Abstract
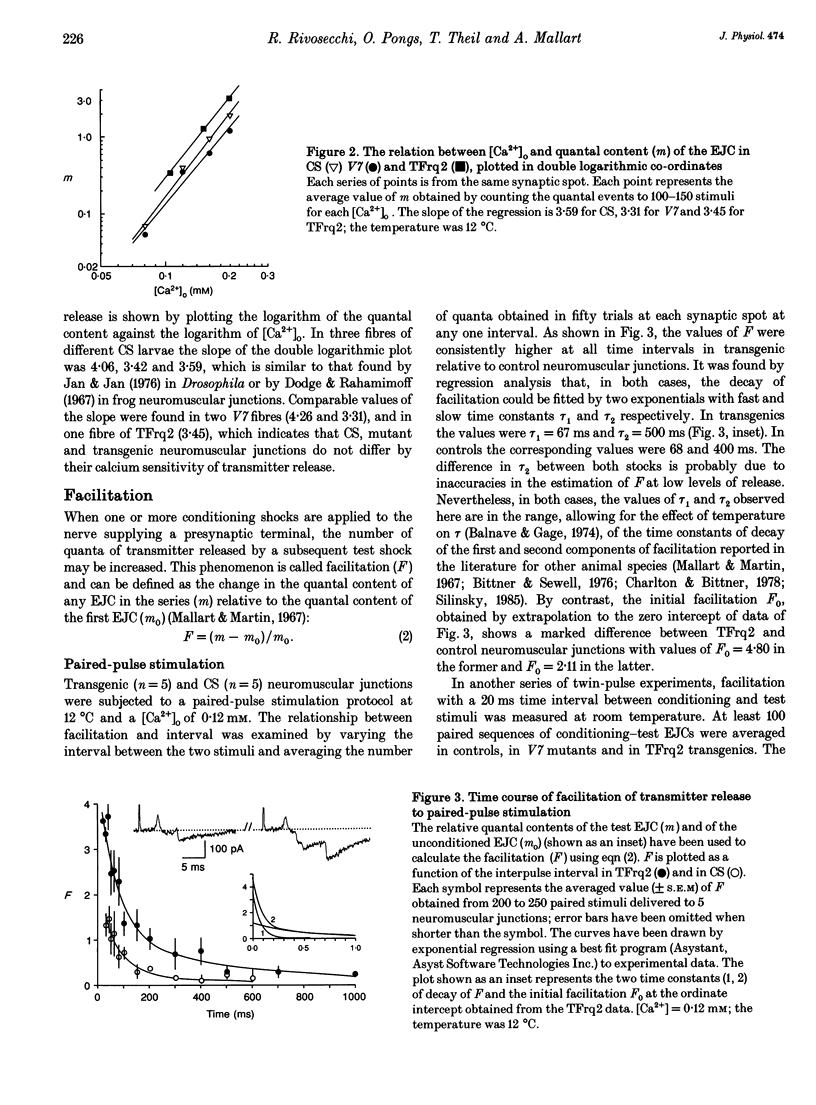
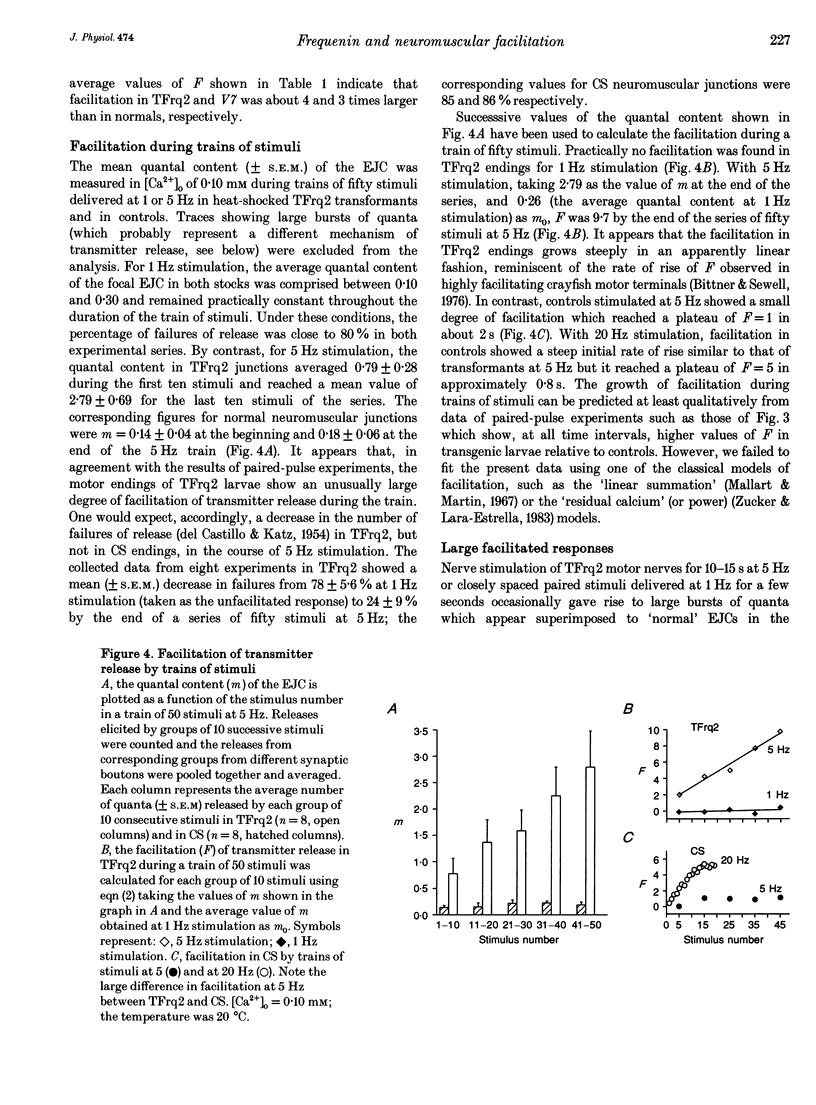
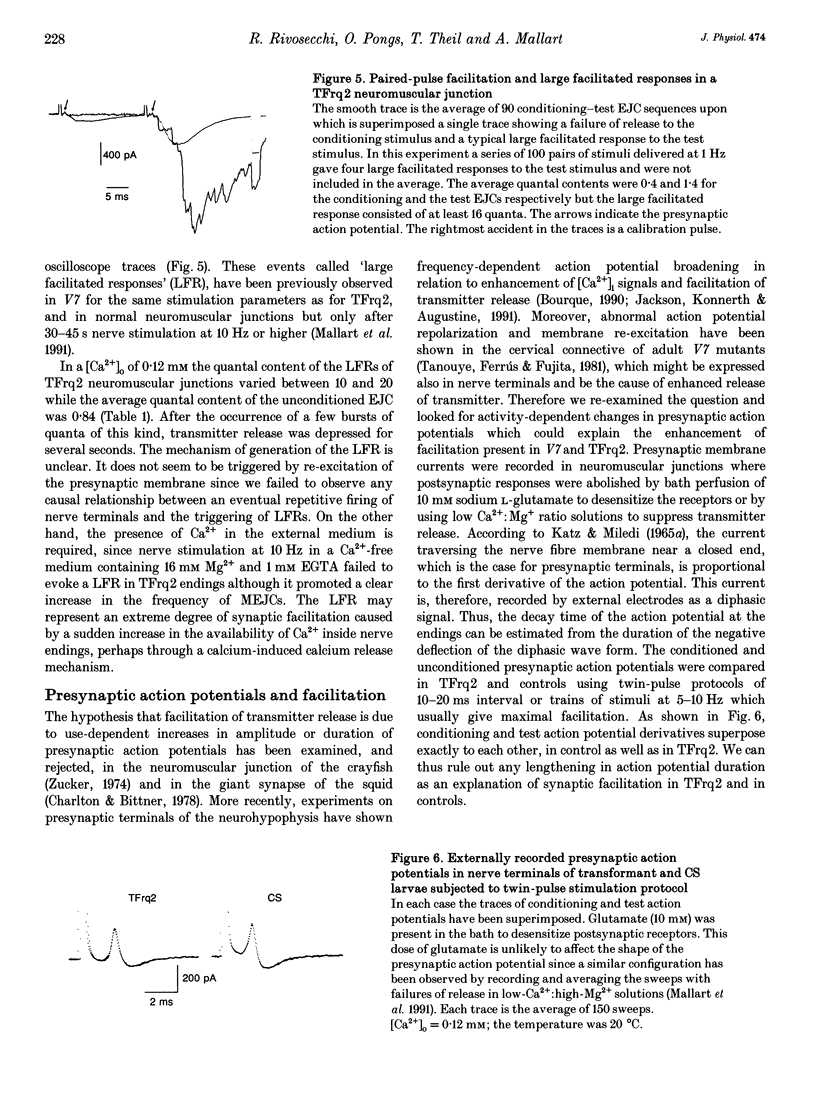
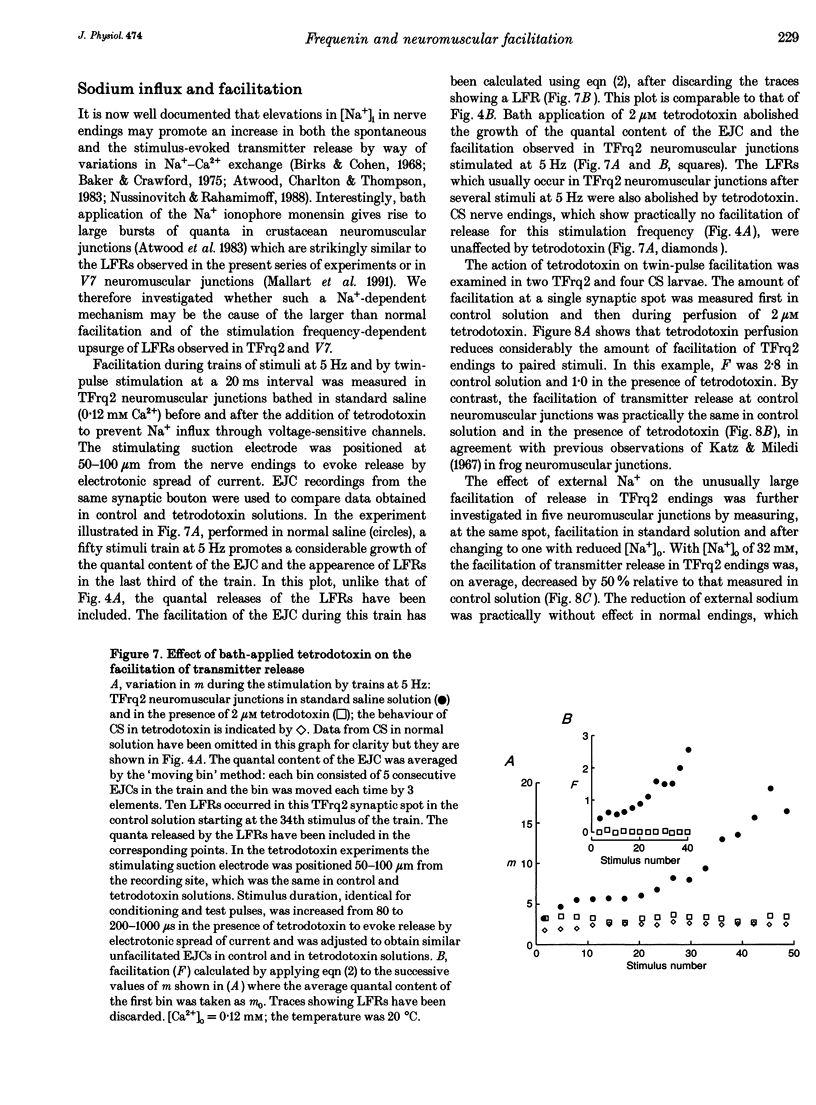
1. We have investigated the possible role of frequenin in the modulation of synaptic facilitation at the larval Drosophila neuromuscular junctions. Excitatory junctional currents (EJCs) and presynaptic nerve terminal currents were recorded by external electrodes in normal larvae and in transgenic larvae carrying an extra insertion of the frequenin cDNA. 2. Motor nerve stimulation by twin pulses or trains of stimuli provoked EJC facilitation which was about three times higher in transgenic larvae compared to controls. Unconditioned EJCs revealed, however, similar quantal content and Ca2+ sensitivity in both Drosophila strains. 3. Differences between normal and transgenic Drosophila in the quantal content of the facilitated EJC do not depend on differences in the duration of the repolarization phase of the presynaptic action potential. 4. Perfusion of tetrodotoxin or of low-Na+ solutions abolished the enhancement of the EJC facilitation observed in the transformants. These treatments only slightly affected the facilitation of normal junctions. 5. These results suggest that (i) internal Na+ accumulation can enhance facilitation of transmitter release in Drosophila neuromuscular junctions overexpressing frequenin, and (ii) this effect possibly depends on a modulation of the activity of the Na(+)-Ca2+ exchanger by frequenin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Charlton M. P., Thompson C. S. Neuromuscular transmission in crustaceans is enhanced by a sodium ionophore, monensin, and by prolonged stimulation. J Physiol. 