Abstract
Open-lung ventilation during cardiopulmonary bypass (CPB) in patients undergoing heart transplantation (HTx) is a potential strategy to mitigate postoperative acute respiratory distress syndrome (ARDS). We utilized an ovine HTx model to investigate whether open-lung ventilation during CPB reduces postoperative lung damage and complications. Eighteen sheep from an ovine HTx model were included, with ventilatory interventions randomly assigned during CPB: the OPENVENT group received low tidal volume (VT) of 3 mL/kg and positive end-expiratory pressure (PEEP) of 8 cm H20, while no ventilation was provided in the NOVENT group as per standard of care. The recipient sheep were monitored for 6 h post-surgery. The primary outcome was histological lung damage, scored at the end of the study. Secondary outcomes included pulmonary shunt, driving pressure, hemodynamics and inflammatory lung infiltration. All animals completed the study. The OPENVENT group showed significantly lower histological lung damage versus the NOVENT group (0.22 vs 0.27, p = 0.042) and lower pulmonary shunt (19.2 vs 32.1%, p = 0.001). In addition, the OPENVENT group exhibited a reduced driving pressure (9.6 cm H2O vs. 12.8 cm H2O, p = 0.039), lower neutrophil (5.25% vs 7.97%, p ≤ 0.001) and macrophage infiltrations (11.1% vs 19.6%, p < 0.001). No significant differences were observed in hemodynamic parameters. In an ovine model of HTx, open-lung ventilation during CPB significantly reduced lung histological injury and inflammatory infiltration. This highlights the value of an open-lung approach during CPB and emphasizes the need for further clinical evidence to decrease risks of lung injury in HTx patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40635-024-00669-w.
Keywords: Ventilation, Cardiopulmonary bypass, Heart transplant
Introduction
Cardiopulmonary bypass (CPB) is an extracorporeal cardio-respiratory support strategy during cardiac surgical procedures. CPB allows the heart to be isolated from the systemic circulation, creating a bloodless surgical field while maintaining control of perfusion, gas exchange and temperature. Despite this support, mild–moderate acute respiratory distress syndrome (ARDS) is a common postoperative complication following cardiac surgery and is associated with increased morbidity and mortality [1, 2].
The pathophysiology of ARDS post CPB is complex and multifactorial [3]. CPB-related systemic inflammatory response syndrome (SIRS) is triggered through exposure of blood to the extracorporeal circuit and contributes to the parenchymal damage and pulmonary capillary dysfunction [4–7]. Localized lung damage can be caused by ischemia during surgery and the subsequent ischemia–reperfusion injury following the restoration of blood flow [8, 9]. Lung deflation is commonly implemented during CPB to optimize the surgical field, and can further amplify local damage leading to atelectasis and decreased alveolar surfactant production [4, 10]. Consequently, following CPB, patients may present with substantial derangements in pulmonary mechanics and gas exchange which lead to ARDS [11, 12].
Patients requiring heart transplantation (HTx) represent an especially vulnerable surgical cohort. The prolonged use of CPB, transplanted heart function, and intraoperative long-term immunosuppression all contribute to a significantly increased risk of postoperative pulmonary complications [1, 2, 13]. The risk of ARDS associated with HTx surgery could be mitigated by preventing lung deflation, through implementation of open-lung ventilation strategies during CPB [14]. The combination of positive end-expiratory pressure (PEEP) and low tidal volume (VT) optimizes alveolar recruitment, while minimizing lung movement and ventilator-induced lung injury (VILI). Recent animal and human studies have demonstrated that continuation of open-lung ventilation through CPB can improve gas exchange, while reducing inflammation, endothelial dysfunction and the occurrence of postoperative complications [11, 15–22]. Despite this promising evidence, the recent large-scale PROVECS and MECANO trials reported no improvement in postoperative pulmonary complications, after the implementation of an open-lung ventilation strategy [23, 24]. While these trials recruited patients undergoing a variety of cardiac surgical procedures, patients undergoing HTx procedures were not included. The evidence regarding the effectiveness of open-lung ventilation during CPB to reduce postoperative pulmonary complications is mixed, and there is a paucity of data regarding its specific use during HTx surgery.
We have previously developed a clinically relevant sheep model of orthotopic HTx to investigate the application of hypothermic oxygenated perfusion (HOPE) to donor heart preservation [25, 26]. During the HOPE preservation study, we simultaneously investigated the application of open-lung ventilation during CPB, hypothesizing that it could further reduce postoperative pulmonary complications. We performed a single-blinded, randomized controlled trial, using an ovine HTx model to confirm that open-lung ventilation could mitigate histologically confirmed postoperative lung damage and decrease short-term postoperative pulmonary complications.
Methods
Animals and ethics
An ovine HTx model was performed using pairs of matched female sheep. In this model, donor sheep were either brain stem death (BD) or brain stem viable (sham) and monitored for 24 h prior to cardiac surgery. After heart excision, the donor heart was preserved in either static cold storage (SCS) for 2 h or HOPE for 2 or 8 h. Further details regarding the HTx model and the novel HOPE methods have been previously reported [26].
Eighteen recipient sheep from the ovine HTx model were included in our study. Animals reported in this study represent a subset of those presented in the previously published HOPE trial [25, 26]. All recipient animals were placed on CPB during surgery, and all surgical procedures were performed by qualified personnel. The project was approved by Queensland University of Technology (QUT) Animal Ethics Committee (Approval #16–1109). Ratified by the University of Queensland AEC (QUT/393/17/QUT), experiments were performed in accordance with the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (8th Edition 2013), the Animal Care and Protection Act 2001 (QLD) and complied with the ARRIVE Guidelines.
Anesthesia and surgery
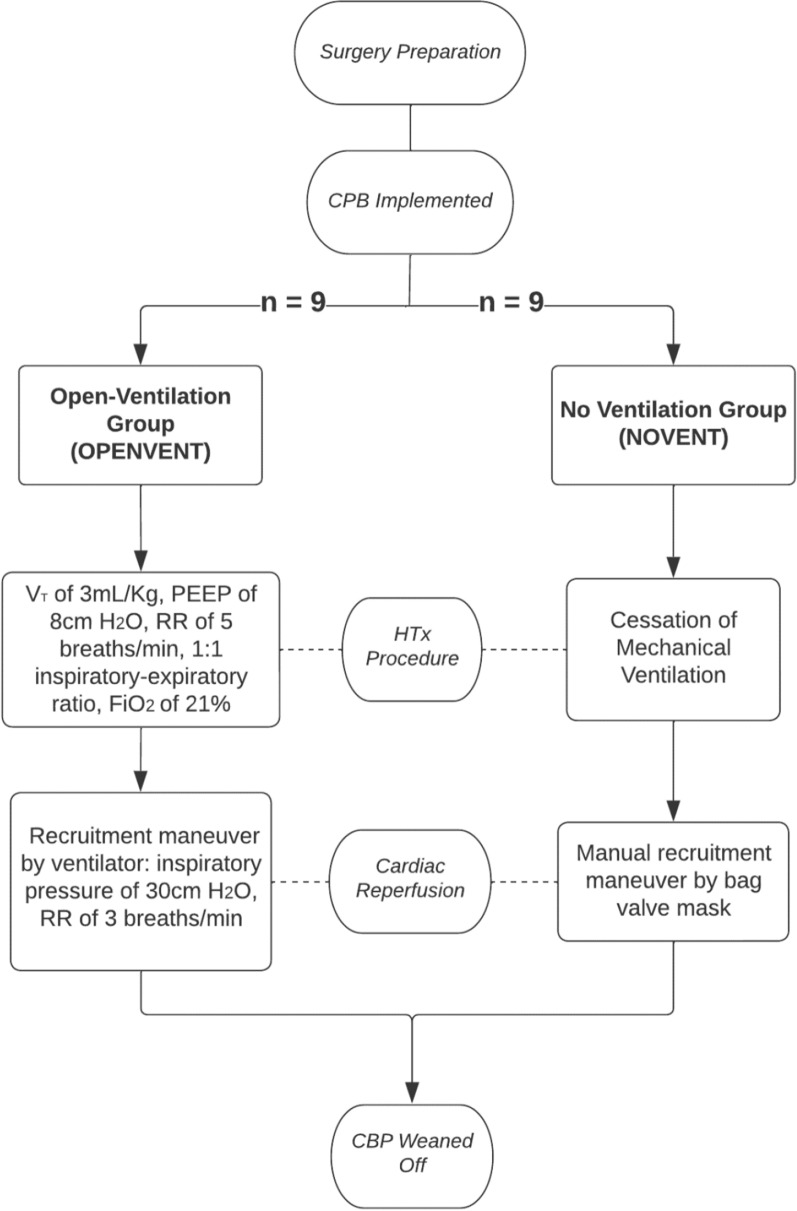
Electrocardiography (ECG), pulse oximetry, and capnography were monitored throughout the study. Anesthesia in recipient sheep was induced by intravenous propofol injection (3–4 mg/kg), followed by orotracheal intubation and placement on a surgical table in the supine position. Anesthesia was maintained with continuous intravenous infusions of fentanyl (5-15 µg/kg/h), midazolam (0.5–0.8 mg/kg/h), and ketamine (2.5–7.5 mg/kg/h). Initially, the animals were mechanically ventilated with a VT of 8 ml/kg and PEEP of 5 cm H2O to achieve arterial oxygen saturation of 92% [27, 28]. Respiratory rate (RR) and fraction of inspired oxygen (FiO2) were further titrated to normalize pH, arterial partial pressure of carbon dioxide (PaCO2) and arterial partial pressure of oxygen (PaO2). A Swan–Ganz catheter was advanced from the right jugular vein to the pulmonary artery to continuously measure cardiac output and mixed venous oxygen saturation. Pulmonary artery pressure and pulmonary vascular resistance were recorded throughout the study. Following administration of vecuronium (0.1 mg/kg IV) and optimization of anesthesia, a median sternotomy was performed. Heparin was administered (100–300 U/kg) to achieve an activated clotting time > 400 s. After cannulation of the inferior vena cava, superior vena cava and aortic arch, CPB was commenced. The target flow rate was 50–60 ml/kg to achieve a mean arterial pressure of > 60 mmHg. Once CPB was stabilized within these parameters, the aorta was cross-clamped and both vena cavae were snared allowing excision of the native heart. The donor heart, from another cross-matched, immune-compatible sheep was then orthotopically transplanted. Using pentobarbitone (0.5 mL/kg), both donor and recipient animals were humanely euthanized at completion of study. During CPB and the transplant procedure in the recipient sheep, ventilatory interventions were applied to the sheep according to the randomly assigned experimental groups (Fig. 1).
