Abstract
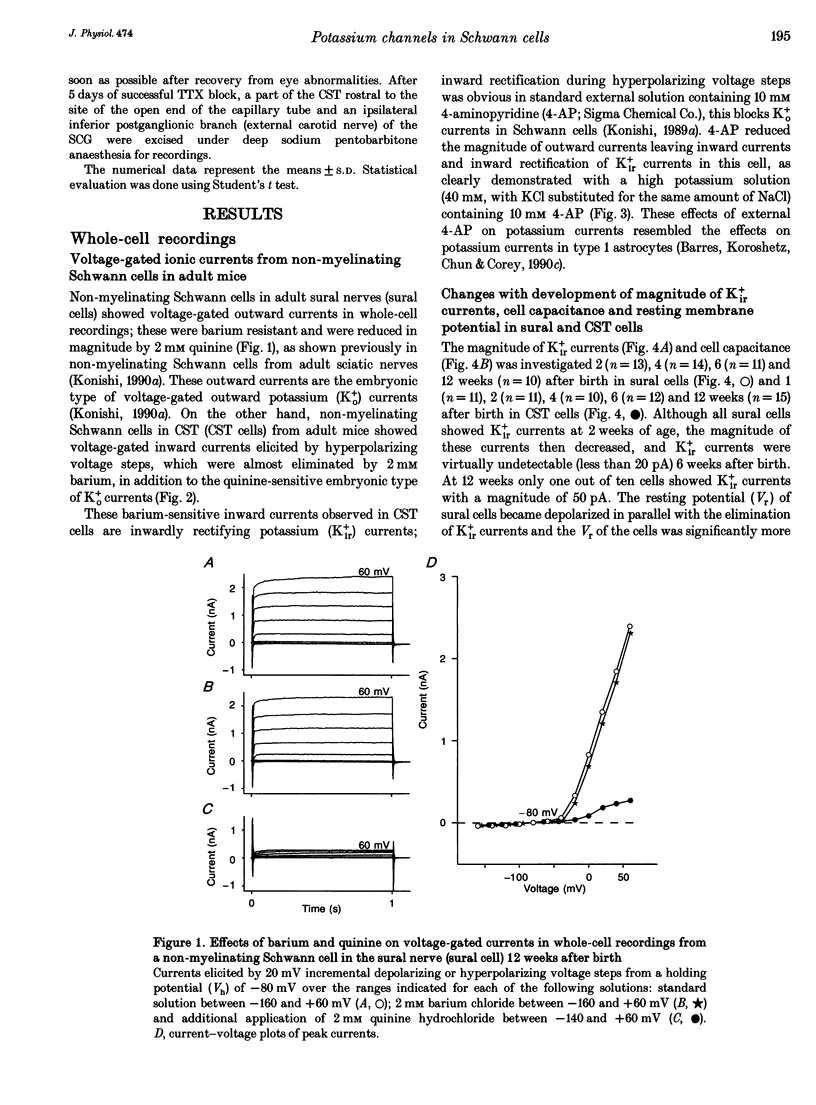
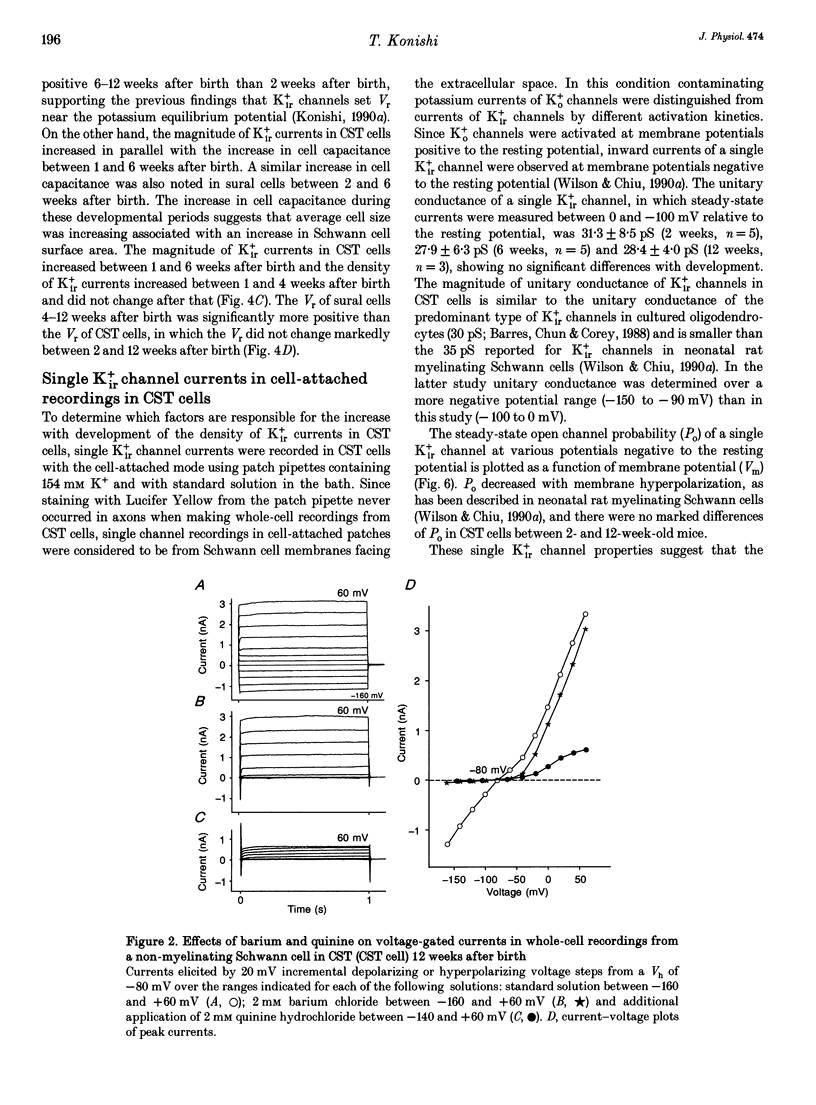
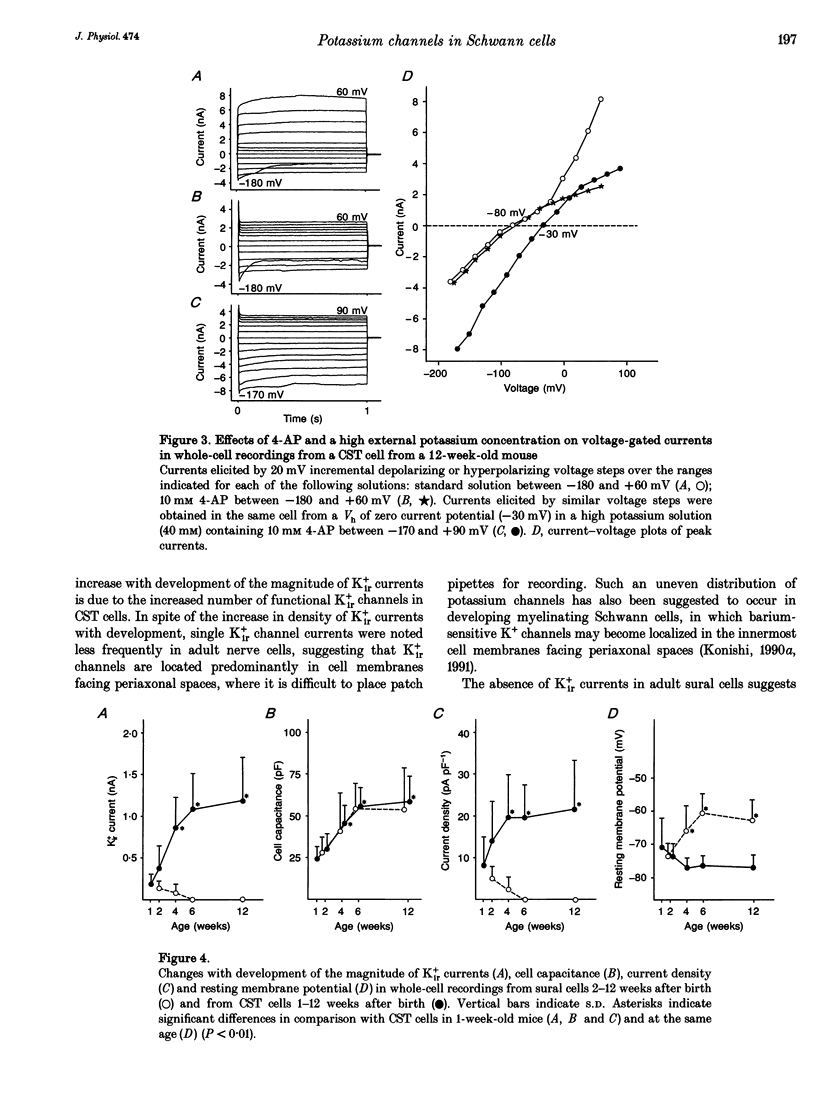
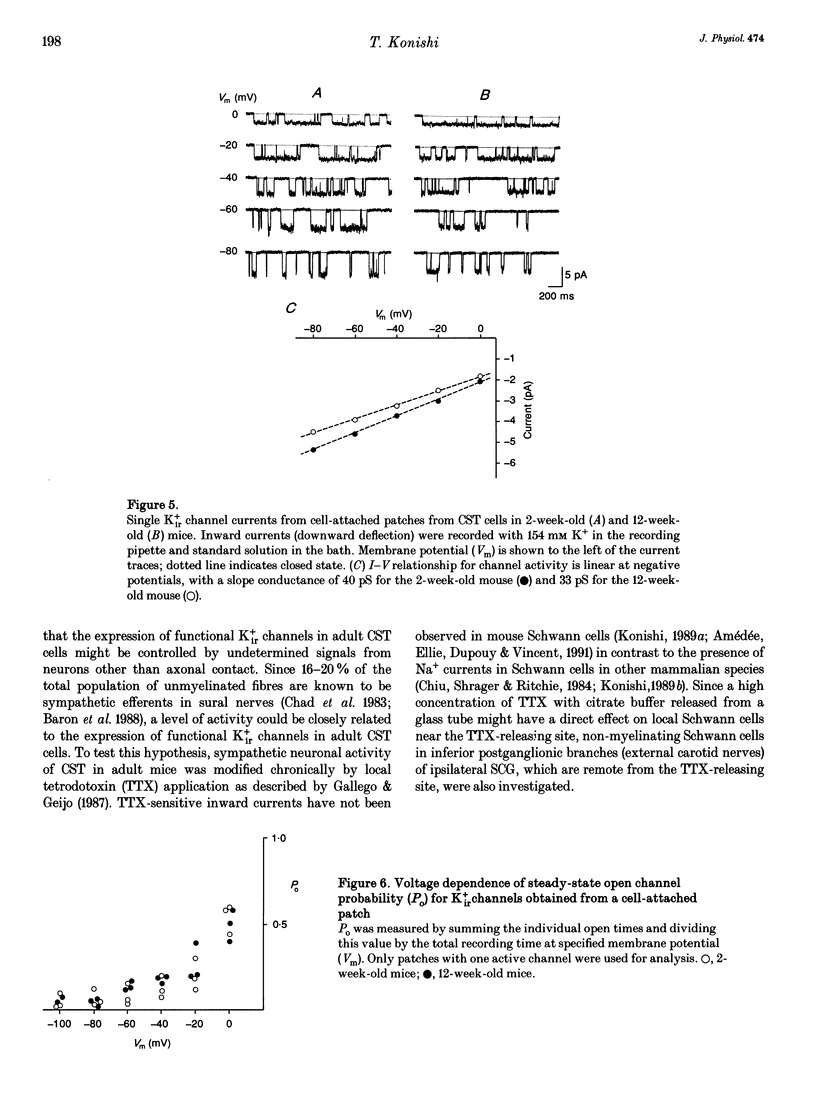
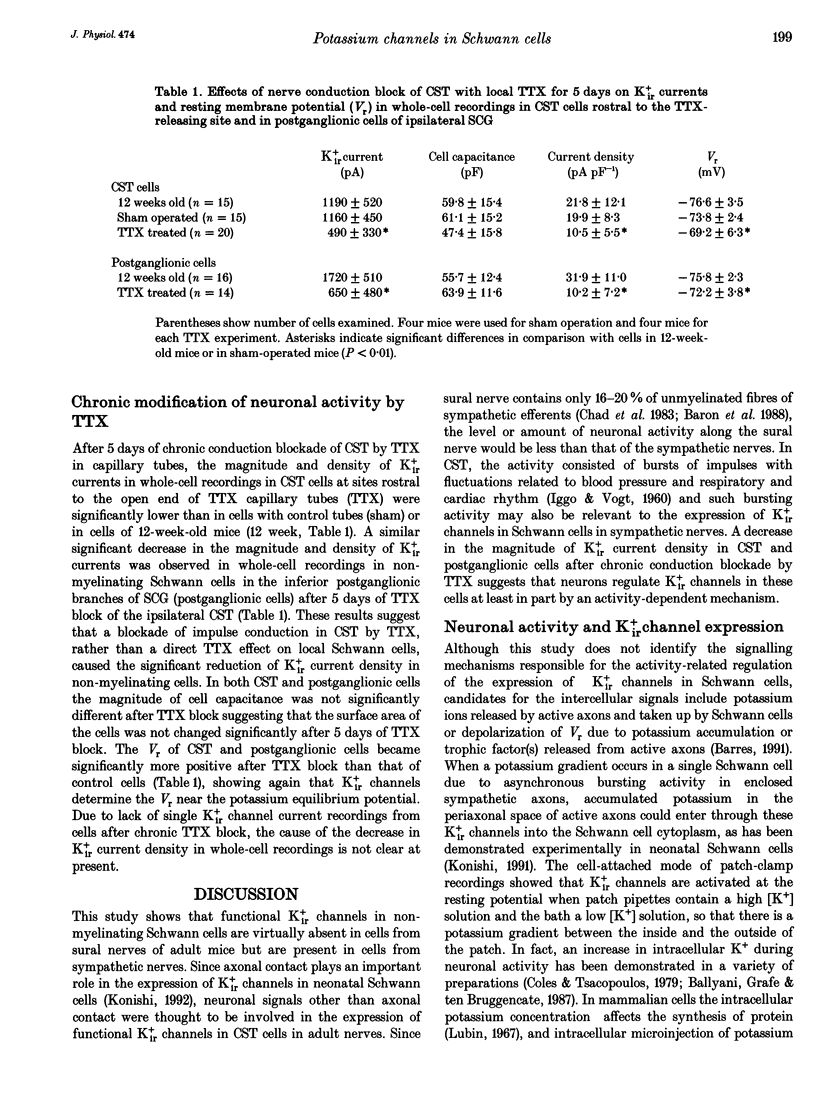
1. Voltage-gated potassium currents were recorded from freshly dissociated non-myelinating Schwann cells of sural and sympathetic nerves from 1- to 12-week-old mice using the whole-cell or a single channel variation of the patch-clamp technique. 2. All sural cells from 2-week-old mice showed inwardly rectifying potassium (Kir+) currents in whole-cell recordings. Kir+ currents were virtually undetectable in sural cells from mice more than 6 weeks old, which also showed depolarization of the resting membrane potential. On the other hand, the magnitude of Kir+ currents increased in cervical sympathetic trunk (CST) cells in parallel with an increase of cell capacitance 1-6 weeks after birth. The density of Kir+ currents in CST cells increased 1-4 weeks after birth and then stayed constant for up to 12 weeks. 3. The unitary conductance of a single Kir+ channel in CST cells was 30 pS 2-12 weeks after birth; this was recorded in a cell-attached configuration with 154 mM K+ in the pipette. The steady-state open channel probability of single Kir+ channels in CST cells decreased with membrane hyperpolarization, but was not markedly changed 2-12 weeks after birth. 