Abstract
Trace elements, specifically magnesium (Mg), zinc (Zn), and selenium (Se) have been linked with immunomodulatory properties. This research delves into identifying the potential impression of serum levels Mg, Zn, and Se on the protective immunity arisen through Sinopharm COVID-19 vaccine in the vaccinated subjects. Levels of Mg, Zn, and Se, cytokine and antibody production as well as virus neutralization potency were investigated in 75 males and 75 females before and 2 weeks after first and second dose of vaccination. Level of Mg, Zn, and Se did not change significantly before and 2 weeks after first and second dose of vaccine administration. Concentrations of Interleukin (IL)-2, IL-6, and Interferon (IFN)-γ were significantly higher in the supernatant of peripheral blood mononuclear cells (PBMCs) obtained from subjects 2 weeks after both first and second dose of vaccination compared to before vaccination. Serum level IgG was significantly higher 2 weeks after first and second dose of vaccination compared to before vaccination. Serum level IgM was only higher after first dose of vaccination compared with before vaccine. Also, 2 weeks after both first and second dose of vaccination compared to before vaccination, FRNT50 titer was significantly higher. Levels of Mg, Zn, and Se did not significantly correlate with levels of IL-2, IL-6, IFN-γ, IgM and IgG, and FRNT50 after first and second dose of vaccination. No severe unwanted clinical symptoms were detected after vaccination. Mg, Zn, and Se do not play a role in modulating protective immunity during Sinopharm COVID-19 vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80787-3.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Magnesium, Zinc, Selenium
Subject terms: Antimicrobial responses, Cytokines, Vaccines
Introduction
The ongoing global effort to combat the SARS-CoV-2 pandemic has led to the rapid development and deployment of COVID-19 vaccines. These vaccines have proven to be indispensable tools in mitigating disease transmission and reducing the severity of COVID-19 cases1. As vaccination campaigns progress, there is a growing interest in deciphering the intricate factors that modulate the generation of protective immune responses post-vaccination2,3. Emerging among these factors are trace elements, notably zinc (Zn), magnesium (Mg), and selenium (Se), which play indispensable roles in immune function, cytokine signaling, and antioxidant defense mechanisms4,5.
Zinc, an essential trace element, is pivotal in regulating both innate and adaptive immune responses6. It is integral for the development and function of immune cells, modulating the production of pro-inflammatory and anti-inflammatory cytokines. Furthermore, Zn is recognized for its role in preserving immune cell integrity, antigen presentation, and antibody production7. Mg, another trace element of paramount importance, is implicated in immune cell activation, T cell differentiation, and antibody formation8. Moreover, Mg functions as a cofactor for various enzymes involved in immune signaling pathways9. Selenium, an essential micronutrient with potent antioxidant properties, is indispensable for the activity of selenoproteins that regulate immune responses, oxidative stress, and inflammation10.
The Sinopharm COVID-19 vaccine is designed to stimulate specific immune responses, including the generation of neutralizing antibodies and cellular immunity, against the SARS-CoV-2 virus11. While the vaccine’s efficacy, safety, and potential adverse events have been thoroughly investigated, the influence of trace element status on vaccine-induced immunity remains a critical area that warrants comprehensive exploration. Perturbations in Zn, Mg, and Se levels have the potential to profoundly impact immune cell function, cytokine production, and the overall vaccine response9.
This study seeks to delve into the intricate interplay between blood concentrations of Zn, Mg, and Se and the multifaceted immune response engendered by the Sinopharm COVID-19 vaccine. Furthermore, we endeavor to discern whether fluctuations in trace element levels are associated with the occurrence of vaccine-related side effects. By delineating the potential connections between trace element bioavailability, vaccine-induced immunity, and adverse reactions, our investigations aspire to offer nuanced insights that can guide refined vaccination strategies. In so doing, our research contributes to an enriched understanding of the multifactorial determinants of vaccine outcomes, fostering the formulation of targeted interventions to optimize vaccine efficacy and ensure safety in the context of the ongoing pandemic.
Materials and methods
Recruitment of study subjects
This study was conducted on 150 individuals candidate for Sinopharm COVID-19 vaccination referred to vaccination centers of Rafsanjan city, Kerman, Iran during the first six months of 2022. The Sinopharm COVID-19 vaccine is a two-dose, inactivated vaccine formulated with an aluminum hydroxide adjuvant that is administered on a schedule of 0 and 21–28 days12. Blood sampling was conducted in 4 time points; before and after administration of first vaccine dose, before and after administration of second vaccine dose. The inclusion criteria were administration of two Sinopharm COVID-19 vaccine doses, absence of autoimmune diseases, lack of any form of cancer, absence of any inflammatory diseases (such as metabolic disorders, cardiovascular diseases, hormonal disorders, rheumatic disorders, etc.), no use of immunosuppressive drugs, no organ transplantation, no receipt of any blood or blood products, no recent infection within the past 1 month, stability and good physical health status. Additionally, no previous infection with the SARS-CoV-2 virus was among the entry criteria, and this was be determined by examining the level of serum antibodies, detection of SARS-CoV-2 genome by Real-time PCR, and also by questioning the individuals. Baseline data, demographic characteristics, and laboratory data of the study subjects are shown in Table 1.
Table 1.