The Open-Lung Ventilation Group (OPENVENT) was ventilated from the commencement of CPB with a VT = 3 mL/kg, PEEP = 8 cm H2O, RR = 5 breaths/min, 21% FiO2, and 1:1 inspiratory/expiratory ratio [23, 24]. These settings ensured minimal disturbance to the surgical field, while allowing the lungs to remain open during CPB. During donor heart reperfusion, the ventilator was used to perform recruitment maneuvers with an inspiratory pressure of 30 cm H2O and RR of 3 breaths/min. Inspiratory/expiratory ratio of 1:1 was maintained during the procedure.
The No Ventilation Group (NOVENT) received no ventilatory intervention during CPB, resulting in lung deflation, and no airway pressure monitoring was applied. During cardiac reperfusion, recruitment maneuvers were performed manually with a bag valve mask connected to the endotracheal tube and with PEEP valve set at 7.5 cm H2O. Notably, as in clinical practice, airway pressure was not monitored during the recruitment phase. Instead, we ensured lung re-expansion by observing the surgical field.
Fig. 1.
Flowchart describing experimental groups (RR respiratory rate, VT tidal volume, PEEP positive end-expiratory pressure, HTx heart transplant). Created with LucidCharts.com
Upon completion of the anastomoses, the animals were rewarmed using CPB and methylprednisolone (250 mg) was administered intravenously. The aortic cross clamp was removed to reperfuse the cardiac allograft. The heart was rested for 30 min, with inotropic, chronotropic, and vasopressor supports administered as dictated by echocardiographic and hemodynamic cardiac function. Following weaning from CPB, protamine was administered and mechanical ventilation resumed in both groups during the postoperative monitoring period with the following settings: VT = 6 mL/kg, RR = 12–20 breaths/min to achieve PaCO2 of 36-44 mmHg, 1:2 inspiratory/expiratory ratio, and PEEP/FiO2 adjusted to achieve at least PaO2 = 100 mmHg [29, 30]. Lung-protective ventilation with abovementioned settings were applied to reduce risk of VILI in the postoperative period. The sternotomy wound and chest wall was left open during the postoperative period. Monitoring and management of recipient sheep continued for 6 h following separation from CPB, with regular assessment of pulmonary and cardiac functions. Animals were humanely euthanized at the end of the 6-h monitoring period, which was determined by the orthotopic HTx and HOPE preservation main study.
Sampling and analyses
Data were collected from preoperative baseline recordings (BSL) and hourly in the 6-h postoperative monitoring period following completion of transplant: T0 (successful weaning from CPB), T0.5, T1, T2, T3, T4, T6 (6 h post-transplant). Arterial and mixed venous blood gases, ventilation and hemodynamics were recorded for all timepoints. Blood gas analyses were recorded using the ABL800 Flex Blood Gas Analyzer (Radiometer). Full blood counts and biochemistry of whole blood (EDTA) and plasma samples were assessed externally (IDEXX Laboratories, Brisbane, Australia). Hematological profiles and blood biochemistry were analyzed on a Sysmex XT2000i-V hematology analyzer, and a Beckman Coulter AU680 ISE chemistry analyzer, respectively. A PowerLab data acquisition system (model ML880) was utilized in conjunction with Labchart 7 (AD Instruments, Bella Vista, Australia) to record hemodynamics. Continuous cardiac output, mean arterial pressure, heart rate, end tidal carbon dioxide (ETCO2), oxygen saturation (SPO2) and mixed venous oxygen saturation (SVO2) were continuously measured through both the Vigilance II Monitor (Edward Lifesciences CA, USA) and the Marquette Solar 8000 (GE Healthcare ILL USA). Following sheep euthanasia, the lungs were excised and dissected. Histological and frozen samples were taken from each lobe for further analysis (right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), left lower lobe (LLL)).
Outcomes
Primary outcome
The primary outcome was histological lung damage. Tissue samples for histological analysis were dissected immediately after excision of lungs from euthanized recipient sheep and fixed in 10% buffered formalin for 24 h. 5 samples were taken from each lobe. Processed samples were embedded in paraffin, sectioned to 5 μm thickness and stained with hematoxylin and eosin (H&E). A Brightfield light microscope was used by a single senior veterinary pathologist blinded to treatment allocation to examine histological lung damage, using a modified scoring system from the American Thoracic Society [31]. Macroscopically, examination and sampling were targeted to the most damaged regions. Eleven criteria were utilized in a scoring system adapted from Kulkarni et al. [31], which assessed the debris in airspaces, alveolar epithelial injury and thickening, presence of neutrophils, thrombi, capillary damage, atelectasis and septal muscle hypertrophy. The scoring system is shown in greater detail in Table E2 (Supplementary Material). Lung slides were scored from 0 to 2 dependent on their features, and 10 lung fields were assessed to produce a mean damage score for each lobe.
Secondary outcomes
Secondary outcomes comprised several postoperative pulmonary complications, which can be broadly defined as any complication involving the respiratory system occurring after surgery. Pulmonary inflammation was determined through quantification of neutrophil and macrophage infiltration, using immunohistochemistry (IHC) and ImageJ. ImageJ is a widely used Java-based image processing program that was developed by the National Institutes of Health, Bethesda, MD, USA. It is an open-source software, and facilitates the visualization, inspection and quantification of scientific image data [32–35]. Images were collected using the AxioImager.Z1 motorized upright microscope (Carl Zeiss) and visualized using ZEN 2.0 (blue edition) (Carl Zeiss). In-house enzyme linked immunosorbent assays (ELISAs) were performed to detect interleukin 8 (IL-8) per previously published protocols [36]. Ventilatory parameters were recorded via the Te Hamilton-G5 ventilator at 100 Hz, and functional measurements of gas exchange and pulmonary mechanics were calculated. Epicardial echocardiography was performed in recipients at baseline (BSL), and during the post-monitoring period at T0, T1, T3 and T6 (completion of transplant). An X5-1 transducer with a spacer connected to an IE-33 ultrasound scanner (Philips, Bothell, WA, USA) was used for image obtainment. Analysis was performed to collect end-diastolic area (EDA), end-systolic area (ESA), fractional area change (FAC), endo-myocardial global circumferential strain (EndoGCS) and global radial strain (GRS). Further data were collected regarding hemodynamics, oxygen delivery/consumption, arterial lactate, and base excess. Extended description of the methods and calculations used to measure the secondary outcomes can be found in the Supplementary Material.
Statistical analysis
This study was conducted alongside the primary study; thus, a predetermined sample size was not calculated. Baseline characteristics were summarized using means and standard errors for normally distributed data, and medians with interquartile ranges for non-normally distributed data. Between-group differences in baseline characteristics were compared using unpaired t-tests and Mann–Whitney tests. We performed a mixed-effects analysis using the PROC MIXED procedure in SAS to assess the effects of OPENVENT and NOVENT on the histology score, while accounting for the repeated measures across pulmonary lobes. A variance components covariance structure was applied to model the random effects. For multiple pairwise comparisons among lobes, Sidak's adjustment was utilized to control the family-wise error rate. Two-way ANOVA was used to analyze secondary outcomes of neutrophil and macrophage lung tissue infiltration. Ventilation strategy and lobe were the two categorical variables, with NOVENT set as reference level for strategy. A two-way interaction term was also specified. Secondary outcomes including gas exchange, pulmonary mechanics, hemodynamic parameters and epicardial echocardiography were analyzed by linear mixed modeling. Ventilation strategy and time point were included in the model as categorical fixed effects, with NOVENT and BSL set as reference levels for strategy and time points, respectively. A two-way interaction term was also specified. A random effect was specified per sheep to account for repeated measurements. Post hoc multiple comparisons were performed for primary and secondary endpoints in cases of a statistically significant interaction term. Sidak’s correction was applied to adjust the family-wise error rate. Residual assumptions were examined through quantile–quantile (QQ) plots, to inform appropriate outcome transformations. Data for respiratory system compliance was non-normally distributed, and natural log transformation was applied. Statistical significance was defined as a p-value less than 0.05. Analysis was performed utilizing SAS 9.4 (SAS Institute Inc., Cary, NC, USA.) and Graphpad Prism 9.0 (GraphPad Software, Boston, Massachusetts USA). Finally, a post hoc power calculation was performed for the primary outcome of the study by simulating from the fitted mixed model, with and without ventilation strategy included as a fixed effect. This simulation therefore tested the hypothesis of no difference between ventilation strategies. The simulation was completed using the simr R package in R version 4.3.3 and it is reported in the supplementary results.
