Abstract
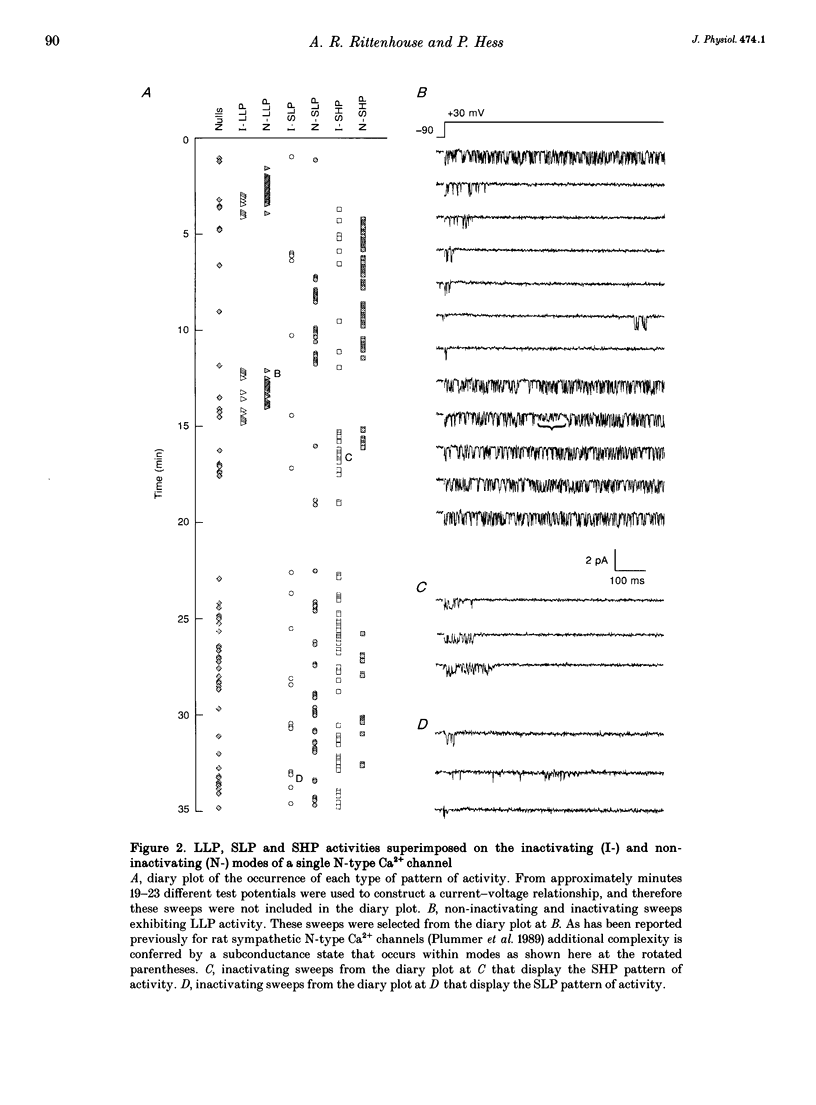
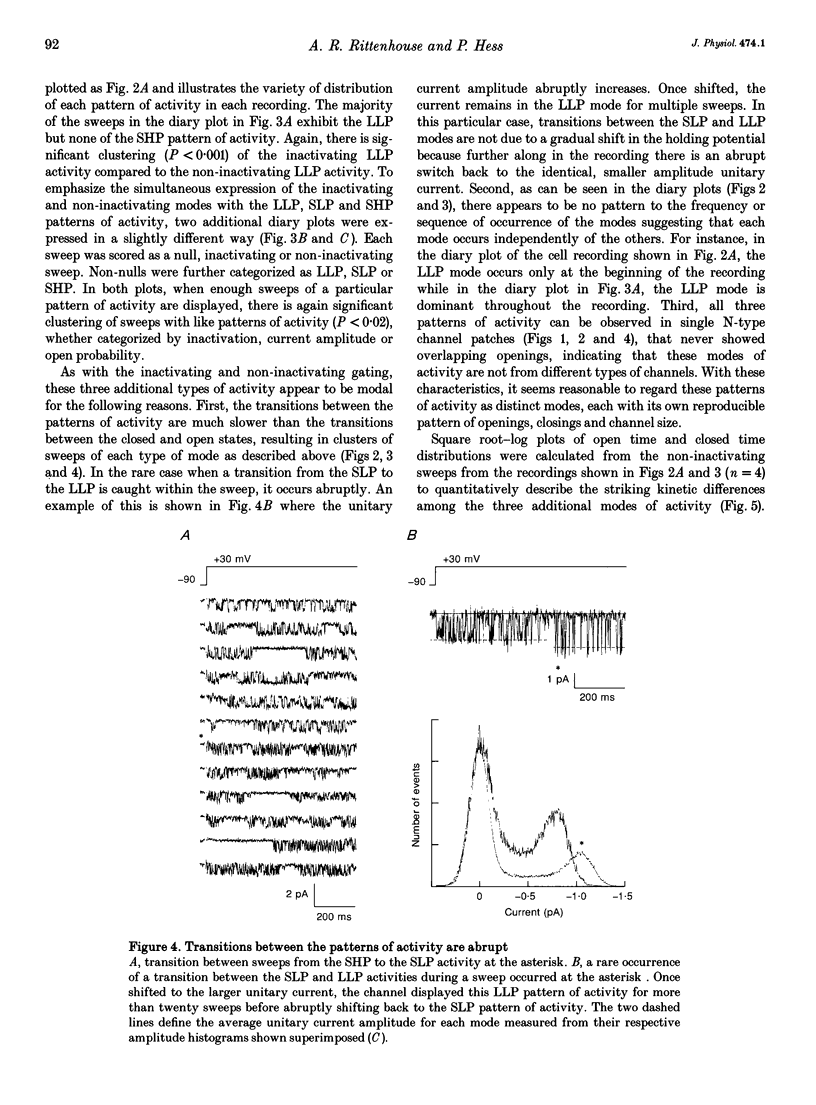
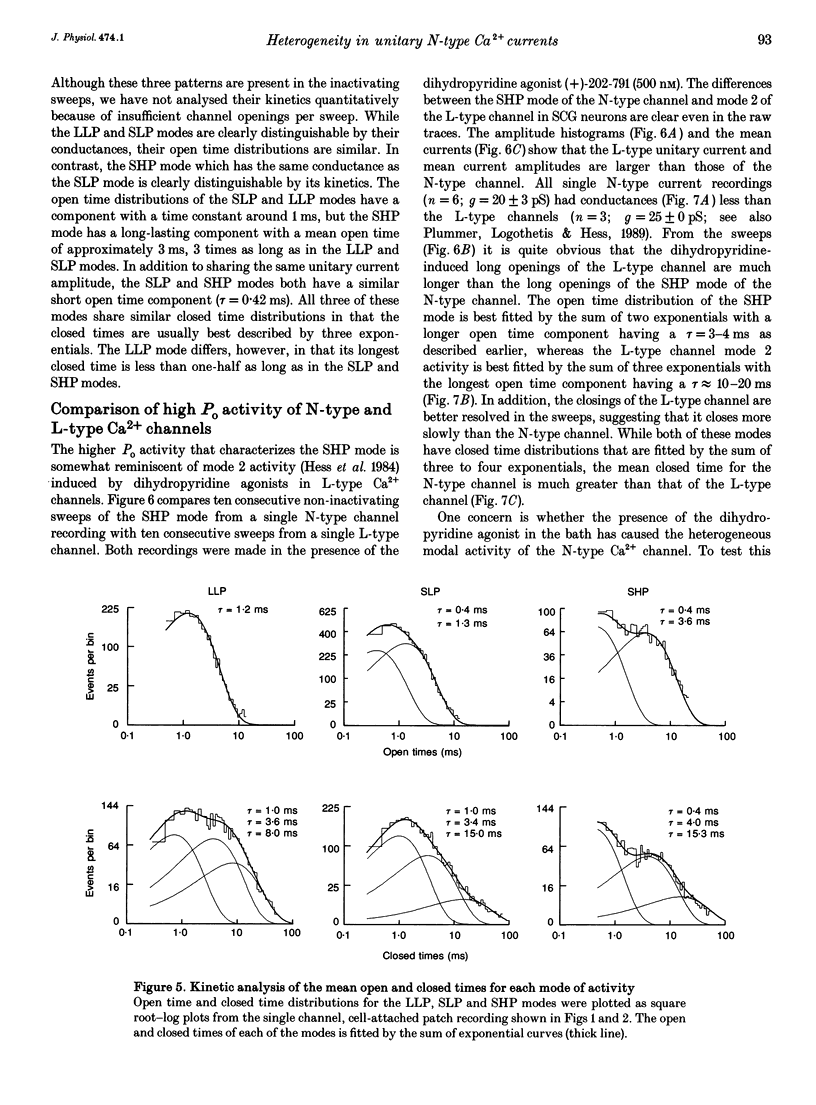
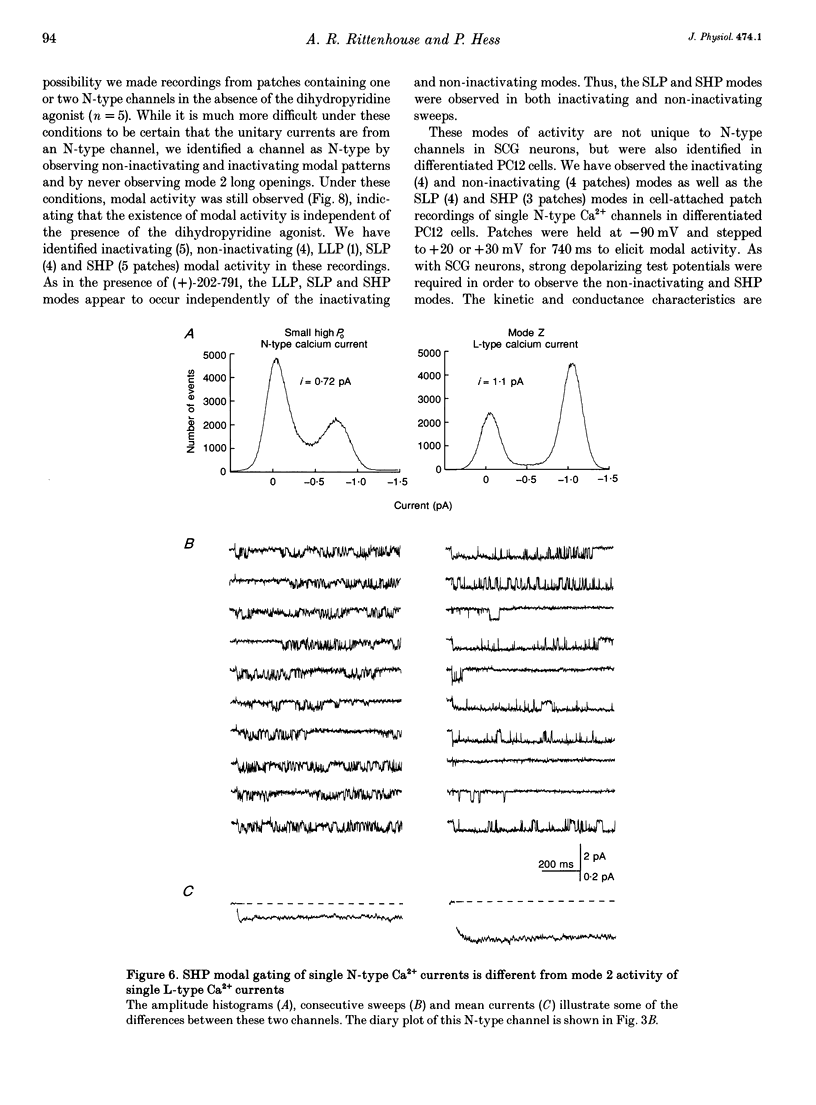
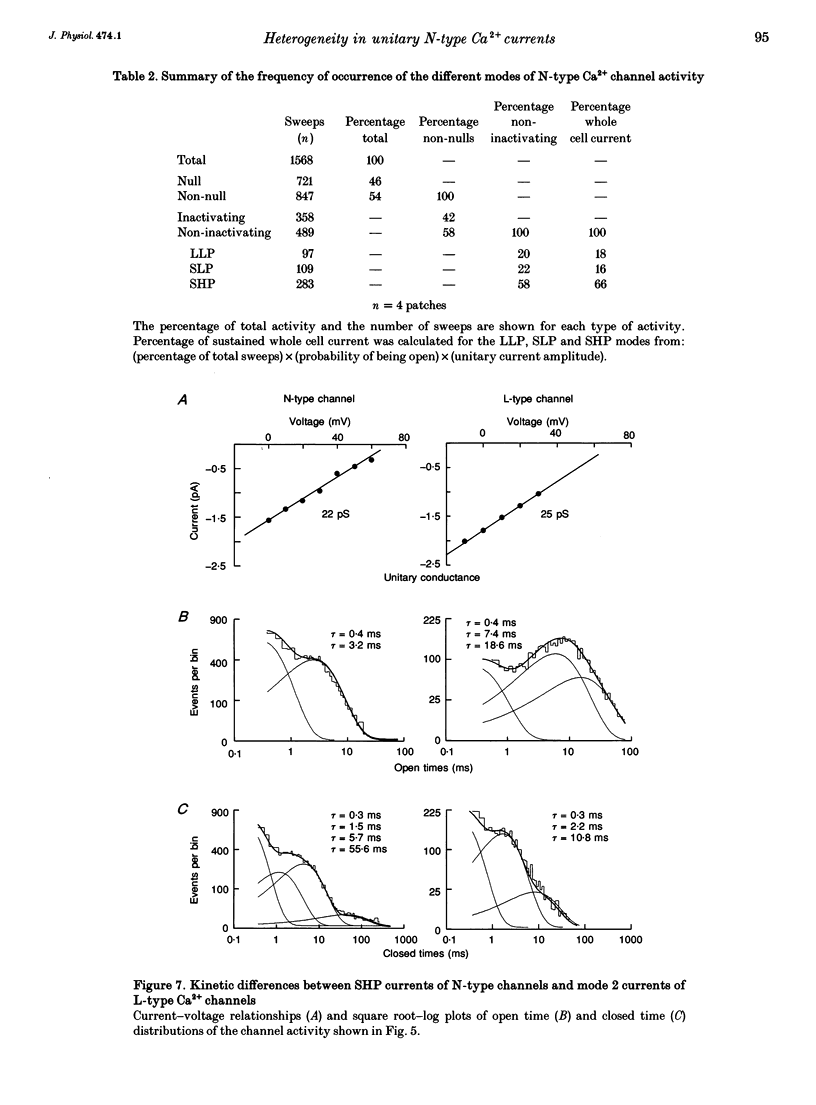
1. Single N-type calcium (Ca2+) channels in rat superior cervical ganglion neurons display complex patterns of activity in both inactivating and non-inactivating gating modes. Unitary currents were elicited by holding the patch at -90 mV and stepping to +30 mV for 740 ms. Barium (110 mM) was used as the charge carrier. The dihydropyridine agonist (+)-202-791 was included in the bath to ensure that single channel recordings showed no L-type Ca2+ channel mode 2 activity. Using this protocol, we characterized three additional patterns of N-type Ca2+ channel activity named: (1) LLP for large unitary current amplitude (i = -0.92 pA) and low open probability (Po = 0.26); (2) SLP for small unitary current amplitude (i = -0.77 pA) and low open probability (Po = 0.25); and (3) SHP for its small unitary current (i = -0.77 pA) and higher open probability (Po = 0.39). 