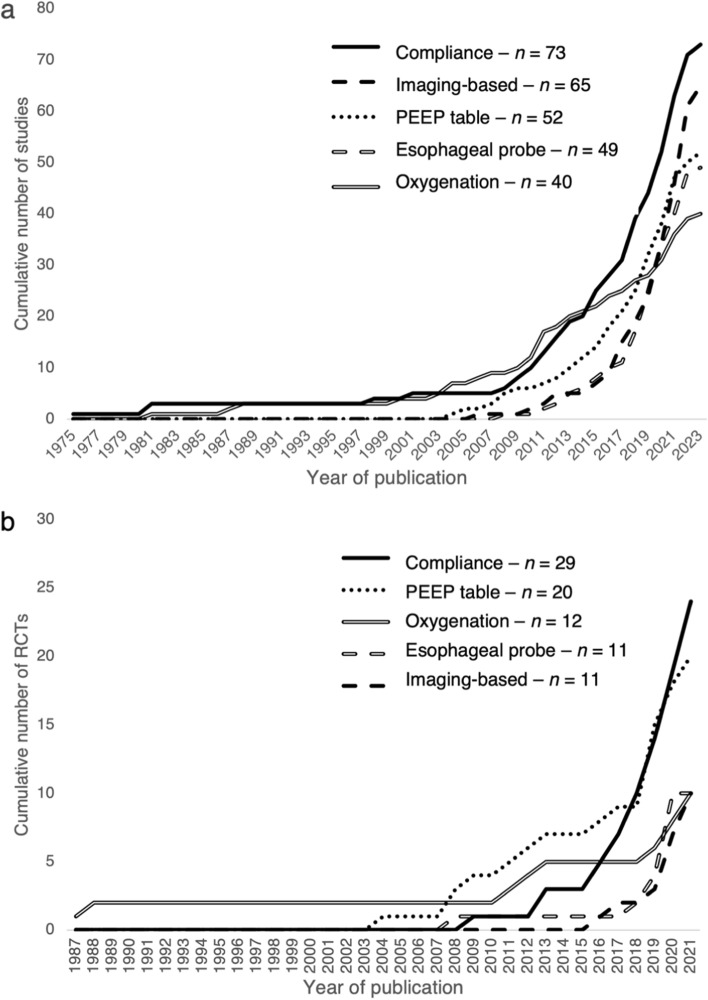
We identified 22 different methods of PEEP selection (Table 2). We briefly describe each method along with strengths and limitations in Table 3.7–10,25–97 All methods were studied in the ICU, whereas only seven of the 22 methods were studied in the OR (Table 2). More than one method was studied in 101 (37%) studies (eTable 6). In the ICU, the three most studied methods included the use of a PEEP-inspired fraction of oxygen (FIO2) table (n = 52 studies), imaging-based methods (n = 51 studies), and compliance-based methods (n = 46 studies). In the OR, the three most studied methods included compliance-based (n = 27 studies), imaging-based (n = 14 studies), and esophageal probe-based (n = 8 studies). Imaging-based methods of setting PEEP included electrical impedance tomography (EIT), ultrasound, and computed tomography. Of these, EIT was the most studied, comprising 83% of the imaging-based studies (n = 54) (eTable 7). Figure 2 shows the cumulative trend in publication over time among the five most studied methods of determining optimal PEEP in terms of overall studies and RCTs. The number of publications by five-year period among studies overall and RCTs can be seen in ESM eFig. 2.
Table 2.
Number of studies published assessing different methods of determining optimal positive end-expiratory pressure
| PEEP method* | Overall, n/total N (%) | Setting, n/total N (%)* | RCTs, n/total N (%)† | |
|---|---|---|---|---|
| ICU | OR | |||
| All studies | 271 | 216 | 55 | 73 |
| Compliance | 73/271 (27%) | 46/216 (21%) | 27/55 (49%) | 29/73 (40%) |
| Imaging-based | 65/271 (24%) | 51/216 (24%) | 14/55 (26%) | 11/73 (15%) |
| PEEP-FIO2 table | 53/271 (20%) | 52/216 (25%) | 0/55 (0%) | 21/73 (29%) |
| Esophageal probe | 49/271 (18%) | 41/216 (19%) | 8/55 (15%) | 11/73 (15%) |
| Oxygenation | 40/271 (15%) | 35/216 (16%) | 5/55 (9%) | 12/73 (16%) |
| Pressure-volume curves | 23/271 (9%) | 23/216 (11%) | 0/55 (0%) | 7/73 (10%) |
| Plateau pressure | 12/271 (4%) | 12/216 (6%) | 0/55 (0%) | 2/73 (3%) |
| Driving pressure | 11/271 (4%) | 5/216 (2%) | 6/55 (11%) | 7/73 (10%) |
| Computer-based | 11/271 (4%) | 11/216 (5%) | 0/55 (0%) | 1/73 (1%) |
| Shunt | 10/271 (4%) | 10/216 (5%) | 0/55 (0%) | 2/73 (3%) |
| Auto-PEEP | 8/271 (3%) | 8/216 (4%) | 0/55 (0%) | 0/73 (0%) |
| EELV/FRC | 7/271 (3%) | 5/216 (2%) | 2/55 (4%) | 1/73 (1%) |
| Dead space | 6/271 (2%) | 4/216 (2%) | 2/55 (4%) | 0/73 (0%) |
| Intra-abdominal pressure | 4/271 (2%) | 3/216 (1%) | 1/55 (2%) | 0/73 (0%) |
| Stress index | 3/271 (1%) | 3/216 (1%) | 0/55 (0%) | 0/73 (0%) |
| Oxygen delivery | 2/271 (1%) | 2/216 (0.9%) | 0/55 (0%) | 0/73 (0%) |
| Airway opening pressure | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
| Airway occlusion pressure | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
| NAVA | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
| R/I ratio | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
| Time constant | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
| Weight | 1/271 (0.4%) | 1/216 (0.5%) | 0/55 (0%) | 0/73 (0%) |
Studies that published assessing different methods of determining optimal positive end-expiratory pressure are stratified by overall, study setting, and number of RCTs for each method
*Sum of columns is greater than total as some articles fit more than one category
†Percentages are for total studies within given column
EELV = end-expiratory lung volume; FRC = functional residual capacity; ICU = intensive care unit; NAVA = neurally adjusted ventilatory assist; OR = operating room; PEEP = positive end-expiratory pressure; RCT = randomized controlled trial; R/I = recruitment to inflation
Table 3.
