Abstract
The association between lumbar bone mineral density (BMD) and triglyceride (TG) levels has been extensively studied; however, the results remain controversial. Therefore, this research aimed to elucidate the association of TG levels with lumbar BMD in patients with osteoporotic fractures (OPFs) who have undergone surgery. This cross-sectional study analyzed 3,558 OPF patients (aged 50 years and above) who were admitted to the First People’s Hospital of Kunshan and assessed their TG levels, lumbar BMD, and other variables. The outcome variable of this research was bone density, whereas the baseline glycerol trihydrate levels were considered as the exposure variable. An analysis adjustment was conducted for various covariates, including age, gender, body mass index (BMI), and other baseline laboratory and clinical results. Furthermore, the potential non-linear relationships were assessed via the smooth curve fitting, and threshold effect analyses. The mean age of 3,558 included OPF patients was 68.87 ± 10.55 years. In the fully adjusted multivariate regression analysis, a positive correlation was found between TG levels and lumbar BMD (β = 0.015, 95% CI: 0.001–0.028, p = 0.033). Furthermore, the threshold effect analysis revealed a curvilinear relationship between TG levels and lumbar BMD, with a turning point at 1.26 mmol/L. Moreover, on both sides of the turning point, different patterns were observed. On the left side, TG levels were positively correlated with lumbar BMD. However, despite higher TG levels, the differences in lumbar BMD on the right side of the turning point, were not statistically significant, indicating a lack of significant association (p = 0.712). In summary, this research indicated that in OPF patients, higher TG levels were significantly positively associated with lumbar BMD. Furthermore, there was a threshold value of 1.26 mmol/L, indicating that TG levels in OPF patients with concomitant hypertriglyceridemia should be maintained within the normal range, and reducing TG levels below 1.26 mmol/L requires continuous monitoring. This approach effectively controls TG levels without adversely impacting lumbar BMD.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78926-x.
Keywords: Triglyceride, Lumbar bone mineral density, Osteoporotic fracture
Subject terms: Endocrine system and metabolic diseases, Metabolic disorders
Introduction
Osteoporosis (OP) is one of the most prevalent metabolic bone disorders in elderly population1. It is primarily characterized by bone microarchitecture deterioration and reduced bone mass/mineral density (BMD)2–4. OP increases the risk of fractures in older adults, and osteoporotic fractures (OPFs) are a common cause of morbidity and mortality5,6. Because of fragility fractures, OP is considered a major health concern, significantly affecting morbidity, socioeconomic status, and mortality7–9. The 2D method of dual-energy X-ray absorptiometry is the gold standard technique for OP diagnosis10,11.
It has been observed that in the elderly population arteriosclerosis and OP are co-morbidities12. Furthermore, dyslipidemia, disruptions in triglyceride (TG) metabolism, has been indicated as a major cause of arteriosclerosis13,14. However, the association between TG and BMD has remain elusive.
Some studies have suggested a negative TG and BMD association in specific populations and propose that higher TG levels might be correlated with lower BMD and increased risk of OP. This hypothesis is supported by the notion that dyslipidemia can disrupt the balance of bone remodeling, affecting the activity and production of osteoclasts and osteoblasts, the cells responsible for bone resorption and formation15–17.
However, several other studies have indicated a positive correlation between BMD and serum TG levels, proposing that higher TG levels may indicate better overall metabolic health, in turn positively affecting BMD. It is hypothesized that TG, as an energy source, essentially supports the metabolic demands of bone tissue and promotes bone mineralization13,18.
Thus, understanding the precise role of TG in OP could significantly help the diagnosis, prevention, and management of this debilitating condition. Therefore, this investigation aimed to elucidate the association between BMD and TG levels in OPF patients aged ≥ 50 years.
Materials and methods
Ethical consideration
The study was authorized by the Ethical Board of the Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China (approval # 2021-06-015-K01) and followed the Helsinki Declaration. To ensure an unbiased investigation, patients’ identities were not revealed and a signed written consent form was acquired from all the participants.
Study population
This retrospective cross-sectional investigation analyzed the data of 3558 OPF patients who underwent surgery or were hospitalized at the Kunshan Hospital, affiliated with Jiangsu University, Suzhou, China, between January 2017 to July 2022. All patient’s blood was tested during hospitalization. The OP was diagnosed based on the following parameters: (1) The presence of bone fractures and instability without any accompanying bone metabolic disorders, and (2) The standard BMD T-score of -2.5 or below, even in the absence of a bone fracture19. Furthermore, patients (1) with missing or incomplete records, (2) with multiple or pathological hip fractures, (3) who are diagnosed with other diseases that interfere with bone metabolism, (4) with prolonged use of drugs that affect bones, such as glucocorticoids, and (5) TG < 6 were excluded from the study13.