Results
Population
Eighteen experiments were completed, nine of those were enrolled into the OPENVENT group and nine into the NOVENT group. The donor hearts were retrieved from sham or BD donors (BD: 9 experiments, Sham: 9 experiments), and the preservation method varied. The allocation of preservation method was randomized between intervention groups. The NOVENT group contained 3 × 2 h HOPE, 5 × 8 h HOPE and 1 SCS while the OPENVENT group contained 3 × 2 h HOPE, 4 × 8 h HOPE and 2 SCS (Table E1, Supplementary Material). There were no statistical differences between recipient groups in preoperative clinical measurements, intraoperative characteristics, or baseline respiratory parameters (Table 1).
Table 1.
Data are presented as mean ± SE and median (IQR)
| NOVENT group (n = 9) | OPENVENT group (n = 9) |
P value | |
|---|---|---|---|
| Preoperative characteristics | |||
| Weight (kg) |
52.0 ± 2.62 52 (9.5) |
51.1 ± 1.29 51 (4.5) |
0.987 |
| Temperature (°C) * |
38.3 ± 0.29 38.6 (0.98) |
38.8 ± 0.12 38.8 (0.4) |
0.436 |
| Mean arterial pressure (mmHg) |
101 ± 3.71 105 (18) |
94.6 ± 3.74 98 (18) |
0.901 |
| Heart rate (bpm) |
86.3 ± 8.46 82.5 (38.8) |
91.8 ± 7.86 87 (31.5) |
0.921 |
| Surgical characteristics | |||
| CPB time (mins) |
180 ± 5.95 183 (21.8) |
190 ± 3.97 190 (15) |
0.905 |
| Aortic cross clamp time (mins) |
73.5 ± 10.2 65 (33) |
63.7 ± 1.86 65 (6) |
0.625 |
| Blood transfusions (mL) |
181.9 ± 91.9 0 (500) |
211 ± 151 0 (300) |
0.592 |
| Baseline respiratory parameters | |||
| PaCO2 (mmHg) |
39.3 ± 1.3 38.8 (5.85) |
45.2 ± 3.61 41.3 (7.15) |
0.914 |
| Driving pressure (cm H2O) |
11.3 ± 2.77 11.5 (15.8) |
7.87 ± 2.82 9 (8) |
0.954 |
| A-a gradient (mmHg) |
90.1 ± 23.5 77 (77.2) |
90.6 ± 25.1 65.5 (140) |
0.993 |
| PaO2/FiO2 (mmHg) |
439 ± 47.4 462 (169) |
435 ± 44.3 492 (255) |
0.939 |
| Respiratory system compliance (mL/cm H2O)* |
33.1 ± 3.71 30.9 (21.9) |
47.46 ± 14.4 36.9 (36.1) |
0.645 |
| Shunt (%) |
18.7 ± 2.71 14.7 (11.7) |
19.9 ± 1.22 20.3 (4.58) |
0.987 |
| Physiological dead space (%) |
9.53 ± 4.19 7.2 (21.7) |
6.01 ± 7.66 − 0.38 (40.3) |
0.951 |
| Minute volume (L/min) |
6.00 ± 0.314 5.9 (1.72) |
6.70 ± 0.151 6.59 (0.88) |
0.990 |
*Non-normally distributed data. CPB, cardiopulmonary bypass; PaCO2, arterial partial pressure of carbon dioxide; A-a, alveolar–arterial; PaO2/FiO2, ratio between arterial partial pressure of oxygen and inspiratory fraction of oxygen
Primary outcome
Atelectasis, interstitial infiltration by neutrophils, thickening of septa, muscle hypertrophy were the most common histological features of injury. Overall, the OPENVENT group showed a significantly lower histological lung damage score when compared to the NOVENT group (0.221 vs 0.267; F = 5.14, p = 0.042). Among all tested lobes, right and left lower lobes showed higher injury score (F = 19.2, p < 0.001). See Fig. 2 for details, and Fig. 3 for histological images.
Fig. 2.
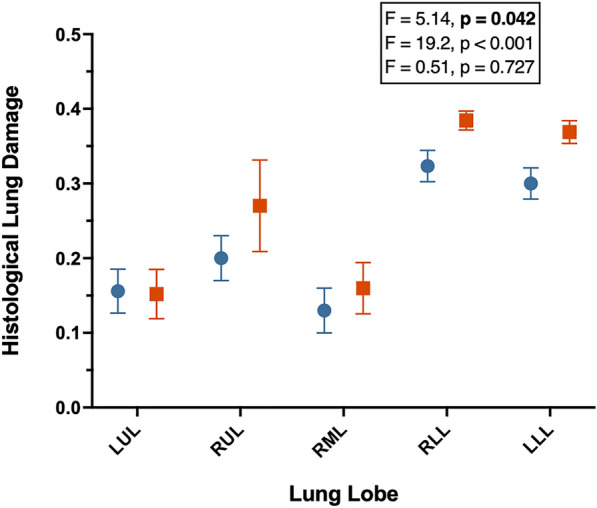
A Histological Lung Injury Score from recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data were clustered into lobes and shown as mean ± SEM with n = 8 for both groups. The effect size and p-values of the mixed analysis are reported from top to bottom of factor ventilatory strategy, lobe and interaction between these two factors. LUL left upper lobe, RUL right upper lobe, RML right middle lobe, RLL right lower lobe and LLL left lower lobe
Fig. 3.
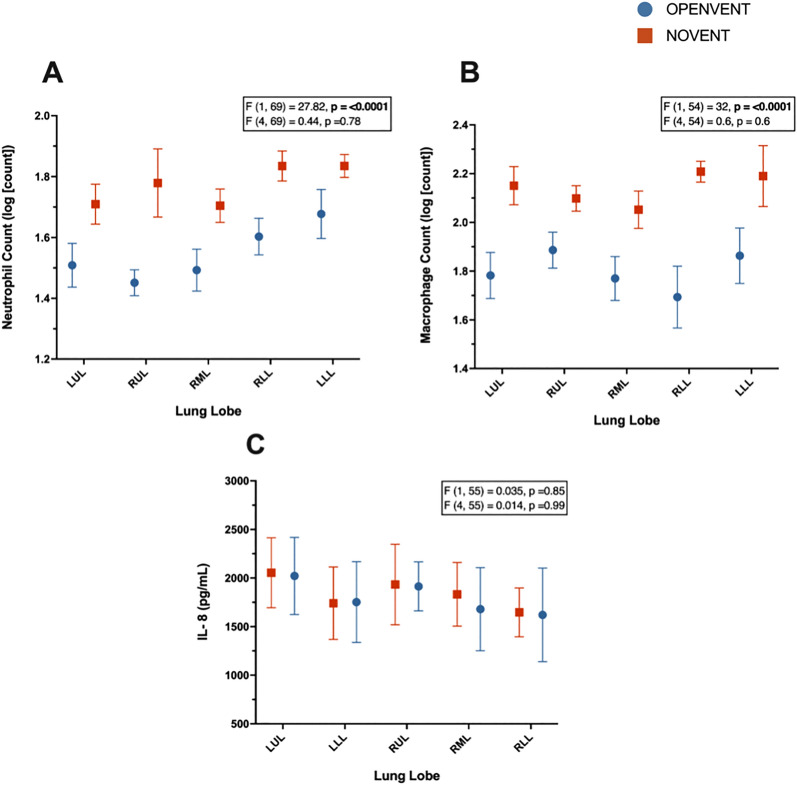
A Neutrophil counts (log [count]), B macrophage counts (log [count]), C interleukin-8 (pg/mL) concentrations in postoperative lung samples from recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data were segmented into lobes, and shown as mean ± SEM with n = 8 for both groups. F (a, b) = c, a represents the between group variance, b the within-group variance, the F value (c) is the ratio of the variation between sample means/ variation within samples. Top F and p values refer to effect of ventilation strategy on outcome, and bottom F and p values refer to the combined effects of ventilation strategies and lung lobe (interaction term). LUL left upper lobe, RUL right upper lobe, RML right middle lobe, RLL right lower lobe and LLL left lower lobe
Secondary outcomes
Pulmonary inflammation
The OPENVENT group presented a significantly lower neutrophil count in the lung tissue (reported as the log of the absolute number of neutrophils), in comparison to the NOVENT group (1.55 vs 1.77; difference between groups = − 0.23, 95% CI [-0.31 to -0.14]; F (1, 69) = 27.8, p ≤0.001). Similarly, the OPENVENT group contained a significantly lower macrophage count in the lung tissue (reported as log of absolute number of macrophages) in comparison to the NOVENT group (1.78 vs 2.14; difference between groups = − 0.34, 95% CI [− 0.46 to -0.22]; F (1, 54) = 32, p < 0.001). Finally, no difference in IL-8 concentration was found between ventilation groups. See Fig. 4 for details and Fig. 5 for histological images.