Methods of positive end-expiratory pressure selection
| Method of PEEP titration | Description | Strengths | Limitations | RCTs, n |
|---|---|---|---|---|
| Compliance | Selection based on highest static or dynamic compliance.26 Assessed by either incremental or decremental stepwise PEEP trial with or without a recruitment maneuver. | Able to be calculated with any ventilator at the bedside. No additional equipment. | Patient must be passive on ventilator. Takes time to do incremental or decremental trials. | 2925–41,43–48,82,87,90,91,93,94 |
| PEEP/FIO2 table | Selected via table that assigns a PEEP (or range of PEEPs) for a given FIO2. There are low PEEP and high PEEP/FIO2 tables, as seen in ALVEOLI trial.7 | Simple, requires no equipment, can be reassessed regularly, minimal time investment | Does not take into consideration patient’s mechanics | 217,9,25–32,49–59 |
| Oxygenation | Selection by change in oxygenation (SpO2 or PaO2) during either a decremental56 or incremental64 stepwise trial, or maximal oxygenation within a defined range65 | Able to be calculated with any ventilator at the bedside | Requires arterial blood gas for PaO2. Does not assess mechanics. | 1245,47,54–56,62,64–67,84,88 |
| Esophageal probe | Pressure transduced from esophageal balloon as surrogate for pleural pressure50 PEEP is titrated to end-expiratory transpulmonary pressure > 0 cm H2O | Partitioning of lung and chest wall mechanics, measuring true pressures affecting the lungs | Special equipment, ventilators, and education | 1130,33,48–50,52,60–63,85 |
| Imaging—EIT | Maps out areas of collapse, normal aeration, and overdistension within the lung.10 PEEP set to minimize both collapse and overdistension51 or to lowest RVDI.68 | Noninvasive bedside method of determining degree of collapse and overdistension | Requires special equipment, monitoring supplies, and education | 830,43,51,68–71,89 |
| Pressure-volume curves | Curve generated with single breath69 or plotting breaths of varying volumes.72 PEEP set above the lower inflection point72 or at the point of maximal hysteresis.69 | Provides information about respiratory system mechanics | Patient must be passive on ventilator. Plotting curve takes time. Few ventilators can do single breath curve. | 769,72–76,92 |
| Driving pressure | Difference between plateau pressure and PEEP. PEEP can be set at the level that corresponds to the lowest driving pressure during an incremental trial.42 | Able to be calculated with any ventilator at the bedside. No additional equipment. | Patient must be passive on ventilator. Takes time to do incremental trials. | 742,57,79,83,86,95,97 |
| Imaging—ultrasound | PEEP adjusted to optimize aeration as assessed by presence or absence of artifact on lung ultrasound. Can be incremental121 or decremental.109 | Able to be measured independent of ventilator mode | Requires training in ultrasound and interrater reliability can be an issue | 358,59,122 |
| Plateau pressure | For a given tidal volume, PEEP can be increased until a plateau pressure of 28–30 cm H2O is achieved8 | Able to be calculated with any ventilator at the bedside. Can be reassessed quickly. | Patient must be passive on ventilator | 28,53 |
| Shunt | Shunt fraction (Qs/Qt) can be calculated using blood from an arterial catheter and a PA catheter. PEEP adjusted for reduction in shunt fraction66 or an absolute value.64 | Best measure of oxygenation of the lungs overall. Patient does not need to be paralyzed. | Requires PA catheter. Does not take into consideration patient’s mechanics. | 264,66 |
| EELV | EELV (absolute or change) is measured by several techniques including plethysmography123 and nitrogen multiple breath washout technique67 | Direct measure of recruitment with different levels of PEEP | Requires specialized equipment | 167 |
| Computer-based | Certain ventilators have software such as Intellivent-ASV80 that will automatically adjust variables including PEEP based on certain inputs | Automated methods require little workforce and can adjust as conditions change | Modes are proprietary to different ventilators and may not be available for all patients | 196 |
| Airway occlusion pressure (P0.1) | P0.1 is pressure measured in first 0.1 sec of inhalation against occluded airway and is surrogate of respiratory drive can be set to keep P0.1 within a range | Useful in patients weaning. Noninvasive. Most modern ventilators can perform. | Not useful in deeply sedated or paralyzed patients | 0 |
| AOP | Low-flow inflation maneuver is done with PEEP 0 cm H2O. Inflection in slope of pressure-time curve is noted as AOP. PEEP is set at or above that level. | Can be measured with any ventilator. Assesses lung mechanics. | Patient must be passive on ventilator. Does not consider hysteresis. | 0 |
| Auto-PEEP | Calculated by subtracting total PEEP (measured with end-expiratory hold) from applied PEEP. PEEP is set to a percentage of auto-PEEP between 50% and 100%. | Can be measured with any ventilator. Considers mechanics and can aid in work of breathing. | Limited value in patients without airflow obstruction. Must be passive on ventilator to perform. | 0 |
| Dead space | Dead space can be calculated using volumetric capnography124–126 and Bohr’s equation. PEEP can be set to reduce or minimize dead space fraction | Continuously monitored. Patient can breathe spontaneously. Measures ventilatory efficiency. | Volumetric capnography requires special equipment. Does not consider mechanics. | 0 |
| Imaging—CT | CT is done at a baseline PEEP, after recruitment, and images are taken as PEEP is gradually decreased. PEEP is set above the level at which lung closure occurs. | Accurate way to measure recruitment and visualize overdistension | Patient must be passive on ventilator. Resource intensive and requires transporting patients. | 0 |
| IAP | IAP is measured (via bladder pressure) and PEEP is set at a percentage (from 50%127 to 125%128) of IAP | Simple to perform. Can easily trend. Accounts for mechanics. | Limited value in patients with normal IAP. Patient must be passive to measure accurate IAP. | 0 |
| NAVA | NAVA is a mode of ventilation that measures the EAdi with an esophageal catheter. PEEP can be set at the level that has optimal EAdi.81 | Accurate way to ensure good patient-ventilator synchrony and assist | Requires special equipment and invasive monitors | 0 |
| Oxygen delivery | DO2 is calculated with PaO2 and cardiac output using transesophageal doppler129 or echocardiography.130 PEEP is adjusted to maximize DO2. | Considers oxygenation as well as the hemodynamic consequences of PEEP | Requires special equipment and training. Does not consider mechanics. | 0 |
| R/I ratio | Recruitment between two levels of PEEP is inferred based on changes in mechanics and change in EELV. PEEP set based on recruiter vs nonrecruiter. | Can be measured with any ventilator | Patient must be passive on ventilator | 0 |
| Stress index | Shape of pressure-time waveform. Upslope at end-inspiration indicates overdistension, and downslope indicates recruitment. PEEP is set to target linear or decreasing index. | Can be measured with any ventilator. Can be monitored continuously. Assesses mechanics. | Patient must be passive on ventilator | 0 |
| Time constant | Using a constant driving pressure, PEEP is adjusted and set at the level corresponding to the highest time constant | Can be measured with any ventilator | Patient must be passive on ventilator | 0 |
| Weight | PEEP set based on BMI. Stratified BMI by < 30 kg·m−2, 30–50 kg·m−2, and > 50 kg·m−2. | Simple method. May compensate for higher pleural pressures in patients with obesity. | BMI does not consider distribution of body mass. Does not measure mechanics or oxygenation. | 0 |
AOP = airway opening pressure; ASV = adaptive supportive ventilation; BMI = body mass index; CT = computed tomography; DO2 = oxygen delivery; EAdi = electrical activity of the diaphragm; EELV = end-expiratory lung volume; EIT = electrical impedance tomography; FIO2 = inspired fraction of oxygen; IAP = intra-abdominal pressure; NAVA = neurally adjusted ventilatory assist; PaO2 = partial pressure of oxygen in arterial blood; PEEP = positive end-expiratory pressure; PA = pulmonary artery; Qs = pulmonary physiologic shunt (mL·min−1); Qt = cardiac output (mL·min−1); R/I = recruitment to inflation; RCT = randomized controlled trial; Ref = reference; RVDI = regional ventilation delay index; SpO2 = peripheral oxygen saturation
Fig. 2.
Cumulative number of studies assessing methods of determining optimal positive end-expiratory pressure over time, stratified by method. (a) Overall number of studies published; (b) randomized controlled trials published.
PEEP = positive end-expiratory pressure; RCT = randomized controlled trials
Most common methods of determining optimal positive end-expiratory pressure
We present detailed descriptions of evidence supporting the eight most studied methods for determining optimal PEEP. These methods represent over 85% of the studies included in this scoping review (231/271 studies). Furthermore, these eight methods are studied in 72 of the 73 RCTs identified.
Compliance
Using respiratory system compliance to determine optimal PEEP was first reported in 197598 and was the most studied method (n = 73). Positive end-expiratory pressure was adjusted in an incremental or decremental fashion to maximize static or dynamic compliance. In many studies, this was preceded by recruitment maneuvers of varying intensity and frequency. This method can be used with any ventilator and no extra equipment or training is needed. Nevertheless, measuring static compliance requires patients to be passive on a ventilator, and serial measurements at different levels of PEEP can be time consuming. In addition, incremental and decremental PEEP trials can give different results. In the ICU setting, there were nine RCTs, two of which were multicentre trials. Both multicentre trials compared a compliance strategy with a low PEEP-FIO2 table. One found no difference in mortality or ventilator-free days (VFD).25 The other found an increase in mortality with the compliance strategy,26 although that arm was also accompanied by high pressure recruitment maneuvers. A large retrospective study compared PEEP determined by either compliance or pressure-volume curves in patients with ARDS being supported with extracorporeal membrane oxygenation (ECMO).99 The compliance group had a shorter duration of mechanical ventilation, ICU length of stay (LOS), and hospital LOS. In the OR setting there were 20 RCTs, two of which were multicentre trials. One study randomized patients to a compliance strategy vs a fixed low PEEP.35 The other study was a pilot RCT and compared compliance with PEEP set with an esophageal balloon or with a fixed low PEEP.48 Both found no difference in postoperative complications.
PEEP-FIO2 tables
PEEP-FIO2 tables were first used along with low tidal volume ventilation in the landmark ARDSNet trial in 2000.100 The tables were developed based on the clinical practice of the study sites participating in the trial.101 The tables were not prospectively tested as a method until 2004 when the same authors published an RCT comparing low and high PEEP-FIO2 tables.7 Since then, most large multicentre RCTs in patients with ARDS have used a PEEP-FIO2 table as the comparator. This method involves a table that specifies a value or range of values for PEEP for a given FIO2 that the patient requires to maintain oxygen saturation within a certain range. The higher the required FIO2, the higher the PEEP specified. This method has the advantage that it can be used without any extra equipment and in patients who are spontaneously breathing. It is quick and simple to implement with little cost. This method does not consider a patient’s respiratory mechanics. It has been assessed in 53 studies. In the ICU setting, 21 RCTs were published, seven of which were multicentre RCTs. All seven compared a PEEP-FIO2 table to another method of determining optimal PEEP (two of the studies compared high vs low tables7,9) in patients with ARDS. All had a primary outcome of either mortality, VFD, or a composite of the two, and only one had a statistically significant difference showing reduced mortality with a low PEEP-FIO2 table compared with a method using compliance and lung recruitment maneuvers.25 A retrospective cohort study of patients with ARDS due to COVID-19 compared those who had PEEP set at levels close to the low PEEP-FIO2 table and those who had PEEP set at levels close to the high PEEP-FIO2 table.102 After propensity score matching, the higher PEEP group was associated with more VFD but also had a greater likelihood of acute kidney injury and renal replacement therapy. This method has not been studied in an OR setting to date.