4. Conduction block of CST for 5 days induced by local application of tetrodotoxin (TTX) resulted in a significant decrease in both the magnitude and the density of Kir+ currents in whole-cell recordings in CST cells rostral to the sites of TTX block. Similar changes of Kir+ currents in whole-cell recordings were observed in cells in the inferior postganglionic branch of a superior cervical ganglion after 5 days of TTX block of CST. 5. These results suggest that neuronal activity regulates the expression of functional Kir+ channels in non-myelinating Schwann cells in adult nerves. The activity-dependent regulation of the expression of glial potassium channels could play an important role in the regulation of the potassium microenvironment around active axons to maintain impulse conduction in unmyelinated fibres.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Ellie E., Dupouy B., Vincent J. D. Voltage-dependent calcium and potassium channels in Schwann cells cultured from dorsal root ganglia of the mouse. J Physiol. 1991 Sep;441:35–56. doi: 10.1113/jphysiol.1991.sp018737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P., ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol. 1987 Jan;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Jänig W., Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988 Sep 15;275(3):460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990 Oct;5(4):527–544. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990 Apr;4(4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad D., Bradley W. G., Rasool C., Good P., Reichlin S., Zivin J. Sympathetic postganglionic unmyelinated axons in the rat peripheral nervous system. Neurology. 