Abstract
Aims
Osimertinib is a third‐generation, irreversible, central nervous system‐active, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with efficacy in EGFR‐mutated non‐small cell lung cancer (NSCLC). We assessed the relationship between plasma osimertinib levels and its efficacy and safety events.
Methods
Comprehensive pharmacokinetics exposure–response (E–R) modelling was performed utilizing steady state area under the curve (AUCss) data from first‐line, ≥second‐line and adjuvant studies from the osimertinib clinical development programme (20–240 mg once‐daily dosing; N = 1689 patients). Analyses were conducted for survival using a proportional hazard model; for interstitial lung disease (ILD) and left ventricular ejection fraction (LVEF) events using a penalized logistic regression model and graphical analysis of potential confounding factors; and for rash and diarrhoea events using descriptive analysis.
Results
E–R modelling analyses indicated no clear trend of increasing efficacy with increasing osimertinib AUCss; efficacy in all exposure quartiles was significantly better than the control arm (comparator EGFR‐TKI, chemotherapy or placebo) irrespective of treatment line. Model‐based analysis suggested a potential relationship between increased osimertinib exposure and increased probability of ILD events, predominantly in Japanese patients. Additionally, there were increased probabilities of rash or diarrhoea with increasing osimertinib exposure. The probability of LVEF events showed overlapping confidence intervals for osimertinib ≤80 mg and control.
Conclusions
E–R modelling in patients with EGFR‐mutated NSCLC demonstrated that increased osimertinib exposure was unlikely to increase efficacy but may increase occurrence of certain adverse events. Hence, long‐term treatment with doses ≥80 mg was not expected to provide additional benefit.
Keywords: anticancer drugs, lung cancer, modelling and simulation, pharmacokinetic‐pharmacodynamic, population analysis
What is already known about this subject
Osimertinib has proven efficacy in EGFR‐mutated NSCLC.
Previous exposure–response modelling showed no relationship between osimertinib exposure and efficacy, and a linear relationship with rash and diarrhoea incidence.
We expanded our model to examine the relationship between osimertinib exposure and efficacy and safety endpoints across several treatment settings.
What this study adds
There was no significant relationship between osimertinib exposure and efficacy in any line of therapy.
There was a potential correlation between osimertinib exposure and probability of interstitial lung disease, especially in Japanese patients.
Probabilities of rash and diarrhoea increased with osimertinib exposure.
Correlation between osimertinib exposure and LVEF events was confounded.
1. INTRODUCTION
Epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) are standard of care for first‐line treatment of EGFR‐mutated (EGFRm) advanced non‐small cell lung cancer (NSCLC). 1 Osimertinib is a third‐generation, irreversible, central nervous system (CNS)‐active, oral EGFR‐TKI that potently and selectively inhibits EGFR‐TKI sensitizing and EGFR T790M resistance mutations, with demonstrated efficacy in EGFRm NSCLC, including CNS metastases. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
Osimertinib was originally approved for treatment of T790M‐positive advanced NSCLC. 12 The phase III AURA3 study (NCT02151981) demonstrated significantly improved progression‐free survival (PFS) vs. platinum‐based chemotherapy in patients with T790M‐positive advanced NSCLC that had progressed on first‐line EGFR‐TKI therapy. 2 First‐line osimertinib treatment for EGFRm advanced NSCLC was assessed in the phase III FLAURA study (NCT02296125), finding significantly improved PFS and overall survival (OS) vs. comparator EGFR‐TKIs (erlotinib or gefitinib). 10 , 11 As a result, osimertinib is the preferred first‐line treatment for EGFRm advanced NSCLC. 1 Osimertinib is also approved for adjuvant treatment of patients with resected EGFRm stage IB–IIIA NSCLC based on significant improvements in disease‐free survival (DFS) vs. placebo in the ADAURA (NCT02511106) phase III study, with DFS benefits translating to significant overall survival improvement. 5 , 13 , 14 , 15
Osimertinib has a manageable and consistent adverse event (AE) profile across phase III clinical studies; commonly reported AEs include rash and diarrhoea. 2 , 5 , 11 Other AEs of interest include interstitial lung disease (ILD), a rare but serious AE that has been reported in patients receiving EGFR‐TKIs, 16 and events related to cardiac function. 17
An exposure–response (E–R) analysis for osimertinib (20–240 mg once‐daily [QD]) and its main metabolite, AZ5104, was previously published using data from two studies in patients with EGFRm advanced NSCLC previously treated with an EGFR‐TKI (AURA [NCT01802632] and AURA2 [NCT02094261]). The analysis showed no statistically significant relationship between osimertinib exposure and efficacy, but there was a linear QT interval change corrected by Fridericia's formula. 18
In this analysis, we expanded upon the previously published data to comprehensively examine the relationship between systemic plasma osimertinib exposure and efficacy and safety endpoints in the first, second, and later lines of treatment in advanced NSCLC and adjuvant setting in resectable NSCLC. We also performed a model‐based analysis of osimertinib exposure and incidences of ILD and left ventricular ejection fraction (LVEF) events and a descriptive analysis of rash and diarrhoea incidences across lines of therapy and/or studies. We used pooled data from the AURA (first‐line and second‐line or later cohorts), AURA extension, AURA2, AURA3, FLAURA and ADAURA studies. 2 , 5 , 11 , 19 , 20 , 21 , 22
2. METHODS
2.1. Patient population
The E–R (safety/efficacy) modelling utilized data from different patient populations (first‐line [N = 338], second‐line or later [N = 1026], and adjuvant [N = 325] cohorts) across the osimertinib clinical development programme (Tables S1, S2 and S3). The E–R efficacy analysis included patients who received ≥1 dose of study treatment. For first‐line therapy, patients who received osimertinib in the AURA (20–240 mg QD) and FLAURA studies (80 mg QD) or comparator EGFR‐TKI in FLAURA were included. For second‐line or later‐line therapy, patients who received osimertinib in AURA (20–240 mg QD), AURA extension (80 mg QD), AURA2 (80 mg QD) and AURA3 (80 mg QD), and those who received chemotherapy in AURA3, were included. For adjuvant therapy, patients who received osimertinib (80 mg QD) or placebo in the ADAURA study were included.
All analyses were conducted in NONMEM (version 7.3) and the runs for the bootstrap analysis were conducted in a Linux environment (CentOS 5, equivalent to Red Hat Enterprise Linux 5) with GFortran FORTRAN Compiler, (version 4.7.3; GNU Compiler Collection, GCC), NONMEM (version 7.3), and MATLAB R2014b for pre‐ and post‐processing analysis. NONMEM code is provided in the Supplementary Methods.
2.2. Exposure–response analysis
2.2.1. Estimation of plasma exposure
Osimertinib pharmacokinetic (PK) samples were collected across the studies as shown in Table S1. Area under the curve at steady state (AUCss) of osimertinib and/or AZ5104 were used as the exposure metric in the analyses. AUCss was derived for each patient based on the individual apparent clearances (CL/F) estimated in the population PK (popPK) analysis 23 and the dose level in each patient as: osimertinib AUCss = dose/clearance. For E–R efficacy analyses, the most prevalent/typical dose level in a patient was used to calculate the individual AUCss, whereas the first given dose was used for the E–R safety analysis. Shrinkage estimates for clearances for osimertinib and metabolite were small (<5%), therefore the derived AUCss values were informative.