Exposure and outcome variables
The serum TG levels were assessed in 759 OPF patients using the GPO-POD method via the Beckman AU5800 automated biochemical analyzer. Furthermore, lumbar BMD was also measured in these patients using dual-energy X-ray absorptiometry (DXA) with a Hologic dual-energy X-ray bone density instrument (Discovery Wi, Hologic Inc, USA). For TG content and lumbar BMD assessment, fasting blood was collected within 24 h of admission, before surgery. A skilled operator collected the samples using identical equipment according to established protocols. BMD analysis was performed in lumbar fracture patients and their average of the vertebral bodies was assessed other than the affected vertebral body.
Covariate variables
The covariate variables such as age, sex, body mass index (BMI), magnesium, sodium, phosphorus, platelet, neutrophil, monocyte, apolipoprotein A, creatinine (CR), uric acid (UA), American Society of Anesthesiologists (ASA) score and fracture category were assessed. Fasting blood samples were collected and all the clinical indicators were assessed within 3 days after admission.
Statistical analyses
The demographics, laboratory tests, and clinical outcomes data were indicated as median with the interquartile range (the 25th and 75th percentiles) or mean ± standard deviation (SD). For each category, the data were presented in the form of frequencies (expressed as percentages). For categorical data analysis, Pearson’s chi-square or Fisher’s exact test was carried out for univariate analysis. For normally and not-normal distributed continuous data, independent samples t-test and Mann-Whitney U test were carried out utilized, respectively. The association between the attributes of OPFs and the lumbar BMD was also investigated using univariate analysis.
The generalized estimating equation (GEE) was employed to appropriately adjust for covariates and investigate the independent association between lumbar BMD and TG levels in OPF patients. The developed models included unadjusted (Model 1), slightly adjusted (Model 2), and fully adjusted (Model 3) models. First, the presence of collinearity among the covariates was detected via the variance inflation factor (VIF) analysis. Then, these elements were modified according to the following parameters: (1) A modification in the matched odds ratio (OR) by ≥ 10% upon the addition or removal of covariates in the basic or full model, respectively, and (2) Variables satisfying criterion 1 or had a univariate model with p-value < 0.120. Model 3 employed both criteria 1 and criteria 2 to adjust the covariates. Thus, three models were ultimately developed: Model 1 (left unadjusted), Model 2 (minimally adjusted), which required covariate adjustments for age, and gender, and Model 3, which additionally included covariates such as magnesium, sodium, phosphorus, platelet, neutrophil, monocyte, apolipoprotein A, CR, UA, ASA score and fracture category.
The probable non-linear associations were assessed via the Generalized Additive Model (GAM). Afterward, the resulting smoothing curve’s threshold effects were evaluated using a two-piecewise linear regression model. Furthermore, the inflection point was determined with the help of a recursive approach, and a maximum likelihood model was employed when these curves indicated a clear ratio21. Subgroup analyses were conducted to assess the study’s robustness and examine differences among patient subgroups by their stratification based on specific covariates. Likelihood ratio tests (LRT) were employed to analyze the interactions and modifications within the subgroups.
All the statistical measurements were performed using Empower Stats from X&Y Solutions, Inc., MA, USA (http://www.empowerstats.com) and R packages from The R Foundation (http://www.R-project.org). The significant threshold of the two-tail test was p < 0.05.
Results
Clinical and demographic traits of subjects
According to the eligibility criteria depicted in Figs. 1 and 759 patients from January 1, 2017, to July 27, 2022 were included in this research analysis. The baseline characteristics of the hospitalized patients (n = 759, 72.46% female and 27.54% male, mean age 68.87 ± 10.55 years) are summarized in Table 1. The subjects in this study indicated mean TG and lumbar BMD values of 1.21 ± 0.72 mmol/L and 0.74 ± 0.15 g/cm2, respectively.
Fig. 1.
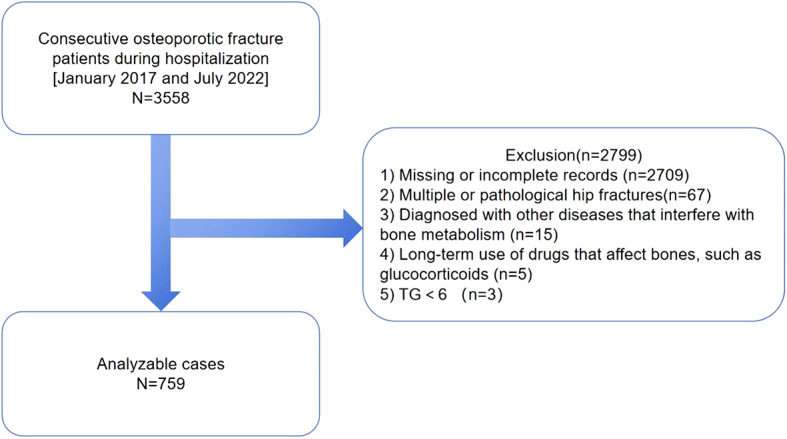
A schematic representation of the study design.