1983 Jul;33(7):841–847. doi: 10.1212/wnl.33.7.841. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Schrager P., Ritchie J. M. Neuronal-type Na+ and K+ channels in rabbit cultured Schwann cells. Nature. 1984 Sep 13;311(5982):156–157. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- Coles J. A., Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol. 1979 May;290(2):525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Ransom B. R., Kunis D. M., Gutnick M. J. Activity-dependent K+ accumulation in the developing rat optic nerve. Science. 1982 Jun 18;216(4552):1341–1343. doi: 10.1126/science.7079771. [DOI] [PubMed] [Google Scholar]
- David G., Barrett J. N., Barrett E. F. Evidence that action potentials activate an internodal potassium conductance in lizard myelinated axons. J Physiol. 1992 Jan;445:277–301. doi: 10.1113/jphysiol.1992.sp018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968 Oct;27(4):546–570. [PubMed] [Google Scholar]
- Gallego R., Geijo E. Chronic block of the cervical trunk increases synaptic efficacy in the superior and stellate ganglia of the guinea-pig. J Physiol. 1987 Jan;382:449–462. doi: 10.1113/jphysiol.1987.sp016377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoppe D., Chvatal A., Kettenmann H., Orkand R. K., Ransom B. R. Characteristics of activity-dependent potassium accumulation in mammalian peripheral nerve in vitro. Brain Res. 1991 Jun 21;552(1):106–112. doi: 10.1016/0006-8993(91)90666-j. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Lau Y. T. A function that relates protein synthetic rates to potassium activity in vivo. J Cell Physiol. 