For E–R safety analyses, all patients who received at least one dose of randomized osimertinib were included. For both E–R (safety/efficacy) analyses, only osimertinib‐treated patients for whom exposure data (AUCss) could be computed from the popPK model were included. The comparator EGFR‐TKI, chemotherapy or placebo data were used in plots for comparative purposes only and were not included in the modelling analysis.
Since osimertinib and AZ5104 exposures were highly correlated (Figure S1), 23 they were not included simultaneously in one model to avoid problems of multicollinearity.
2.2.2. Model‐based efficacy analysis
E–R efficacy analyses were performed separately for first‐line, second‐line, and adjuvant patient populations. Initial exploratory Kaplan–Meier (KM) analyses of PFS or DFS were followed by model‐based analyses using a Cox proportional hazard model (see Supplementary Methods). Survival analyses were stratified by AUCss quartiles to identify potential underlying relationships between steady‐state exposure and PFS or DFS.
2.2.3. Model based exposure‐ILD/LVEF analysis
ILD‐like events included ILD, pneumonitis, acute interstitial pneumonitis, alveolitis, diffuse alveolar damage, idiopathic pulmonary fibrosis, lung disorder, pulmonary toxicity and pulmonary fibrosis. LVEF events were defined as post‐baseline decreases in LVEF of ≥10 percentage points (pp), leading to values below 50%. The number of patients experiencing ILD/LVEF events was relatively small. In such cases, the maximum likelihood estimation of the logistic regression model is known to suffer from small sample bias and the degree of bias is strongly dependent on the number of cases in the less frequent of the two outcome categories. To address this bias, a penalized logistic regression model 24 utilizing a penalized likelihood was applied to reduce small‐sample bias. The model‐based analysis only included ILD/LVEF data from osimertinib‐treated patients with available PK exposure information. Since a possible exposure dependency might be explained by other confounding risk factors, a graphical analysis of patients treated with chemotherapy or comparator EGFR‐TKI was performed to assess correlation of exposure metrics with covariates of interest. Details of model form and covariate inclusion are summarized in the Supplementary Methods. The final models were simulated to assess differences in predicted probabilities of ILD/LVEF events in osimertinib‐treated and placebo‐treated patients (assumed 0 mg dose). The simulation algorithm consisted of a bootstrapping approach (N = 1000) and is detailed in the Supplementary Methods.
The final model that was developed based on AURA3, FLAURA and ADAURA was then used as validation for the model's ability to predict ILD/LVEF events in patients treated with adjuvant osimertinib in the ADAURA study.
2.2.4. Descriptive analysis for rash and diarrhoea incidence
A previously published model‐based analysis performed using data from 748 patients in the AURA and AURA2 studies, across dose levels from 20 to 240 mg, showed a clear exposure‐dependent increase in the incidence of rash and diarrhoea. 18 The subsequent phase III studies (FLAURA, AURA3 and ADAURA) included only the 80 mg dose. Hence, it was assumed that the exposure range studied in the previous analysis, which predominantly included the 80 mg dose level, was sufficient to explain the exposure relationship. The current analysis compared the incidences of rash and diarrhoea, descriptively, across lines of therapy/studies.
2.3. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2023/24.
3. RESULTS
Demographics and baseline characteristics for patients who received osimertinib (N = 1689) or control treatment (N = 756) are provided in Tables S2–S4. Based on previous popPK analyses, there was no impact of age, gender, body weight, race/ethnicity or line of therapy on osimertinib or AZ5104 PK exposure. 18
3.1. Model‐based efficacy analysis
An exploratory KM analysis evaluated PFS (for first‐line and second‐line) or DFS (for adjuvant treatment) as a function of osimertinib and AZ5104 AUCss quartiles. For each patient population, there was no clear trend of increased efficacy with increasing AUCss of osimertinib or AZ5104. Additionally, median PFS or DFS and the associated 95% confidence intervals (CIs) overlapped across exposure quartiles for osimertinib and AZ5104 (Figure 1). However, in all patient populations, there was a clear difference in PFS or DFS between the control group (chemotherapy or comparator EGFR‐TKI or placebo) and all the quartiles of osimertinib and AZ5104 AUCss (Figure 1).
FIGURE 1.
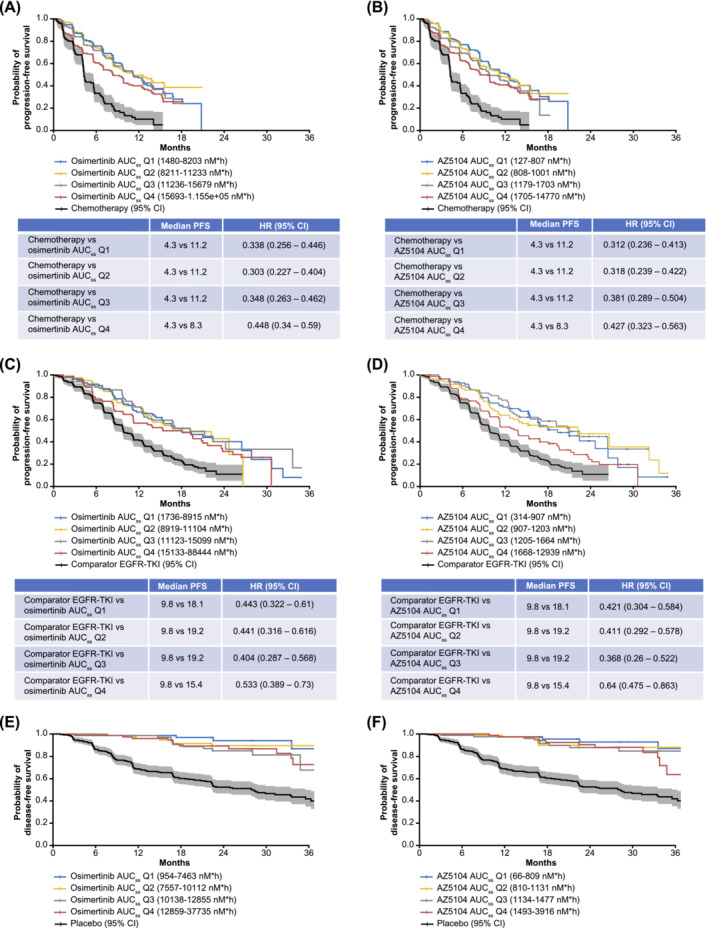
Kaplan–Meier estimates of progression‐free survival or disease‐free survival* for osimertinib and its metabolite, AZ5104, respectively, in patients receiving osimertinib in ≥second‐line (A and B), first‐line (C and D) or adjuvant settings (E and F). Curves were stratified by quartiles of osimertinib or AZ5104 AUCss. The solid black line shows progression‐free survival or disease‐free survival for the comparator treatment (platinum‐pemetrexed in AURA3, no comparator in AURA or AURA2, comparator epidermal growth factor receptor‐tyrosine kinase inhibitors in FLAURA and placebo in ADAURA). The shaded area represents the 95% CI range. *In ADAURA, the median disease‐free survival was not reached at the 17 January 2020 data cut‐off; therefore, median disease‐free survival could not be calculated for the osimertinib and AZ5104 AUCss quartiles. AUCss, area under the curve at steady state; CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
A Cox proportional hazard model (for first‐line and second‐line patients only) was used to assess the relative hazard ratios (HRs) between control treatment and the AUCss quartiles of osimertinib and AZ5104. These results suggest a reduced risk of disease progression with osimertinib treatment (Figure 1). In patients receiving adjuvant osimertinib, the median DFS was not reached at the 17 January 2020 data cut‐off for ADAURA; as such, median DFS could not be calculated for both osimertinib and AZ5104 AUCss quartiles.