Table 1.
Characteristics of study participants.
| Characteristics | Mean (SD) Median (Q1-Q3)a |
|---|---|
| Age, years | 68.87 (10.55) 68.00 (61.00–77.00) |
| BMI, kg/m2 | 22.95 (3.30) 22.89 (20.69–24.99) |
| Magnesium, mmol/L | 0.89 (0.10) 0.89 (0.82–0.96) |
| Sodium, mmol/L | 140.99 (2.93) 141.20 (139.50-142.60) |
| Phosphorus, mmol/L | 1.08 (0.20) 1.08 (0.97–1.19) |
| Platelet, ×109/L | 175.62 (63.95) 166.00 (135.00-208.00) |
| Neutrophil, ×109/L | 6.41 (3.15) 5.76 (4.20-8.00) |
| Monocyte, ×109/L | 0.49 (0.24) 0.50 (0.30–0.60) |
| Apolipoprotein A, g/L | 1.20 (0.23) 1.17 (1.04–1.33) |
| CR, umol/L | 67.63 (31.92) 63.00 (53.00–75.00) |
| UA, umol/L | 282.78 (96.00) 272.00 (219.00-331.50) |
| TG, mmol/L | 1.21 (0.72) 0.98 (0.73–1.45) |
| Lumbar BMD, g/cm2 | 0.74 (0.15) 0.73 (0.64–0.83) |
| N (%) | |
| Sex, N (%) | |
| Female | 550 (72.46%) |
| Male | 209 (27.74%) |
| ASA score, N (%) | |
| 1 | 67 (8.83%) |
| 2 | 521 (68.64%) |
| ≥3 | 171 (22.53%) |
| Fracture category, N (%) | |
| Thoracic vertebra | 149 (19.63%) |
| Lumbar vertebra | 293 (38.60%) |
| Wrist | 17 (2.24%) |
| Proximal humerus | 57 (7.51%) |
| Thighbone | 243 (32.02%) |
aFor continuous variables.
Abbreviations: SD, standard deviation; Q1, first quartile; Q3, third quartile; CI, confidence interval; BMI, body mass index; CR, creatinine; UA, uric acid; TG, triglycerides; BMD, bone mineral density; ASA, American Society of Anesthesiologists.
Univariate analysis of lumbar BMD
To elucidate the association of lumbar BMD with covariate variables, a univariate analysis was carried out (Table 2), which indicated no significant relationships between the investigated variables and lumbar BMD in OPF patients.
Table 2.
Univariate analysis for lumbar BMD.
| Characteristics | Statisticsa | Lumbar BMD β (95%CI) P-value |
|---|---|---|
| Age, years | 68.87 ± 10.55 | -0.004 (-0.005, -0.003) < 0.001 |
| BMI, kg/m2 | 22.95 ± 3.30 | 0.011 (0.008, 0.014) < 0.001 |
| Magnesium, mmol/L | 0.89 ± 0.10 | 0.073 (-0.031, 0.177) 0.169 |
| Sodium, mmol/L | 140.99 ± 2.93 | -0.003 (-0.007, 0.000) 0.061 |
| Phosphorus, mmol/L | 1.08 ± 0.20 | -0.007 (-0.059, 0.046) 0.803 |
| Platelet, ×109/L | 175.62 ± 63.95 | 0.000 (-0.000, 0.000) 0.962 |
| Neutrophil, ×109/L | 6.41 ± 3.15 | 0.001 (-0.002, 0.005) 0.435 |
| Monocyte, ×109/L | 0.49 ± 0.24 | 0.002 (-0.041, 0.045) 0.925 |
| Apolipoprotein A, g/L | 1.20 ± 0.23 | 0.072 (0.027, 0.116) 0.002 |
| CR, umol/L | 67.63 ± 31.92 | -0.000 (-0.001, -0.000) 0.017 |
| UA, umol/L | 282.78 ± 96.00 | 0.000 (-0.000, 0.000) 0.475 |
| TG, mmol/L | 1.21 ± 0.72 | 0.016 (0.002, 0.030) 0.029 |
| Sex, N (%) | ||
| Female | 55 (72.46%) | Reference |
| Male | 209 (27.54%) | 0.124 (0.102, 0.145) < 0.001 |
| ASA score, N (%) | ||
| 1 | 67 (8.83%) | Reference |
| 2 | 521 (68.64%) | -0.066 (-0.102, -0.029) < 0.001 |
| ≥ 3 | 171 (22.53%) | -0.106 (-0.147, -0.065) < 0.001 |
| Fracture category, N (%) | ||
| Thoracic vertebra | 149 (19.63%) | Reference |
| Lumbar vertebra | 293 (38.60%) | 0.040 (0.011, 0.068) 0.007 |
| Wrist | 17 (2.24%) | 0.078 (0.005, 0.151) 0.036 |
| Proximal humerus | 57 (7.51%) | 0.063 (0.019, 0.108) 0.005 |
| Thighbone | 243 (32.02%) | 0.036 (0.007, 0.066) 0.017 |
aFor continuous variables.
