Abstract
Background
Breast cancer (BC) is the leading cause of cancer-related deaths in women worldwide. There has been a significant increase in the incidence of BC in Pakistan. Family history, older age, obesity, tobacco use, oral contraceptive use, early menarche, and hormonal replacement therapy are among the major risk factors. The most common histological subtype of BC is invasive ductal carcinoma (IDC). Molecular subtypes of BC include mainly Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER-2) enriched, and triple-negative BC subtypes, with the triple-negative subtype having the worst prognosis. CK5/6 serves as a basal keratin biomarker. This aimed to assess the expression of CK5/6 in IDC of the breast belonging to different molecular classes and to compare its expression with traditionally defined prognostic factors for different molecular subtypes.
Methodology
A cross-sectional, observational study was conducted at the Chughtai Institute of Pathology after approval from the Institutional Review Board (approval number: 1198/IRB/CIP). All cases during a period of six months (April 2023 to September 2023) were sampled using non-probability convenient sampling. All mastectomy samples diagnosed as IDC were included in the study. After standard tissue processing, paraffin tissue blocks and slides were prepared followed by hematoxylin & eosin staining. Hormonal receptors (estrogen receptor, progesterone receptor, HER-2) were assessed for cases to segregate them into molecular subtypes. CK5/6 antibody was then applied and the data were collected on a pre-designed proforma. SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used for data analysis.
Results
Of a total of 85 cases, 19 (22.3%) were positive for CK5/6. Of these 19 cases, the majority (68%, p = 0.001) belonged to the triple-negative class of tumors, comprising 13 cases. No case from the Luminal A class showed expression for CK5/6 stain (p = 0.028). Overall, four cases of the Luminal B subtype showed CK5/6 positivity (10.8%, p = 0.022) while two cases of the HER-2-enriched subtype were positive for the stain (33.3%, p > 0.05). These results were analyzed in relation to different prognostic factors. The majority of CK5/6-positive cases showed lymphovascular invasion (42%) and belonged to grade 3 tumors (57.8%).
Conclusions
The expression of CK5/6 in IDC of the breast is associated with poor prognostic factors such as triple-negative molecular subtypes, high histological grade, lymphovascular invasion, positive nodal status, and high pathological stage.
Keywords: ck5/6, her-2-enriched, luminal a, luminal b, triple-negative breast cancer, triple-negative breast carcinoma
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer-related deaths in women worldwide [1,2]. In 2023, the cancer statistics report showed 300,590 new cases of BC and 43,700 deaths related to BC in the United States [3]. The overall incidence of BC is comparatively lower in Asia than in Western countries; however, there has been a drastic increase in the incidence of BC in Pakistan with an estimate of one in nine women having a lifetime risk of developing BC [4]. Family history, older age, obesity, tobacco use, oral contraceptive use, early menarche, and hormonal replacement therapy are among the major risk factors for the development of BC [2]. It is a highly heterogeneous disease with great variability in the clinical treatment and prognosis among different patients [5]. The most common histological subtype of BC is invasive ductal carcinoma (IDC), also referred to as invasive breast carcinoma of no special type (IBC NST) [6,7].
The treatment strategies for IBC NST include a surgical approach, radiotherapy, chemotherapy, hormonal therapy, and immune therapy which largely depend on the expression of hormone receptors in the tumor cells, including estrogen (ER), progesterone (PR), and human epidermal growth factor (HER-2) receptors [8]. Based on hormone receptors, IBC NST is divided into four distinct molecular subtypes, including Luminal A, Luminal B, and HER-2-enriched, and triple-negative class (TNC) [9]. Luminal A tumors have the best prognosis while TNC has the worst prognosis [10].
CK5/6 is a basal keratin biomarker whose expression in BC has been linked with aggressive behavior and has traditionally been reported in IDC belonging to TNC. It has been linked to worse prognostic characteristics such as high histological grade, larger tumor size, and decreased disease-free survival [11-14]. This study aimed to assess the expression of CK5/6 in IDC belonging to different molecular classes and to establish a correlation with traditionally defined prognostic factors.