Figures 1A–D suggested that in first‐line and second‐line patients, PFS was shorter in those with the highest exposure levels (quartile 4 for osimertinib and AZ5104). Hence, we performed post‐hoc analyses of AURA3 data to further explore factors confounding exposure. Results suggested that the shorter PFS observed in the highest exposure quartile was likely due to a higher proportion of patients with poor prognostic features (higher World Health Organization [WHO] performance status and lower baseline albumin levels; Figures S2A–D; Figure S3; Table S5).
A similar analysis was performed for patients receiving first‐line osimertinib. Potential predictors (age, baseline albumin levels, baseline sum of longest tumour diameter [BSLD], weight, WHO performance status, presence of brain metastases at study entry, gender, smoking status at study entry, statin use, race and medical history of hypertension) for PFS were explored using a Cox regression analysis, and BSLD was identified as a covariate influencing PFS. However, BSLD did not account for the slightly shorter median PFS among patients in the highest exposure quartiles compared with lower exposure quartiles. Further exploratory analysis suggested that the shorter median PFS in the highest exposure quartiles in FLAURA may be due to shorter treatment duration as there were more dose interruptions and patients discontinuing in the highest vs. lowest exposure quartiles (Figure S4, Table S6). Furthermore, the Kaplan–Meier analysis including only the uncensored patients (i.e., those who progressed or died) showed no differences in PFS between patients in different AUCss quartiles (osimertinib and AZ5104) suggesting censoring might have influenced this PFS outcome (Figure S5).
3.2. Model‐based ILD analysis
3.2.1. Model development (first‐ and ≥second‐line treatment)
The observed proportion of ILD events stratified by osimertinib AUCss quartile is shown in Figure 2A. These data show that the probability of ILD events increases with increasing osimertinib AUCss. A similar relationship was seen for AZ5104 (Figure S6A). Penalized logistic regression was used to further evaluate the relationship between osimertinib or AZ5104 AUCss and ILD‐like events.
FIGURE 2.
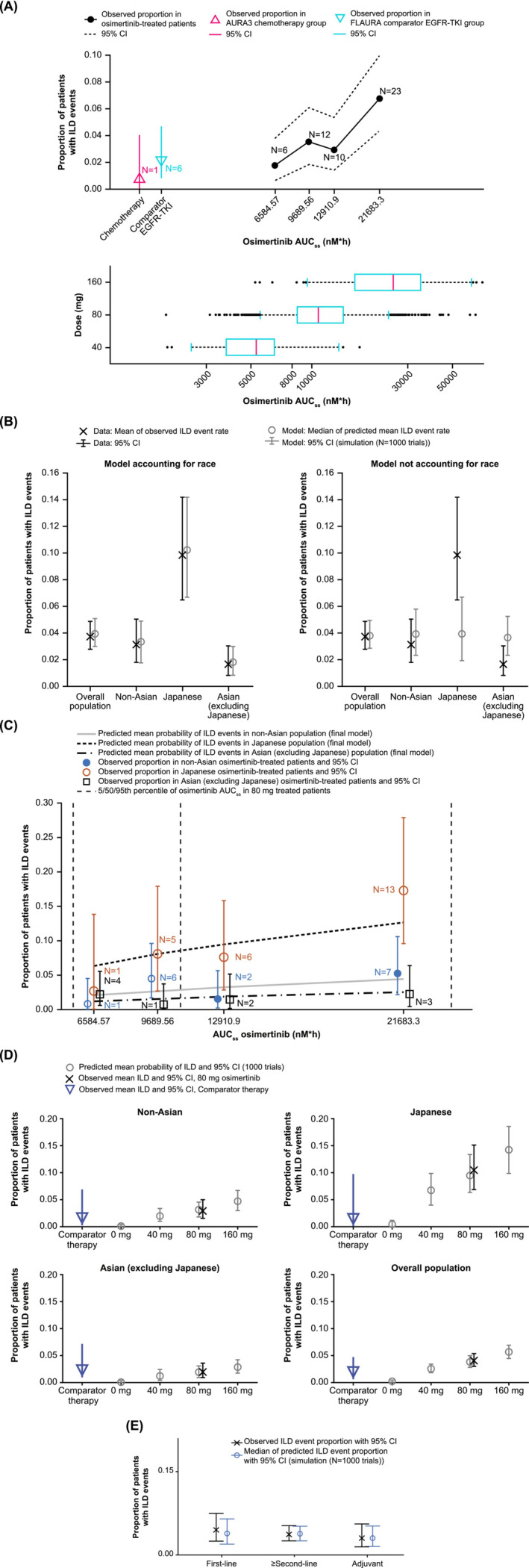
Model‐based exposure and ILD analysis. Proportion of patients with ILD events stratified by osimertinib AUCss quartiles (A) and visual predictive checks for the frequency of ILD events (B) and the predicted mean probability and observed proportion of patients with ILD events (C) in different populations. Simulation of the frequency of ILD events for patients treated with 0, 40, 80, or 160 mg osimertinib and patients treated with comparator therapy in different racial populations (D) and prediction of ILD events for patients according to line of therapy (E). AUCss, area under the curve at steady state; CI, confidence interval; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; ILD, interstitial lung disease.