bThe dependent variable was bone turnover markers and β is the result of univariate analysis for lumbar BMD.
Abbreviations: TG, triglycerides; BMD, bone mineral density; BMI, body mass index; CR, creatinine; UA, uric acid; ASA, American Society of Anesthesiologists.
Relationship analysis between triglyceride and lumbar BMD
Altogether, 3 models were utilized to analyze the correlation between TG levels and lumbar BMD in OPF patients (Table 3). The unadjusted Model 1 indicated a significant correlation between lumbar BMD and TG levels (β = 0.016, 95% CI: 0.002–0.030, p = 0.029). In Model 2, after adjusting the variables (age and sex), consistent relationships were observed and TG was significantly associated with lumbar BMD (β = 0.014, 95% CI: 0.001–0.027, p = 0.036). Model 3 included further adjustments for magnesium, sodium, phosphorus, platelet, neutrophil, monocyte, apolipoprotein A, CR, UA, ASA score, and fracture category, which consistently indicated a significant positive correlation between TG levels and lumbar BMD (β = 0.015, 95% CI: 0.001–0.028, p = 0.033).
Table 3.
Association between TG and lumbar BMD in different models.
| Model 1aN = 759 β (95%CI) P-value |
Model 2bN = 759 β (95%CI) P-value |
Model 3cN = 757 β (95%CI) P-value |
|
|---|---|---|---|
| Lumbar BMD |
0.016 (0.002, 0.030) 0.029 |
0.014 (0.001, 0.027) 0.036 |
0.015 (0.001, 0.028) 0.033 |
aNo adjustment.
bAdjusted for age, sex.
cAdjusted for age, sex, BMI, magnesium, sodium, phosphorus, platelet, neutrophil, monocyte, apolipoprotein A, CR, UA, ASA, fracture category.
Abbreviations: CI, confidence interval; TG, triglycerides; BMD, bone mineral density; BMI, body mass index; CR, creatinine; UA, uric acid; ASA, American Society of Anesthesiologists.
Further subgroup analysis was performed to assess the robustness of Model 3 by categorizing OPF patients based on various characteristics such as age, sex, BMI, magnesium, sodium, phosphorus, platelet, neutrophil, monocyte, apolipoprotein A, CR, UA, ASA score, and fracture. Covariates that were not utilized for stratification were adjusted (Table 4). Furthermore, the interaction analysis indicated that UA levels influenced the correlation between TG levels and lumbar BMD (p = 0.007) (Fig. 2). In patients with low UA levels (≤ 271 µmol/L), the β coefficient (β = 0.033, 95% CI: 0.013–0.054, p < 0.001) representing the correlation between TG and lumbar BMD was significantly consistent with the main findings of this study. However, in high UA patients (> 271 µmol/L), the association of increasing TG levels with corresponding increasing lumbar BMD was not clarified. In addition, the results of other analyses were also consistent with the pattern observed in the main study result, and no significant interactions due to stratification were detected.
Table 4.
Subgroup analysis between TG and lumbar BMD.
