The commentary by Malkoc et al. highlights possible arguments explaining divergent findings of how mitochondrial metabolism in blood cells is related to whole-organism resting metabolic rate (RMR) in birds across the three studies that have addressed this relationship (Casagrande et al., 2023; Malkoc et al., 2021; Thoral et al., 2024). The authors argue that the lack of correlation between ROUTINE respiration in blood and RMR in Thoral et al. (2024), but the presence of such in their previous papers (Casagrande et al., 2023; Malkoc et al., 2021), can be explained by methodology (intact versus permeabilized blood cells), acclimation temperature, or the stress status of the individuals. In our response, we argue why the concerns expressed by Malkoc et al. should be interpreted with caution, and comment on the conclusions that can be drawn from studying both individual and mitochondrial metabolisms.
Firstly, we agree that permeabilization status can explain the absence or presence of a relationship between mitochondrial respiration traits in blood cells and RMR. However, this alone cannot explain why ROUTINE respiration was uncorrelated to RMR in Thoral et al. (2024), but correlated to RMR in Malkoc et al. (2021) and Casagrande et al. (2023), since blood cells are not permeabilized when ROUTINE is measured (Gnaiger, 2020). We therefore disagree that a relationship between ROUTINE respiration in intact blood cells and RMR is a general phenomenon and that the absence of such reflects neglectful experimentation. In line with this, a study on Japanese quail (Coturnix japonica) in our laboratory found no relationships between these traits (Fig. 1). We suggest that permeabilized experiments may bypass any rate-limiting steps influencing mitochondrial function in intact cells (Djafarzadeh and Jakob, 2017), such as substrate availability (Kyriazis et al., 2022; Leverve, 2007), transmembrane transport rate (Osellame et al., 2012), or any interactions between mitochondria and other cell components (Boldogh and Pon, 2007; Wieckowski et al., 2009). Thus, even though the measurement conditions in intact cell experiments may be more similar to the in vivo situation, permeabilization could be necessary to reveal a link between cellular and organismal metabolic rate. We propose that future studies apply complete SUIT protocols (substrate – uncoupler – inhibitor titration protocols; Gnaiger, 2020) in both intact and permeabilized samples from the same individual. The resultant improved understanding of mechanisms underlying phenotypic changes observed at the individual level would provide a more comprehensive ecological context.
Fig. 1.
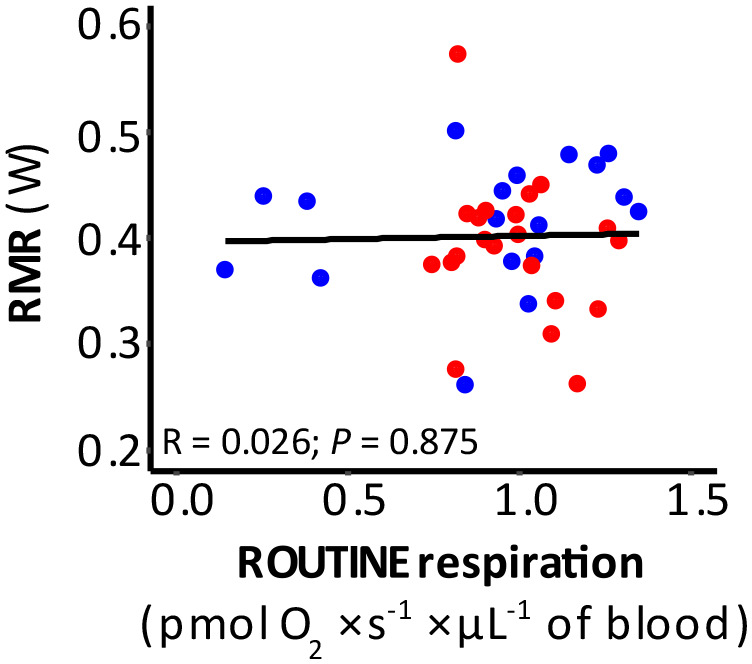
Relationship between RMR and ROUTINE respiration obtained in intact blood cells from Japanese quail (Coturnix japonica). Half of the birds were acclimated at 10°C (blue dots) while the other half was acclimated at 30°C (red dots) for 11 weeks. RMR was measured at 30°C, which is thermoneutral in Japanese quail, and blood respiration was measured at 41°C. A Pearson correlation test on pooled data revealed no significant association between RMR and ROUTINE. Nor was this relationship significant within groups (cold birds: R=0.255, P=0.306; warm birds: R=−0.250, P=0.275).