A higher incidence of ILD was also observed in Japanese patients compared with Asian (non‐Japanese) and non‐Asian patients (Figure S6B); Japanese patients also had a lower body weight compared with the other racial groups and higher osimertinib and AZ5104 AUCss. Therefore, in addition to AUCss, the influence of race (Japanese and non‐Japanese Asian) on ILD incidence was assessed using penalized logistic regression (Table 1). In the base model (Model 1), the relationship between osimertinib AUCss and ILD occurrence was identified as significant (change in objective function value [OBJ] of 10.1 for osimertinib and 9.62 for AZ5104; a decrease of 10.83 [one degree of freedom, P < .001 χ2, df = 1] was required for statistical significance; Table 1 and Table S7). Exposure–response relationships for ILD events were examined by introducing various models on each parameter of the base logistic model and changes in model‐predicted ILD incidence were observed. Addition of Japanese race as covariate (Model 2) considerably improved the description of the data, showing a decrease in OBJ of 32.7 for osimertinib and 32.9 for AZ5104, which was greater than that required for statistical significance (13.8 [P < .001, χ2, df = 2]). In Model 3, inclusion of Asian race (excluding Japanese) as a covariate on the intercept lead to a modest decrease in OBJ compared to Model 2 (1.6 for osimertinib and 1.5 for AZ5104; Table 1 and Table S7). Nevertheless, the impact of Asian race (excluding Japanese) as a covariate on the intercept was retained in the final model. Model 4 included Japanese race as a covariate and excluded PK exposure (AUCss) metrics and Asian race (excluding Japanese) to further compare the effect of exposure and Japanese race on ILD incidence. The difference in the optimal penalized log‐likelihood was smaller between Model 2 (accounting for exposure and Japanese race) and Model 4 (6.9 for osimertinib and 7.1 for AZ5104) than between Model 1 (accounting for exposure alone) and the model with intercept only (10.1 for osimertinib and 9.62 for AZ5104), suggesting that the effect of exposure was statistically non‐significant when accounting for Japanese race in the models. However, a more conservative approach for a safety analysis is to include exposure dependency of ILD, and therefore Model 3 (with osimertinib or AZ5104 exposure dependency) was taken forward for additional covariate modelling.
TABLE 1.
Assessment of osimertinib dependency of ILD events using penalized logistic regression.
| Model | Intercept (95% CI) | Exposure (95% CI) | Japanese (95% CI) | Asian (non‐Japanese) (95% CI) | Change in OBJ |
|---|---|---|---|---|---|
| 1 | −10.3 (−14.5, −5.93) | 0.741 (0.288, 1.18) | – | – | −10.1 |
| 2 | −9.83 (−14.3, −5.29) | 0.649 (0.172, 1.11) | 1.42 (0.85, 1.99) | – | −32.7 |
| 3 | −9.35 (−13.8, −4.76) | 0.628 (0.149, 1.09) | 1.14 (0.510, 1.80) | −0.567 (−1.38, 0.206) | −34.3 |
| 4 | −3.71 (−4.12, −3.35) | – | 1.51 (0.949, 2.08) | – | −25.8 |
Note: Dashes denote variables that were not included in respective models.
Abbreviations: CI, confidence interval; ILD, interstitial lung disease; OBJ, objective function value.
None of the additional covariates evaluated (age, weight, body mass index, sex, WHO performance status, history of hypertension, line of therapy, smoking and statin use) appeared to improve the model when incorporated (Tables S8 and S9). There was no apparent difference in the relationship between osimertinib exposure and ILD between patients treated with first‐line osimertinib and those in the second‐line setting (data not shown).
3.2.2. Model evaluation (first‐ and ≥second‐line treatment)
The adequacy of the final osimertinib model to describe ILD event probability was assessed for different patient sub‐populations. A comparison of model predictions to observations is shown for both the models including and excluding Japanese race as a covariate (Figure 2B). While the model without ethnicity as a covariate described the mean in the total population reasonably well, it lacked the ability to describe ILD event probability for the Japanese and the Asian (excluding Japanese) subgroups. Hence, the differences in exposure (AUCss) alone in these groups could not account for the differences in ILD event probability.
The final model (accounting for racial differences) was used to simulate the expected probability of patients experiencing ILD events over the available range of AUCss values for different race groups (Figure 2C). Figure 2C suggests that model‐predicted probabilities of ILD for different racial populations were similar to the observed ILD probabilities and that the model was appropriate for describing the data. For the Japanese population, the estimated ILD probability appeared consistently higher than that for the non‐Asian population over the range of considered AUCss values. In contrast, for the Asian (excluding Japanese) population, the predicted ILD probability was consistently lower than that for the non‐Asian population. However, the uncertainty of ILD event probability was high, which should be considered when drawing conclusions from these simulations. Similar results were observed for ILD events based on AZ5104 AUCss values (Figure S7).
3.2.3. Model simulations (first‐ and ≥second‐line treatment)
The final model was used to simulate ILD event probability in osimertinib‐treated (40, 80 or 160 mg dose) and comparator therapy‐treated (chemotherapy or comparator EGFR‐TKIs) patients (Figure 2D). The simulated results for patients on 80 mg osimertinib corresponded well to the observed data across populations. The error bars for patients treated with osimertinib 80 mg and those treated with chemotherapy or comparator EGFR‐TKIs were not completely separated, although ILD event rate tended to be higher in Japanese patients treated with osimertinib 80 mg than in Japanese patients who received comparator treatment. The predicted ILD event probability in osimertinib‐treated patients appeared to increase with increasing dose and was the lowest in comparator therapy‐treated patients. The predicted ILD probabilities in placebo‐treated patients (AUCss = 0) would be 0.009% from the osimertinib model and 0.06% from the AZ5104 model (calculated from the intercept estimates in Table 1 and Table S7). However, it needs to be considered that the prediction at AUCss = 0 is based on a model that was inferred from data from osimertinib‐treated patients only (there were no placebo‐treated patients in the analysis).
3.2.4. Predicting ILD incidence for adjuvant patients in ADAURA
The adequacy of the final ILD model to describe ILD event probability was re‐assessed for different sub‐populations of patients including the ADAURA study. The good alignment between observed and simulated data distributions (Figure 2E) confirms that both previously developed models (with either osimertinib AUCss or AZ5104 AUCss as exposure predictor variable) adequately described the ILD event probability in the available data from AURA, AURA extension, AURA2, AURA3, FLAURA and ADAURA in different sub‐populations.
3.3. Model‐based exposure and LVEF analysis
There were 1/94 (1.1%) and 4/256 (1.6%) patients with LVEF events in the chemotherapy and comparator EGFR‐TKI groups, respectively, and 0/4 (0%), 35/909 (3.9%), 1/21 (4.8%), and 0/2 (0%) in the osimertinib 40 mg, 80 mg, 160 mg and 240 mg groups, respectively.
The number and proportion of patients with LVEF events, stratified by the quartiles of osimertinib and AZ5104 AUCss values, are shown in Figure 3A and Figure S8, respectively. There was a trend for increased LVEF event frequency among patients in higher osimertinib AUCss quartiles, though the 95% CIs for the first and fourth quartile were overlapping. Hence, a penalized logistic regression analysis was conducted to assess if this result was still valid without the stratification based on exposure quartiles, and to make sure that the results were not confounded by covariates. The initial base model evaluated the relationship between continuous AUCss of osimertinib/AZ5104 and LVEF events, and the final model was derived by assessing the potential relationship between LVEF event incidence and a number of covariates. Additional details on covariate model building are provided in Tables S10 and S11. The covariate models were constructed by including each parameter on the intercept of the osimertinib base model and the results are presented in Table 2. None of the tested covariates except baseline LVEF was significant (prespecified threshold for statistical significance was P < .001). Hence, the model using log‐transformed osimertinib or AZ5104 AUCss, with baseline LVEF as a covariate, was chosen as the final model.