1988 Jun;135(3):425–434. doi: 10.1002/jcp.1041350309. [DOI] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A., VOGT M. Preganglionic sympathetic activity in normal and in reserpine-treated cats. J Physiol. 1960 Jan;150:114–133. doi: 10.1113/jphysiol.1960.sp006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq C. B., Coggeshall R. E. Effects of sciatic nerve regeneration on axonal populations in tributary nerves. Brain Res. 1984 Mar 12;295(1):91–100. doi: 10.1016/0006-8993(84)90819-9. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Malenka R. C., Waxman S. G. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol. 1983 Jan;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Dye coupling between mouse Schwann cells. Brain Res. 1990 Jan 29;508(1):85–92. doi: 10.1016/0006-8993(90)91121-v. [DOI] [PubMed] [Google Scholar]
- Konishi T. Potassium channel-dependent changes in the volume of developing mouse Schwann cells. Brain Res. 1991 Nov 22;565(1):57–66. doi: 10.1016/0006-8993(91)91736-k. [DOI] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in cultured mammalian Schwann cells. Brain Res. 1989 Oct 16;499(2):273–280. doi: 10.1016/0006-8993(89)90775-0. [DOI] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in mouse Schwann cells. J Physiol. 1989 Apr;411:115–130. doi: 10.1113/jphysiol.1989.sp017564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Voltage-gated potassium currents in myelinating Schwann cells in the mouse. J Physiol. 