| Subgroup | N | Lumbar BMDa β (95% CI) P-value |
P-value for interaction |
|---|---|---|---|
| Age, years, N (%) | 0.835 | ||
| Low | 397 | 0.012 (-0.006, 0.030) 0.202 | |
| High | 362 | 0.018 (-0.002, 0.038) 0.083 | |
| Sex, N (%) | 0.586 | ||
| Female | 550 | 0.012 (-0.004, 0.027) 0.141 | |
| Male | 209 | 0.020 (-0.010, 0.043) 0.225 | |
| BMI, kg/m2, N (%) | 0.815 | ||
| Low | 378 | 0.019 (-0.001, 0.038) 0.061 | |
| High | 381 | 0.013 (-0.006, 0.032) 0.181 | |
| Magnesium, mmol/L | 0.651 | ||
| Low | 344 | 0.009 (-0.012, 0.030) 0.402 | |
| High | 415 | 0.020 (0.003, 0.038) 0.025 | |
| Sodium, mmol/L | 0.748 | ||
| Low | 341 | 0.013 (-0.007, 0.034) 0.208 | |
| High | 418 | 0.018 (0.000, 0.036) 0.048 | |
| Phosphorus, mmol/L | 0.159 | ||
| Low | 367 | 0.027 (0.006, 0.048) 0.014 | |
| High | 392 | 0.006 (-0.011, 0.023) 0.501 | |
| Platelet, ×109/L | 0.651 | ||
| Low | 379 | 0.014 (-0.007, 0.034) 0.201 | |
| High | 378 | 0.015 (-0.003, 0.033) 0.102 | |
| Neutrophil, ×109/L | 0.569 | ||
| Low | 399 | 0.020 (0.001, 0.039) 0.043 | |
| High | 358 | 0.008 (-0.011, 0.027) 0.414 | |
| Monocyte, ×109/L | 0.052 | ||
| Low | 378 | 0.004 (-0.014, 0.022) 0.685 | |
| High | 379 | 0.030 (0.010, 0.050) 0.004 | |
| Apolipoprotein A, g/L | 0.509 | ||
| Low | 365 | 0.018 (-0.004, 0.039) 0.107 | |
| High | 394 | 0.012 (-0.005, 0.030) 0.175 | |
| CR, umol/L | 0.041 | ||
| Low | 375 | 0.033 (0.013, 0.054) 0.001 | |
| High | 384 | -0.000 (-0.018, 0.018) 0.975 | |
| UA, umol/L | 0.007 | ||
| Low | 377 | 0.041 (0.020, 0.063) < 0.001 | |
| High | 382 | 0.002 (-0.019, 0.014) 0.801 | |
| ASA score, N (%) | 0.671 | ||
| 1 | 67 | 0.034 (-0.008, 0.075) 0.112 | |
| 2 | 521 | 0.011 (-0.005, 0.027) 0.164 | |
| ≥3 | 171 | 0.023 (-0.008, 0.055) 0.153 | |
| Fracture category, N (%) | 0.303 | ||
| Thoracic vertebra | 149 | 0.006 (-0.026, 0.039) 0.706 | |
| Lumbar vertebra | 293 | 0.004 (-0.017, 0.025) 0.710 | |
| Wrist | 17 | 0.111 (0.012, 0.210) 0.273 | |
| Proximal humerus | 57 | 0.002 (-0.041, 0.046) 0.920 | |
| Thighbone | 243 | 0.039 (0.014, 0.064) 0.003 |
aPatients were stratified based on age; sex; BMI; magnesium; sodium; phosphorus; platelet; neutrophil; monocyte; apolipoprotein A; CR; UA; ASA; fracture category, and additional covariates not included in the stratification were adjusted for in the analysis.
Abbreviations: CI, confidence interval; BMI, body mass index; CR, creatinine; UA, uric acid; TG, triglycerides; BMD, bone mineral density; ASA, American Society of Anesthesiologists.
Fig. 2.
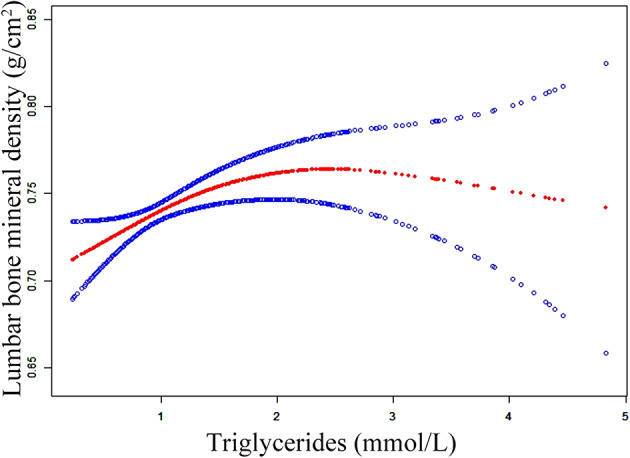
In the high uric acid group (≤ 271umol/L) and low uric acid group (>271umol/L), TG exhibited distinct trends in relation to lumbar BMD.
Spline smoothing plot and threshold analysis
Graphical techniques were also employed to assess whether the correlation between TG and lumbar BMD was linear or nonlinear (Fig. 3). The GAM estimation revealed that, after accounting for covariate variables, there were distinct nonlinear relationships between TG and lumbar BMD in OPF patients. These associations were modeled using segmented linear regression, with the identified breakpoints (K-values) of 1.26 (Table 5). To the left of the thresholds, a strong positive correlation between TG and lumbar BMD [0.061 (95% CI: 0.024–0.098, p = 0.001)] was observed. However, the effect size on the right side of this threshold was − 0.004 (95% CI: -0.023–0.016, p = 0.712).
Fig. 3.