Fig. 4.
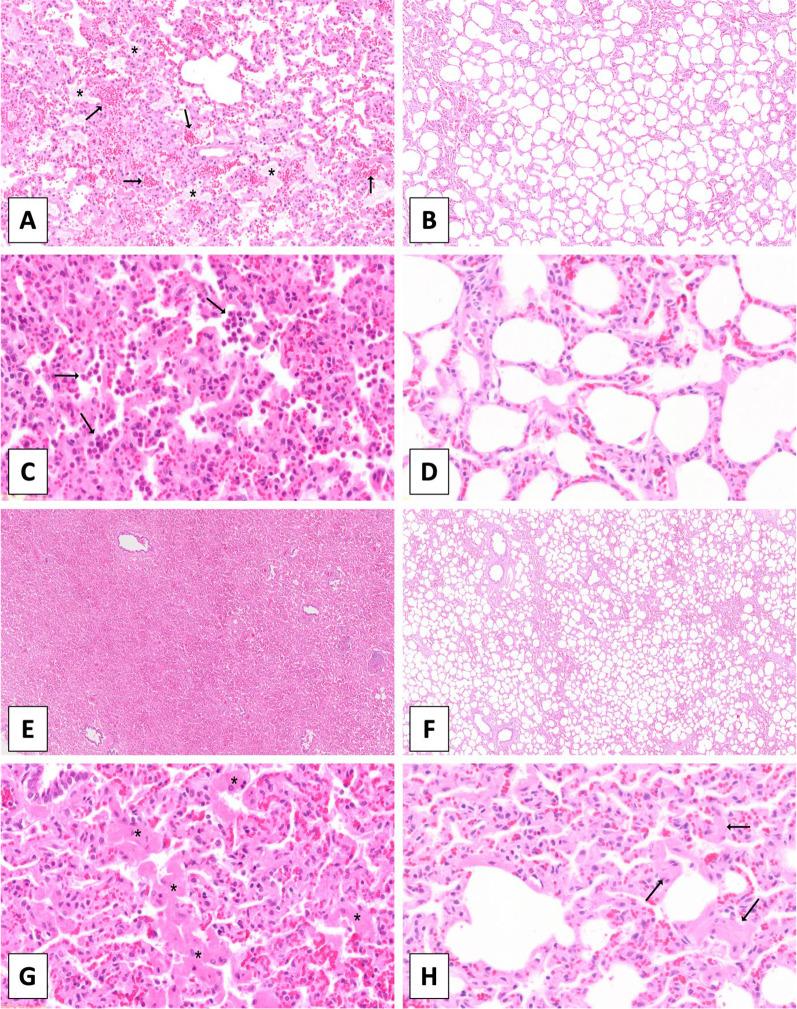
Histology images (H&E staining), demonstrating typical histological features. A Presence of mild intra-alveolar edema (asterisks) and hemorrhage (arrows). B Absence of edema or intra-alveolar hemorrhagic material. C Intra-alveolar accumulation of neutrophils (arrows) and mild increase in the numbers of interstitial neutrophils. D Low numbers of neutrophils within the interstitial space. E Severe diffuse atelectasis with alveolar collapse. F Normally aerated alveoli. G Severe multifocal thickening of the alveolar septa caused by hypertrophy of smooth muscle cells (asterisks). (H) Mild septal smooth muscle hypertrophy (asterisks). A, B Magnification 5x. C, D, G, H Magnification 20x. E, F Magnification 2x
Fig. 5.
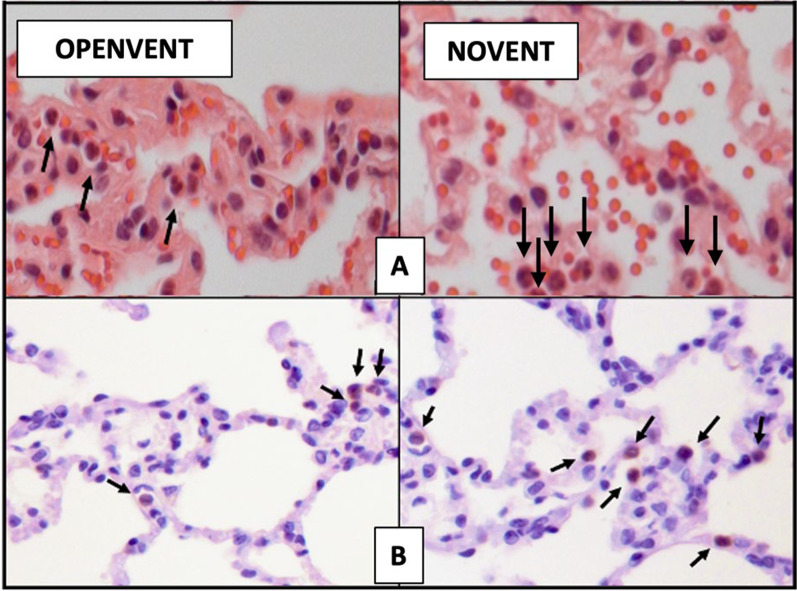
A Histology images (H&E staining) used for neutrophil quantification in tissue samples. Brightfield light microscope used to capture images, with arrows indicating neutrophils. Magnification 40X. B Images from IHC staining used for macrophage quantification in postoperative samples. Brightfield light microscope used to capture images, with arrows indicating macrophages. Magnification 40X
Gas exchange
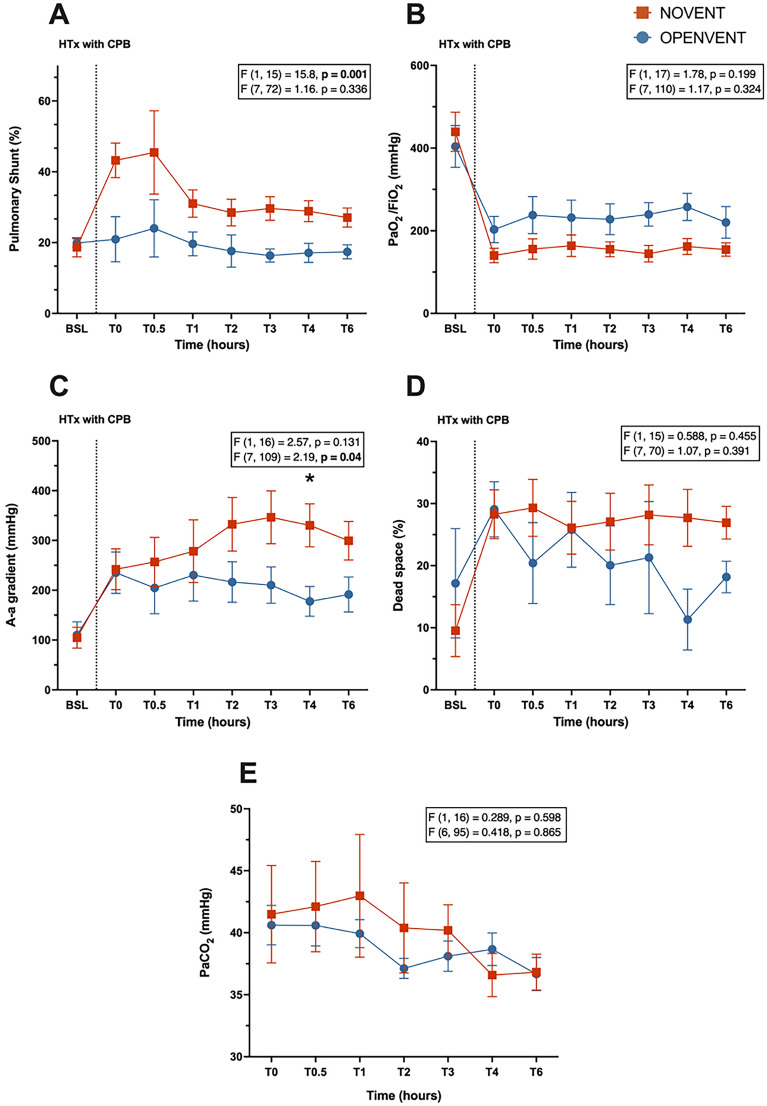
The OPENVENT ventilation strategy was associated with a reduced pulmonary shunt (32.1% vs 19.2%; difference between groups = 12.9%, 95% CI [5.97% to 19.8%]; F (1, 15) = 15.8, p = 0.001). The OPENVENT group resulted in an improved A-a gradient over time (interaction term: F (7, 109) = 2.19, p = 0.040). A statistically significant difference for the A-a gradient was found between ventilation groups at T4 (334 mmHg vs 178 mmHg; Difference between groups at T4 = 173 ± 52.4 mmHg; p = 0.045). No significant differences between ventilation groups were found for PaO2/FiO2 (PF) ratio, PaCO2 or physiological dead space ventilation (Fig. 6).
Fig. 6.