1990 Dec;431:123–139. doi: 10.1113/jphysiol.1990.sp018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. cAMP-mediated expression of inwardly rectifying potassium channels in cultured mouse Schwann cells. Brain Res. 1992 Oct 30;594(2):197–204. doi: 10.1016/0006-8993(92)91126-y. [DOI] [PubMed] [Google Scholar]
- Lau Y. T., Yassin R. R., Horowitz S. B. Potassium salt microinjection into Xenopus oocytes mimics gonadotropin treatment. Science. 1988 Jun 3;240(4857):1321–1323. doi: 10.1126/science.3375816. [DOI] [PubMed] [Google Scholar]
- Lubin M. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature. 1967 Feb 4;213(5075):451–453. doi: 10.1038/213451a0. [DOI] [PubMed] [Google Scholar]
- Mills R. G., Bray J. J. A slow-release technique for inducing prolonged paralysis by tetrodotoxin. Pflugers Arch. 1979 Dec;383(1):67–70. doi: 10.1007/BF00584476. [DOI] [PubMed] [Google Scholar]
- Newman E. A., Frambach D. A., Odette L. L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984 Sep 14;225(4667):1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A. Voltage-dependent calcium and potassium channels in retinal glial cells. 1985 Oct 31-Nov 6Nature. 317(6040):809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romine J. S., Bray G. M., Aguayo A. J. Schwann cell multiplication after crush injury of unmyelinated fibers. Arch Neurol. 1976 Jan;33(1):49–54. doi: 10.1001/archneur.1976.00500010051008. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M., Devilliers G., Bock E., Porte A. Biochemical and ultrastructural studies of cultured rat astroglial cells: effect of brain extract and dibutyryl cyclic AMP on glial fibrillary acidic protein and glial filaments. Differentiation. 