Electric impedance tomography
Studies using imaging to determine optimal PEEP have gained considerable interest in the last decade, most commonly with EIT (54 studies). The method was first described in an OR setting in 2006103 and subsequently in an ICU setting in 2015.104 In less than ten years, 42 papers on setting PEEP with EIT in the ICU have been published. Electrical impedance tomography measures the impedance between leads placed across a patient’s chest to map areas of the lungs with normal aeration, overdistension, and collapse. Studies have set PEEP by determining the optimal level that minimizes both overdistension and collapse as determined by EIT. This method has shown promise as a noninvasive bedside test that can help clinicians assess regional differences within the lungs. Nevertheless, it has several limitations. Most notably, it requires purchasing equipment and conducting training, and in nearly all studies required patients to be passive on the ventilator. In the ICU setting, there were two published single-centre RCTs, neither of which found a difference in outcome between the EIT arm and either a low PEEP-FIO2 table51 (mortality) or use of a pressure-volume curve69 (physiologic measures). A prospective observational study compared patients with ARDS whose PEEP was determined using EIT with a historical cohort that used a pressure-volume to determine optimal PEEP.105 The EIT group had improved compliance but no difference in mortality. In the OR setting, there were five single-centre RCTs. Most used physiologic endpoints except for one, which found a reduction in postoperative hypoxemia compared with a fixed low-PEEP strategy.89
Esophageal balloons
Esophageal balloons were first used to determine optimal PEEP in a single-centre RCT in 200850 and, to date, 50 studies have assessed this method. These devices measure esophageal pressure, which can estimate the pleural pressure and calculate transpulmonary pressure. The PEEP is set to ensure transpulmonary PEEP is zero or slightly positive. This method could be more accurate in reflecting lung physiology vs other methods that assess mechanics using airway pressures, especially if the chest wall pressures are abnormal. One advantage of this method is that the balloon can detect pressures generated by the diaphragm; therefore, this method can accurately estimate distending pressures in patients who are spontaneously breathing. Nevertheless, it requires extra equipment and training to use, and insertion can be time consuming. In the ICU setting, seven RCTs were published, one of which was a multicentre RCT in patients with ARDS comparing esophageal balloon with a high PEEP-FIO2 table.49 It found no difference in the composite primary outcome of mortality and VFD. A post hoc analysis of that trial found that, among patients with lower Acute Physiology and Chronic Health Evaluation II (APACHE-II) scores, the esophageal balloon group had lower mortality. The inverse relationship was true in those with higher APACHE-II scores, although the relationship was not statistically significant (P = 0.08).106 This method was tested in the only RCT of patients being weaned from mechanical ventilation,33 likely because of its utility in spontaneously breathing patients. There was no difference in duration of mechanical ventilation between this method and PEEP determined by best compliance. A retrospective study compared PEEP determined with an esophageal balloon with a fixed PEEP of 10 cm H2O in patients with ARDS being supported by ECMO.107 The esophageal balloon group had less hemodynamically significant right ventricular dysfunction and improved survival to ECMO decannulation. In the OR setting, four RCTs were published, one of which was a multicentre RCT. All four RCTs had primary physiologic endpoints. Three found a significant difference favouring the esophageal balloon, with improvements in driving pressure,48,60,62 compliance,48,62 and oxygenation62 compared with either an oxygenation62 or low fixed PEEP48,60 strategy. One of the studies also had a third arm using a compliance strategy and found similar outcomes in terms of respiratory mechanics.48
Oxygenation
Oxygenation was first used as a target for setting PEEP in 1981, when it was studied in postoperative surgical ICU patients.108 There were 40 studies assessing this method. Several variations exist that use oxygenation or change in oxygenation to determine optimal PEEP. Incremental or decremental stepwise trials are done with or without lung recruitment maneuvers beforehand, with PEEP set at the level with the highest level of oxygenation or change in oxygenation. This can be measured via pulse oximetry or blood gas analysis. This method does not require any special equipment but may require an arterial line and frequent blood gas sampling if PaO2 is used. The method is less resource intensive with pulse oximetry but could be time consuming if incremental or decremental stepwise trials are done. In the ICU setting, there were 11 RCTs, three of which were multicentre RCTs. These were done mainly in patients with ARDS or acute hypoxemic respiratory failure (AHRF). Two multicentre trials—one done in patients with ARDS with a low PEEP-FIO2 table comparator56 and the other in patients without ARDS with a PEEP 0–5 cm H2O comparator65—found no difference in their primary outcome of VFD. The third multicentre RCT compared oxygenation with the use of a pressure-volume loop to determine optimal PEEP, but its primary endpoint was biochemical markers.92 The pressure-volume loop group had a reduction in cytokines and inflammatory markers. A prospective nonrandomized trial studied patients with ARDS and atelectasis, comparing PEEP set with oxygenation and PEEP set with lung ultrasound.109 The lung ultrasound group had improved oxygenation, but no change in compliance, and a greater drop in blood pressure during the procedure. In the OR setting, there were three single-centre RCTs, all with primary physiologic endpoints. One compared oxygenation with the use of an esophageal balloon and found better oxygenation, compliance, and driving pressure in the esophageal balloon group.62 The other two compared either oxygenation alone84 or a combination of oxygenation and compliance47 with a low fixed PEEP and found these strategies superior to fixed PEEP in terms of oxygenation.
Pressure-volume loops
The pressure-volume loop was first used for determining optimal PEEP in 1,987 in patients with AHRF,110 and 23 studies have since assessed this method. In that study and others in the period, pressure-volume loops were measured using a large 2-L syringe attached to a pressure transducer. In more recent studies, the loops are generated with ventilators either by manually plotting the curve with increasing tidal volumes, or with a single breath technique. The latter is only possible with certain ventilators. The level of PEEP is typically set at or just above the lower inflection point of the inspiratory limb of the pressure-volume loop or is sometimes set at the point of maximum hysteresis between the inspiratory and expiratory limbs. This technique assesses many aspects of a patient’s respiratory mechanics but can be time consuming, requires extra training and equipment, and the patient must be passive on the ventilator. In the ICU setting, seven RCTs were completed in patients with ARDS and one RCT in postcardiac surgery patients. Two of these were multicentre RCTs, both studying patients with ARDS. One mentioned above compared oxygenation with pressure-volume loop methods, but had biochemical markers as the primary endpoint.92 The pressure-volume loop group had a reduction in cytokines and inflammatory markers. The other compared PEEP set with a pressure-volume loop to PEEP set at the clinician’s preference. This study found a benefit in ICU mortality in the pressure-volume loop arm.74 Nevertheless, tidal volumes differed between arms, making it difficult to assess the effect of the PEEP strategy alone. This method has not been studied in an OR setting.
Driving pressure
Driving pressure was first used to determine optimal PEEP in 2016 in patients with obesity without ARDS.111 There were 11 studies assessing this method. PEEP can be adjusted to the highest level that keeps driving pressure under a given threshold (in one study ≤ 14 cm H2O57) or can be adjusted within a given range to a value that yields the lowest driving pressure. Driving pressure is inversely correlated to compliance. For a fixed tidal volume, an increase in compliance will result in a proportional decrease in driving pressure. This method can be performed with any ventilator without extra training or equipment. Nevertheless, testing driving pressure at several PEEP levels can be time consuming, and patients must be passive on the ventilator to measure an accurate driving pressure. In the ICU setting, there was only one RCT in patients with ARDS.57 It was a single-centre study comparing use of driving pressure with a low PEEP-FIO2 table to determine optimal PEEP. There was a statistically significant difference in 28-day mortality favouring the driving pressure group. In the OR setting, there were five single-centre RCTs, all comparing the driving pressure method with a low fixed PEEP. Of the two that had a clinical endpoint of postoperative complications, both favoured driving pressure.42,86
Plateau pressure
Plateau pressure was first used to determine optimal PEEP in 2003 in patients with ARDS.112 Positive end-expiratory pressure is increased until the patient’s plateau pressure hits a certain level, typically between 28 and 30 cm H2O. This method can be done with any ventilator without extra training and is fast. Nevertheless, to obtain an accurate plateau pressure, patients must be passive on the ventilator. In the ICU setting, there were two RCTs, both of which were large multicentre trials of patients with ARDS. One study compared this method to a fixed PEEP of 5–9 cm H2O8 and the other used a low PEEP-FIO2 table.53 Mortality was the primary endpoint in both studies, and neither found a difference between the two arms, although the first study showed a reduction in their secondary outcome of VFD in the plateau pressure group.8 This method has not been studied in an OR setting.
Description of randomized controlled trials
Eleven of the 22 methods were studied with at least one RCT and we identified 73 RCTs overall with 10,708 patients. Table 4 shows the characteristics of the included RCTs. Four of the RCTs were reported in abstract format and have not yet been published. The remaining 69 RCTs were published in 46 different medical journals (ESM eTable 8). Of the 73 RCTs, 31 directly compared two different methods of determining optimal PEEP (ESM eTable 6). The sample sizes of the RCTs ranged from 12 to 1,012 patients with a median [IQR] of 60 [40–115]. The distribution in the number of patients enrolled in RCTs among ICU and OR studies is summarized in ESM eFig. 3. The RCTs included participants from 35 different countries. Six RCTs contained participants from multiple countries. The four countries with the largest number of trials conducted were China (n = 18), USA (n = 9), Brazil (n = 7), and Spain (n = 7) (ESM eTable 9). Over half of the studies were publicly funded (38/73, 52%). Most RCTs were single-centred, with 19% being multicentred (n = 14). Among the 39 RCTs done in an ICU setting, 82% involved patients with ARDS (n = 32). Among the 34 RCTs done in the OR, 91% involved participants with normal lungs (n = 31). In 58% of the RCTs (n = 42), a difference in the primary outcome was reported, which was considered statistically significant (ESM eTable 10). Tidal volumes were standardized within treatment arms in 74% of the RCTs (n = 54). Of these, all but one used low tidal volume ventilation whereas the other arms were ventilated initially at 12 cc/kg.66
Table 4.