Secondly, the authors argue that acclimation temperature may affect metabolism at different biological scales, altering the relationship between RMR and mitochondrial respiration. However, since the effect of environmental temperature on RMR is linear below the lower critical temperature (Scholander et al., 1950), it can be argued that an acute organismal metabolic response would only affect the intercept of the regression, not the slope. In support of this, the relationship between organismal metabolic rate and mitochondrial respiration parameters were largely identical when RMR was measured at 7.5°C and 20°C in our study [Fig. S4 in Thoral et al. (2024)]. Even if the timeline of changes to cellular and organismal metabolic rate differ [e.g. if acclimation time could be too short or too stochastic to cause either intrinsic changes in mitochondrial functioning or increased presence of a new blood cell phenotype (Voss et al., 2010)], it seems unlikely that variation in the temporal response would completely negate a potential underlying biological relationship. However, we agree with the incentive that “…effort should be directed to investigate how environmental temperature, acclimatization state, body and assay temperatures affect RMR and mitochondrial metabolic rate”. Such a study has already been performed: García-Díaz et al. (2023) exposed winter-acclimated great tits to −15°C, +5°C or +25°C for 13-15 h overnight whilst measuring RMR and body temperature, and mitochondrial respiration in intact blood cells at a normothermic (41°C) and a hypothermic (35°C) assay temperature the morning after. While RMR was nearly twice as high in −15°C compared to +25°C, all mitochondrial respiration traits were unaffected by acclimation temperature. Moreover, while ROUTINE and LEAK respirations, and associated flux control ratios, were strongly affected by assay temperature, body temperature was unrelated to all mitochondrial respiration traits but the E-R control efficiency (an index of respiratory reserve capacity; Gnaiger, 2020; García-Díaz et al., 2023).
Thirdly, Malkoc et al. argue that the stress status of individuals can affect both individual and mitochondrial metabolism, potentially changing the relationship between the two. While consideration of glucocorticoid levels may sometimes reveal biologically interesting relationships otherwise not apparent (Malkoc et al., 2021), other studies report that circulating stress hormone levels are unrelated to both organismal and blood cell respiration (Casagrande et al., 2023). Hence, while we agree that the physiological status of individuals, including affective state, may impact metabolism, such relationships are arguably of limited effect compared to the main issue of relating metabolic rate at different biological scales.
At any point in time, organismal metabolism can be considered a sum of mitochondrial respiration across the body (Salin et al., 2016), with some oxidative tissues contributing more heavily to the energy expenditure than others (Casagrande et al., 2023). However, even if mitochondrial metabolism affects organismal performance (Koch et al., 2021) and can thus be related to fitness (Hood et al., 2018), mitochondrial respiration differs between tissues (Casagrande et al., 2023; Salin et al., 2016, 2019), and subpopulations of distinct mitochondrial phenotypes may be present within single tissues (Scott et al., 2018). Moreover, mitochondrial metabolism is affected by multiple biotic and abiotic factors (Sokolova, 2018), and the conditions under which mitochondrial metabolism is measured, whether with intact or permeabilized cells, are always far from the physiological conditions experienced by cells within organisms (i.e. hormonal status, connection with the other tissues, substrate availability, supra-physiological oxygen concentrations, etc.). Organismal metabolic rate is equally labile when extrinsic and intrinsic conditions change (Versteegh et al., 2008; Norin and Malte, 2011; White et al., 2013; Welcker et al., 2015; Burton et al., 2011). Thus, the relationship between organismal metabolic rate and blood cell respiration remains a physiological parameter that must be interpreted with caution as prediction of RMR from mitochondrial respiration in specific tissues may be imprecise (Thoral et al., 2024).