FIGURE 3.
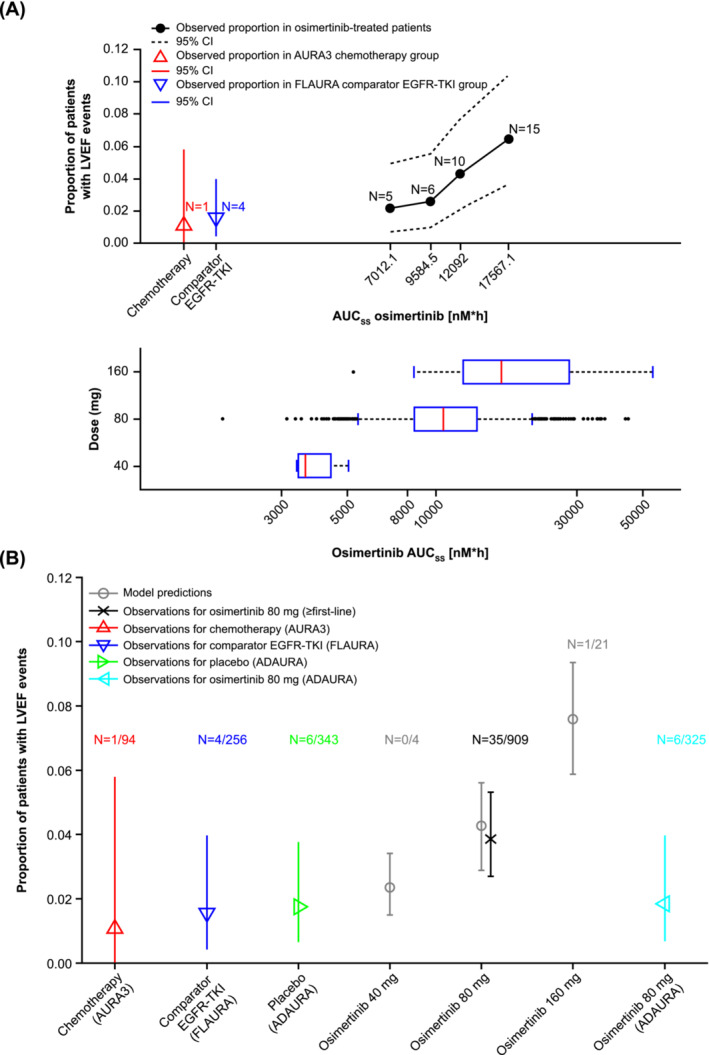
Model‐based exposure and left ventricular ejection fraction analysis. Proportion of patients with LVEF events stratified by osimertinib AUCss quartiles (A) and the predicted probability of LVEF events for different osimertinib doses and comparison to observed data (B). Observed data were not plotted for 40 mg and 160 mg due to limited sample size (N = 4 and N = 21, respectively). Vertical bars represent 95% CI. AUCss, area under the curve at steady state; CI, confidence interval; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; LVEF, left ventricular ejection fraction.
TABLE 2.
Parameter estimates assessing the influence of exposure on LVEF events.
| Exposure | Model | Parameter | Estimate (95% CI) | P‐value |
|---|---|---|---|---|
| Osimertinib | Base model | Intercept | −12.8 (−19.3, −6.02) | <.001 |
| Slope | 1.02 (0.304, 1.7) | .006 | ||
| Final model | Intercept | −6.36 (−13.6, 1.01) | .090 | |
| Slope | 0.921 (0.209, 1.61) | .012 | ||
| Baseline LVEF | −0.087 (−0.134, −0.042) | <.001 | ||
| AZ5104 | Base model | Intercept | −10.5 (−15.1, −5.9) | <.001 |
| Slope | 1.02 (0.382, 1.64) | .002 | ||
| Final model | Intercept | −4.38 (−9.88, 1.07) | .115 | |
| Slope | 0.927 (0.292, 1.56) | .004 | ||
| Baseline LVEF | −0.087 (−0.134, −0.041) | <.001 |
Note: The base models used AUCss of osimertinib/AZ5104 as the predictor variable and the final models used AUCss of osimertinib/AZ5104 with baseline LVEF as a covariate.
Abbreviations: CI, confidence interval; LVEF, left ventricular ejection fraction.
The final model was used to simulate LVEF event probability in patients treated with osimertinib (40, 80 or 160 mg dose) (Figure 3B). For the osimertinib 80 mg dose, the model‐predicted LVEF event probability was similar to the observed number of LVEF events. The observed LEVF event frequency in patients receiving osimertinib 80 mg in ADAURA appeared to be lower than in patients receiving osimertinib 80 mg in previous studies (first‐line and ≥second‐line studies) but the CIs were overlapping (Figure 3B). Modelling results suggest 95% CIs based on the simulations are overlapping and there is no clear evidence of a difference in LVEF events across osimertinib exposure. While LVEF event probability was predicted to be higher for 160 mg‐treated patients than that observed for non‐osimertinib‐treated patients (chemotherapy, comparator EGFR‐TKIs, adjuvant placebo), the CIs for patients treated with osimertinib 80 mg and those receiving comparator treatment largely overlapped (Figure 3B). Furthermore, this relationship is confounded by the small number of events across doses and the baseline LVEF values.
3.4. Descriptive analysis for rash and diarrhoea
In general, patients treated with first‐line osimertinib 80 mg showed a higher incidence and severity of rash or diarrhoea than those treated in later‐line or adjuvant settings (Figure 4A–C). Overall, the incidence and severity of rash was lower in patients treated with osimertinib 80 mg compared with patients receiving comparator EGFR‐TKI, whereas the incidence and severity of diarrhoea was similar between these groups (Figure 4B and C). First‐line (including osimertinib 80 mg and 160 mg doses) and adjuvant patients in the highest osimertinib exposure quartiles tended to have high frequencies of moderate‐to‐severe rash or diarrhoea events (Figure 4D and E).
FIGURE 4.