2. Transitions among these patterns of activity occur more slowly than transitions between closed and open states, resulting in significant clustering of like sweeps. Thus, the complicated gating of single N-type Ca2+ channels can be dissected into multiple, independent modes, each with the same reproducible pattern of activity. 3. This heterogeneous activity is not unique to sympathetic neurons, for inactivating (4), non-inactivating (4), SLP (4) and SHP (3 patches) gating modes were also observed in cell-attached patch recordings (n = 4) of single N-type Ca2+ channels in differentiated phaeochromocytoma (PC12) cells. 4. The 1568 sweeps from four single N-type Ca2+ channel recordings that used the same voltage protocol were categorized by mode to determine the frequency of occurrence of each. Of the 54% of sweeps that showed activity, 42% were inactivating and 58% were non-inactivating. The contribution by each mode to the sustained current was estimated using the equation: I = NPoi, where N is the frequency of occurrence of each mode and Po and i are the mean values of open probability and unitary current amplitude respectively. The LLP mode contributed 18%, the SLP mode 16%, and the SHP mode 66% of the sustained whole cell N-type Ba2+ current. 5. The variability in the incidence among these modes in other cell types may resolve some of the controversy surrounding the characterization of N- and L-type whole cell Ca2+ current components in peripheral neurons. In addition, the number of different modes provides a source of plasticity that may be a target of modulation by neurotransmitters and cellular signals.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991 Apr;6(4):619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- Delcour A. H., Lipscombe D., Tsien R. W. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J Neurosci. 1993 Jan;13(1):181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. R., Sah P., Buckett K. J., Gage P. W. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990 Jun;95(6):1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Patterson P. H. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Jacobs L. S. Dihydropyridine actions on calcium currents of frog sympathetic neurons. J Neurosci. 1990 Jul;10(7):2261–2267. doi: 10.1523/JNEUROSCI.10-07-02261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Kinetic properties of single sodium channels in rat heart and rat brain. J Gen Physiol. 1989 Jan;93(1):85–99. doi: 10.1085/jgp.93.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Kinetic diversity of Na+ channel bursts in frog skeletal muscle. J Gen Physiol. 1989 Aug;94(2):279–301. doi: 10.1085/jgp.94.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., Vandenberg C. A., Jong D. S., Bezanilla F. Single channel studies of the phosphorylation of K+ channels in the squid giant axon. I. Steady-state conditions. J Gen Physiol. 1991 Jul;98(1):1–17. doi: 10.1085/jgp.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Rittenhouse A., Kanevsky M., Hess P. Neurotransmitter modulation of calcium channels in rat sympathetic neurons. J Neurosci. 1991 Aug;11(8):2339–2348. doi: 10.1523/JNEUROSCI.11-08-02339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Scroggs R. S., Fox A. P. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992 Jan;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. E., Brust P. F., Feldman D. H., Patthi S., Simerson S., Maroufi A., McCue A. F., Veliçelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992 Jul 17;257(5068):389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G. Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction. Trends Pharmacol Sci. 1990 Jan;11(1):38–44. doi: 10.1016/0165-6147(90)90040-f. [DOI] [PubMed] [Google Scholar]
- Zhou J. Y., Potts J. F., Trimmer J. S., Agnew W. S., Sigworth F. J. Multiple gating modes and the effect of modulating factors on the microI sodium channel. Neuron. 1991 Nov;7(5):775–785. doi: 10.1016/0896-6273(91)90280-d. [DOI] [PubMed] [Google Scholar]