A Pulmonary shunt (%), B PaO2/FiO2 ratio (mmHg), C A-a gradient (mmHg), D physiological dead space (%), E PaCO2 (mmHg) during 6-h post-transplantation monitoring period in recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data shown as mean ± SEM from preoperative baseline (BSL) through to 6 h postoperative (T6), with n = 9 for both groups. F (a, b) = c, a represents the between group variance, b the within-group variance, the F value (c) is the ratio of the variation between sample means/ variation within samples. Top F and p values refer to effect of ventilation strategy on outcome, and bottom F and p values refer to the combined effects of ventilation strategies and time (interaction term)
Pulmonary mechanics and hemodynamic parameters
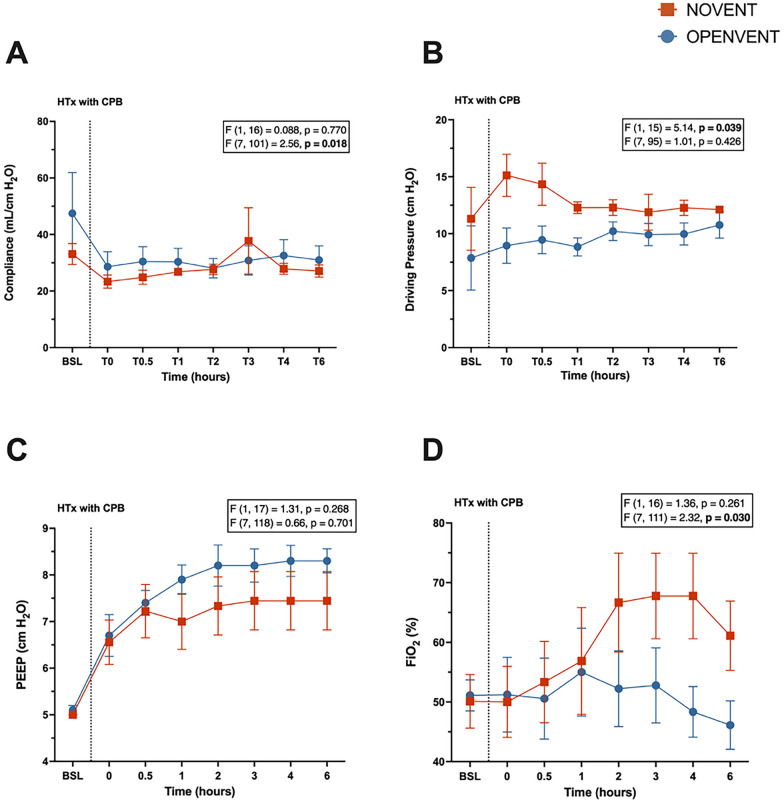
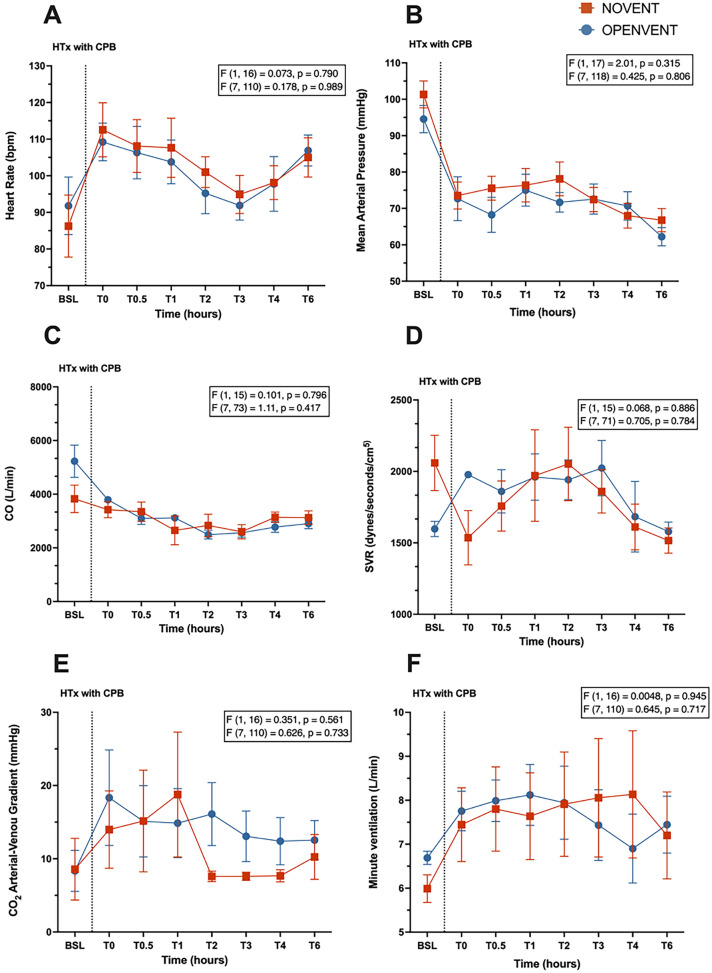
A lower driving pressure was seen in the OPENVENT strategy group (12.8 cm H2O vs 9.64 cm H2O; difference between groups = 3.17 cm H2O, 95% CI [0.188 cm H2O to 6.15 cm H2O]; p = 0.039). While the interaction term was significant (F (7, 101) = 2.56, p = 0.018), the respiratory system compliance did not differ between groups at any timepoint (Fig. 7). Similarly, the interaction term for postoperative FiO2 was found to be significant (F (7, 111) = 2.32, p = 0.030), but no significant changes were found between groups at any timepoints despite a trend for increased FiO2 in the NOVENT group. No significant differences between timepoints were seen in PEEP (Fig. 7), hemodynamic parameters (Fig. 8), ventilatory settings, oxygen delivery and consumption, lactate and acid–base status (Figure E1 and E2 Supplementary Material).
Fig. 7.
A Compliance (mL/ cm H2O), B driving pressure (cm H2O), C positive end-expiratory pressure (PEEP) (cm H2O), D FiO2 (%) during 6-h post-transplantation monitoring period in recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data shown as mean ± SEM from preoperative baseline (BSL) through to 6 h postoperative (T6), with n = 9 for both groups. F (a, b) = c, a represents the between group variance, b the within-group variance, the F value (c) is the ratio of the variation between sample means/ variation within samples. Top F and p values refer to effect of ventilation strategy on outcome, and bottom F and p values refer to the combined effects of ventilation strategies and time (interaction term)
Fig. 8.
A Heart rate (bpm), B mean arterial pressure (mmHg), C cardiac output (L/min), D systemic vascular resistance (dynes/second/cm5) and E CO2 arterial–venous gradient (mmHg), F minute ventilation (L/min) during 6-h post-transplantation monitoring period in recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data shown as mean ± SEM from preoperative baseline (BSL) through to 6 h postoperative (T6), with n = 9 for both groups. F (a, b) = c, a represents the between group variance, b the within-group variance, the F value (c) is the ratio of the variation between sample means/ variation within samples. Top F and p values refer to effect of ventilation strategy on outcome, and bottom F and p values refer to the combined effects of ventilation strategies and time (interaction term)
Epicardial echocardiography
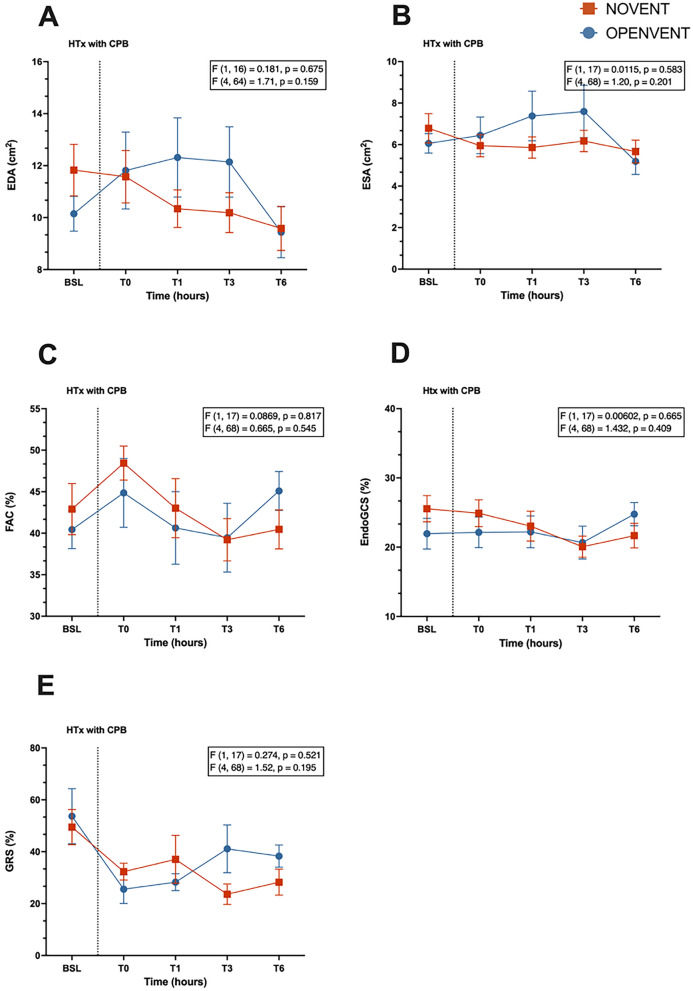
No significant differences were seen in any measurements of heart function and contractility (Fig. 9).
Fig. 9.