The biochemical presentations of the study participants pre and post vaccination.
| Index | Before first dose | 2 weeks after first does | P value (before vs. after first dose) | Before second dose | 2 weeks after first second | P value (before vs. after second dose) |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg, mean ± SD) | 111.25 ± 18.54 | 110.84 ± 18.13 | < 0.05 | 108.36 ± 17.09 | 109.55 ± 17.13 | < 0.05 |
| Diastolic BP (mmHg, mean ± SD) | 82.16 ± 4.11 | 80.23 ± 4.18 | < 0.05 | 79.79 ± 4.29 | 80.24 ± 4.33 | < 0.05 |
| WBC count (cell/mm3, mean ± SD) | 5564 ± 841 | 11,988 ± 1,246 | < 0.0001 | 9511 ± 1,011 | 13,516 ± 1,984 | < 0.0001 |
| Platelet count (cells/mm3; Mean ± SD | 234,632 ± 19,507 | 236,299 ± 19,850 | < 0.05 | 230,411 ± 18.966 | 229,614 ± 19.374 | < 0.05 |
| Hb (g/dL; mean ± SD) | 14.08 ± 2.44 | 13.88 ± 2.59 | < 0.05 | 14.26 ± 3.12 | 14.62 ± 3.31 | < 0.05 |
| CRP (mg/L; mean ± SD) | 1.42 ± 0.26 | 6.02 ± 1.49 | < 0.0001 | 2.46 ± 1.36 | 7.53 ± 1.79 | < 0.0001 |
| ESR (mm/h; mean ± SD) | 4.28 ± 2.04 | 8.32 ± 4.51 | < 0.0001 | 5.13 ± 2.20 | 9.14 ± 4.95 | < 0.0001 |
| ALP (IU/L; mean ± SD) | 72.11 ± 18.23 | 73.16 ± 19.16 | < 0.05 | 70.23 ± 18.02 | 72.12 ± 17.08 | < 0.05 |
| AST (IU/L; mean ± SD) | 23.11 ± 6.17 | 24.45 ± 6.22 | < 0.05 | 27.75 ± 7.27 | 27.24 ± 6.40 | < 0.05 |
| ALT (IU/L; mean ± SD) | 22.08 ± 5.48 | 23.21 ± 6.48 | < 0.05 | 22.48 ± 6.53 | 23.45 ± 6.28 | < 0.05 |
| LDH (IU/L; mean ± SD) | 159.14 ± 38.20 | 162.11 ± 37.58 | < 0.05 | 163.263 ± 38.25 | 166.25 ± 37.86 | < 0.05 |
| Total cholesterol (mg/dL; mean ± SD) | 125.11 ± 35.1086 | 126.35 ± 36.40 | < 0.05 | 128.11 ± 37.59 | 129.13 ± 40.80 | < 0.05 |
| TG (mg/dL; mean ± SD) | 94.11 ± 18.32 | 98.03 ± 17.22 | < 0.05 | 98.32 ± 17.60 | 97.26 ± 18.22 | < 0.05 |
| LDL (mg/dL; mean ± SD) | 87.12 ± 13.44 | 88.27 ± 14.06 | < 0.05 | 85.49 ± 14.66 | 86.42 ± 14.34 | < 0.05 |
| HDL (mg/dL; mean ± SD) | 41.16 ± 5.30 | 42.21 ± 6.70 | < 0.05 | 41.82 ± 5.46 | 42.25 ± 5.23 | < 0.05 |
| Creatinine (mg/dL; mean ± SD) | 0.95 ± 0.16 | 0.94 ± 0.36 | < 0.05 | 0.97 ± 0.45 | 0.95 ± 0.34 | < 0.05 |
| BUN (mg/dL; mean ± SD) | 13.23 ± 5.44 | 13.63 ± 5.46 | < 0.05 | 14.11 ± 4.67 | 14.48 ± 5.50 | < 0.05 |
| FBS (mg/dL; mean ± SD) | 89.40 ± 13.69 | 88.14 ± 13.71 | < 0.05 | 89.15 ± 15.30 | 90.14 ± 15.06 | < 0.05 |
| BMI (kg/m2; mean ± SD) | 22.84 ± 2.60 | 22.79 ± 2.59 | < 0.05 | 22.82 ± 2.58 | 22.77 ± 2.64 | < 0.05 |
SD standard deviation, WBC white blood cell, BP blood pressure, Hb hemoglobin, IL interleukin, CRP C-reactive protein, ESR erythrocyte sedimentation rate, ALP alkaline phosphatase, AST aspartate transaminase, ALT alanine transaminase, LDH lactate dehydrogenase, TG triglyceride, LDL low-density lipoprotein, HDL high-density lipoprotein, BUN blood urea nitrogen, FBS fasting blood sugar, BMI body-mass index.
Significant values are in bold.
To evaluate the severity of clinical presentations and potential adverse effects in vaccinated individuals, follow-up telephone calls were conducted 2 to 4 days post-vaccination. During these calls, participants were inquired to report the presence or absence of any symptoms, which were systematically recorded using a checklist. Possible symptoms included pain, fever, chills, redness, or swelling at the injection site, fatigue, headache, joint pain, muscle pain, nausea, vomiting, and swollen lymph nodes, among others. If needed, a physician conducted a further examination of the participant.
Prior to sample collection, all participants provided written informed consent. Approximately 10 mL of peripheral blood was collected from each subject via venipuncture at four distinct time points. The study protocol received ethical approval from the Rafsanjan University of Medical Sciences Ethics Committee (IR.RUMS.REC.1400.165). This study was conducted in full compliance with the Declaration of Helsinki for research involving human subjects.
Laboratory measurements
The serum concentrations of Alkaline phosphatase (ALP), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Lactate dehydrogenase (LDH), were determined using the enzymatic colorimetric methods (BT3000, Biotecnica, Italy). ESR was measured using the automated ESR analyzer (YHLO Vision, China). C-reactive protein (CRP) levels were assessed using Enzyme-linked immunosorbent assay (ELISA).
Trace elements’ measurement
The colorimetric method was used to measure the levels of Mg (limit of detection: ~0.01–0.1 mg/dL) in serum of subjects through biochemistry autoanalyzer (BT3000, Biotecnica, Italy). Serum Zn (limit of detection: ~0.1–0.5 µg/dL) and Se (limit of detection: ~0.5–2 ng/mL) levels were measured using the Atomic Absorption Spectrophotometry (AA240 Agilent, Germany).
Antibody measurement
Levels of anti-spike IgM and IgG in serum samples were determined by sandwich ELISA approach using local commercial kits (Padtan Gostar Isar Co., Tehran, Iran) before and two weeks after each dose of vaccination. The specification for IgM ELISA kit were Analytical sensitivity of 6.12 mg/dL, Inter-assay coefficient of variation (CV) < 10%, and Intra-assay CV < 10%, assay range: 4.15–3000 mg/dL, while the specification for IgG ELISA kit were Analytical sensitivity of 5.89 mg/dL, Inter-assay CV < 10%, and Intra-assay CV < 10%, assay range: 5.99–5000 mg/dL. The positive cut-off was set at an OD of 0.3 for IgG and 0.2 for IgM, based on assay validation. The optical density (OD) was determined by a microplate reader (Tecan Spectra, Austria).