1980;17(1):51–61. doi: 10.1111/j.1432-0436.1980.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Kume-Iwaki A., Goldman J. E. Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia. 1988;1(5):346–354. doi: 10.1002/glia.440010507. [DOI] [PubMed] [Google Scholar]
- Steward O., Torre E. R., Tomasulo R., Lothman E. Neuronal activity up-regulates astroglial gene expression. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6819–6823. doi: 10.1073/pnas.88.15.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Identification of afferent C units in intact human skin nerves. Brain Res. 1974 Mar 8;67(3):387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Tse F. W., Fraser D. D., Duffy S., MacVicar B. A. Voltage-activated K+ currents in acutely isolated hippocampal astrocytes. J Neurosci. 1992 May;12(5):1781–1788. doi: 10.1523/JNEUROSCI.12-05-01781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. F., Chiu S. Y. Ion channels in axon and Schwann cell membranes at paranodes of mammalian myelinated fibers studied with patch clamp. J Neurosci. 1990 Oct;10(10):3263–3274. doi: 10.1523/JNEUROSCI.10-10-03263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. F., Chiu S. Y. Potassium channel regulation in Schwann cells during early developmental myelinogenesis. J Neurosci. 1990 May;10(5):1615–1625. doi: 10.1523/JNEUROSCI.10-05-01615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