Characteristics of included randomized controlled trials
| Study characteristic | Overall, n/total N (%) | Setting, n/total N (%)* | |
|---|---|---|---|
| ICU | OR | ||
| Overall | 73 | 39 | 34 |
| Study design, n/total N (%) | |||
| Multicentred | 14/73 (19%) | 12/39 (31%) | 2/34 (6%) |
| Multinational | 6/73 (8%) | 6/39 (15%) | 0/34 (0%) |
| Number of individuals randomized, median [IQR] | 60 [40–115] | 61 [39–123] | 56 [41–91] |
| Funding, n/total N (%) | |||
| Publicly funded | 38/73 (52%) | 19/39 (49%) | 19/34 (56%) |
| Not reported | 26/73 (36%) | 17/39 (44%) | 9/34 (27%) |
| No funding | 8/73 (11%) | 2/39 (5%) | 6/34 (18%) |
| Industry funded | 1/73 (1%) | 1/39 (3%) | 0/34 (0%) |
| Patient population, n/total N (%) | |||
| ARDS | 32/73 (44%) | 32/39 (82%) | 0/34 (0%) |
| Normal | 31/73 (43%) | 0/39 (0%) | 31/34 (91%) |
| Laparoscopic | 22/73 (30%) | 0/39 (0%) | 22/34 (65%) |
| Obese | 4/73 (6%) | 1/39 (3%) | 3/34 (9%) |
| Postoperative | 3/73 (4%) | 3/39 (8%) | 0/34 (0%) |
| AHRF | 2/73 (3%) | 2/39 (5%) | 0/34 (0%) |
| ECMO | 2/73 (3%) | 2/39 (5%) | 0/34 (0%) |
| Mixed | 2/73 (3%) | 2/39 (5%) | 0/34 (0%) |
| Outcomes, n/total N (%) | |||
| Type of primary outcome | |||
| Physiologic | 39/73 (53%) | 16/39 (41%) | 23/34 (68%) |
| Oxygenation | 27/73 (37%) | 12/39 (31%) | 15/34 (44%) |
| Compliance | 12/73 (16%) | 4/39 (10%) | 8/34 (24%) |
| Mortality | 14/73 (19%) | 14/39 (36%) | 0/34 (0%) |
| Other | 8/73 (11%) | 4/39 (10%) | 4/34 (12%) |
| Postoperative complication | 6/73 (8%) | 0/39 (0%) | 6/34 (18%) |
| Ventilator-free days | 4/73 (6%) | 4/39 (10%) | 0/34 (0%) |
| Not reported | 2/73 (3%) | 2/39 (5%) | 0/34 (0%) |
| Duration of ventilation | 1/73 (1%) | 1/39 (3%) | 0/34 (0%) |
| Reported by any study | |||
| Safety outcomes | 52/73 (71%) | 28/39 (72%) | 24/34 (71%) |
| Length of stay | 37/73 (51%) | 24/39 (62%) | 13/34 (38%) |
| Mortality | 36/73 (49%) | 30/39 (77%) | 6/34 (18%) |
| Duration of ventilation | 34/73 (47%) | 16/39 (41%) | 18/34 (53%) |
| Postoperative complication | 23/73 (32%) | 0/39 (0%) | 23/34 (68%) |
| Ventilator-free days | 19/73 (26%) | 19/39 (49%) | 0/34 (0%) |
| Costs/feasibility | 4/73 (6%) | 3/39 (8%) | 1/34 (3%) |
| Tidal volumes between arms, n/total N (%) | |||
| Same tidal volumes | 54/73 (74%) | 27/39 (69%) | 27/34 (79%) |
| Different tidal volumes | 13/73 (18%) | 8/39 (21%) | 5/34 (15%) |
| Not reported | 6/73 (8%) | 4/39 (10%) | 2/34 (6%) |
Included randomized controlled trials are stratified by overall and trial setting
*Percentages are for total studies within given column
AHRF = hypoxemic respiratory failure; ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IQR = interquartile ratio; OR = operating room
Outcomes among randomized controlled trials
Most of the RCTs had a physiologic measure as the primary outcome (n = 39, 53%). This was more common among RCTs in the OR (23/34, 68%) compared with RCTs in the ICU (16/39, 41%). The most common physiologic measures used as a primary outcome were oxygenation and compliance. Among the RCTs with a physiologic measure as the primary outcome, the difference in the primary endpoint was statistically significant in 69% of studies (n = 27) (ESM eTable 10).
Only RCTs conducted in the ICU had mortality as a primary outcome (n = 14, 36%). Among RCTs, mortality was recorded as an outcome (whether primary or secondary) in 77% (30/39) of studies done in the ICU compared with 18% (6/34) of studies done in the OR. The most common measures of mortality were 28-day mortality (n = 21, 29%), hospital mortality (n = 19, 26%), and ICU mortality (n = 15, 21%) (ESM eTable 11). Among the RCTs that used mortality as the primary outcome, the difference in the primary endpoint was statistically significant in 36% of studies (n = 5) (ESM eTable 10).25,57,66,72,74
For other clinically relevant outcomes, 77% (30/39) of RCTs conducted in the ICU measured a ventilation outcome compared with 53% (18/34) of RCTs done in the OR. The two most common measures were duration of mechanical ventilation (n = 34, 47%) and VFD at 28 days, which is a composite of mortality and ventilation (n = 16, 22%) (ESM eTable 11). Length-of-stay outcomes were measured in 62% (24/39) of ICU studies and 38% (13/34) of OR studies. The most common measures were ICU LOS (n = 31, 43%) and hospital LOS (n = 29, 40%) (ESM eTable 11). Most studies (n = 52, 71%) reported some safety outcome. The most common safety outcome was barotrauma in ICU studies (26/39, 67%) and hemodynamic instability in OR studies (24/34, 71%) (ESM eTable 11). Four studies assessed outcomes related to costs and/or feasibility of certain strategies. One study qualitatively discussed the cost-effectiveness of a certain strategy,35 while three others measured the time involved with certain strategies.29,58,96
Discussion
In this scoping review, we synthesized 271 articles published over 48 years that reported assessments of methods of determining optimal PEEP in hospitalized individuals receiving mechanical ventilation. Among these studies, there were 22 different methods of selecting PEEP. Only 11 of these methods were studied with an RCT. Patients with ARDS in an ICU were most studied with 162 studies and 32 RCTs. There is a growing body of literature looking at patients ventilated in the OR, especially in the last decade, with 55 studies and 34 RCTs. The majority of RCTs set in the ICU measured a clinically relevant primary outcome such as mortality or duration of mechanical ventilation. Most RCTs done in the OR used a physiologic endpoint such as oxygenation or compliance as the primary outcome.
It is unsatisfying that, despite the large number of published studies, the very large number of participants studied, and a diversity of methods for determining optimal PEEP, the ideal strategy remains elusive. This is reflected in recommendations from recent guidelines on ARDS, which abstain from recommending which method should be used to set PEEP.113 This review illustrates several explanations as to why there is lack of consensus on which method is the best. One major driver is the variation in quantity and quality of research for each method, which makes it challenging to determine if any given one may be superior. For example, 11 methods have not been studied with an RCT. These methods were investigated with observational studies, many without comparator groups. It is difficult to evaluate these methods due to the higher risk of bias and confounding with these study designs. Furthermore, 83% (166/199) of the nonrandomized studies had fewer than 50 participants, which may limit power to detect differences in clinically meaningful outcomes. Nevertheless, these studies can be helpful in generating hypotheses that can be tested with randomized trials. Among those studied with an RCT, there is variability in population, setting, comparators, and outcomes, which limits the ability to pool and meta-analyze these studies.
Many systematic reviews have attempted to synthesize the data within specific populations, including patients with ARDS,11–15 ICU patients without ARDS,114,115 and patients undergoing surgery.116,117 Nevertheless, they often include studies that arbitrarily set PEEP at a certain level and have not directly compared specific strategies or methods of determining optimal PEEP. Instead, many systematic reviews elect to group PEEP levels into high or low groups. Li et al. is the only meta-analysis that compared “individualized PEEP” with PEEP set at arbitrary low, moderate, or high levels in the intraoperative setting, but did not distinguish between different methods used to individualize.116 The establishment of a superior method was not the objective of this scoping review; however, the breadth of methods in this scoping review and its associated variability in population, setting, and outcomes studied shows why equipoise remains.