Assessment of immune system activation
To determine immune system activation, level of IL-2, IL-6, and Interferon (IFN)-γ was measured in the supernatant of Peripheral blood mononuclear cells (PBMCs) before and two weeks after each dose of vaccination. To this end, PBMCs were isolated from peripheral blood samples using Ficoll solution (Lymphodex, inno-Train, Germany) and subsequent gradient centrifugation. About 2 × 104 PBMCs were seeded in 24-well plates in RPMI media supplemented by Pen/Strep and L-glutamine (all from Gibco, Germany). Next day, cells were treated with purified and deactivated SARS-CoV-2 (Pasteur Institute of Iran, Tehran, Iran) for 48 h. Deactivation of virus was conducted through heat inactivation (incubation of virus samples at 56 °C for 30 min) followed by UV irradiation (exposing the virus to UV light for around 5–10 min). Afterwards, levels of IL-2 (Analytical sensitivity: 7 pg/mL, Inter-assay CV < 6%, and Intra-assay CV < 6%, assay range: 31.2–2,000 pg/mL), IL-6 (Analytical sensitivity: 0.7 pg/mL, Inter-assay CV < 7%, and Intra-assay CV < 8%, assay range: 3.1–300 pg/mL), and IFN-γ (Analytical sensitivity: 2.56 pg/mL, Inter-assay CV < 10%, and Intra-assay CV < 5%, assay range: 31.3–2,000 pg/mL) were assayed using ELISA by commercial kits (Biotech R&D systems, China). The OD was measured by a microplate reader (Tecan Spectra, Austria).
Virus neutralizing test
The serum potency of vaccinated individuals in neutralizing the virus was assessed using the Focus Reduction Neutralization Test (FRNT) two weeks before and after the administration of the first and second vaccine doses as described previously13. To this end, serial dilutions of individuals’ serum were mixed with infectious cloned SARS-CoV2 and exposed to Vero C1008 cells (Pasteur Institute of Iran, Tehran, Iran). The key concept of the FRNT is that the presence of neutralizing antibodies prevents the virus from infecting cells. As a result, areas of cell cultures that have been treated with sera containing effective neutralizing antibodies exhibit fewer infected cells, which are referred to as “foci”. Hence, antibody neutralization was quantified through enumerating the number of foci by the Viridot program14 and FRNT50 titers was determined. The positive cut-off for neutralizing antibodies was defined as an FRNT50 titer of ≥ 200 for neutralization efficacy.
Statistical analysis
Initially, the Kolmogorov–Smirnov test was employed to assess the normality of quantitative data distribution. For scale variables that did not follow a normal distribution, the Wilcoxon and Friedman tests were applied. Correlations between variables were determined using Spearman’s rho test. Quantitative data are presented as mean ± standard deviation (SD), and nominal data as frequency (percentage). Statistical significance was defined as P < 0.05. Data analysis and graph design were performed using GraphPad Prism v.8 for Windows (CA, USA).
Results
Baseline characteristics and biochemical data
The study participants comprised of 150 subjects, involving 75 (50%) males and 75 (50%) females. The mean age of the participants was 39.77 ± 18.29 years. The interval between the first and second vaccine administration was 2.5 ± 0.3 months. Table 1 indicate the biochemical data of the study subjects before and after first and second vaccine injection. Frequency of WBC, level of CRP, and ESR score were significantly higher 2 weeks after vaccine injection compared to before vaccine injection both at first and second doses.
Levels of trace elements
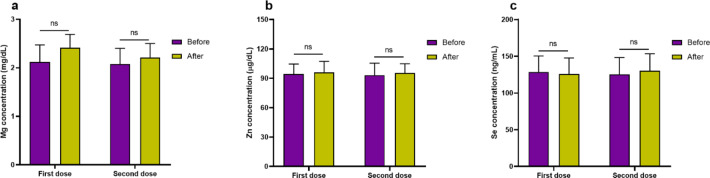
Concentration of Mg (Fig. 1a) before and 2 weeks after first dose of vaccination was 2.12 ± 0.35 mg/dL and 2.41 ± 0.28 mg/dL, respectively, that was not different statistically (P = 0.284). Moreover, concentration of Mg did not have significant difference before (2.08 ± 0.32 mg/dL) and 2 weeks after (2.21 ± 0.29 mg/dL) second dose of vaccination (P = 0.506). Zn concentration (Fig. 1b) before and 2 weeks after first dose of vaccination was 94.32 ± 10.25 µg/dL and 96.14 ± 11.21 µg/dL, respectively, that was not different statistically (P = 0.450). Also, Zn concentration did not have significant difference before (93.18 ± 12.25 µg/dL) and 2 weeks after (95.45 ± 9.41 µg/dL) second dose of vaccination (P = 0.281). Concentration of Se (Fig. 1c) before and 2 weeks after first dose of vaccination was 128.65 ± 21.65 ng/mL and 125.65 ± 22.13 ng/mL, respectively, that was not different statistically (P = 0.284). Moreover, concentration of Mg did not have significant difference before (125.21 ± 23.14 ng/mL) and 2 weeks after (130.21 ± 23.16 ng/mL) second dose of vaccination (P = 0.506). It should be mentioned that, based on analysis, levels of Mg, Zn, and Se did not have significant differences between males and female subjects at before and after first and second doses of vaccination.
Fig. 1.
Bar graphs indicate levels of Mg (a), Zn (b), and Se (c) before and after vaccination for first and second dose (ns non-significant).