1983 Feb;335:179–195. doi: 10.1113/jphysiol.1983.sp014527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. On facilitation of transmitter release at the toad neuromuscular junction. J Physiol. 1974 Jun;239(3):657–675. doi: 10.1113/jphysiol.1974.sp010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Bittner G. D., Schatz R. A. An examination of the residual calcium theory for facilitation of transmitter release. Brain Res. 1981 Apr 6;210(1-2):431–436. doi: 10.1016/0006-8993(81)90922-7. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990 Feb;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Bittner G. D. Presynaptic potentials and facilitation of transmitter release in the squid giant synapse. J Gen Physiol. 1978 Oct;72(4):487–511. doi: 10.1085/jgp.72.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251(4996):915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Ohshima N., Tawada-Iwata Y., Shigekawa M. Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem. 1991 Jul 5;266(19):12337–12341. [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics. 1982 Apr;100(4):597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Konnerth A., Augustine G. J. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976 Oct;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Genetic dissection of short-term and long-term facilitation at the Drosophila neuromuscular junction. Proc Natl Acad Sci U S A. 1978 Jan;75(1):515–519. doi: 10.1073/pnas.75.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht H. G., Koch K. W. A 26 kd calcium binding protein from bovine rod outer segments as modulator of photoreceptor guanylate cyclase. EMBO J. 1991 Apr;10(4):793–798. doi: 10.1002/j.1460-2075.1991.tb08011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. A., Richardson N. P., Taylor P., Donatsch P. Increasing intracellular sodium triggers calcium release from bound pools. Nature. 