Materials and methods
A cross-sectional, prospective study was conducted at the Chughtai Institute of Pathology after obtaining approval from the institutional review board (approval number: 1198/IRB/CIP). This study was conducted over a six-month period, i.e., from April 2023 to September 2023. Sampling was done using non-probability convenient sampling, and all cases booked during the above-mentioned study period were included in the study. A total of 733 cases were booked in this period, and 85 cases were included which fulfilled the study criteria. All mastectomy specimens diagnosed as IDC were included in the study. All other types of BCs, incisional biopsies, and poorly preserved samples with autolyzed morphologies were excluded from the study.
After standard processing of tissue samples, paraffin tissue blocks were made and slides were prepared followed by hematoxylin & eosin staining. Hormonal receptors (ER, PR, HER-2) were assessed for all cases using the Autostainer Link 48 provided by DAKO. The data were then grouped into molecular classes based on hormone receptor expression as per Allred scoring. CK5/6 antibody (Monoclonal Mouse Anti-human Cytokeratin 5/6 Clone D5/16 B4 from DAKO) was applied to all cases using the same protocol. Membranous staining of more than 10% of tumor cells with CK5/6 antibody was considered positive. Variables such as age and gender, histological grade (G), pathological tumor stage (pT), regional lymph node status, lymphovascular invasion (LVI), and perineural invasion (PNI) were noted. All data were collected on a pre-designed proforma and the results were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
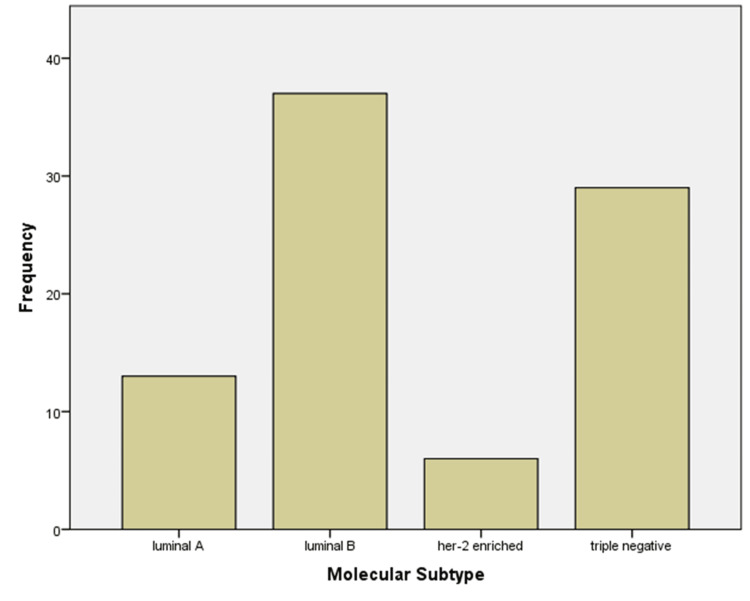
A total of 85 cases of IDC were included in the study. The most common molecular subtype was Luminal B (n = 37, 43.53%), followed by TNC (n = 29, 34.12%), Luminal A (n = 13, 15.29%), and HER-2-enriched (n = 6, 7.06%) (Figure 1).
Figure 1. Frequency of molecular subtypes of invasive ductal carcinoma in the study population.
Of a total of 85 cases, 19 (22.3%) were positive for CK5/6. Overall, 13 out of these 19 cases fell in the TNC category (68%, p = 0.001). No case from the Luminal A class showed expression for CK5/6 (0%, p = 0.028). Four cases of Luminal B class showed CK5/6 positivity (10.8%, p = 0.022) while two cases of HER-2-enriched class were positive for CK5/6 (33.3%, p > 0.05) (Table 1).
Table 1. Frequency of CK5/6 expression in different molecular subtypes.
HER-2: human epidermal growth factor receptor 2
| CK5/6 expression | Molecular subtype | Total | |||
| Luminal A | Luminal B | HER-2 enriched | Triple-negative | ||
| Negative | 13 | 33 | 4 | 16 | 66 |
| Positive | 0 | 4 | 2 | 13 | 19 |
| Total | 13 | 37 | 6 | 29 | 85 |
Among the CK5/6-positive cases, the most common histological grade was grade 3 (G3) (57.8%, p = 0.20) and the pathological stage was pT3 (57.8%, p = 0.049).