This scoping review has helped identify important patient populations that could benefit from further study. We found an abundance of studies of patients with ARDS in the ICU (75% of overall studies and 82% of RCTs), but other populations were lacking in robust RCTs. For example, very few studies examined ICU patients without ARDS, those who were weaning from mechanical ventilation, or patients with chronic obstructive pulmonary disease (COPD). Only one RCT in the past 20 years has assessed a method of determining optimal PEEP in patients without ARDS in the ICU65 and one RCT of patients with obesity weaning from mechanical ventilation.33 Given that patients without ARDS or those who are weaning may be on spontaneous modes of mechanical ventilation, many of the methods described are difficult or impossible to perform in these clinical settings. We identified ten studies in patients with COPD, most of which used a patient’s auto-PEEP to determine the set PEEP.118–120 Neither that population nor method has been studied with a randomized trial. Future studies in these populations could inform guidelines and potentially simplify care if different methods of determining optimal PEEP have no influence on the outcomes of these patients.
This scoping review illustrates that many of the previous trials and systematic reviews have used mortality as the primary outcome.11,12,14 Of the RCTs that used similar tidal volumes between arms and measured mortality as the primary outcome, only two out of ten found a significant difference.25,57 In contrast to mortality, alternative outcomes like the duration of mechanical ventilation or a composite outcome such as VFD may be more illustrative of its benefits given the effects PEEP has on compliance, oxygenation, and recruitment. Ventilator-free days were measured in many RCTs, and this outcome has only been synthesized in terms of high vs low PEEP11,15 but not synthesized by method of determining optimal PEEP.
Many other outcomes are also important but are rarely measured. Cost-effectiveness and ease of implementation and use between methods is relevant, especially if there is equipoise between two methods on mortality or other clinical outcomes. For example, a PEEP-FIO2 table requires no extra equipment and takes little to no time to set PEEP. Contrast this with EIT, which requires special equipment and takes longer or a compliance-based method, which requires patients to be deeply sedated or paralyzed to prevent spontaneous efforts. If two methods have equivalent efficacy on mortality and other clinical outcomes but one is quicker and cheaper to implement, it could be argued that it is the superior method. We identified only three RCTs that measured the time associated with a specific PEEP method29,58,96 and none did a formal cost-analysis. In centres with limited staff and limited resources, these differences could be important for implementation and should be studied further.
Our review should be interpreted in the context of its limitations. We were unable to access several papers because of difficulties either obtaining or translating them (ESM eTable 12). Nevertheless, we were thorough and comprehensive in our attempts. For some articles that were inaccessible via our institutional access, we e-mailed authors (if an e-mail address was available) to request a copy of the manuscript. For non-English articles, we translated any PDFs that were compatible with Google Translate. Some articles could not be translated and thus were not included in the qualitative analysis. Another limitation is that, because we chose to perform a scoping review, we did not perform risk of bias assessments on the included studies or synthesize any of the outcomes as this is not the goal or purpose of a scoping review. In addition, although we included a large number of studies, many of the included studies were observational and not RCTs. Observational studies may show association but not causality and may also be at risk of bias because they cannot adjust for unmeasured confounders.
Our review also has several strengths. We developed a rigorous search strategy with a librarian and searched five databases. We also developed a framework and used predefined inclusion and exclusion criteria and assessed consistency in screening, inclusion, and data abstraction. We described not only RCTs but also nonrandomized studies pertaining to methods of determining optimal PEEP, thereby including many more methods. With the RCTs, we abstracted detailed information about the important primary and secondary outcomes, including less reported outcomes such as safety, feasibility, and cost.
Using scoping review methodology, we identified a spectrum of methods of determining optimal PEEP among mechanically ventilated patients. We included studies using 22 different methods from a variety of settings, with different populations, different study designs, and assessing different outcomes. We identified important gaps in the literature, including more robust nonmortality outcomes such as ventilator-free survival among patients with ARDS, as well as populations that warrant further study including patients with normal lungs as well as those on spontaneous modes of ventilation who are weaning from mechanical ventilation. Future studies should address these gaps and should incorporate measures of feasibility when comparing different methods.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Author contributions
Stefan Edginton, Ken Kuljit S. Parhar, and Helen Lee Robertson designed the search strategy. Stefan Edginton, Ken Kuljit S. Parhar, and Natalia Kruger performed the search and screening of articles. All authors contributed to development of the study protocol. Stefan Edginton and Ken Kuljit S. Parhar wrote the manuscript and all authors reviewed and edited the final manuscript. Ken Kuljit S. Parhar takes responsibility for the contents of the manuscript including all data and analysis.
Acknowledgements
We would like to thank Zahra A. Premji PhD MLIS (Health Research Librarian, Advanced Research Services, University of Victoria Libraries) for her help as the second librarian for the PRESS during the development of our search strategy.
Disclosures
All other authors declare that they have no financial disclosures or conflicts of interest.
Funding statement
This project did not receive any funding.
Prior conference presentations
Critical Care Canada Forum 2022 (23–25 November, Toronto, ON, Canada).
Editorial responsibility
This submission was handled by Dr. Patricia S. Fontela, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mélot C. Contribution of multiple inert gas elimination technique to pulmonary medicine. 5. Ventilation-perfusion relationships in acute respiratory failure. Thorax 1994; 49: 1251–8. 10.1136/thx.49.12.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2014; 370: 980. 10.1056/nejmc1400293 [DOI] [PubMed] [Google Scholar]
- 3.Ball L, Serpa Neto A, Trifiletti V, et al. Effects of higher PEEP and recruitment manoeuvres on mortality in patients with ARDS: a systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Intensive Care Med Exp 2020; 8: 39. 10.1186/s40635-020-00322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhurani RE, Oeckler RA, Franco PM, Jenkins SM, Gajic O, Pannu SR. Refractory hypoxemia and use of rescue strategies. A U.S. national survey of adult intensivists. Ann Am Thorac Soc 2016; 13: 1105–14. 10.1513/annalsats.201508-560oc [DOI] [PubMed] [Google Scholar]
- 5.Dickel S, Grimm C, Popp M, et al. A nationwide cross-sectional online survey on the treatment of COVID-19-ARDS: high variance in standard of care in German ICUs. J Clin Med 2021; 10: 3363. 10.3390/jcm10153363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dushianthan A, Cusack R, Chee N, Dunn JO, Grocott MP. Perceptions of diagnosis and management of patients with acute respiratory distress syndrome: a survey of United Kingdom intensive care physicians. BMC Anesthesiol 2014; 14: 87. 10.1186/1471-2253-14-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351: 327–36. 10.1056/nejmoa032193 [DOI] [PubMed] [Google Scholar]
- 8.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299: 646–55. 10.1001/jama.299.6.646 [DOI] [PubMed] [Google Scholar]
- 9.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299: 637–45. 10.1001/jama.299.6.637 [DOI] [PubMed] [Google Scholar]
- 10.Millington SJ, Cardinal P, Brochard L. Setting and titrating positive end-expiratory pressure. Chest 2022; 161: 1566–75. 10.1016/j.chest.2022.01.052 [DOI] [PubMed] [Google Scholar]
- 11.Dianti J, Tisminetzky M, Ferreyro BL, et al. Association of positive end-expiratory pressure and lung recruitment selection strategies with mortality in acute respiratory distress syndrome: a systematic review and network meta-analysis. Am J Respir Crit Care Med 2022; 205: 1300–10. 10.1164/rccm.202108-1972oc [DOI] [PubMed] [Google Scholar]
- 12.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010; 303: 865–73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 13.Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Respir Care 2011; 56: 568–75. 10.4187/respcare.01011 [DOI] [PubMed] [Google Scholar]
- 14.Sud S, Friedrich JO, Adhikari NKJ, et al. Comparative effectiveness of protective ventilation strategies for moderate and severe acute respiratory distress syndrome. A network meta-analysis. Am J Respir Crit Care Med 2021; 203: 1366–77. 10.1164/rccm.202008-3039oc [DOI] [PubMed] [Google Scholar]
- 15.Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 2017; 14: S297–303. 10.1513/annalsats.201704-338ot [DOI] [PubMed] [Google Scholar]
- 16.Amog K, Pham B, Courvoisier M, et al. The web-based "Right Review" tool asks reviewers simple questions to suggest methods from 41 knowledge synthesis methods. J Clin Epidemiol 2022; 147: 42–51. 10.1016/j.jclinepi.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 17.Edginton S, Kruger N, Stelfox HT, et al. Methods for determination of optimal positive end-expiratory pressure: a protocol for a scoping review. BMJ Open 2023; 13: e071871. 10.1136/bmjopen-2023-071871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32. 10.1080/1364557032000119616 [Google Scholar]
- 20.Peters MD, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020; 18: 2119–26. 10.11124/jbies-20-00167 [DOI] [PubMed] [Google Scholar]
- 21.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–73. 10.7326/m18-0850 [DOI] [PubMed] [Google Scholar]
- 22.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Google Translate. Homepage. Available from URL: https://translate.google.ca (accessed July 2024).