Cytokine levels to determine activation of the immune system
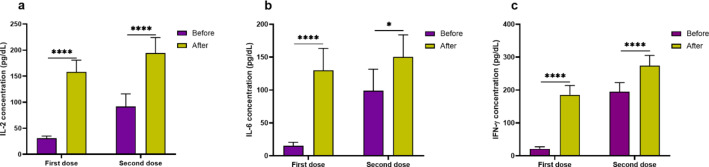
Experiments revealed that IL-2 level was significantly higher (P < 0.0001) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after (158.25 ± 22.49 pg/dL) first dose of vaccination compared to before vaccination (30.71 ± 4.36 pg/dL). Also, IL-2 level was significantly higher (P < 0.0001) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after (194.32 ± 29.69 pg/dL) second dose of vaccination compared to before second dose vaccination (91.64 ± 24.32 pg/dL; Fig. 2a). It was seen that IL-2 level did not have significant difference between female and male subjects both before and after first and second dose of vaccination. It was detected that IL-6 level was significantly higher (P < 0.0001) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after (130.16 ± 33.25 pg/dL) first dose of vaccination compared to before vaccination (15.17 ± 5.46 pg/dL). Also, IL-6 level was significantly higher (P = 0.024) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after (150.25 ± 33.68 pg/dL) second dose of vaccination compared to before second dose vaccination (99.15 ± 32.62 pg/dL; Fig. 2b). It was seen that IL-6 level did not have significant difference between female and male subjects both before and after first and second dose of vaccination. We observed that IFN-γ level was significantly higher (P < 0.0001) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after first (185.62 ± 28.14 pg/dL) dose of vaccination compared to before (21.25 ± 6.25 pg/dL) first dose vaccination. Furthermore, IFN-γ level was significantly higher (P < 0.0001) in the supernatant of PBMCs isolated from peripheral blood of subjects 2 weeks after (274.25 ± 31.29 pg/dL) second dose of vaccination compared to before (195.25 ± 27.41 pg/dL) second dose of vaccination (Fig. 2c). IFN-γ level did not differ significantly between female and male subjects both before and after first and second dose of vaccination.
Fig. 2.
Levels of IL-2 (a), IL-6 (b) and IFN-γ (c) in the supernatant. To assess an activated situation of immune system, levels of IL-2, IL-6, and IFN-γ was assessed in the supernatant of PBMCs isolated from peripheral blood samples and treated with purified and deactivated SARS-CoV-2 for 48 h. Levels of IL-2, IL-6, and IFN-γ was higher significantly after vaccination at first and second doses.
Immune response; anti-spike IgM and IgG levels
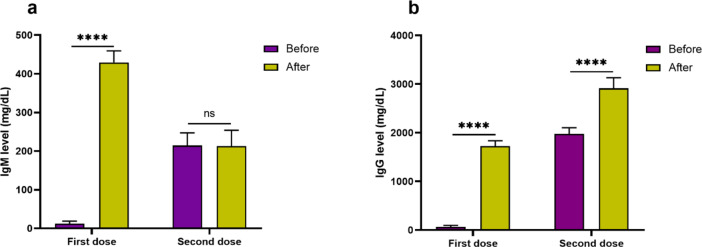
Serum level of IgM (429.25 ± 29.98 mg/dL) after first dose of vaccination was significantly higher (P < 0.0001) compared to its level before first dose of vaccination (12.18 ± 6.74 mg/dL). On the contrary, it was detected that serum IgM level after second dose of vaccination (213.25 ± 40.62 mg/dL) did not increase significantly (P = 0.274) compared to before (214.32 ± 32.85 mg/dL) second dose of vaccination (Fig. 3a). IgM levels did not differ significantly between female and male subjects both before and after first and second dose of vaccination.
Fig. 3.
Bar graphs show the levels of IgM (a) and IgG (b) before and after vaccination for first and second dose. Level of IgM increased significantly after first dose of vaccination but not after second dose of vaccine. Level of IgG increased significantly after both first and second dose of vaccine.
Level of IgG in serum after first dose of vaccination (1724.36 ± 112.36 mg/dL) was significantly higher (P < 0.0001) compared to its level before first vaccination dose (63.45 ± 32.38 mg/dL). Moreover, serum IgG level 2 weeks after (2912.36 ± 219.74 mg/dL) second dose of vaccination was significantly higher (P < 0.0001) compared to before (1978.36 ± 124.28 mg/dL) second vaccination (Fig. 3b). The analysis divulged that levels of IgG did not differ significantly between female and male subjects both before and after first and second dose of vaccination.
Neutralizing capacity of vaccine-induced serum antibodies
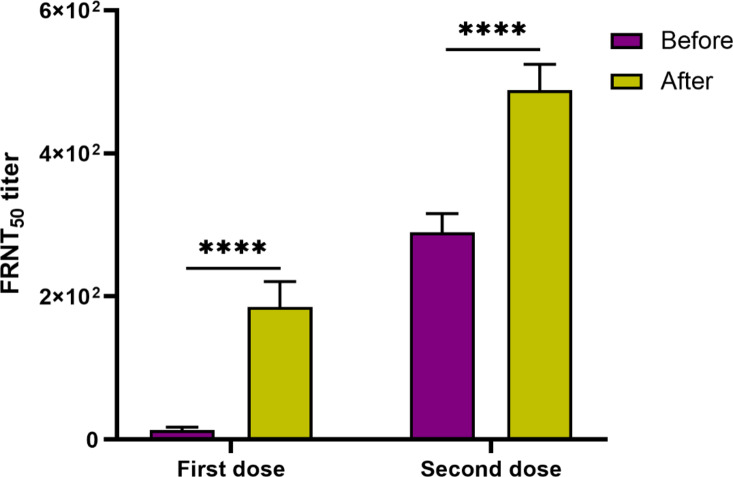
It was detected that FRNT50 titer was higher significantly (P < 0.0001) 2 weeks after first dose of vaccination in comparison to before first vaccine dose administration. In addition, it was seen that FRNT50 titer increased significantly (P < 0.0001) 2 weeks after second dose of vaccination compared with before second vaccination (Fig. 4). Analysis demonstrated that FRNT50 titer did not significantly differ between female and male subjects both before and after first and second dose of vaccination.
Fig. 4.