To uncover the ecological and mechanistic explanations for why there is a relationship between blood cell respiration and RMR, we believe that future studies would benefit from addressing: 1) the relative aerobic level of bird blood, by estimating the proportion of ATP produced via the oxidative and glycolytic pathways compared to the situation in other tissues; and 2), as already initiated by Casagrande et al. (2023), partitioning the total contributions of mitochondrial respiration across key metabolic tissues towards RMR, when substrate availability, environmental conditions, and affective state of individuals vary; and 3) determining whether the response to thermal acclimation over short and long time periods is comparable between blood cells and tissues with more clearly defined roles in energy metabolism and thermogenesis.
Footnotes
Funding
This research was supported by the Swedish Research Council (grant no. 2020-04686, to A.N.). Open Access funding provided by The Company of Biologists Ltd, Lund University and University of Jyväskylä. Deposited in PMC for immediate release.
References
- Boldogh, I. R. and Pon, L. A. (2007). Mitochondria on the move. Trends Cell Biol. 17, 502-510. 10.1016/j.tcb.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Burton, T., Killen, S. S., Armstrong, J. D. and Metcalfe, N. B. (2011). What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 278, 3465-3473. 10.1098/rspb.2011.1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande, S., Dzialo, M., Trost, L., Malkoc, K., Sadowska, E. T., Hau, M., Pierce, B., Mcwilliams, S. and Bauchinger, U. (2023). Mitochondrial metabolism in blood more reliably predicts whole-animal energy needs compared to other tissues. iScience 26, 108321. 10.1016/j.isci.2023.108321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djafarzadeh, S. and Jakob, S. M. (2017). High-resolution respirometry to assess mitochondrial function in permeabilized and intact cells. J. Vis. Exp. 120, 54985. 10.3791/54985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Díaz, C. C., Chamkha, I., Elmér, E. and Nord, A. (2023). Plasticity of mitochondrial function safeguards phosphorylating respiration during. FASEB J. 37, e22854. 10.1096/fj.202201613R [DOI] [PubMed] [Google Scholar]
- Gnaiger, E. (2020). Mitochondrial pathways and respiratory control - an introduction to OXPHOS analysis. In: Bioenerg. Commun, 5th edn, 1-23. [Google Scholar]
- Hood, W. R., Austad, S. N., Bize, P., Jimenez, A. G., Montooth, K. L., Schulte, P. M., Scott, G. R., Sokolova, I., Treberg, J. R. and Salin, K. (2018). The mitochondrial contribution to animal performance, adaptation, and life-history variation. Integr. Comp. Biol. 58, 480-485. 10.1093/icb/icy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, R. E., Buchanan, K. L., Casagrande, S., Crino, O., Dowling, D. K., Hill, G. E., Hood, W. R., Mckenzie, M., Mariette, M. M., Noble, D. W. A., Pavlova, A., Seebacher, F., Sunnucks, P., Udino, E., White, C. R., Salin, K. and Stier, A. (2021). Integrating mitochondrial aerobic metabolism into ecology and evolution. Trends Ecol. Evol. 36, 1-12. 10.1016/j.tree.2020.11.011 [DOI] [PubMed] [Google Scholar]
- Kyriazis, I. D., Vassi, E., Alvanou, M., Angelakis, C., Skaperda, Z. O. I., Tekos, F., Naga, V. and Garikipati, S. (2022). The impact of diet upon mitochondrial physiology (Review). Int. J. Mol. Med. 50, 135. 10.3892/ijmm.2022.5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverve, X. M. (2007). Mitochondrial function and substrate availability. Crit. Care Med. 35, S454-S460. 10.1097/01.CCM.0000278044.19217.73 [DOI] [PubMed] [Google Scholar]