A End-diastolic area (EDA, cm H2O), B end-systolic area (ESA, cm H2O), C fractional area change (FAC, %), D endo-myocardial global circumferential strain (EndoGCS, %) and E global radial strain (GRS, %) during 6-h post-transplantation monitoring period in recipient sheep treated with either no ventilation (NOVENT ■) or open-lung ventilation (OPENVENT ●) during cardiopulmonary bypass. Data shown as mean ± SEM from preoperative baseline (BSL) through to 6 h postoperative (T6), with n = 9 for both groups. F (a, b) = c, a represents the between group variance, b the within-group variance, the F value (c) is the ratio of the variation between sample means/ variation within samples. Top F and p values refer to effect of ventilation strategy on outcome, and bottom F and p values refer to the combined effects of ventilation strategies and time (interaction term)
Discussion
In this ovine HTx model, open-lung ventilation throughout CPB demonstrated reduction in lung histological injury. Improvements in other secondary endpoints were also observed in the open-lung ventilation group, including pulmonary shunt, A-a gradient, inflammatory cell infiltration, and lung mechanics.
Our findings indicate an improved pulmonary shunt in the OPENVENT group, resulting in a reduction of postoperative lung dysfunction and ventilation/perfusion (V/Q) mismatching. Lung deflation during CPB causes significant regional atelectasis and pulmonary edema, with resumption of ventilation seldom leading to complete lung re-expansion [37]. Maintaining ventilation during CPB has been demonstrated by Kirmani and colleagues to prevent perioperative deflation, and significantly decrease atelectasis after surgery in patients undergoing coronary artery bypass grafts (CABG) [38]. Multiple trials have concluded that residual atelectasis significantly contributes to deterioration in postoperative oxygenation measurements, such as A-a gradient and pulmonary shunt [39, 40]. Our findings corroborate those made by Magnusson and colleagues, as their porcine model demonstrated an increase in shunt fraction after CPB with lung deflation, strongly correlating with atelectasis [11]. Further, the link between the increased shunt fraction and postoperative atelectasis is strengthened by our findings of histological damage in the lower lobes of the NOVENT group. This suggests an increase in alveolar collapse, as histology scoring primarily assessed the presence of atelectasis, along with other vascular, bronchiolar, and extravascular aspects. Since the samples were taken at the end of the monitoring period, we cannot determine whether the atelectasis occurred primarily due to the CPB-related deflation or developed at a later stage. Previous data from D’Angelo and colleagues suggest a combination of both likely occurred in these settings, indicating that alveolar collapse associated with perioperative deflation caused epithelial damage, impairing surfactant secretion and subsequently increasing the likelihood of further atelectasis at later stages [41]. Regarding the increased damage observed in the lower lobes, explanations for this phenomenon can only be speculative. It is plausible that the prominent atelectasis, induced by diaphragmatic splinting might contribute to this effect. Additionally, recruitment and overdistension could be another factor influencing the higher damage observed in the lower lobes.
Maintaining ventilation through CPB appeared to mitigate respiratory dysfunction, with the OPENVENT group exhibiting significantly lower A-a gradients. These results corroborate data from previous clinical trials and could have been caused by V/Q mismatch in the collapsed lower areas of the NOVENT group [38, 42, 43]. Mitigating atelectasis through the continuation of ventilation minimized the downstream wash out and impairment of surfactant production, ultimately alleviating the continued a-/dystelectasis, shunt and poor oxygenation [44]. Similar conclusions were drawn in a meta-analysis from Chi et al. who demonstrated that improvements in PF ratio and A-a gradient were attributed to the ventilation-mediated mitigation of atelectasis and V/Q mismatch [16].
The postoperative ARDS observed in the NOVENT group could have been exacerbated by VILI. The increased regional atelectasis and lower lobe collapse in the NOVENT group appears to have led to the uneven distribution of ventilation through the upper lobes during the postoperative monitoring period. Recruitment maneuvers performed at the end of surgery could have also contributed, as bag ventilation performed in the NOVENT group was more likely to cause overdistension due to the difficulty in measuring the exact volumes applied [45]. This is reflected in our findings of higher driving pressures in this group, signifying an increasing susceptibility to VILI and over inflation [44]. Of note, the chest wall was maintained open during the entire postoperative monitoring period, implying that all the pressure created by the ventilator was completely applied to isolated lungs. Hence, the values presented in this paper likely overestimate the extent of the driving pressure seen in typical patients, as the closure of the chest wall in these patients would prevent lung overinflation and excessive strain [46]. Furthermore, our histological lung analysis revealed the presence of hypertrophy in the intralobular septum and thickening of the space between alveolar lining, which can be due to increased strain and respiratory effort. Our results corroborate multiple studies that previously suggested high driving pressures can worsen patient outcome, through either alveolar overdistension, or the repetitive recruitment and re-collapse of unstable tissue in areas impacted by surfactant dysfunction [47–49]. Further, the risk of lung overinflation in cardiac patients is significantly more likely due to abnormal chest wall compliance following sternotomy [50]. To the best of our knowledge, this is the first report appraising driving pressure in this context. Furthermore, our results demonstrate an increase in neutrophil infiltration caused by lung damage associated with alveolar collapse in the lower lobes, as well as overdistension due to VILI in the upper regions. Several previous animal studies confirm this, suggesting that atelectasis-mediated epithelial damage and alveolar overdistension can separately recruit monocytes and neutrophils, and increase cytokine transcription [19, 51–54]. Conversely, ventilation during CPB appeared to lower driving pressures and immune cell infiltration, most likely through mitigation of atelectasis and reduction in VILI. IL-8 concentrations were expected to increase in the NOVENT group, however no differences were seen [55, 56]. Our findings could potentially be explained by the distinctive response of IL-8, a stress-responsive proinflammatory chemokine. Its activation facilitates the influx of neutrophils and subsequent inflammation. Alternatively, our negative results may have been related to the use of corticosteroids post-transplant or lung biopsies, which could potentially underestimate lobar inflammation. In future investigations, it would be prudent to conduct comprehensive evaluations encompassing both pro-inflammatory and anti-inflammatory chemokines. Additionally, employing bronchoalveolar lavage techniques could offer a more thorough characterization of ventilator-induced inflammation [57].
Our study has several noteworthy strengths. Firstly, we employed a clinically relevant sheep model of orthotopic heart transplantation performed 24 h after donor brainstem death. This model has proven to be highly translatable, as evidenced by the first Australian and New Zealand trial, which demonstrated that hypothermic oxygenated perfusion (HOPE) safely and effectively extends acceptable donor heart preservation times in humans [58]. Secondly, we utilized histological appraisal of lung injury rather than relying on surrogate endpoints of lung function. Lastly, we conducted a comprehensive evaluation of potential alternative causes of lung injury, including cardiac function assessed through echocardiography, inflammatory markers, and injurious ventilatory settings. Major limitations of the study included the relatively low number of samples. Secondly, this ventilation study was performed within the limitations of the HTx model and involved different heart preservation strategies and types of death in the donor sheep. These factors all introduced a degree of variability into the condition of the transplanted heart that could not be accounted for and may have influenced postoperative lung function, independent of perioperative ventilation. We introduced a randomized scheme to counter any potential bias, and the distribution of preservation methods between intervention groups was similar (Table E1). However, it is possible that the condition of the transplanted heart, and the influence of postoperative vasoactive support, remained a confounding factor. It must be noted that the sheep were immunosuppressed due to the use of corticosteroids post heart transplantation. This could have limited the accuracy of our IL-8 assays. Further, due to the nature of the animal model, no conclusions could be made regarding important clinical factors, such as time to extubation and length of ICU and hospital stays. While the study provided strong evidence for the use of ventilation during CPB, the restricted postoperative monitoring period limited our ability to determine whether initial ventilation-mediated improvements in respiratory function can lead to long-term benefits in patient outcome. These clinical outcomes have been investigated in the literature, with the PROVECS trial exploring protective ventilation during CPB, but no differences in postoperative complications or other endpoints have been observed [23]. The MECANO trial showed similar results, however the authors did note that ventilation during CPB significantly decreased postoperative complications in isolated patients undergoing CABG [24]. Nguyen and colleagues emphasized that these post hoc subgroup analyses carry less weight as compared to primary analysis, but are of clinical relevance and warrant further investigation [24]. As Schultz and colleagues note, both trials may have been underpowered to show benefit from the maintenance of ventilation, and further investigation is needed [59]. Thirdly, pulmonary perfusion data were not recorded during the study. Although cardiac output did not show a significant difference between groups, pulmonary artery pressure and resistance should be investigated in future studies to understand heart–lung interactions during mechanical ventilation. These parameters could have provided further insight into the extent of lung injury between the groups. Fourthly, additional markers of lung permeability, such as the wet-to-dry lung weight ratio and the lung-to-body weight ratio, were not reported. Since this was a concomitant study, the primary focus was on evaluating donor heart function in the HTx model, and lung assessment was a secondary objective. Lastly, a single senior veterinary pathologist performed the histology assessment of lung injury. While this could increase the risk of bias due to the lack of cross-verification and potential subjectivity, the pathologist was blinded to treatment allocation.
In conclusion, in our ovine HTx model, the continuation of ventilation during CPB significantly improved short-term postoperative oxygenation parameters including pulmonary shunt and A-a gradient, as well as reducing histological damage, inflammatory cell infiltration and driving pressures. These data provide perceptive new pre-clinical insights on the pathophysiology of lung derecruitment during cardiac transplantation and the value of an approach to avoid postoperative ARDS following cardiac surgery.