The serum capacity of vaccinated subjects in neutralizing the virus was assessed using FRNT before and two weeks after the administration of the first and second vaccine doses. First, serial dilutions of individuals’ serum were mixed with infectious cloned SARS-CoV2 and exposed to Vero C1008 cells. Antibody neutralization was determined by FRNT50 titers. The virus neutralizing potency increased significantly after first and second dose of vaccine compared before vaccination.
Correlation analysis
The correlation analysis was performed to assess if basal (before first vaccination) serum levels of Mg, Zn, and Se (at first before first vaccine administration) might impress the immune system activation and immune protective state in vaccinated individuals after first and second dose of vaccination. The analysis demonstrated that serum levels of Mg, Zn, and Se did not have significant correlation with levels of IL-2, IL-6, IFN-γ, IgM, and IgG, and FRNT50 after first and second dose of vaccination (Table 2). In addition, the correlation analysis between serum levels of Mg, Zn, and Se and biochemical indices of the subjects did not have statistically significant correlations (Table 3).
Table 2.
Correlation of the basal levels of mg, zn, and Se with the immunological outcome after first and second vaccine administrations.
| Variable | Mg After first does |
Mg After second does |
Zn After first does |
Zn After second does |
Se After first does |
Se After second does |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| IgM | 0.11 | 0.884 | 0.11 | 0.863 | 0.05 | 0.490 | 0.15 | 0.251 | 0.13 | 0.902 | 0.15 | 0.799 |
| IgG | 0.08 | 0.630 | 0.07 | 0.190 | 0.06 | 0.934 | 0.12 | 0.906 | 0.08 | 0.759 | 0.14 | 0.732 |
| IL-2 | 0.10 | 0.293 | 0.12 | 0.605 | 0.09 | 0.684 | 0.10 | 0.922 | 0.11 | 0.45 | 0.11 | 0.94 |
| IL-6 | 0.06 | 0.936 | 0.15 | 0.304 | 0.18 | 0.284 | 0.08 | 0.231 | 0.14 | 0.549 | 0.08 | 0.870 |
| IFN-γ | 0.11 | 0.537 | 0.17 | 0.582 | 0.16 | 0.845 | 0.18 | 0.979 | 0.12 | 0.494 | 0.17 | 0.704 |
| FRNT50 titer | 0.06 | 0.345 | 0.11 | 0.644 | 0.15 | 0.402 | 0.12 | 0.524 | 0.12 | 0.225 | 0.07 | 0.147 |
FRNT focus reduction neutralization test, IL interleukin, IFN interferon, Mg magnesium, Zn zinc, Se selenium.
Table 3.
Correlation between the levels of mg, zn, and Se and the biochemical data of the vaccinated individuals after first and second vaccination.
| Variable | Mg After first does |
Mg After second does |
Zn After first does |
Zn After second does |
Se After first does |
Se After second does |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Age | 0.09 | 0.777 | 0.06 | 0.245 | 0.1 | 0.868 | 0.05 | 0.435 | 0.14 | 0.860 | 0.05 | 0.270 |
| Systolic BP | 0.12 | 0.658 | 0.18 | 0.284 | 0.18 | 0.161 | 0.07 | 0.396 | 0.12 | 0.669 | 0.14 | 0.269 |
| Diastolic BP | 0.16 | 0.509 | 0.08 | 0.817 | 0.18 | 0.784 | 0.12 | 0.178 | 0.09 | 0.511 | 0.05 | 0.777 |
| WBC count | 0.18 | 0.551 | 0.11 | 0.173 | 0.07 | 0.394 | 0.16 | 0.630 | 0.13 | 0.879 | 0.09 | 0.636 |
| Platelet count | 0.13 | 0.976 | 0.17 | 0.996 | 0.11 | 0.219 | 0.12 | 0.451 | 0.09 | 0.738 | 0.05 | 0.973 |
| Hb | 0.17 | 0.613 | 0.11 | 0.432 | 0.09 | 0.666 | 0.09 | 0.868 | 0.15 | 0.494 | 0.12 | 0.601 |
| CRP | 0.13 | 0.775 | 0.14 | 0.402 | 0.05 | 0.805 | 0.11 | 0.412 | 0.09 | 0.931 | 0.10 | 0.699 |
| ESR | 0.11 | 0.436 | 0.05 | 0.184 | 0.07 | 0.243 | 0.14 | 0.685 | 0.12 | 0.762 | 0.06 | 0.174 |
| FBS | 0.07 | 0.991 | 0.09 | 0.331 | 0.08 | 0.547 | 0.16 | 0.294 | 0.05 | 0.464 | 0.14 | 0.244 |
| ALP | 0.15 | 0.520 | 0.08 | 0.620 | 0.17 | 0.359 | 0.16 | 0.320 | 0.1 | 0.552 | 0.18 | 0.333 |
| AST | 0.13 | 0.137 | 0.10 | 0.327 | 0.12 | 0.370 | 0.16 | 0.701 | 0.08 | 0.251 | 0.15 | 0.335 |
| ALT | 0.06 | 0.795 | 0.11 | 0.653 | 0.07 | 0.432 | 0.11 | 0.523 | 0.13 | 0.633 | 0.13 | 0.222 |
| LDH | 0.07 | 0.309 | 0.09 | 0.962 | 0.17 | 0.699 | 0.16 | 0.524 | 0.07 | 0.413 | 0.12 | 0.347 |
| Total cholesterol | 0.14 | 0.737 | 0.06 | 0.839 | 0.12 | 0.278 | 0.05 | 0.421 | 0.05 | 0.489 | 0.17 | 0.679 |
| TG | 0.18 | 0.660 | 0.16 | 0.323 | 0.16 | 0.226 | 0.15 | 0.246 | 0.08 | 0.464 | 0.15 | 0.588 |
| LDL | 0.16 | 0.309 | 0.11 | 0.105 | 0.07 | 0.627 | 0.09 | 0.514 | 0.09 | 0.209 | 0.07 | 0.745 |
| HDL | 0.06 | 0.718 | 0.16 | 0.784 | 0.10 | 0.208 | 0.18 | 0.292 | 0.06 | 0.904 | 0.18 | 0.102 |
| Creatinine | 0.12 | 0.940 | 0.16 | 0.524 | 0.14 | 0.304 | 0.09 | 0.488 | 0.10 | 0.831 | 0.09 | 0.855 |
| BUN | 0.07 | 0.119 | 0.16 | 0.937 | 0.07 | 0.689 | 0.06 | 0.463 | 0.05 | 0.454 | 0.06 | 0.416 |
| BMI | 0.04 | 0.548 | 0.09 | 0.351 | 0.03 | 0.690 | 0.08 | 0.204 | 0.10 | 0.727 | 0.12 | 0.232 |
WBC white blood cell, BP blood pressure, Hb hemoglobin, IL interleukin, CRP C-reactive protein, ALP alkaline phosphatase, AST aspartate transaminase, ALT alanine transaminase, LDH lactate dehydrogenase, ESR erythrocyte sedimentation rate, FBS fasting blood sugar, TG triglyceride, LDL low-density lipoprotein, HDL high-density lipoprotein, BUN blood urea nitrogen, Mg magnesium, Zn zinc, Se selenium.