- 24.Jackson JL, Kuriyama A, Anton A, et al. The accuracy of Google Translate for abstracting data from non-English-language trials for systematic reviews. Ann Intern Med 2019; 171: 677–9. 10.7326/m19-0891 [DOI] [PubMed] [Google Scholar]
- 25.Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome—a randomized clinical trial. JAMA 2017; 318: 1335–45. 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kacmarek RM, Villar J, Sulemanji D, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med 2016; 44: 32–42. 10.1097/ccm.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 27.Khan NA, Saleem M, Ashfaq A, Yusuf M. Is the lung recruitment and titrated positive end expiratory pressure a better strategy as compare to low PEEP on mortality in patients with acute respiratory distress syndrome. Med Forum Mont 2018; 29: 93–7. Available from URL: http://medicalforummonthly.com/index.php/mfm/article/view/3032 (accessed July 2024).
- 28.Pintado MC, de Pablo R, Trascasa M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care 2013; 58: 1416–23. 10.4187/respcare.02068 [DOI] [PubMed] [Google Scholar]
- 29.Lam NN, Hung TD, Hung DK. Impact of "opening the lung" ventilatory strategy on burn patients with acute respiratory distress syndrome. Burns 2019; 45: 1841–7. 10.1016/j.burns.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 30.Veiga VC, Maia IS, onai C, Pincelli M, Cavalcanti AB. Comparison of 4 methods of positive end expiratory pressure (PEEP) titration in acute respiratory distress syndrome (ARDS): PEEP/FiO2 table, best compliance, esophageal catheter and electric impedance tomography (EIT): a randomized controlled trial. Am J Respir Crit Care Med 2020; 201: A1138. 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1138
- 31.Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care 2009; 13: R22. 10.1186/cc7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kung SC, Hung YL, Chen WL, Wang CM, Chang HC, Liu WL. Effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. J Clin Med 2019; 8: 231. 10.3390/jcm8020231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obi ON, Mazer M, Bangley C, et al. Obesity and weaning from mechanical ventilation—an exploratory study. Clin Med Insights Circ Respir Pulm Med 2018; 12: 1179548418801004. 10.1177/1179548418801004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrando C, Suarez-Sipmann F, Tusman G, et al. Open lung approach versus standard protective strategies: effects on driving pressure and ventilatory efficiency during anesthesia—a pilot, randomized controlled trial. PLoS One 2017; 12: e0177399. 10.1371/journal.pone.0177399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrando C, Soro M, Unzueta C, et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med 2018; 6: 193–203. 10.1016/s2213-2600(18)30024-9 [DOI] [PubMed] [Google Scholar]
- 36.Halawa NM, Elshafie MA, Fernandez JG, Metwally AA, Yassen KA. Respiratory and hemodynamic effects of prophylactic alveolar recruitment during liver transplant: a randomized controlled trial. Exp Clin Transplant 2021; 19: 462–72. 10.6002/ect.2020.0412 [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Wu X, Li J, et al. Individualized PEEP ventilation between tumor resection and dural suture in craniotomy. Clin Neurol Neurosurg 2020; 196: 106027. 10.1016/j.clineuro.2020.106027 [DOI] [PubMed] [Google Scholar]
- 38.Weber J, Gutjahr J, Schmidt J, et al. Effect of individualized PEEP titration guided by intratidal compliance profile analysis on regional ventilation assessed by electrical impedance tomography—a randomized controlled trial. BMC Anesthesiol 2020; 20: 42. 10.1186/s12871-020-00960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruszkai Z, Kiss E, László I, et al. Effects of intraoperative positive end-expiratory pressure optimization on respiratory mechanics and the inflammatory response: a randomized controlled trial. J Clin Monit Comput 2021; 35: 469–82. 10.1007/s10877-020-00519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon HK, Kim BR, Yoon S, Jeong YH, Ku JH, Kim WH. The effect of ventilation with individualized positive end‐expiratory pressure on postoperative atelectasis in patients undergoing robot‐assisted radical prostatectomy: a randomized controlled trial. J Clin Med 2021; 10: 850. 10.3390/jcm10040850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Huang X, Hu S, Meng Z, He H. Individualized lung protective ventilation vs. conventional ventilation during general anesthesia in laparoscopic total hysterectomy. Exp Ther Med 2020; 19: 3051–9. 10.3892/etm.2020.8549 [DOI] [PMC free article] [PubMed]
- 42.Zhang C, Xu F, Li W, et al. Driving pressure-guided individualized positive end-expiratory pressure in abdominal surgery: a randomized controlled trial. Anesth Analg 2021; 133: 1197–205. 10.1213/ane.0000000000005575 [DOI] [PubMed] [Google Scholar]
- 43.He X, Jiang J, Liu Y, et al. Electrical impedance tomography-guided PEEP titration in patients undergoing laparoscopic abdominal surgery. Medicine 2016; 95: e3306. 10.1097/md.0000000000003306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Hecke D, Bidgoli JS, Van der Linden P. Does lung compliance optimization through PEEP manipulations reduce the incidence of postoperative hypoxemia in laparoscopic bariatric surgery? A randomized trial. Obes Surg 2019; 29: 1268–75. 10.1007/s11695-018-03662-x [DOI] [PubMed] [Google Scholar]
- 45.Mahto H, Shenoy A, Shanbag V. Efficacy of recruitment manoeuvre with or without antiderecruitment strategy in ARDS patients: a prospective study. Ind J Resp Care 2013; 2: 284–91. [Google Scholar]
- 46.Liu J, Meng Z, Lv R, Zhang Y, Wang G, Xie J. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy. Braz J Med Biol Res 2019; 52: e8523. 10.1590/1414-431x20198523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Wang C, Lv R, et al. Protective mechanical ventilation with optimal PEEP during RARP improves oxygenation and pulmonary indexes. Trials 2021; 22: 351. 10.1186/s13063-021-05310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Bustamante A, Sprung J, Parker RA, et al. Individualized PEEP to optimise respiratory mechanics during abdominal surgery: a pilot randomised controlled trial. Br J Anaesth 2020; 125: 383–92. 10.1016/j.bja.2020.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-FIO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019; 321: 846–57. 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008; 359: 2095–104. 10.1056/nejmoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He H, Chi Y, Yang Y, et al. Early individualized positive end-expiratory pressure guided by electrical impedance tomography in acute respiratory distress syndrome: a randomized controlled clinical trial. Crit Care 2021; 25: 230. 10.1186/s13054-021-03645-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B, Wu B, Ran YN. A clinical study on mechanical ventilation PEEP setting for traumatic ARDS patients guided by esophageal pressure. Technol Health Care 2019; 27: 37–47. 10.3233/thc-181380 [DOI] [PubMed] [Google Scholar]
- 53.Constantin JM, Jabaudon M, Lefrant JY, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med 2019; 7: 870–80. 10.1016/s2213-2600(19)30138-9 [DOI] [PubMed] [Google Scholar]
- 54.Al Masry A, Boules ML, Boules NS, Ebied RS. Optimal method for selecting PEEP level in ALI/ARDS patients under mechanical ventilation. J Egypt Soc Parasitol 2012; 42: 359–72. 10.12816/0006323 [DOI] [PubMed]
- 55.Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care 2011; 15: R133. 10.1186/cc10249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgson CL, Cooper DJ, Arabi Y, et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP). A phase ii, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med 2019; 200: 1363–72. 10.1164/rccm.201901-0109oc [DOI] [PubMed]
- 57.Hamama KM, Fathy SM, AbdAlrahman RS, Alsherif SE, Ahmed SA. Driving pressure-guided ventilation versus protective lung ventilation in ARDS patients: a prospective randomized controlled study. Egypt J Anaesth 2021; 37: 261–7. 10.1080/11101849.2021.1930401 [Google Scholar]
- 58.Samadder M, Agarwal A, Verma AK, Gehlot S. Comparison of clinical outcome of lung recruitment by PEEP/FIO2 incremental method and by using ultrasonography. Indian J Anaesth 2020; 64: S41–7. Available from URL: https://journals.lww.com/ijaweb/toc/2020/64001 (accessed July 2024).