Supplementary Information
Acknowledgements
This work is supported by the Prince Charles Hospital Foundation, the University of Queensland, Queensland Health (Bionics Project), the Metro North Hospital and Health Service, the Alfred Foundation, The Donald and Joan Wilson Foundation, the National Health and Medical Research Council (GNT1145761—The Dead Heart Project). JS is the recipient of an Advanced Queensland Industry Research Fellowship. KW received a PhD scholarship and a fee waiver from the University of Queensland. SH is the recipient of a Postgraduate Scholarship from the Prince Charles Hospital Foundation and a fee waiver from the University of Queensland.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ARDS
Acute respiratory distress syndrome
- BD
Brain stem death
- BSL
Baseline recordings
- CABG
Coronary artery bypass graft
- CPB
Cardiopulmonary bypass
- EDA
End-diastolic area
- ELISA
Enzyme linked immunosorbent assay
- EndoGCS
Endo-myocardial global circumferential strain
- ESA
End-systolic area
- ETCO
End tidal carbon dioxide
- FAC
Fractional area change
- FiO2
Fraction of inspired O2
- GRS
Global radial strain
- H&E
Hematoxylin and eosin
- HOPE
Hypothermic oxygenated perfusion
- HTx
Heart transplant
- IHC
Immunohistochemistry
- IL-8
Interleukin 8
- IR
Ischemia–reperfusion
- LLL
Left lower lobe
- LTV
Low tidal volume
- LUL
Left upper lobe
- NHMRC
National Health and Medical Research Council
- NOVENT
No ventilation
- OPENVENT
Open-lung ventilation group
- PAO2
Alveolar partial pressure of O2
- PaO2
Arterial partial pressure of O2
- PEEP
Positive end-expiratory pressure
- PVENT
Protective ventilation
Quantile–quantile
- RCTs
Randomized controlled trials
- RLL
Right lower lobe
- RML
Right middle lobe
- RMs
Recruitment maneuvers
- RR
Respiratory rate
- RUL
Right upper lobe
- SCS
Static cold storage
- SIRS
Systemic inflammatory response syndrome
- SPO2
Oxygen saturation
- V/Q
Tidal volume
- VT
Ventilation/perfusion ratio
- VILI
Ventilator induced lung injury
Author contributions
VK, participated in design of the study and coordination and helped to draft the manuscript; SMC, conceived of the study, participated in its design and coordination and helped to draft the manuscript; participated in collection of data and reviewed the initial draft of the manuscript; LR, participated in collection of data and reviewed the initial draft of the manuscript; SH, Participated in the design of the study, participated in collection of data and reviewed the initial draft of the manuscript; LESH, participated in collection of data and reviewed the initial draft of the manuscript; KW, participated in collection of data and reviewed the initial draft of the manuscript; MRP, participated in collection of data and reviewed the initial draft of the manuscript; MB, participated in collection of data and reviewed the initial draft of the manuscript; KS, participated in collection of data and reviewed the initial draft of the manuscript; CA, participated in collection of data and reviewed the initial draft of the manuscript; NB, participated in collection of data and reviewed the initial draft of the manuscript; EW, participated in collection of data and reviewed the initial draft of the manuscript; KH, participated in collection of data and reviewed the initial draft of the manuscript; KS, participated in collection of data and reviewed the initial draft of the manuscript; NGO, participated in collection of data and reviewed the initial draft of the manuscript; CM, participated in collection of data and reviewed the initial draft of the manuscript; SL, participated in collection of data and reviewed the initial draft of the manuscript; GA, participated in collection of data and reviewed the initial draft of the manuscript reviewed the initial draft of the manuscript; AH, participated in collection of data and reviewed the initial draft of the manuscript; JSJ, participated in collection of data and reviewed the initial draft of the manuscript; NS, participated in collection of data and reviewed the initial draft of the manuscript; LJ, participated in collection of data and reviewed the initial draft of the manuscript; BL, participated in collection of data and reviewed final draft of the manuscript; NW, supported statistical analysis and reviewed the final draft of the manuscript; CP, performed histology analysis and reviewed the final draft of the manuscript; MB, participated in collection of data and reviewed the initial draft of the manuscript; JYS, participated in collection of data and reviewed the final draft of the manuscript; DCM, participated in collection of data and reviewed the final draft of the manuscript; JFF, participated in design of the study and reviewed the initial draft of the manuscript; and GLB, conceived the study, participated in its design and coordination and helped to draft the manuscript and conducted the statistical analysis. All authors read and approved the final manuscript. JFF and GLB were the primary supervisors, and equally contributed to this work.
Data availability
Raw data will be made available upon reasonable request.
Declarations
Ethics approval and consent to participate
The project was approved by Queensland University of Technology (QUT) Animal Ethics Committee (Approval #16-1109). Ratified by the University of Queensland AEC (QUT/393/17/QUT), experiments were performed in accordance with the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (8th Edition 2013), the Animal Care and Protection Act 2001 (QLD) and complied with the ARRIVE Guidelines.
Consent for publication
Not applicable.
Competing interests
Professor John Fraser is the CEO of the Quantum Medical Innovation Fund and De Motu Cordis Pty Limited. He is also the co-founder of BiVACOR™ Pty Ltd. In addition, Prof. Fraser and his research group collaborate with Australian Red Cross Lifeblood, Fisher and Paykel healthcare, Mallinckrodt Pharmaceuticals and CSL. Professor Fraser receives reimbursement for travel costs when presenting research created collaboration with Fisher and Paykel Healthcare. Professor David McGiffin provides consultancy services to Abbott. This has no direct impact on the contents of the manuscript. The other authors do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Varun Karnik and Sebastiano Maria Colombo equally contributed to this work.
John F. Fraser and Gianluigi Li Bassi equally contributed to this work.
References
- 1.Camkiran Firat A, Komurcu O, Zeyneloglu P, Turker M, Sezgin A, Pirat A (2015) Early postoperative pulmonary complications after heart transplantation. Transplant Proc 47(4):1214–1216 [DOI] [PubMed] [Google Scholar]
- 2.Lenner R, Padilla ML, Teirstein AS, Gass A, Schilero GJ (2001) Pulmonary complications in cardiac transplant recipients. Chest 120(2):508–513 [DOI] [PubMed] [Google Scholar]
- 3.Bignami E, Guarnieri M, Saglietti F, Maglioni EM, Scolletta S, Romagnoli S et al (2017) Different strategies for mechanical VENTilation during CardioPulmonary Bypass (CPBVENT 2014): study protocol for a randomized controlled trial. Trials 18(1):264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H (2001) Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest 119(1):31–36 [DOI] [PubMed] [Google Scholar]
- 5.Reber A, Budmiger B, Wenk M, Haefeli WE, Wolff T, Bein T et al (2000) Inspired oxygen fraction after cardiopulmonary bypass: effects on pulmonary function with regard to endothelin-1 concentrations and venous admixture. Br J Anaesth 84(5):565–570 [DOI] [PubMed] [Google Scholar]
- 6.Utley JR (1990) Pathophysiology of cardiopulmonary bypass: current issues. J Card Surg 5(3):177–189 [DOI] [PubMed] [Google Scholar]
- 7.Zheng XM, Yang Z, Yang GL, Huang Y, Peng JR, Wu MJ (2022) Lung injury after cardiopulmonary bypass: Alternative treatment prospects. World J Clin Cases 10(3):753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolakis E, Filos KS, Koletsis E, Dougenis D (2010) Lung dysfunction following cardiopulmonary bypass. J Card Surg 25(1):47–55 [DOI] [PubMed] [Google Scholar]
- 9.Ovechkin AV, Lominadze D, Sedoris KC, Robinson TW, Tyagi SC, Roberts AM (2007) Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem 113(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy JH, Tanaka KA (2003) Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 75(2):S715–S720 [DOI] [PubMed] [Google Scholar]
- 11.Magnusson L, Zemgulis V, Wicky S, Tyden H, Thelin S, Hedenstierna G (1997) Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 87(5):1153–1163 [DOI] [PubMed] [Google Scholar]
- 12.Vargas FS, Terra-Filho M, Hueb W, Teixeira LR, Cukier A, Light RW (1997) Pulmonary function after coronary artery bypass surgery. Respir Med 91(10):629–633 [DOI] [PubMed] [Google Scholar]
- 13.Pons S, Sonneville R, Bouadma L, Styfalova L, Ruckly S, Neuville M et al (2019) Infectious complications following heart transplantation in the era of high-priority allocation and extracorporeal membrane oxygenation. Ann Intensive Care 9(1):17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Delgado M, Navarrete-Sanchez I, Colmenero M (2014) Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol 27(2):146–152 [DOI] [PubMed] [Google Scholar]
- 15.Chaney MA, Nikolov MP, Blakeman BP, Bakhos M (2000) Protective ventilation attenuates postoperative pulmonary dysfunction in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth 14(5):514–518 [DOI] [PubMed] [Google Scholar]
- 16.Chi D, Chen C, Shi Y, Wang W, Ma Y, Zhou R et al (2017) Ventilation during cardiopulmonary bypass for prevention of respiratory insufficiency: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 96(12):e6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N (2004) Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med 30(4):620–626 [DOI] [PubMed] [Google Scholar]