The correlation analysis of pre-vaccination (both first and second doses) levels of Mg, Zn, and Se and fold increase of IgM and IgG (after vaccination vs. before vaccination at both first and second doses) revealed no statistically significant correlations (Supplementary Table 1).
The correlation analysis was also performed separately in male and female subjects. Same as the overall correlation analysis results, the analysis did not indicate a significant correlation between serum levels of Mg, Zn, and Se and immune system activation and immune protective state as well as biochemical data in male and female subjects.
Side effect analysis
It was observed that vaccine receivers did not show severe unwanted clinical symptoms, and therefore serum levels of Mg, Zn, and Se level did not associate with severe clinical presentations in the vaccinated cases.
Discussion
The primary objective of this research was, for the first time, to investigate the potential influence of trace elements, specifically Mg, Zn, and Se on immune responses and the occurrence of adverse symptoms subsequent to the administration of the Sinopharm COVID-19 vaccine. As the global population continues to be vaccinated against the COVID-19 virus, there is a growing interest in understanding the factors that could modulate vaccine efficacy and post-vaccination reactions. We previously reported that serum levels of Dehydroepiandrosterone sulfate (DHEA-S) was not instrumental in the protective immune response developed after Oxford-AstraZeneca COVID-19 vccine15. This study aimed to contribute to the existing knowledge by focusing on the role of these essential trace elements in shaping the immune response and determining the severity of adverse effects after receiving the Sinopharm COVID-19 vaccine.
Several research have assessed the role of trace elements in the pathogenesis of COVID-19. As well, we previously indicated that zinc deficiency may confer an increased susceptiblity towards SARS-CoV-2 infection16. Anuk et al.. demonstrated a correlation between changes in serum Zn, copper, Mg levels, and acute phase reactants in response to COVID-19 infection in pregnant women17. Research by Thomas et al.. did not show a significant reduction in COVID-19 symptom duration or severity with high-dose Zn gluconate, ascorbic acid, or their combination compared to standard treatment18. It was demonstrated that oxidative stress is a characteristic of COVID-19 and is associated with immunopathological disorders observed in severe COVID-19 cases. Se deficiency is associated with excessive oxidative stress and inflammation observed in critical conditions and severe COVID-19 cases. Thus, Se supplementation at appropriate doses may act as supportive treatment in COVID-1919. Tan et al.. demonstrated that the combination of vitamin D, magnesium, and vitamin B12 in elderly COVID-19 patients was associated with a significant reduction in clinical severity20. Therefore, these evidence suggests that supplements could potentially alleviate the severity of symptoms and improve outcomes in COVID-19 patients.
In current study, the absence of discernible alterations in these trace element concentrations following vaccination suggests several noteworthy considerations. First, the stability of Mg, Zn, and Se levels before and after vaccination underscores the potential robustness of these trace element concentrations within the studied population. The lack of substantial fluctuations in these elements indicates that the act of vaccination itself might not precipitate immediate changes in their systemic availability. Second, the study’s findings suggest that the administration of the Sinopharm COVID-19 vaccine might not be a prominent determinant of short-term fluctuations in Mg, Zn, and Se levels. These elements, known to play roles in immune function and homeostasis, might be influenced by factors beyond vaccination, and their immediate response might not be a primary consideration in the context of this particular vaccine. Third, the lack of significant alterations in trace element levels post-vaccination might suggest that the pre-existing baseline concentrations of Mg, Zn, and Se do not strongly correlate with immediate vaccine-induced immune responses. While these trace elements can impact immune function, the transient changes due to vaccination might be overshadowed by the broader and more complex mechanisms governing vaccine-induced immunity. The study’s focus on short-term (2 weeks) post-vaccination trace element levels might not fully capture potential long-term effects of vaccination on trace element metabolism. Additionally, trace element status can be influenced by dietary intake, overall health status, and underlying conditions, which could indirectly impact vaccine responses over time.
In the study by Giurgea et al.., it was revealed that Mg in vaccinated mice with non-combined vaccines could induce strong antibody titers against N1 and N2 antigens of the influenza virus. After challenge with influenza A virus, viral titers decreased and antiviral and inflammatory responses improved21. Our experiments, on the other hand, divulged that Mg concentration in serum of vaccinated individuals did not modulate the levels of IgM and IgG as well as the serum capacity in neutralizing the SARS-CoV-2. The discrepancies observed in the outcomes might arise from several factors, including differences in the pathogens targeted, vaccine technologies employed, and the complexities of immune responses in various contexts. One key distinction lies in the pathogens under investigation. Influenza and SARS-CoV-2 differ significantly in their antigenic properties, genetic makeup, and immune interactions. The N1 and N2 antigens of the influenza virus targeted in the former study might induce responses that are uniquely modulated by Mg. On the other hand, the spike protein of SARS-CoV-2 elicits a distinct set of immune responses, which could potentially explain why Mg did not significantly impact IgM, IgG levels, or serum neutralization capacity in our study. Immune responses are complex and multifaceted, involving various cell types, signaling pathways, and molecules. The immune response to one pathogen or vaccine might not necessarily mirror that to another. While Mg might modulate specific immune pathways related to the N1 and N2 antigens of influenza in the Giurgea study, these pathways might not be as critical for the immune response to the spike protein of SARS-CoV-2. Factors such as the kinetics of immune cell activation, cytokine profiles, and interactions between immune components can vary widely. These factors could play a role in explaining why impact of Mg on immune responses differs between the studies.