- Malkoc, K., Casagrande, S. and Hau, M. (2021). Inferring whole-organism metabolic rate from red blood cells in birds. Front. Physiol. 12, 691633. 10.3389/fphys.2021.691633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norin, T. and Malte, H. (2011). Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J. Exp. Biol. 214, 1668-1675. 10.1242/jeb.054205 [DOI] [PubMed] [Google Scholar]
- Osellame, L. D., Blacker, T. S. and Duchen, M. R. (2012). Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 26, 711-723. 10.1016/j.beem.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin, K., Auer, S. K., Rudolf, A. M., Anderson, G. J., Selman, C. and Metcalfe, N. B. (2016). Variation in metabolic rate among individuals is related to tissue-specific differences in mitochondrial leak respiration. Physiol. Biochem. Zool. 89, 511-523. 10.1086/688769 [DOI] [PubMed] [Google Scholar]
- Salin, K., Villasevil, E. M., Anderson, G. J., Lamarre, S. G., Melanson, C. A., Mccarthy, I., Selman, C. and Metcalfe, N. B. (2019). Differences in mitochondrial efficiency explain individual variation in growth performance. Proc. R. Soc. B Biol. Sci. 286, 20191466. 10.1098/rspb.2019.1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander, P. F., Hock, R., Walters, V. and Irving, L. (1950). Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic Rate. Biol. Bull. 99, 259-271. 10.2307/1538742 [DOI] [PubMed] [Google Scholar]
- Scott, G. R., Guo, K. H. and Dawson, N. J. (2018). The mitochondrial basis for adaptive variation in aerobic performance in high-altitude deer mice. Integr. Comp. Biol. 58, 506-518. 10.1093/icb/icy056 [DOI] [PubMed] [Google Scholar]
- Sokolova, I. (2018). Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr. Comp. Biol. 58, 519-531. 10.1093/icb/icy017 [DOI] [PubMed] [Google Scholar]
- Thoral, E., García-Díaz, C. C., Chamkha, I., Elmér, E., Ruuskanen, S. and Nord, A. (2024). The relationship between mitochondrial respiration, resting metabolic rate and blood cell count in great tits. Biol. Open 13, bio060302. 10.1242/bio.060302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegh, M. A., Helm, B., Dingemanse, N. J. and Tieleman, B. I. (2008). Repeatability and individual correlates of basal metabolic rate and total evaporative water loss in birds: a case study in European stonechats. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 150, 452-457. 10.1016/j.cbpa.2008.05.006 [DOI] [PubMed] [Google Scholar]
- Voss, M., Shutler, D. and Werner, J. (2010). A hard look at blood sampling of birds. Auk 127, 704-708. 10.1525/auk.2010.10033 [DOI] [Google Scholar]
- Welcker, J., Speakman, J. R., Elliott, K. H., Hatch, S. A. and Kitaysky, A. S. (2015). Resting and daily energy expenditures during reproduction are adjusted in opposite directions in free-living birds. Funct. Ecol. 29, 250-258. 10.1111/1365-2435.12321 [DOI] [Google Scholar]
- White, C. R., Schimpf, N. G., Cassey, P., Van Ekeris, L., Linser, P. J. and Besansky, N. J. (2013). The repeatability of metabolic rate declines with time. J. Exp. Biol. 216, 1763-1765. 10.1242/jeb.087189 [DOI] [PubMed] [Google Scholar]
- Wieckowski, M. R. M. R., Giorgi, C., Lebiedzinska, M., Duszynski, J. and Pinton, P. (2009). Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 4, 1582-1590. 10.1038/nprot.2009.151 [DOI] [PubMed] [Google Scholar]