- 18.Lamarche Y, Gagnon J, Malo O, Blaise G, Carrier M, Perrault LP (2004) Ventilation prevents pulmonary endothelial dysfunction and improves oxygenation after cardiopulmonary bypass without aortic cross-clamping. Eur J Cardiothorac Surg 26(3):554–563 [DOI] [PubMed] [Google Scholar]
- 19.Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW et al (2001) Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med 164(3):494–498 [DOI] [PubMed] [Google Scholar]
- 20.Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, Feelders R et al (2005) Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg 28(6):889–895 [DOI] [PubMed] [Google Scholar]
- 21.Tutun U, Parlar AI, Altinay L, Topcu DI, Babaroglu S, Yay K et al (2011) Does on-pump normothermic beating-heart valve surgery with low tidal volume ventilation protect the lungs? Heart Surg Forum 14(5):E297-301 [DOI] [PubMed] [Google Scholar]
- 22.Zamani MM, Najafi A, Sehat S, Janforooz Z, Derakhshan P, Rokhtabnak F et al (2017) The effect of intraoperative lung protective ventilation vs conventional ventilation, on postoperative pulmonary complications after cardiopulmonary bypass. J Cardiovasc Thorac Res 9(4):221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagier D, Fischer F, Fornier W, Huynh TM, Cholley B, Guinard B et al (2019) Effect of open-lung vs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: the PROVECS randomized clinical trial. Intensive Care Med 45(10):1401–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LS, Estagnasie P, Merzoug M, Brusset A, Law Koune JD, Aubert S et al (2021) Low tidal volume mechanical ventilation against no ventilation during cardiopulmonary bypass in heart surgery (MECANO): a randomized controlled trial. Chest 159(5):1843–1853 [DOI] [PubMed] [Google Scholar]
- 25.See Hoe LE, Li Bassi G, Wildi K, Passmore MR, Bouquet M, Sato K et al (2023) Donor heart ischemic time can be extended beyond 9 hours using hypothermic machine perfusion in sheep. J Heart Lung Transplant 42(8):1015–1029 [DOI] [PubMed] [Google Scholar]
- 26.See Hoe LE, Wildi K, Obonyo NG, Bartnikowski N, McDonald C, Sato K et al (2021) A clinically relevant sheep model of orthotopic heart transplantation 24 h after donor brainstem death. Intensive Care Med Exp 9(1):60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bickenbach J, Zoremba N, Fries M, Dembinski R, Doering R, Ogawa E et al (2009) Low tidal volume ventilation in a porcine model of acute lung injury improves cerebral tissue oxygenation. Anesth Analg 109(3):847–855 [DOI] [PubMed] [Google Scholar]
- 28.de Haro C, Neto AS, Goma G, Gonzalez ME, Ortega A, Forteza C et al (2023) Effect of a low versus intermediate tidal volume strategy on pulmonary complications in patients at risk of acute respiratory distress syndrome-a randomized clinical trial. Front Med (Lausanne) 10:1172434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guay J, Ochroch EA, Kopp S (2018) Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in adults without acute lung injury. Cochrane Database Syst Rev 7(7):CD011151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D, Grant MC, Stone A, Wu CL, Wick EC (2016) A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Ann Surg 263(5):881–887 [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni HS, Lee JS, Bastarache JA, Kuebler WM, Downey GP, Albaiceta GM et al (2022) Update on the features and measurements of experimental acute lung injury in animals: an official American Thoracic Society Workshop Report. Am J Respir Cell Mol Biol 66(2):e1–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abràmoff M, Magalhães PJ, Ram SJ (2004) Image processing with imageJ. Biophotonics International 11(7):36–41 [Google Scholar]
- 33.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW (2015) The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82(7–8):518–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9(7):671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder AB, Dobson ETA, Rueden CT, Tomancak P, Jug F, Eliceiri KW (2021) The ImageJ ecosystem: open-source software for image visualization, processing, and analysis. Protein Sci 30(1):234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouquet M, Passmore MR, See Hoe LE, Tung J-P, Simonova G, Boon AC et al (2020) Development and validation of ELISAs for the quantitation of interleukin (IL)-1β, IL-6, IL-8 and IL-10 in ovine plasma. J Immunol Methods 486:112835 [DOI] [PubMed] [Google Scholar]
- 37.Niyayeh Saffari NH, Nasiri E, Mousavinasab SN, Ghafari R, Soleimani A, Esmaeili R (2015) Frequency rate of atelectasis in patients following coronary artery bypass graft and its associated factors at Mazandaran Heart Center in 2013–2014. Glob J Health Sci. 7(7):97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirmani SM, Bashir I, Ahmad K, Masroor R, Khan S, Javaid R, Abbas S. Effects of continuous ventilation during cardiopulmonary bypass in preventing post-operative pulmonary complications in open heart surgery. Pak Armed Forces Med J. 2022;66.
- 39.Loeckinger A, Kleinsasser A, Lindner KH, Margreiter J, Keller C, Hoermann C (2000) Continuous positive airway pressure at 10 cm H(2)O during cardiopulmonary bypass improves postoperative gas exchange. Anesth Analg 91(3):522–527 [DOI] [PubMed] [Google Scholar]
- 40.Verheij J, van Lingen A, Raijmakers PG, Spijkstra JJ, Girbes AR, Jansen EK et al (2005) Pulmonary abnormalities after cardiac surgery are better explained by atelectasis than by increased permeability oedema. Acta Anaesthesiol Scand 49(9):1302–1310 [DOI] [PubMed] [Google Scholar]
- 41.D’Angelo E, Pecchiari M, Gentile G (2007) Dependence of lung injury on surface tension during low-volume ventilation in normal open-chest rabbits. J Appl Physiol 102(1):174–182 [DOI] [PubMed] [Google Scholar]
- 42.Beer L, Warszawska JM, Schenk P, Debreceni T, Dworschak M, Roth GA et al (2015) Intraoperative ventilation strategy during cardiopulmonary bypass attenuates the release of matrix metalloproteinases and improves oxygenation. J Surg Res 195(1):294–302 [DOI] [PubMed] [Google Scholar]
- 43.Nyrén S, Radell P, Lindahl Sten GE, Mure M, Petersson J, Larsson Stig A et al (2010) Lung ventilation and perfusion in prone and supine postures with reference to anesthetized and mechanically ventilated healthy volunteers. Anesthesiology 112(3):682–687 [DOI] [PubMed] [Google Scholar]
- 44.Chiumello D, Carlesso E, Brioni M, Cressoni M (2016) Airway driving pressure and lung stress in ARDS patients. Crit Care 20:276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulus F, Binnekade JM, Vroom MB, Schultz MJ (2012) Benefits and risks of manual hyperinflation in intubated and mechanically ventilated intensive care unit patients: a systematic review. Crit Care 16(4):R145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–64. [DOI] [PubMed]
- 47.Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA et al (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372(8):747–755 [DOI] [PubMed] [Google Scholar]
- 48.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM et al (2004) Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 32(9):1817–1824 [DOI] [PubMed] [Google Scholar]
- 49.Sahetya SK, Mallow C, Sevransky JE, Martin GS, Girard TD, Brower RG et al (2019) Association between hospital mortality and inspiratory airway pressures in mechanically ventilated patients without acute respiratory distress syndrome: a prospective cohort study. Crit Care 23(1):367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragnarsdottir M, KristjAnsdottir A, Ingvarsdottir I, Hannesson P, Torfason B, Cahalin L (2004) Short-term changes in pulmonary function and respiratory movements after cardiac surgery via median sternotomy. Scand Cardiovasc J 38(1):46–52 [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal NR, King LS, D’Alessio FR (2014) Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306(8):L709–L725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bregeon F, Roch A, Delpierre S, Ghigo E, Autillo-Touati A, Kajikawa O et al (2002) Conventional mechanical ventilation of healthy lungs induced pro-inflammatory cytokine gene transcription. Respir Physiol Neurobiol 132(2):191–203 [DOI] [PubMed] [Google Scholar]
- 53.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M et al (2006) Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177(3):1817–1824 [DOI] [PubMed] [Google Scholar]
- 54.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T et al (2005) The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol 175(3):1884–1893 [DOI] [PubMed] [Google Scholar]
- 55.Halbertsma FJ, Vaneker M, Scheffer GJ, van der Hoeven JG (2005) Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 63(10):382–392 [PubMed] [Google Scholar]
- 56.Yang R, Zhou L, Chen Z, He S, Lian S, Shen Y et al (2023) Effect and mechanical mechanism of spontaneous breathing on oxygenation and lung injury in mild or moderate animal ARDS. BMC Pulm Med 23(1):428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomassetti S, Colby TV, Wells AU, Poletti V, Costabel U, Matucci-Cerinic M. Bronchoalveolar lavage and lung biopsy in connective tissue diseases, to do or not to do? Ther Adv Musculoskelet Dis. 2021;13:1759720X211059605. [DOI] [PMC free article] [PubMed]
- 58.McGiffin DC, Kure CE, Macdonald PS, Jansz PC, Emmanuel S, Marasco SF et al (2024) Hypothermic oxygenated perfusion (HOPE) safely and effectively extends acceptable donor heart preservation times: Results of the Australian and New Zealand trial. J Heart Lung Transplant 43(3):485–495 [DOI] [PubMed] [Google Scholar]
- 59.Schultz MJ, Zochios V, Serpa NA (2021) Ventilation during cardiopulmonary bypass: can we, must we, should we individualize It? Chest 159(5):1703–1705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data will be made available upon reasonable request.