Previously, a number of researchrs aimed to aaseess the effect of Zn levels in serum on the efficacy of different vaccines. Braga et al. aimed to determine the effect of oral Zn supplementation on antibody titers and seroconversion capacity following vaccination in colorectal cancer patients undergoing chemotherapy. The results indicated that Zn supplementation did not alter antibody titers post-vaccination. Nonetheless, the lower seroconversion rate in the chemotherapy-Zn group suggests an effect of Zn on the protective response induced by vaccination22. Moreover, serum Zn concentration had a positive effect on the level of antibody titers in children after vaccination against tetanus23. Our study revealed that levels of Zn in the vacinated subjects were not correlated with anti-spike IgM and IgG as well as the potential serum in neutrilizing SARS-CoV-2. The observation that serum Zn concentration had a positive effect on antibody titers in children after tetanus vaccination underscores the potential importance of Zn in enhancing the immune response following certain vaccines. However, the varying effects of Zn on different vaccine responses also point to the complexity of these interactions.
In a study by Janbakhsh et al.. in 2013, the impact of Se on immune response to hepatitis B vaccine (HBV) in insulin-dependent diabetic patients was evaluated. The results demonstrated that Se could increase the protection level and anti-HBsAg antibody titer through rapid vaccination. Ultimately, the group concluded that supplementing Se with standard HBV vaccination in insulin-dependent diabetic patients was recommended to enhance the antibody response24. In 2017, Ivory and colleagues reported that Se supplementation in healthy adults led to dual effects (both beneficial and adverse) on cellular immunity against influenza, and these effects might be influenced by the type of supplement, supplement dosage, and the immune background25. Our results indicated that Se level did not impress the levels of IgM and IgG as well as the serum potency to neutrilize the SARS-CoV-2 after Sinopharm COVID-19 vaccination. It appears that the effects of Se on immune responses can vary depending on the pathogen or vaccine. The implications are that the impact of Se might be pathogen-specific, and its potential benefits may not extend uniformly to all immune responses. In addition, the differing outcomes emphasize the importance of considering the context in which trace elements are studied. Positive influence of Se on the HBV vaccine response in diabetic patients illustrates the potential for tailored interventions to enhance immunity in vulnerable populations. On the other hand, the lack of significant impact on immune responses during Sinopharm COVID-19 vaccination suggests that the effects of Se might not be as relevant or pronounced in the context of this vaccine. This underscores the necessity of conducting targeted research to understand how trace elements function in specific vaccinations scenarios.
Compared to Sinopharm’s inactivated virus approach, mRNA and viral vector COVID-19 vaccines may be more sensitive to trace elements due to their reliance on intracellular processes and robust innate immune activation. mRNA vaccines, like Pfizer-BioNTech and Moderna, heavily depend on trace elements (e.g., magnesium, manganese) for mRNA stability and immune response26, while viral vector vaccines (e.g., AstraZeneca, Johnson & Johnson) rely on cellular mechanisms influenced by elements like zinc27. Protein-based vaccines might experience moderate trace element influence due to their use of pre-formed viral proteins, which need elements for proper folding28. In contrast, Sinopharm’s approach primarily stimulates immune responses through antigen presentation29, likely making it the least influenced by trace element variations.
One of the aims of the current research was to assess if any potential unwanted clinical preseantation du to vaccine might be associate with levels of Mg, Zn, and Se in serum as thse trace elements posssess immunomodulatory properties. The lack of adverse symptoms in vaccine recipients is generally positive news, as it suggests that the Sinopharm COVID-19 vaccine did not result in immediate or obvious negative reactions in the subjects we studied. This contributes to the overall body of evidence supporting the safety profile of the vaccine. The absence of adverse symptoms in relation to serum Mg, Zn, and Se levels might suggest that these specific minerals may not be directly linked to acute adverse reactions to the vaccine. This information could help guide future research into the potential mechanisms behind adverse effects related to COVID-19 vaccines.
It is important to acknowledge the limitations of our study. While we did not observe adverse symptoms in our cohort, the absence of such symptoms could be influenced by various factors, such as the size of sample, the specific population studied, and the study duration. It is possible that longer-term effects or effects in a larger population might still be worth investigating. Adverse reactions to vaccines can be complex and multifactorial. While mineral levels might play a role in certain adverse effects, they might not be the sole determinant. Other factors such as genetics, underlying health conditions, and individual immune responses could also contribute to adverse reactions. Our findings suggest that other factors might be more relevant in triggering adverse reactions to the Sinopharm COVID-19 vaccine. This opens the door for further research to explore potential associations with different biomarkers, genetic factors, or pre-existing health conditions that could contribute to adverse events.
Conclusion
In conclusion, the current research does not support that serum levels of Mg, Zn, and Se might modulate protective immunity arisen upon Sinopharm COVID-19 vaccine. The findings help establish that the effectiveness of the Sinopharm COVID-19 vaccine might not be significantly influenced by the baseline levels of Mg, Zn, and Se. This consistency in vaccine efficacy across different trace element levels could enhance the vaccine’s reliability in diverse populations with varying trace element statuses. As the research suggests that serum levels of these trace elements do not notably impact vaccine response, public health interventions aimed solely at adjusting these trace element levels might not be a primary focus for enhancing vaccine effectiveness. Other factors, such as age, pre-existing immunity, and vaccine dosing, could play more prominent roles. The results underscore the complexity of immune responses and how various factors interact to determine vaccine outcomes. Immune responses are influenced by an array of components beyond trace elements, including genetics, individual health status, and the vaccine’s intrinsic properties. While this research provides valuable insights, it does not definitively rule out potential roles for trace elements in different vaccine scenarios or with different vaccine technologies. Further research could explore the effects of trace elements on other vaccines or vaccine platforms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful of the patients and the healthy individuals for their participation in the study.