LVI was present in eight of the CK5/6-positive cases (42%, p = 0.035). While none of the cases showed PNI (0%, p > 0.05). Regional lymph node metastasis was noted in 12 CK5/6-positive cases (63%, p > 0.05) (Table 2).
Table 2. Different prognostic parameters in relation to CK5/6-positive and negative expression.
| Clinical parameters | CK5/6 expression | P-value | |
| Positive (n = 19) | Negative (n = 66) | ||
| Lymphovascular invasion | 8 (42%) | 12 (18%) | 0.035 |
| Perineural invasion | 0 (0%) | 11(16%) | >0.05 |
| Histological grade | |||
| I | 0 (0%) | 0 (0%) | |
| II | 8 (42%) | 36 (54.5%) | >0.05 |
| III | 11 (57.8%) | 30 (45%) | >0.05 |
| Pathological stage | |||
| pT1 | 0 (0%) | 4 (0.06%) | >0.05 |
| pT2 | 5 (25%) | 32 (42%) | >0.05 |
| pT3 | 11 (57.8%) | 22 (33%) | 0.049 |
| pT4 | 4 (15.7%) | 8 (12%) | >0.05 |
| Regional lymph node status | 12 (63%) | 28 (42%) | >0.05 |
Discussion
BC is one of the most common malignancies globally. The incidence of BC has increased by 123% between 1990 and 2017. The death toll of BC is high, with an estimated 1,503,694 deaths worldwide in 2025, suggesting that the global disease burden of BC will remain severe for some time to come [15].
BC comprises a heterogeneous group of tumors with variable clinical presentation, histological subtypes, biological behavior, prognosis, and treatment response [16]. The World Health Organization recognizes 18 different histological subtypes with IBC NST, previously known as IDC, being the most frequent type accounting for 40-80% of all BC cases. Other histological variants include invasive lobular carcinoma, mucinous carcinoma (types A and B), neuroendocrine, papillary, and salivary gland-type tumors [17-19].
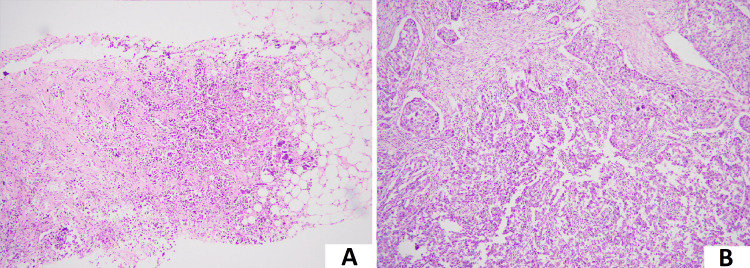
The IBC NST is historically classified into different grades (G) with varying prognostic and predictive values. Prognostically, G1 is the best, while G3 is the worst. However, this grading is based on histological features only (as shown in Figure 2) and cannot accurately predict tumor biology and behavior.
Figure 2. Histological features of invasive breast carcinoma.
(A) Low-power image of invasive breast carcinoma of no special type, histological grade 2, demonstrating nests of tumor cells showing tubule formation and moderate pleomorphism (100× magnification).
(B) Low-power image of invasive breast carcinoma of no special type, histological grade 3, demonstrating sheets of tumor cells showing marked nuclear pleomorphism (100× magnification).
Hence, these are further stratified based on mRNA gene expression in four main molecular subtypes, including Luminal A, Luminal B, HER-2-enriched, and TNC, which have better prognostic and predictive values [20,21]. TNC includes a spectrum of tumors with variable prognosis; however, the most common type within this class is grade 3 IBC NST which has poor response to conventional treatment regimens and overall prognosis [22-25]. Based on this variability, the triple-negative BC (TNBC) class has been extensively investigated for further histological or molecular signatures which may help in an improved understanding of tumor biology and help guide appropriate treatment options [26,27].