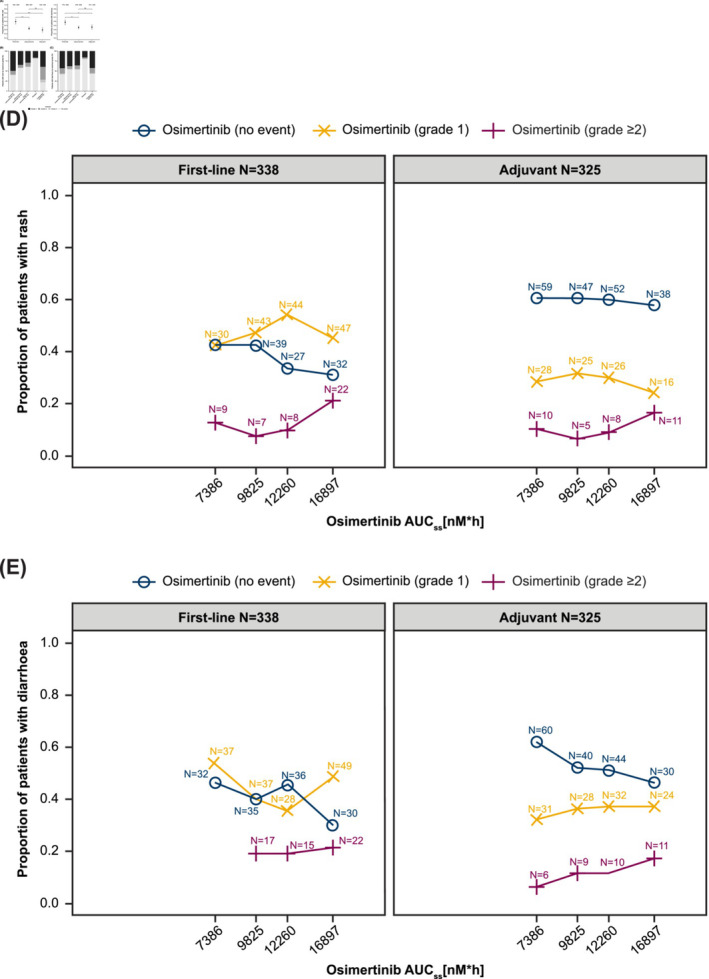
Descriptive analysis of rash and diarrhoea. Incidence of all‐grade rash and diarrhoea with osimertinib 80 mg across different lines of therapy (A). Distribution of maximum CTCAE grades of rash (B) and diarrhoea (C) occurring with osimertinib 80 mg, comparator EGFR‐TKI, and placebo. Proportion of CTCAE rash grades (D) and CTCAE diarrhoea grades (E) by osimertinib AUCss quartiles. AUCss, area under the curve at steady state; CTCAE, Common Terminology Criteria for Adverse Events; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; ns, not significant.
4. DISCUSSION
This was the first study to assess the relationship between osimertinib plasma exposure and its efficacy and safety across different NSCLC treatment settings using pooled data from the AURA, AURA extensions, AURA2, AURA3, FLAURA and ADAURA studies. In a previous analysis of patients with EGFR T790M‐positive NSCLC, a once‐daily osimertinib dose of 80 mg was shown to provide a positive benefit–risk profile that maximized clinical activity while minimizing the likelihood of adverse reactions across various population‐based covariates. It should also be noted that age, gender, body weight, race/ethnicity, renal or hepatic impairment, smoking status, or line of therapy had no impact on osimertinib or AZ5104 PK exposure. 18
Across all treatment settings, the E–R analysis indicated that increased osimertinib exposure was not likely to increase efficacy, and that efficacy with osimertinib in all exposure quartiles was significantly better than in the control arm. Hence, osimertinib 80 mg was considered the optimal dose, and doses above 80 mg were not likely to provide additional benefit.
The ILD analysis showed that increasing osimertinib (or AZ5104) exposure (and dose) increased the probability that patients develop ILD or ILD‐like events. However, this relationship was not statistically significant (at the pre‐defined criterion of P < .001). At a similar exposure (AUCss), Japanese patients were predicted to have a higher probability of experiencing ILD and ILD‐like events compared to other races. While the reason for the difference in the incidence of ILD between Japanese, non‐Japanese Asians and non‐Asians is unknown, it may relate to physiological and environmental factors that are specific to Japan or Japanese patients. Moreover, the high rate of ILD detection in Japanese patients receiving EGFR‐TKIs may contribute to this observation. 25 , 26
None of the additional covariates (age, weight, BMI, gender, WHO performance status, history of hypertension, line of therapy, nicotine use and statin use) showed any statistically significant effect on ILD event occurrence. We also considered the possibility of extrapolating the ILD incidence rate to placebo‐treated patients, under the assumption that placebo treatment corresponds to zero osimertinib exposure (AUCss = 0). The predicted probabilities of ILD in placebo‐treated patients would be 0.009% from the osimertinib model and 0.06% from the AZ5104 model, which were considerably lower than previously reported ILD incidence levels in placebo‐treated patients. In the gefitinib case–control study, the observed incidence of ILD‐like events in Japanese patients with NSCLC treated with chemotherapy was 2.1% (unadjusted for imbalances in risk factors between treatments). 25 In a phase III study of gefitinib vs. placebo (plus best supportive care in both arms) in patients with advanced NSCLC, the incidence of ILD or ILD‐like events was approximately 1% among 562 patients in the placebo arm. 27 Hence, it is likely that the current model considerably underestimates the placebo effect and, thereby, potentially overestimates the effect of osimertinib treatment on ILD events.
The relationship between osimertinib exposure and LVEF event incidence was evaluated using a similar approach to the ILD analysis. Modelling results suggest 95% CIs based on the simulations largely overlapped across doses and exposure and there was no clear evidence of a difference in LVEF events across osimertinib dose and exposure. Furthermore, this relationship was confounded by small number of events across different doses and the baseline LVEF values.
We additionally performed a descriptive analysis of rash and diarrhoea events, which showed an increased incidence and severity of these events in the first‐line setting compared with later‐line and adjuvant settings. However, rash incidence and severity were lower in patients treated with osimertinib 80 mg than in those treated with comparator EGFR‐TKIs, whereas diarrhoea incidence and severity were comparable. Based on these data, long‐term treatment at 80 mg appears to be appropriate.
While our study provides a comprehensive assessment of the relationship between systemic plasma exposure of osimertinib and efficacy and safety endpoints across a range of treatment settings, it is limited by most data being available from patients receiving the osimertinib 80 mg daily dose. This might contribute to the lack of a dose–exposure response relationship for the efficacy and safety endpoints.
Across the clinical programme, patients with the lowest osimertinib exposure at 80 mg QD dose were found to achieve an AUCss that was no greater than two‐fold the geometric mean AUCss for the 20 mg QD dose. 18 , 23 Thus, the 80 mg QD dose ensures patients will attain exposures above 20 mg QD, the lowest dose evaluated in the clinical studies that demonstrated clinical activity, while still allowing prescribers the option to dose reduce in response to toxicity. Moreover, taking into consideration the PK variability in exposure, the 40 mg QD dose may result in some patients having similar exposure to the lowest dose studied (20 mg QD), providing limited scope to reduce dose.
In summary, the 160 mg QD dose was less tolerable than the 80 mg QD dose, with no perceivable additional benefit in efficacy. Given the inter‐individual PK variability 18 , 23 in the patient population, and that osimertinib is likely to be administered for a prolonged period, it is vital to utilize a dose that will achieve adequate systemic and CNS exposure to osimertinib. Based on E–R modelling, the recommended daily dose of osimertinib 80 mg demonstrated a positive benefit–risk profile that maximizes clinical activity in patients with EGFRm NSCLC. Importantly, this dose ensures that patients receive a clinically active dose regardless of inter‐individual variability and allows prescribers to reduce the dose if needed. The results from the AURA3, FLAURA and ADAURA phase III studies consistently showed a positive benefit–risk profile for patients at the 80 mg QD dose of osimertinib. 2 , 5 , 11
AUTHOR CONTRIBUTIONS
The PK E–R analysis was conceived by M.J., K.V. and H.S. Analysis was performed by M.J., E.V.M., H.S. and M.S. All authors contributed to interpretation. All coauthors provided critical revisions of the manuscript. M.J. was responsible for drafting the manuscript and incorporating the suggestions of the coauthors, and is the guarantor of the manuscript. M.J. certifies that the manuscript is an honest, accurate and transparent account of the analyses being reported; that no important aspects have been omitted.