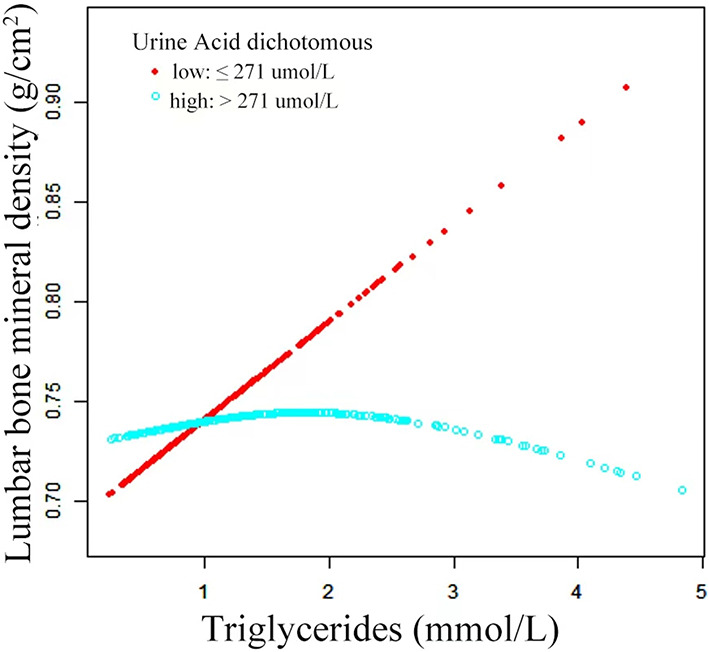
Adjusted Smoothed Curve Analysis Revealing the Interplay Between TG and Lumbar BMD: GAM identified a threshold non-linear correlation between TG and lumbar BMD in OPFs. The upper and bottom curves depict the extent of the 95% confidence interval, whereas the middle curve illustrates the link between TG and lumbar BMD. The models were adjusted for covariate variables. The middle curve exhibited a point of inflection (K) at positions 1.26.
Table 5.
Threshold analyses examining the relationship between TG and lumbar BMD.
| Model 3a | |
|---|---|
| Lumbar BMD | |
| Model Ab | |
| One line slope | 0.015 (0.001, 0.028) 0.030 |
| Model Bc | |
| TG turning point (K) | 1.26 |
| < K | 0.061 (0.024, 0.098) 0.001 |
| > K | -0.004 (-0.023, 0.016) 0.712 |
| Slope 2-Slope 1 | -0.065 (-0.113, -0.016) 0.009 |
| LRTd | 0.008 |
aAdjusted for age, sex, BMI, magnesium; sodium; phosphorus; platelet; neutrophil; monocyte; apolipoprotein A; CR; UA; ASA; fracture category.
bLinear analysis, P value < 0.05 indicates a linear relationship.
cNonlinear analysis.
dP value < 0.05 means Model B is significantly different from Model A, which indicates a nonlinear relationship.
Abbreviations: TG, triglycerides; BMD, bone mineral density; BMI, body mass index; CR, creatinine; UA, uric acid;, ASA, American Society of Anesthesiologists.
Discussion
This study analyzed the data of OPF patients (age ≥ 50) enrolled at Kunshan Hospital affiliated with Jiangsu University collected between January 2017 to July 2022 to elucidate the correlation between TG and lumbar BMD. The results demonstrated a significant positive correlation between TG and lumbar BMD on the left side of the inflection point (1.26 mmol/L), while a non-significant negative correlation on the right side.
Hypertriglyceridemia is one of the most common lipid abnormalities22. Several studies have been performed on elevated TG levels23–25. The association between TG and BMD has been extensively investigated in the general population and has yielded varying conclusions. Furthermore, a significant correlation between elevated TG levels and decreased BMD or OP has been observed13. In this research, a multiple regression equation was employed to elucidate the relationship between TG and lumbar BMD, which indicated a positive correlation (β = 0.015, 95% CI: 0.001–0.028, p = 0.033). Furthermore, the smooth curve fitting and threshold effect analyses revealed a turning point in the association between TG and lumbar BMD. When TG levels were below 1.26 mmol/L, an elevated TG level was significantly associated with increased lumbar BMD. At one side of the inflection point, a slight negative correlation was observed.
Fat is an essential component of the human body and reflects the nutritional status of an individual. Here, a positive correlation was observed between lower TG levels and increased lumbar BMD, consistent with the findings of previous research26–28. Furthermore, increased TG levels are often associated with endocrine disruption, potentially influencing the levels of various hormones, including sex, thyroid, and growth hormones, as well as cortisol. These hormonal disruptions can have negative effects on the skeletal system, leading to bone loss and decreased BMD29,30. Moreover, high TG levels may trigger inflammatory responses and oxidative stress31, both of which are closely associated with disturbances in bone metabolism.