- 59.Salem MS, Eltatawy HS, Abdelhafez AA, Alsherif SE. Lung ultrasound- versus FiO2-guided PEEP in ARDS patients. Egypt J Anaesth 2020; 36: 31–7. 10.1080/11101849.2020.1741253 [Google Scholar]
- 60.Falde S, Gali B, Brown D, et al. Mechanical ventilation guided by transpulmonary and airway driving pressures in the setting of intraabdominal hypertension. Chest 2020; 157: A379. 10.1016/j.chest.2020.05.424 [Google Scholar]
- 61.Wang R, Sun B, Li X, et al. Mechanical ventilation strategy guided by transpulmonary pressure in severe acute respiratory distress syndrome treated with venovenous extracorporeal membrane oxygenation. Crit Care Med 2020; 48: 1280–8. 10.1097/ccm.0000000000004445 [DOI] [PubMed] [Google Scholar]
- 62.Cammarota G, Lauro G, Sguazzotti I, et al. Esophageal pressure versus gas exchange to set PEEP during intraoperative ventilation. Respir Care 2020; 65: 625–35. 10.4187/respcare.07238 [DOI] [PubMed] [Google Scholar]
- 63.Piriyapatsom A, Phetkampang S. Effects of intra-operative positive end-expiratory pressure setting guided by oesophageal pressure measurement on oxygenation and respiratory mechanics during laparoscopic gynaecological surgery: a randomised controlled trial. Eur J Anaesthesiol 2020; 37: 1032–9. 10.1097/eja.0000000000001204 [DOI] [PubMed] [Google Scholar]
- 64.Nelson LD, Civetta JM, Hudson-Civetta J. Titrating positive end-expiratory pressure therapy in patients with early, moderate arterial hypoxemia. Crit Care Med 1987; 15: 14–9. 10.1097/00003246-198701000-00003 [DOI] [PubMed] [Google Scholar]
- 65.Algera AG, Pisani L, Serpa Neto A, et al. Effect of a lower vs higher positive end-expiratory pressure strategy on ventilator-free days in ICU patients without ARDS: a randomized clinical trial. JAMA 2020; 324: 2509–20. 10.1001/jama.2020.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll GC, Tuman KJ, Braverman B, et al. Minimal positive end-expiratory pressure (PEEP) may be "best PEEP." Chest 1988; 93: 1020–5. 10.1378/chest.93.5.1020 [DOI] [PubMed] [Google Scholar]
- 67.Rollas K, Hanci P, Topeli A. Effects of end-expiratory lung volume versus PaO2 guided PEEP determination on respiratory mechanics and oxygenation in moderate to severe ARDS. Exp Lung Res 2022; 48: 12–22. 10.1080/01902148.2021.2021326 [DOI] [PubMed] [Google Scholar]
- 68.Nestler C, Simon P, Petroff D, et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 2017; 119: 1194–205. 10.1093/bja/aex192 [DOI] [PubMed] [Google Scholar]
- 69.Hsu HJ, Chang HT, Zhao Z, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: a randomized trial in moderate to severe ARDS. Physiol Meas 2021; 42: 014002. 10.1088/1361-6579/abd679 [DOI] [PubMed] [Google Scholar]
- 70.Pereira SM, Tucci MR, Morais CC, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 2019; 129: 1070–81. 10.1097/aln.0000000000002435 [DOI] [PubMed] [Google Scholar]
- 71.Girrbach F, Petroff D, Schulz S, et al. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: a prospective, randomised controlled clinical trial. Br J Anaesth 2020; 125: 373–82. 10.1016/j.bja.2020.05.041 [DOI] [PubMed] [Google Scholar]
- 72.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338: 347–54. 10.1056/nejm199802053380602 [DOI] [PubMed] [Google Scholar]
- 73.Carvalho CR, Barbas CS, Medeiros DM, et al. Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med 1997; 156: 1458–66. 10.1164/ajrccm.156.5.9604081 [DOI] [PubMed] [Google Scholar]
- 74.Villar J, Kacmarek RM, Perez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006; 34: 1311–8. 10.1097/01.ccm.0000215598.84885.01 [DOI] [PubMed] [Google Scholar]
- 75.Saxena S, Tripathi M, Kumar V, Malviya D, Harjai M, Rai S. Study of tidal volume and positive end-expiratory pressure on alveolar recruitment using spiro dynamics in mechanically ventilated patients. Anesth Essays Res 2020; 14: 154–9. 10.4103/aer.aer_10_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dyhr T, Laursen N, Larsson A. Effects of lung recruitment maneuver and positive end-expiratory pressure on lung volume, respiratory mechanics and alveolar gas mixing in patients ventilated after cardiac surgery. Acta Anaesthesiol Scand 2002; 46: 717–25. 10.1034/j.1399-6576.2002.460615.x [DOI] [PubMed] [Google Scholar]
- 77.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372: 747–55. 10.1056/nejmsa1410639 [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Zhang M, Wang X, Shang G, Dong Y. Individualized positive end-expiratory pressure in patients undergoing thoracoscopic lobectomy: a randomized controlled trial. Braz J Anesthesiol 2021; 71: 565–71. 10.1016/j.bjane.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mini G, Ray BR, Anand RK, et al. Effect of driving pressure-guided positive end-expiratory pressure (PEEP) titration on postoperative lung atelectasis in adult patients undergoing elective major abdominal surgery: a randomized controlled trial. Surgery 2021; 170: 277–83. 10.1016/j.surg.2021.01.047 [DOI] [PubMed] [Google Scholar]
- 80.Komnov R, Eremenko A. Intellivent-ASV mode is superior to conventional ventilation modes after uncomplicated cardiac surgery during all phases of postoperative respiratory support. Intensive Care Med Exper 2020; 8: 73. 10.1186/s40635-020-00354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Passath C, Takala J, Tuchscherer D, Jakob SM, Sinderby C, Brander L. Physiologic response to changing positive end-expiratory pressure during neurally adjusted ventilatory assist in sedated, critically ill adults. Chest 2010; 138: 578–87. 10.1378/chest.10-0286 [DOI] [PubMed] [Google Scholar]
- 82.Blecha S, Hager A, Gross V, et al. Effects of individualised high positive end-expiratory pressure and crystalloid administration on postoperative pulmonary function in patients undergoing robotic-assisted radical prostatectomy: a prospective randomised single-blinded pilot study. J Clin Med 2023; 12: 1460. 10.3390/jcm12041460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ernest E, Bhattacharjee S, Baidya DK, et al. Effect of incremental PEEP titration on postoperative pulmonary complications in patients undergoing emergency laparotomy: a randomized controlled trial. J Clin Monit Comput 2024; 38: 445–54. 10.1007/s10877-023-01091-5 [DOI] [PubMed] [Google Scholar]
- 84.Gao L, Yang L, Pan L, Cui Y, Zhang J. Optimal positive end-expiratory pressure obtained with titration of a fraction of inspiratory oxygen: a randomized controlled clinical trial. Ann Transl Med 2023; 11: 203. 10.21037/atm-22-4357 [DOI] [PMC free article] [PubMed]
- 85.Guervilly C, Fournier T, Chommeloux J, et al. Ultra-lung-protective ventilation and biotrauma in severe ARDS patients on veno-venous extracorporeal membrane oxygenation: a randomized controlled study. Crit Care 2022; 26: 383. 10.1186/s13054-022-04272-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin D, Liu H, Kong X, et al. Effects of driving pressure-guided ventilation on postoperative pulmonary complications in prone-positioned patients undergoing spinal surgery: a randomized controlled clinical trial. J Invest Surg 2022; 35: 1754–60. 10.1080/08941939.2022.2107250 [DOI] [PubMed] [Google Scholar]
- 87.Li J, Ma S, Chang X, et al. Effect of pressure-controlled ventilation-volume guaranteed mode combined with individualized positive end-expiratory pressure on respiratory mechanics, oxygenation and lung injury in patients undergoing laparoscopic surgery in Trendelenburg position. J Clin Monit Comput 2022; 36: 1155–64. 10.1007/s10877-021-00750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moghaddas-Ghahfarrokhi M, Rashidi M, Asadizaker M, Adineh M, Ghanbari S. The effect of adjustment of positive end-expiratory pressure (PEEP) and fraction of inspired oxygen (FiO2) based on arterial blood oxygen pressure on the care and treatment outcomes of patients in intensive care unit (ICU). J Isfahan Med School 2022; 39: 894–900. 10.22122/jims.v39i651.14317
- 89.Pan LL, Gao LL, Yang L, et al. Effect of EIT-guided individualized PEEP setting on the incidence of hypoxemia in elderly patients undergoing robot-assisted radical prostatectomy [Chinese]. Zhonghua Yi Xue Za Zhi 2022; 102: 3727–33. 10.3760/cma.j.cn112137-20220415-00818 [DOI] [PubMed] [Google Scholar]
- 90.Peyton PJ, Aitken S, Wallin M. Effects of an open lung ventilatory strategy on lung gas exchange during laparoscopic surgery. Anaesth Intensive Care 2022; 50: 281–8. 10.1177/0310057x211047602 [DOI] [PubMed] [Google Scholar]
- 91.Qian M, Yang F, Zhao L, Shen J, Xie Y. Individualized positive end-expiratory pressure titration on respiration and circulation in elderly patients undergoing spinal surgery in prone position under general anesthesia. Am J Transl Res 2021; 13: 13835–44. [PMC free article] [PubMed] [Google Scholar]