One of the most investigated areas in the TNBC class is the identification of tumors with basal-like phenotype which commonly expresses basal keratin CK5/6 [28-30]. Expression of CK5/6 has been widely investigated in other tumors, e.g., carcinomas of the urinary bladder and prostate, where it has conventionally been linked to poor prognosis [31-33]. For high-grade IBC NST, it is suggested in the literature to use CK5/6 as a surrogate marker to identify basal-like carcinomas and define poor prognosis [34,35].
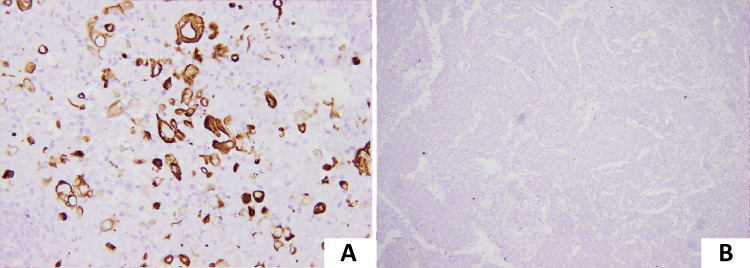
In this study, we evaluated the frequency of expression of CK5/6 in IBC NST cases within different molecular subtypes of BC. We also aimed to establish a correlation between adverse pathological features and the expression of CK5/6 (Figure 3).
Figure 3. Expression of CK5/6.
(A) Strong membranous expression of CK5/6 in tumor cells on immunohistochemistry.
(B) Lack of membranous expression of CK5/6 in tumor cells on immunohistochemistry.
In our study, we observed that CK5/6 expression was most frequent in TNBC, which is concordant with the findings of Kumar et al. and Khan et al. with CK5/6 expression in 85.7% and 40% of TNBC cases, respectively [36,37]. This finding is also concordant with Munirah et al. who reported CK5/6 expression in 100% of TNBC cases [38]. The Luminal B class had the second most common expression of CK 5/6 (10.8%) in our study which is concordant with Munirah et al. who reported it to be 7.1% while discordant with the findings of Kumar et al. who reported it to be 0% [36,38]. Luminal A showed no expression of CK5/6 in our study which is in concordance with the findings of Kumar et al. [36]. The HER-2-enriched class showed a 10% expression of CK5/6 which is concordant with the findings of Kumar et al. [36].
Expression of CK5/6 directly correlated with high histological grade (G3) which is concordant with the findings of Kumar et al. and Sowjanya et al. while discordant with the findings of Ara et al. [36,39,40].
Expression of CK5/6 showed a direct correlation with positive nodal status in our study. This finding is concordant with the findings of Sowjanya et al., Constantinou et al., and Tiwari et al., demonstrating 66%, 56.8%, and 91% of the cases, respectively, with CK5/6 expression and positive nodal status [40-42]. No correlation of CK5/6 expression was established with PNI in our study.
The majority of the cases with CK5/6 expression were high-stage tumors (pT3) in our study. The majority of cases with CK5/6 expression had a high pathological stage at the time of diagnosis. This is a significant finding that has not been adequately discussed in previous literature.
LVI was found in less than half of CK5/6-positive cases in our study which is concordant with the findings of Khan et al. [37]. However, Kumar et al. reported it to be in more than half of CK5/6-positive cases [36].