1976 Mar 25;260(5549):337–338. doi: 10.1038/260337a0. [DOI] [PubMed] [Google Scholar]
- Luther P. W., Yip R. K., Bloch R. J., Ambesi A., Lindenmayer G. E., Blaustein M. P. Presynaptic localization of sodium/calcium exchangers in neuromuscular preparations. J Neurosci. 1992 Dec;12(12):4898–4904. doi: 10.1523/JNEUROSCI.12-12-04898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Angaut-Petit D., Bourret-Poulain C., Ferrús A. Nerve terminal excitability and neuromuscular transmission in T(X;Y)V7 and Shaker mutants of Drosophila melanogaster. J Neurogenet. 1991 Feb;7(2-3):75–84. doi: 10.3109/01677069109066212. [DOI] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J. G., Quastel D. M. A voltage clamp study of the glutamate responsive neuromuscular junction in Drosophila melanogaster. Can J Physiol Pharmacol. 1988 Apr;66(4):321–327. doi: 10.1139/y88-055. [DOI] [PubMed] [Google Scholar]
- Misler S., Hurlbut W. P. Post-tetanic potentiation of acetylcholine release at the frog neuromuscular junction develops after stimulation in Ca2+-free solutions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):315–319. doi: 10.1073/pnas.80.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Posttetanic potentiation at the crayfish neuromuscular junction is dependent on both intracellular calcium and sodium ion accumulation. J Neurosci. 1992 Nov;12(11):4327–4336. doi: 10.1523/JNEUROSCI.12-11-04327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch I., Rahamimoff R. Ionic basis of tetanic and post-tetanic potentiation at a mammalian neuromuscular junction. J Physiol. 