CONFLICT OF INTEREST STATEMENT
M.J. and H.T. report employment at AstraZeneca at the time of this study and ownership of stocks/shares in AstraZeneca. Y.‐W.L. reports employment at Certara, ownership of stocks/shares in Certara and grants or funds from the US National Institutes of Health. H.S. reports employment and consulting fees from IntiQuan AG (IntiQuan AG received consulting fees for the modelling work on osimertinib). M.S. reports employment at AstraZeneca AB and ownership of stocks/shares in AstraZeneca AB. E.V.M. reports no conflict of interest to declare. X.H., Y.R. and K.V. report employment at AstraZeneca and ownership of stocks/shares in AstraZeneca.
Supporting information
Figure S1. Scatter plot of AUCss values for osimertinib and AZ5104 for patients treated with osimertinib in the safety population. AUCss, area under the curve at steady state.
Figure S2. Kaplan–Meier representation of PFS in the AURA3 study stratified by baseline albumin (A), WHO performance status (B), gender (C) and body weight (D) categories. BALB, baseline albumin; PFS, progression‐free survival; WHO, World Health Organization; WT, body weight.
Figure S3. Kaplan–Meier representation of PFS in the AURA3 study stratified by WHO performance status and median BALB. The median of BALB in the dataset was 39 g/L. WHO denotes the WHO performance status. Kaplan–Meier survival curves were estimated for the four groups, as indicated in the legend of the figure. The baseline hazard function for the simulations was approximated with a Burr distribution that was fitted for the subset of patients with a WHO performance status of 0. The simulations for the different groups were performed by determining the hazard ratios from the Cox regression model in which (BALB‐39)/(WHO> = 1) were used as regressors. The BALB values for simulation of each group were determined as the median BALB values in each group. BALB, baseline albumin; PFS, progression‐free survival; WHO, World Health Organization.
Figure S4. Actual duration of osimertinib treatment in months stratified by study and osimertinib (A) and AZ5104 (B) AUCss quartiles. AUCss, area under the curve at steady state.
Figure S5. Kaplan–Meier representation of PFS stratified by quartiles of osimertinib (A) and AZ5104 (B) AUCss for uncensored patients only (first‐line AURA and FLAURA patients). AUCss, area under the curve at steady state; PFS, progression‐free survival.
Figure S6. Proportion of patients with ILD events across AZ5104 AUCss quartiles (A). Proportion of ILD events and distributions of bodyweight and exposure in patients with ILD events in different populations (B). AUCss, area under the curve at steady state; ILD, interstitial lung disease.
Figure S7. Predicted mean probability of ILD events in different populations based on AZ5104 AUCss values. A‐NJ, Asian non‐Japanese; AUCss, area under the curve at steady state; ILD, interstitial lung disease; JAP, Japanese; N‐A, non‐Asian.
Figure S8. Proportion of patients with LVEF events across AZ5104 AUCss quartiles. AUCss, area under the curve at steady state; Chemo, chemotherapy; LVEF, left ventricular ejection fraction; SoC, standard of care.
Table S1. Summary of studies included in the exposure–response analysis.
Table S2. Baseline demographics for patients receiving osimertinib in AURA, AURA extension, AURA2, AURA3, FLAURA and ADAURA.
Table S3. Baseline demographics for patients receiving osimertinib by line of therapy.
Table S4. Baseline demographics for patients receiving control therapies (chemotherapy in AURA3, comparator EGFR‐TKIs in FLAURA and placebo in ADAURA).
Table S5. Assessment of PFS–exposure relationship using Cox regression (AURA3).
Table S6. Dose interruption and discontinuation across osimertinib AUCss and AZ5104 AUCss quartiles based on FLAURA data.
Table S7. Assessment of AZ5104 dependency of ILD events. Change in objective function value compares the restricted model (model with intercept only) to the full model (model with intercept and variables for exposure and racial covariates [in Models 2 and 3 only]). Dashes denote variables that were not included in respective models. ILD, interstitial lung disease.
Table S8. Evaluation of the significance of single covariates in the osimertinib ILD model. ILD, interstitial lung disease.
Table S9. Evaluation of the significance of single covariates in the AZ5104 ILD model using penalized logistic regression. ILD, interstitial lung disease.
Table S10. Evaluation of the significance of single covariates in the osimertinib LVEF model using penalized logistic regression. LVEF, left ventricular ejection fraction.
Table S11. Evaluation of the significance of single covariates in the AZ5104 LVEF model using penalized logistic regression. LVEF, left ventricular ejection fraction.
ACKNOWLEDGEMENTS
Thanks to all the patients and their families from the studies that provided data that informed this analysis. The authors would like to thank Anthe Zandvliet for her valuable contribution to this analysis. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Sally Cotterill, PhD, CMPP, and Hedley Coppock, PhD, CMPP of Ashfield MedComms, an Inizio company, and was funded by AstraZeneca in accordance with Good Publications Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022).
Johnson M, Lin Y‐W, Schmidt H, et al. Exposure–response modelling of osimertinib in patients with non‐small cell lung cancer. Br J Clin Pharmacol. 2024;90(12):3263‐3276. doi: 10.1111/bcp.16199
Funding information This study was funded by AstraZeneca, Cambridge, UK.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
REFERENCES
- 1. Hendriks LE, Kerr KM, Menis J, et al. Oncogene‐addicted metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2023;34(4):339‐357. doi: 10.1016/j.annonc.2022.12.009 [DOI] [PubMed] [Google Scholar]
- 2. Mok TS, Wu Y‐L, Ahn M‐J, et al. Osimertinib or platinum–pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376(7):629‐640. doi: 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation‐positive non‐small‐cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538‐547. doi: 10.1200/JCO.19.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M‐positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15(4):637‐648. doi: 10.1016/j.jtho.2019.12.113 [DOI] [PubMed] [Google Scholar]
- 5. Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2020;383(18):1711‐1723. doi: 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 6. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M‐positive advanced non‐small‐cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702‐2709. doi: 10.1200/JCO.2018.77.9363 [DOI] [PubMed] [Google Scholar]
- 7. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36(33):3290‐3297. doi: 10.1200/JCO.2018.78.3118 [DOI] [PubMed] [Google Scholar]
- 8. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046‐1061. doi: 10.1158/2159-8290.CD-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum‐pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR‐tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536‐1544. doi: 10.1016/j.annonc.2020.08.2100 [DOI] [PubMed] [Google Scholar]
- 10. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41‐50. doi: 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 11. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113‐125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration. Osimertinib (TAGRISSO) . https://www.fda.gov/drugs/resources-information-approved-drugs/osimertinib-tagrisso. Accessed August 7, 2024. [Correction added on 30 August 2024, after first online publication: Reference 12 has been updated in this version.]