Conclusions
The expression of CK5/6 in IDC of the breast is associated with poor prognostic factors such as the triple-negative molecular subtype, high histological grade, LVI, positive nodal status, and high pathological stage at the time of diagnosis.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Review Board, Chughtai Institute of Pathology issued approval 1198/IRB/CIP.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Rafeya Yasin, Ghazi Zafar, Fatima Rooman Ali Syed, Zonaira Rathore, Akhtar Chughtai, Anila Chughtai
Acquisition, analysis, or interpretation of data: Rafeya Yasin, Ghazi Zafar, Sameen Afzal, Maryam Fatima
Drafting of the manuscript: Rafeya Yasin, Ghazi Zafar, Fatima Rooman Ali Syed, Zonaira Rathore, Akhtar Chughtai, Anila Chughtai
Critical review of the manuscript for important intellectual content: Rafeya Yasin, Sameen Afzal, Maryam Fatima, Akhtar Chughtai, Anila Chughtai
Supervision: Rafeya Yasin, Anila Chughtai
References
- 1.Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Lei S, Zheng R, Zhang S, et al. Cancer Commun (Lond) 2021;41:1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer statistics, 2023. Siegel RL, Miller KD, Wagle NS, Jemal A. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 4.Estimates of past and future time trends in age-specific breast cancer incidence among women in Karachi, Pakistan: 2004-2025. Zaheer S, Shah N, Maqbool SA, Soomro NM. BMC Public Health. 2019;19:1001. doi: 10.1186/s12889-019-7330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triple-negative breast cancer molecular subtyping and treatment progress. Yin L, Duan JJ, Bian XW, Yu SC. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Histological and molecular classification of breast cancer: what do we know? do Nascimento RG, Otoni KM. https://revistamastology.emnuvens.com.br/revista/article/view/945 Mastology. 2020;12:1–8. [Google Scholar]
- 7.Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature) Smolarz B, Nowak AZ, Romanowicz H. Cancers (Basel) 2022;14:2569. doi: 10.3390/cancers14102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molecular classification of breast cancer: a retrospective cohort study. Al-Thoubaity FK. Ann Med Surg (Lond) 2020;49:44–48. doi: 10.1016/j.amsu.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breast cancer molecular subtypes: from TNBC to QNBC. Hon JD, Singh B, Sahin A, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5043099/ Am J Cancer Res. 2016;6:1864–1872. [PMC free article] [PubMed] [Google Scholar]
- 10.Global increase in breast cancer incidence: risk factors and preventive measures. Kashyap D, Pal D, Sharma R, et al. Biomed Res Int. 2022;2022:9605439. doi: 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Molecular subtypes of breast cancer: a review for breast radiologists. Johnson KS, Conant EF, Soo MS. J Breast Imaging. 2021;3:12–24. doi: 10.1093/jbi/wbaa110. [DOI] [PubMed] [Google Scholar]
- 12.Cinical significance of androgen receptor, CK-5/6, KI-67 and molecular subtypes in breast cancer. Kayahan M, İdiz UO, Gucin Z, Erözgen F, Memmi N, Müslümanoğlu M. J Breast Health. 2014;10:201–208. doi: 10.5152/tjbh.2014.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cytokeratin 5/6 and cytokeratin 8/18 expression in triple negative breast cancers: clinicopathologic significance in South-Asian population. Hashmi AA, Naz S, Hashmi SK, et al. BMC Res Notes. 2018;11:372. doi: 10.1186/s13104-018-3477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Characteristics of basal cytokeratin expression in breast cancer. Alshareeda AT, Soria D, Garibaldi JM, Rakha E, Nolan C, Ellis IO, Green AR. Breast Cancer Res Treat. 2013;139:23–37. doi: 10.1007/s10549-013-2518-x. [DOI] [PubMed] [Google Scholar]
- 15.Global trends and forecasts of breast cancer incidence and deaths. Xu Y, Gong M, Wang Y, Yang Y, Liu S, Zeng Q. Sci Data. 2023;10:334. doi: 10.1038/s41597-023-02253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. Fitzmaurice C, Allen C, Barber RM, et al. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Cancers (Basel) 2021;13:4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genetics of breast cancer: a topic in evolution. Shiovitz S, Korde LA. Ann Oncol. 2015;26:1291–1299. doi: 10.1093/annonc/mdv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The 2019 World Health Organization classification of tumours of the breast. Tan PH, Ellis I, Allison K, et al. Histopathology. 2020;77:181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 20.Histology of luminal breast cancer. Erber R, Hartmann A. Breast Care (Basel) 2020;15:327–336. doi: 10.1159/000509025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diversity of breast carcinoma: histological subtypes and clinical relevance. Makki J. Clin Med Insights Pathol. 2015;8:23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. Prat A, Cheang MC, Martín M, et al. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. Ades F, Zardavas D, Bozovic-Spasojevic I, et al. J Clin Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