1988 Feb;396:435–455. doi: 10.1113/jphysiol.1988.sp016971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Lindemeier J., Zhu X. R., Theil T., Engelkamp D., Krah-Jentgens I., Lambrecht H. G., Koch K. W., Schwemer J., Rivosecchi R. Frequenin--a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993 Jul;11(1):15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M., Rahamimoff H. Na(+)-Ca2+ exchange activity in synaptic plasma membranes derived from the electric organ of Torpedo ocellata. Biochim Biophys Acta. 1991 Jul 22;1066(2):208–218. doi: 10.1016/0005-2736(91)90188-e. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J. M., Atwood H. L. Long-term facilitation alters transmitter releasing properties at the crayfish neuromuscular junction. J Neurophysiol. 1986 Mar;55(3):484–498. doi: 10.1152/jn.1986.55.3.484. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Goto K., Kuo C. H., Kondo H., Miki N. Visinin: a novel calcium binding protein expressed in retinal cone cells. Neuron. 1990 Mar;4(3):469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991 Jan 11;251(4990):198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Characteristics of crayfish neuromuscular facilitation and their calcium dependence. J Physiol. 1974 Aug;241(1):91–110. doi: 10.1113/jphysiol.1974.sp010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Lara-Estrella L. O. Post-tetanic decay of evoked and spontaneous transmitter release and a residual-calcium model of synaptic facilitation at crayfish neuromuscular junctions. J Gen Physiol. 1983 Mar;81(3):355–372. doi: 10.1085/jgp.81.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]