- 92.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999; 282: 54–61. 10.1001/jama.282.1.54 [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Gong C, Zhang Y, et al. Intelligent algorithm-based echocardiography to evaluate the effect of lung protective ventilation strategy on cardiac function and hemodynamics in patients undergoing laparoscopic surgery. Comput Math Methods Med 2022; 2022: 9349027. 10.1155/2022/9349027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Wang X, Wang H, Wang H, Li S, Chen L. Intraoperative right heart function with individualized mechanical ventilation in laparoscopic surgery with Trendelenburg positioning: a randomized-controlled study. Heart Lung 2023; 58: 185–90. 10.1016/j.hrtlng.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 95.Xu Q, Guo X, Liu J, et al. Effects of dynamic individualized PEEP guided by driving pressure in laparoscopic surgery on postoperative atelectasis in elderly patients: a prospective randomized controlled trial. BMC Anesthesiol 2022; 22: 72. 10.1186/s12871-022-01613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yeremenko AA, Komnov RD, Koshek EA. The efficacy and safety of automatic modes during respiratory support after cardiac surgery. Obshchaya Reanimatologiya 2022; 18: 21–9. 10.15360/1813-9779-2022-3-21-29
- 97.Zhang W, Liu F, Zhao Z, et al. Driving pressure-guided ventilation improves homogeneity in lung gas distribution for gynecological laparoscopy: a randomized controlled trial. Sci Rep 2022; 12: 21687. 10.1038/s41598-022-26144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 1975; 292: 284–9. 10.1056/nejm197502062920604 [DOI] [PubMed] [Google Scholar]
- 99.Yin C, Gao X, Cao C, Xu L, Lu X. Individualized positive end-expiratory pressure setting in patients with severe acute respiratory distress syndrome supported with veno-venous extracorporeal membrane oxygenation. Perfusion 2020; 36: 374–81. 10.1177/0267659120946728 [DOI] [PubMed] [Google Scholar]
- 100.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8. 10.1056/nejm200005043421801 [DOI] [PubMed] [Google Scholar]
- 101.Kallet RH, Branson RD. Respiratory controversies in the critical care setting. Do the NIH ARDS Clinical Trials Network PEEP/FIO2 tables provide the best evidence-based guide to balancing PEEP and FIO2 settings in adults? Respir Care 2007; 52: 461–75. [PubMed]
- 102.Valk CM, Tsonas AM, Botta M, et al. Association of early positive end-expiratory pressure settings with ventilator-free days in patients with coronavirus disease 2019 acute respiratory distress syndrome: a secondary analysis of the practice of VENTilation in COVID-19 study. Eur J Anaesthesiol 2021; 38: 1274–83. 10.1097/eja.000000000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erlandsson K, Odenstedt H, Lundin S, Stenqvist O. Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery. Acta Anaesthesiol Scand 2006; 50: 833–9. 10.1111/j.1399-6576.2006.01079.x [DOI] [PubMed] [Google Scholar]
- 104.Long Y, Liu DW, He HW, Zhao ZQ. Positive end-expiratory pressure titration after alveolar recruitment directed by electrical impedance tomography. Chin Med J (Engl) 2015; 128: 1421–7. 10.4103/0366-6999.157626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Z, Chang MY, Chang MY, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care 2019; 9: 7. 10.1186/s13613-019-0484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarge T, Baedorf-Kassis E, Banner-Goodspeed V, et al. Effect of esophageal pressure-guided positive end-expiratory pressure on survival from acute respiratory distress syndrome: a risk-based and mechanistic reanalysis of the EPVent-2 trial. Am J Respir Crit Care Med 2021; 204: 1153–63. 10.1164/rccm.202009-3539oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Estoos EM, Jocham KP, Zhang C, Benson LM, Milas A, Zakhary B. Optimal positive end-expiratory pressure reduces right ventricular dysfunction in COVID-19 patients on venovenous extracorporeal membrane oxygenation: a retrospective single-center study. J Crit Care 2023; 75: 154274. 10.1016/j.jcrc.2023.154274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tremper KK, Waxman K, Shoemaker WC. Use of transcutaneous oxygen sensors to titrate PEEP. Ann Surg 1981; 193: 206–9. 10.1097/00000658-198102000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radwan WA, Khaled MM, Salman AG, Fakher MA, Khatab S. Use of lung ultrasound for assessment of lung recruitment maneuvers in patients with ARDS. Open Access Maced J Med Sci 2021; 9: 952–63. 10.3889/oamjms.2021.6883 [Google Scholar]
- 110.Blanch L, Fernández R, Benito S, Mancebo J, Net A. Effect of PEEP on the arterial minus end-tidal carbon dioxide gradient. Chest 1987; 92: 451–4. 10.1378/chest.92.3.451 [DOI] [PubMed] [Google Scholar]
- 111.Pirrone M, Fisher D, Chipman D, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med 2016; 44: 300–7. 10.1097/ccm.0000000000001387 [DOI] [PubMed] [Google Scholar]
- 112.Richard JC, Brochard L, Vandelet P, et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med 2003; 31: 89–92. 10.1097/00003246-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 113.Grasselli G, Calfee CS, Camporota L, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 2023; 49: 727–59. 10.1007/s00134-023-07050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yi H, Li X, Mao Z, et al. Higher PEEP versus lower PEEP strategies for patients in ICU without acute respiratory distress syndrome: a systematic review and meta-analysis. J Crit Care 2022; 67: 72–8. 10.1016/j.jcrc.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 115.Pettenuzzo T, Boscolo A, De Cassai A, et al. Higher versus lower positive end-expiratory pressure in patients without acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care 2021; 25: 247. 10.1186/s13054-021-03669-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li X, Ni ZL, Wang J, et al. Effects of individualized positive end-expiratory pressure combined with recruitment maneuver on intraoperative ventilation during abdominal surgery: a systematic review and network meta-analysis of randomized controlled trials. J Anesth 2022; 36: 303–15. 10.1007/s00540-021-03012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P, Wu L, Shi X, et al. Positive end-expiratory pressure during anesthesia for prevention of postoperative pulmonary complications: a meta-analysis with trial sequential analysis of randomized controlled trials. Anesth Analg 2020; 130: 879–89. 10.1213/ane.0000000000004421 [DOI] [PubMed] [Google Scholar]
- 118.Liang G, Zhang Z. Positive end expiratory pressure titration guided by plateau pressure in chronic obstructive pulmonary disease patients. Clin Respir J 2018; 12: 674–80. 10.1111/crj.12578 [DOI] [PubMed] [Google Scholar]
- 119.Aerts J, van den Berg B, Verbraak AF, Bogaard JM. Elastic work of breathing during continuous positive airway pressure in intubated patients with chronic obstructive pulmonary disease (theoretical analysis and experimental validation). Acta Anaesthesiol Scand 1997; 41: 607–13. 10.1111/j.1399-6576.1997.tb04751.x [DOI] [PubMed] [Google Scholar]
- 120.Rossi A, Santos C, Roca J, Torres A, Félez MA, Rodriguez-Roisin R. Effects of PEEP on VA/Q mismatching in ventilated patients with chronic airflow obstruction. Am J Respir Crit Care Med 1994; 149: 1077–84. 10.1164/ajrccm.149.5.8173744 [DOI] [PubMed] [Google Scholar]
- 121.Tang KQ, Yang SL, Zhang B, et al. Ultrasonic monitoring in the assessment of pulmonary recruitment and the best positive end-expiratory pressure. Medicine (Baltimore) 2017; 96: e8168. 10.1097/md.0000000000008168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elshazly M, Khair T, Bassem M, Mansour M. The use of intraoperative bedside lung ultrasound in optimizing positive end expiratory pressure in obese patients undergoing laparoscopic bariatric surgeries. Surg Obes Relat Dis 2021; 17: 372–8. 10.1016/j.soard.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 123.Cole AG, Weller SF, Sykes MK. Inverse ratio ventilation compared with PEEP in adult respiratory failure. Intensive Care Med 1984; 10: 227–32. 10.1007/bf00256258 [DOI] [PubMed] [Google Scholar]
- 124.Maisch S, Reissmann H, Fuellekrug B, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg 2008; 106: 175–81. 10.1213/01.ane.0000287684.74505.49 [DOI] [PubMed] [Google Scholar]
- 125.Tusman G, Groisman I, Fiolo FE, et al. Noninvasive monitoring of lung recruitment maneuvers in morbidly obese patients: the role of pulse oximetry and volumetric capnography. Anesth Analg 2014; 118: 137–44. 10.1213/01.ane.0000438350.29240.08 [DOI] [PubMed] [Google Scholar]
- 126.Fengmei G, Jin C, Songqiao L, Congshan Y, Yi Y. Dead space fraction changes during PEEP titration following lung recruitment in patients with ARDS. Respir Care 2012; 57: 1578–85. 10.4187/respcare.01497 [DOI] [PubMed] [Google Scholar]
- 127.Regli A, De Keulenaer BL, Palermo A, van Heerden PV. Positive end-expiratory pressure adjusted for intra-abdominal pressure—a pilot study. J Crit Care 2018; 43: 390–4. 10.1016/j.jcrc.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 128.Mazzinari G, Diaz-Cambronero O, Alonso-Iñigo JM, et al. Intraabdominal pressure targeted positive end-expiratory pressure during laparoscopic surgery: an open-label, nonrandomized, crossover, clinical trial. Anesthesiology 2020; 132: 667–77. 10.1097/aln.0000000000003146 [DOI] [PubMed] [Google Scholar]
- 129.Singer M, Bennett D. Optimisation of positive and expiratory pressure for maximal delivery of oxygen to tissues using oesophageal Doppler ultrasonography. BMJ 1989; 298: 1350–3. 10.1136/bmj.298.6684.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chimot L, Fedun Y, Gacouin A, et al. Optimization of positive end-expiratory pressure targeting the best arterial oxygen transport in the acute respiratory distress syndrome: the OPTIPEP study. ASAIO J 2017; 63: 450–5. 10.1097/mat.0000000000000496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.