- 24.Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. Prat A, Carey LA, Adamo B, et al. J Natl Cancer Inst. 2014;106:0. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database. Plasilova ML, Hayse B, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Santonja A, Sánchez-Muñoz A, Lluch A, et al. Oncotarget. 2018;9:26406–26416. doi: 10.18632/oncotarget.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. Pareja F, Geyer FC, Marchiò C, Burke KA, Weigelt B, Reis-Filho JS. NPJ Breast Cancer. 2016;2:16036. doi: 10.1038/npjbcancer.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Badve S, Dabbs DJ, Schnitt SJ, et al. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 29.Molecular stratification within triple-negative breast cancer subtypes. Wang DY, Jiang Z, Ben-David Y, Woodgett JR, Zacksenhaus E. Sci Rep. 2019;9:19107. doi: 10.1038/s41598-019-55710-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molecular classification of breast cancer: an overview with emphasis on ethnic variations and future perspectives. Shawarby MA, Al-Tamimi DM, Ahmed A. Saudi J Med Med Sci. 2013;1:14–19. [Google Scholar]
- 31.CK20 and CK5/6 immunohistochemical staining of urothelial neoplasms: a perspective. Akhtar M, Rashid S, Gashir MB, Taha NM, Al Bozom I. Adv Urol. 2020;2020:4920236. doi: 10.1155/2020/4920236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cytokeratin 5/6 expression in bladder cancer: association with clinicopathologic parameters and prognosis. Hashmi AA, Hussain ZF, Irfan M, Edhi MM, Kanwal S, Faridi N, Khan A. BMC Res Notes. 2018;11:207. doi: 10.1186/s13104-018-3319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Expression of high molecular weight cytokeratin-a novel feature of aggressive and innate hormone-refractory prostatic adenocarcinoma. Lu JG, Lo ET, Williams C, Ma B, Sherrod AE, Xiao GQ. Prostate. 2023;83:462–469. doi: 10.1002/pros.24478. [DOI] [PubMed] [Google Scholar]
- 34.Expression of the immunohistochemical markers CK5, CD117, and EGFR in molecular subtypes of breast cancer correlated with prognosis. Schulmeyer CE, Fasching PA, Häberle L, et al. Diagnostics (Basel) 2023;13:372. doi: 10.3390/diagnostics13030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Nielsen TO, Hsu FD, Jensen K, et al. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 36.Prevalence of molecular subtypes of invasive breast cancer: a retrospective study. Kumar N, Patni P, Agarwal A, Khan MA, Parashar N. Med J Armed Forces India. 2015;71:254–258. doi: 10.1016/j.mjafi.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A comparative study of clinicopathological characteristics and expression of basal markers (CK5/6 & EGFR) in triple negative and non triple negative breast carcinomas in Kashmir Valley. Khan SP, Yasin SB, Khan FP. Ann Appl Bio-Sci. 2017;25:35–40. [Google Scholar]
- 38.Identification of different subtypes of breast cancer using tissue microarray. Munirah MA, Siti-Aishah MA, Reena MZ, et al. http://www.rjme.ro/RJME/resources/files/520211669677.pdf. Rom J Morphol Embryol. 2011;52:669–677. [PubMed] [Google Scholar]
- 39.Determining the expressions of cytokeratin 5/6 by immunohistochemistry in basal like triple negative breast carcinoma and its correlation with histomorphological grade. Ara NJ. SAS J Med. 2021;5:204–207. [Google Scholar]
- 40.Expression of cytokeratin 5/6 in benign and malignant breast lesions. Sowjanya R, Divyagna T, Goud SD, Jyothi C, Swapna B, Kumari KY. Int J Acad Med Pharm. 2023;5:891–896. [Google Scholar]
- 41.Expression and clinical significance of Claudin-7, PDL-1, PTEN, c-Kit, c-Met, c-Myc, ALK, CK5/6, CK17, p53, EGFR, Ki67, p63 in triple-negative breast cancer-a single centre prospective observational study. Constantinou C, Papadopoulos S, Karyda E, Alexopoulos A, Agnanti N, Batistatou A, Harisis H. https://iv.iiarjournals.org/content/32/2/303.short. In Vivo. 2018;32:303–311. doi: 10.21873/invivo.11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breast cancer: correlation of molecular classification with clinicohistopathology. Tiwari S, Malik R, Trichal VK, et al. Sch J App Med Sci. 2015;3:1018–1026. [Google Scholar]