These findings provide valuable insights for clinical practice. In patients with OPF and hypertriglyceridemia, lipid-lowering medications should be employed to control TG levels within the normal range. However, it is not recommended to reduce TG below 1.26 mmol/L as this may have adverse effects on the OPF prognosis and prevent increases in lumbar BMD. Because of the relatively low contribution of TG levels to lumbar BMD, once the concentration exceeds the threshold, patients with hypertriglyceridemia should aim to reduce their TG levels to 1.26 mmol/L. This approach can control blood TG levels effectively while minimizing any detrimental impact on lumbar BMD.
Study advantages and limitations
Compared to previous studies, this research has several advantages. (1) This research comprised OPF patients aged 50 years and above, which is inconsistent with prior research. (2) The results of this investigation indicated a positive correlation between TG and lumbar BMD, with a turning point at 1.26 mmol/L, providing a baseline value for clinical considerations. (3) This investigation identified an interaction effect of UA on the association of TG with lumbar BMD, which has not been previously addressed in the literature.
Despite the advantages, this study has certain limitations. (1) The study was cross-sectional, and thus does not permit inferences concerning time and causality relationships. Further mechanistic insights and large-scale prospective studies are needed to elucidate the specific underlying mechanisms. We plan to conduct longitudinal analyses in our future research, involving long-term patient follow-ups, to obtain more comprehensive and reliable results. This would further advance the understanding of the relationship between serum triglycerides and lumbar spine bone density in the elderly osteoporotic population. (2) Other potential covariates, such as treatment modalities during hospitalization, were not considered. These confounding factors may also influence the association between TG and lumbar BMD. (3) This study had a relatively small sample size of only 760 subjects, highlighting the need for comprehensive investigations of patients with different diseases.
Conclusions
In summary, this research evaluated the relationship between TG and lumbar BMD in OPF patients (≥ 50 age) and revealed a significant positive correlation with a turning point at 1.26 mmol/L. The results suggest that for the management of OPFs in patients with concurrent hypertriglyceridemia, maintaining the TG levels within the normal range and ensuring they do not fall below 1.26 mmol/L is crucial to effectively regulate TG levels without adversely affecting lumbar BMD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Peng Zhou, Lei Liu, Ke Lu, wrote the main manuscript text. Min-zhe Xu, Yao-wei Ye prepared Figs. 1, 2 and 3.Chong Li, Yi Yin prepared Tables 1, 2, 3, 4 and 5. All authors reviewed the manuscript.
Funding
The study was supported by Suzhou City Major Disease Multicenter Clinical Research Project (CN) (DZXYJ202312), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023738), Key Project of the Jiangsu University Medical Education Collaborative Innovation Fund (JDY2022013) and Gusu Health Talent Plan Scientific Research Project (CN) (GSWS2022105).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
We received ethical approval from the Affiliated Kunshan Hospital of Jiangsu University (approval No. 2021-06-015-K01), and was compliant with the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual patients included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Zhou, Lei Liu, Ke Lu, Chong Li and Yi Yin contributed equally to this work.
Contributor Information
Chong Li, Email: lichong1705@163.com.
Yi Yin, Email: yy-19723@163.com.
References
- 1.Xie, W. et al. Neuropeptide Y1 receptor antagonist promotes osteoporosis and microdamage repair and enhances osteogenic differentiation of bone marrow stem cells via cAMP/PKA/CREB pathway. Aging. 12, 8120–8136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon, K., Lee, S. & Cha, J. Bacillus subtilis Fermentation of Malva verticillata leaves enhances antioxidant activity and osteoblast differentiation. Foods. 9, 671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ke, K., Arra, M. & Abu-Amer, Y. Mechanisms underlying bone loss Associated with gut inflammation. Int. J. Mol. Sci.20, 6323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y., Zhu, J., Zhou, Y., Peng, J. & Wang, B. Efficacy and safety of Denosumab in osteoporosis or low bone Mineral Density Postmenopausal women. Front. Pharmacol.12, 588095 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu, K. et al. Toxoplasma Gondii infection as a risk factor for osteoporosis: a case–control study. Parasit. Vectors. 15, 151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, W. C., Ji, X., Nissim, I. & Long, F. Malic enzyme couples mitochondria with aerobic glycolysis in Osteoblasts. Cell. Rep.32, 108108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iolascon, G. et al. Pharmacological therapy of osteoporosis: what’s New? Clin. Interv Aging. 15, 485–491 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jothimurugan, S. et al. Testing the water: osteoporosis management in primary care. Cureus14, e21082 . [DOI] [PMC free article] [PubMed]