Author contributions
MA: Performed the experiments, participated in manuscript preparation, and read the manuscript critically. KK: Performed the statistical analysis, conducted graphical illustrations, participated in manuscript preparation, and read the manuscript critically. GTKS: Contributed in performing the experiments, participated in manuscript preparation and read the manuscript critically. MRH: Participated in manuscript preparation, and read the manuscript critically. ZBH: Developed the main idea, examined the patients, take the financial support, participated in manuscript preparation and read the manuscript critically.
Funding
This study was financially supported by the Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
Data availability
The datasets analyzed and generated during the study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved from the local Ethical Review committee located in Rafsanjan University of Medical Sciences (Permission No. IR.RUMS.REC.1400.165) and written informed consent form was taken by all subjects. All methods were carried out in accordance with relevant guidelines and regulations provided by Rafsanjan University of Medical Sciences. Research carried out here were in compliance with the Helsinki Declaration. The protocol of this study was approved by the Human Research Ethics Committee from the Rafsanjan University of Medical Sciences, Rafsanjna, Iran (Permission No. IR.RUMS.REC.1400.165). Written informed consent forms were obtained from all subjects before blood taking.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lazarus, J. V. et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med.29, 366–375 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Tzenios, N., Chahine, M. & Tazanios, M. Better strategies for coronavirus (COVID-19) vaccination. Special J. Med. Acad. Other Life Sci. 1 (2023).
- 3.Bagheri-Hosseinabadi, Z., Kaeidi, A., Rezvani, M., Sharifi, G. T. K. & Abbasifard, M. Evaluation of the serum levels of CCL2, CCL3, and IL-29 after first and second administrations of the COVID-19 vaccine (Oxford–AstraZeneca). Immunobiology229, 152789 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Wintergerst, E. S., Maggini, S. & Hornig, D. H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 51, 301–323 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Shahi, A., Aslani, S., Ataollahi, M. & Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology27, 649–661 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Dardenne, M. Zinc and immune function. Eur. J. Clin. Nutr.56, S20–S23 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Gammoh, N. Z. & Rink, L. Zinc and the immune system. Nutr. Immun., 127–158 (2019).
- 8.Tam, M., Gomez, S., Gonzalez-Gross, M. & Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr.57, 1193–1197 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Weyh, C., Krüger, K., Peeling, P. & Castell, L. The role of minerals in the optimal functioning of the immune system. Nutrients14, 644 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann, P. R. & Berry, M. J. The influence of selenium on immune responses. Mol. Nutr. Food Res.52, 1273–1280 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallapaty, S. China’s COVID vaccines have been crucial - now immunity is waning. Nature598, 398–399. 10.1038/d41586-021-02796-w (2021). [DOI] [PubMed] [Google Scholar]
- 12.Organization, W. H. The Sinopharm COVID-19 vaccine: What you need to know. WHO Strategic Advisory Group of Experts (Accessed 10 July 2021) (2021).
- 13.Vanderheiden, A. et al. Development of a rapid focus reduction neutralization test assay for measuring SARS-CoV‐2 neutralizing antibodies. Curr. Protocols Immunol.131, e116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzelnick, L. C. et al. Viridot: an automated virus plaque (immunofocus) counter for the measurement of serological neutralizing responses with application to dengue virus. PLoS Negl. Trop. Dis.12, e0006862. 10.1371/journal.pntd.0006862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasifard, M., Dehghan Banadaki, M., Khaje Sharifi, T., Rahnama, G., Bagheri-Hosseinabadi, Z. & A. & Serum dehydroepiandrosterone sulfate (DHEA-S) level and its potential impact on immune responses and symptom severity after Oxford-AstraZeneca COVID-19 vaccination. Int. Immunopharmacol.133, 112057. 10.1016/j.intimp.2024.112057 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Bagheri-Hosseinabadi, Z., Pirsadeghi, A., Rahnama, A., Bahrehmand, F. & Abbasifard, M. Is there any relationship between serum zinc levels and angiotensin-converting enzyme 2 gene expression in patients with coronavirus disease 2019? Meta Gene31, 100991. 10.1016/j.mgene.2021.100991 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anuk, A. T. et al. The relation between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol. Trace Elem. Res.199, 3608–3617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas, S. et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. Open4, e210369–e210369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatiwada, S. & Subedi, A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19). Curr. Nutr. Rep.10, 125–136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan, C. W. et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition79, 111017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giurgea, L. T. et al. The effect of calcium and magnesium on activity, immunogenicity, and efficacy of a recombinant N1/N2 neuraminidase vaccine. NPJ Vaccines6, 48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braga, C. B. M. et al. Effect of zinc supplementation on serological response to vaccination against streptococcus Pneumoniae in patients undergoing chemotherapy for colorectal cancer. Nutr. Cancer67, 926–932 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Das, R. et al. Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka. Vaccine39, 59–67 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janbakhsh, A. et al. Effect of selenium on immune response against hepatitis B vaccine with accelerated method in insulin-dependent diabetes mellitus patients. Caspian J. Intern. Med.4, 603 (2013). [PMC free article] [PubMed] [Google Scholar]
- 25.Ivory, K. et al. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr.36, 407–415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nešić, A. et al. A six-month study of anti-SARS-CoV-2 BNT162b2 mRNA vaccination: a comparative analysis of essential trace elements and anti-RBD IgG sera levels. J. Trace Elem. Med. Biol.74, 127079 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuyama, R. mRNA and Adenoviral Vector Vaccine platforms utilized in COVID-19 vaccines: technologies, Ecosystem, and future directions. Vaccines11, 1737 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian, G., Gao, C., Zhang, M., Chen, Y. & Xie, L. A review of protein-based COVID-19 vaccines: from monovalent to multivalent formulations. Vaccines12, 579 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meo, S. A., ElToukhy, R. A., Meo, A. S. & Klonoff, D. C. Comparison of biological, pharmacological characteristics, indications, contraindications, efficacy, and adverse effects of inactivated whole-virus COVID-19 vaccines Sinopharm, CoronaVac, and Covaxin: an observational study. Vaccines11, 826 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed and generated during the study are available from the corresponding author on reasonable request.