- 13. Herbst RS, Wu YL, John T, et al. Adjuvant osimertinib for resected EGFR‐mutated stage IB‐IIIA non‐small‐cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41(10):1830‐1840. doi: 10.1200/JCO.22.02186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration . FDA approves osimertinib as adjuvant therapy for non‐small cell lung cancer with EGFR mutations. Accessed December 1, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-adjuvant-therapy-non-small-cell-lung-cancer-egfr-mutations [Google Scholar]
- 15. Tsuboi M, Herbst RS, John T, et al. Overall survival with osimertinib in resected EGFR‐mutated NSCLC. N Engl J Med. 2023;389(2):137‐147. doi: 10.1056/NEJMoa2304594 [DOI] [PubMed] [Google Scholar]
- 16. Ohmori T, Yamaoka T, Ando K, et al. Molecular and clinical features of EGFR‐TKI‐associated lung injury. Int J Mol Sci. 2021;22(2):792. doi: 10.3390/ijms22020792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ewer MS, Tekumalla SH, Walding A, Atuah KN. Cardiac safety of osimertinib: a review of data. J Clin Oncol. 2021;39(4):328‐337. doi: 10.1200/JCO.20.01171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown K, Comisar C, Witjes H, et al. Population pharmacokinetics and exposure–response of osimertinib in patients with non‐small cell lung cancer. Br J Clin Pharmacol. 2017;83(6):1216‐1226. doi: 10.1111/bcp.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2016;17(12):1643‐1652. doi: 10.1016/S1470-2045(16)30508-3 [DOI] [PubMed] [Google Scholar]
- 20. Yang JCH, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288‐1296. doi: 10.1200/JCO.2016.70.3223 [DOI] [PubMed] [Google Scholar]
- 21. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med. 2015;372(18):1689‐1699. doi: 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 22. Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36(9):841‐849. doi: 10.1200/JCO.2017.74.7576 [DOI] [PubMed] [Google Scholar]
- 23. Johnson M, Lin YW, Schmidt H. Population pharmacokinetics of osimertinib in patients with non‐small cell lung cancer (in preparation). [Google Scholar]
- 24. Heinze G, Ploner M, Beyea J. Confidence intervals after multiple imputation: combining profile likelihood information from logistic regressions. Stat Med. 2013;32(29):5062‐5076. doi: 10.1002/sim.5899 [DOI] [PubMed] [Google Scholar]
- 25. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case‐control study. Am J Respir Crit Care Med. 2008;177(12):1348‐1357. doi: 10.1164/rccm.200710-1501OC [DOI] [PubMed] [Google Scholar]
- 26. Koo LC, Clark JA, Quesenberry CP, et al. National differences in reporting ‘pneumonia’ and ‘pneumonia interstitial’: an analysis of the WHO International Drug Monitoring Database on 15 drugs in nine countries for seven pulmonary conditions. Pharmacoepidemiol Drug Saf. 2005;14(11):775‐787. doi: 10.1002/pds.1071 [DOI] [PubMed] [Google Scholar]
- 27. Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: results from a randomised, placebo‐controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366(9496):1527‐1537. doi: 10.1016/S0140-6736(05)67625-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatter plot of AUCss values for osimertinib and AZ5104 for patients treated with osimertinib in the safety population. AUCss, area under the curve at steady state.
Figure S2. Kaplan–Meier representation of PFS in the AURA3 study stratified by baseline albumin (A), WHO performance status (B), gender (C) and body weight (D) categories. BALB, baseline albumin; PFS, progression‐free survival; WHO, World Health Organization; WT, body weight.
Figure S3. Kaplan–Meier representation of PFS in the AURA3 study stratified by WHO performance status and median BALB. The median of BALB in the dataset was 39 g/L. WHO denotes the WHO performance status. Kaplan–Meier survival curves were estimated for the four groups, as indicated in the legend of the figure. The baseline hazard function for the simulations was approximated with a Burr distribution that was fitted for the subset of patients with a WHO performance status of 0. The simulations for the different groups were performed by determining the hazard ratios from the Cox regression model in which (BALB‐39)/(WHO> = 1) were used as regressors. The BALB values for simulation of each group were determined as the median BALB values in each group. BALB, baseline albumin; PFS, progression‐free survival; WHO, World Health Organization.
Figure S4. Actual duration of osimertinib treatment in months stratified by study and osimertinib (A) and AZ5104 (B) AUCss quartiles. AUCss, area under the curve at steady state.
Figure S5. Kaplan–Meier representation of PFS stratified by quartiles of osimertinib (A) and AZ5104 (B) AUCss for uncensored patients only (first‐line AURA and FLAURA patients). AUCss, area under the curve at steady state; PFS, progression‐free survival.
Figure S6. Proportion of patients with ILD events across AZ5104 AUCss quartiles (A). Proportion of ILD events and distributions of bodyweight and exposure in patients with ILD events in different populations (B). AUCss, area under the curve at steady state; ILD, interstitial lung disease.
Figure S7. Predicted mean probability of ILD events in different populations based on AZ5104 AUCss values. A‐NJ, Asian non‐Japanese; AUCss, area under the curve at steady state; ILD, interstitial lung disease; JAP, Japanese; N‐A, non‐Asian.
Figure S8. Proportion of patients with LVEF events across AZ5104 AUCss quartiles. AUCss, area under the curve at steady state; Chemo, chemotherapy; LVEF, left ventricular ejection fraction; SoC, standard of care.
Table S1. Summary of studies included in the exposure–response analysis.
Table S2. Baseline demographics for patients receiving osimertinib in AURA, AURA extension, AURA2, AURA3, FLAURA and ADAURA.
Table S3. Baseline demographics for patients receiving osimertinib by line of therapy.
Table S4. Baseline demographics for patients receiving control therapies (chemotherapy in AURA3, comparator EGFR‐TKIs in FLAURA and placebo in ADAURA).
Table S5. Assessment of PFS–exposure relationship using Cox regression (AURA3).
Table S6. Dose interruption and discontinuation across osimertinib AUCss and AZ5104 AUCss quartiles based on FLAURA data.
Table S7. Assessment of AZ5104 dependency of ILD events. Change in objective function value compares the restricted model (model with intercept only) to the full model (model with intercept and variables for exposure and racial covariates [in Models 2 and 3 only]). Dashes denote variables that were not included in respective models. ILD, interstitial lung disease.
Table S8. Evaluation of the significance of single covariates in the osimertinib ILD model. ILD, interstitial lung disease.
Table S9. Evaluation of the significance of single covariates in the AZ5104 ILD model using penalized logistic regression. ILD, interstitial lung disease.
Table S10. Evaluation of the significance of single covariates in the osimertinib LVEF model using penalized logistic regression. LVEF, left ventricular ejection fraction.
Table S11. Evaluation of the significance of single covariates in the AZ5104 LVEF model using penalized logistic regression. LVEF, left ventricular ejection fraction.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.