- 9.Dominguez, L. J. et al. Association between Serum Magnesium and Fractures: a systematic review and Meta-analysis of Observational studies. Nutrients. 15, 1304 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, P. et al. Estimation of prevalence of osteoporosis using OSTA and its correlation with Sociodemographic Factors, disability and comorbidities. Int. J. Environ. Res. Public. Health. 16, 2338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki, Y., Maruyama-Nagao, A., Sakuraba, K. & Kawai, S. Level of serum undercarboxylated osteocalcin correlates with bone quality assessed by calcaneal quantitative ultrasound sonometry in young Japanese females. Exp. Ther. Med.13, 1937–1943 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang, T. L., Li, Y. D., Hsiao, F. T., Chuang, M. H. & Wang, Y. F. FRAX® Fracture Risks Are Associated with Coronary Artery Calcification Score. Dis. Markers 1592598 (2017). (2017). [DOI] [PMC free article] [PubMed]
- 13.Wang, P. et al. High cholesterol and low triglycerides are associated with total lumbar bone mineral density among adults aged 50 years and over: the NHANES 2017–2020. Front. Med.9, 923730 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidemann, B. E., Visseren, F. L., van Setten, J., Marais, A. D. & Koopal, C. The association between a genetic variant in the SULF2 gene, metabolic parameters and vascular disease in patients at high cardiovascular risk. Cardiovasc. Endocrinol. Metab.12, e0278 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu, L., Lai, X., Wang, Y., Zhang, J. & Liu, J. A community-based study of the relationship between calcaneal bone mineral density and systemic parameters of blood glucose and lipids. Med. (Baltim).98, e16096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Maghraoui, A. et al. Osteoporosis, vertebral fractures and metabolic syndrome in postmenopausal women. BMC Endocr. Disord. 14, 93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang, Y. et al. Curcumin protects bone biomechanical properties and microarchitecture in type 2 diabetic rats with osteoporosis via the TGFβ/Smad2/3 pathway. Exp. Ther. Med.20, 2200–2208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, L. J. W., Nayeem, F., Anderson, K. E., Grady, J. J. & Nagamani, M. Lean body Mass, not estrogen or progesterone, predicts peak bone Mineral Density in Premenopausal Women. J. Nutr.139, 250–256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aibar-Almazán, A. et al. Current status of the diagnosis and management of osteoporosis. Int. J. Mol. Sci.23, 9465 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernan, W. N. et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl. J. Med.343, 1826–1832 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Liu, S. et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol. Int.7, 901–909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toor, A., Toor, A., Khalighi, K. & Krishnamurthy, M. Triglyceride Levels Greater Than 10,000 mg/dL in a 49-Year-Old Female without Evidence of Pancreatitis. Case Rep. Endocrinol. 6273196 (2019). (2019). [DOI] [PMC free article] [PubMed]
- 23.Miura, Y. & Suzuki, H. Triglyceride levels Greater Than 10,000 mg/dL in a 49-Year-old female without evidence of pancreatitis. Int. J. Mol. Sci.23, 16224 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen, L. et al. Longitudinal trends in lipid profiles during pregnancy: Association with gestational diabetes mellitus and longitudinal trends in insulin indices. Front. Endocrinol.13, (2022). [DOI] [PMC free article] [PubMed]
- 25.Saadeldin, M. K., Elshaer, S. S., Emara, I. A., Maged, M. & Abdel-Aziz, A. K. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: a case control study. PLoS ONE. 13, e0206761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saoji, R. et al. Association of high-density lipoprotein, triglycerides, and homocysteine with bone mineral density in young Indian tribal women. Arch. Osteoporos.13, 108 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Jia, L. & Cheng, M. Correlation analysis between risk factors, BMD and serum osteocalcin, CatheK, PINP, β-crosslaps, TRAP, lipid metabolism and BMI in 128 patients with postmenopausal osteoporotic fractures. [DOI] [PubMed]
- 28.Zheng, J. et al. The effect of plasma lipids and lipid-lowering interventions on bone Mineral density: a mendelian randomization study. J. Bone Min. Res. Off J. Am. Soc. Bone Min. Res.35, 1224–1235 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Olejarz, M., Szczepanek-Parulska, E. & Ruchala, M. Lipoprotein alterations in endocrine disorders - a review of the recent developments in the field. Front. Endocrinol.15, 1354098 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczepanek-Parulska, E. et al. Lipid profile abnormalities associated with endocrine disorders. Endokrynol Pol.73, 863–871 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Liu, J. et al. Plasma quantitative lipid profiles: identification of CarnitineC18:1-OH, CarnitineC18:2-OH and FFA (20:1) as novel biomarkers for pre-warning and prognosis in Acute myocardial infarction. Front. Cardiovasc. Med.9, 848840 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


