Abstract
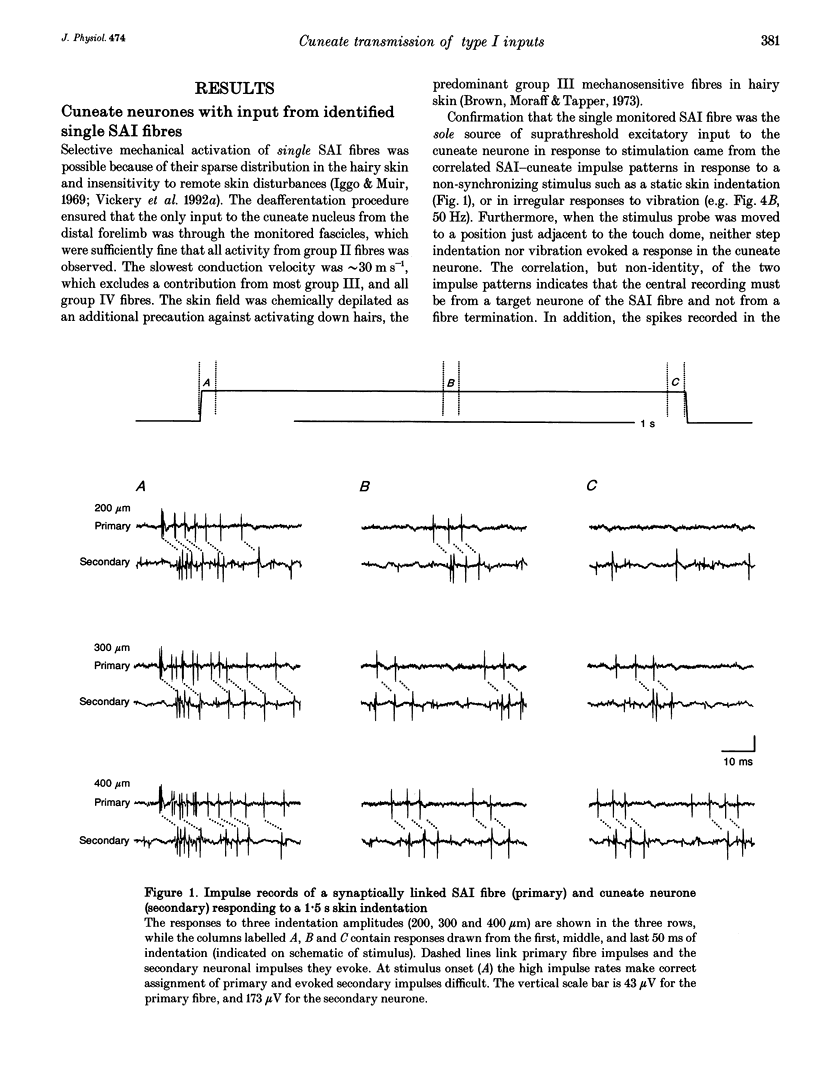
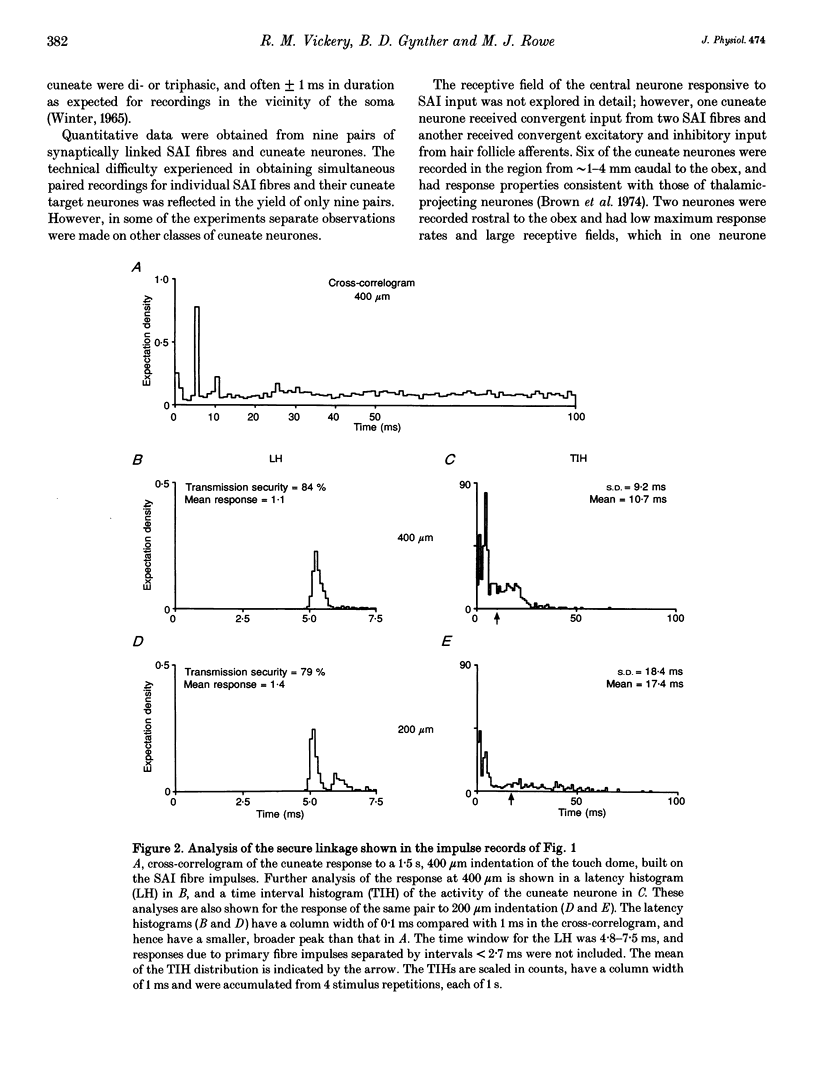
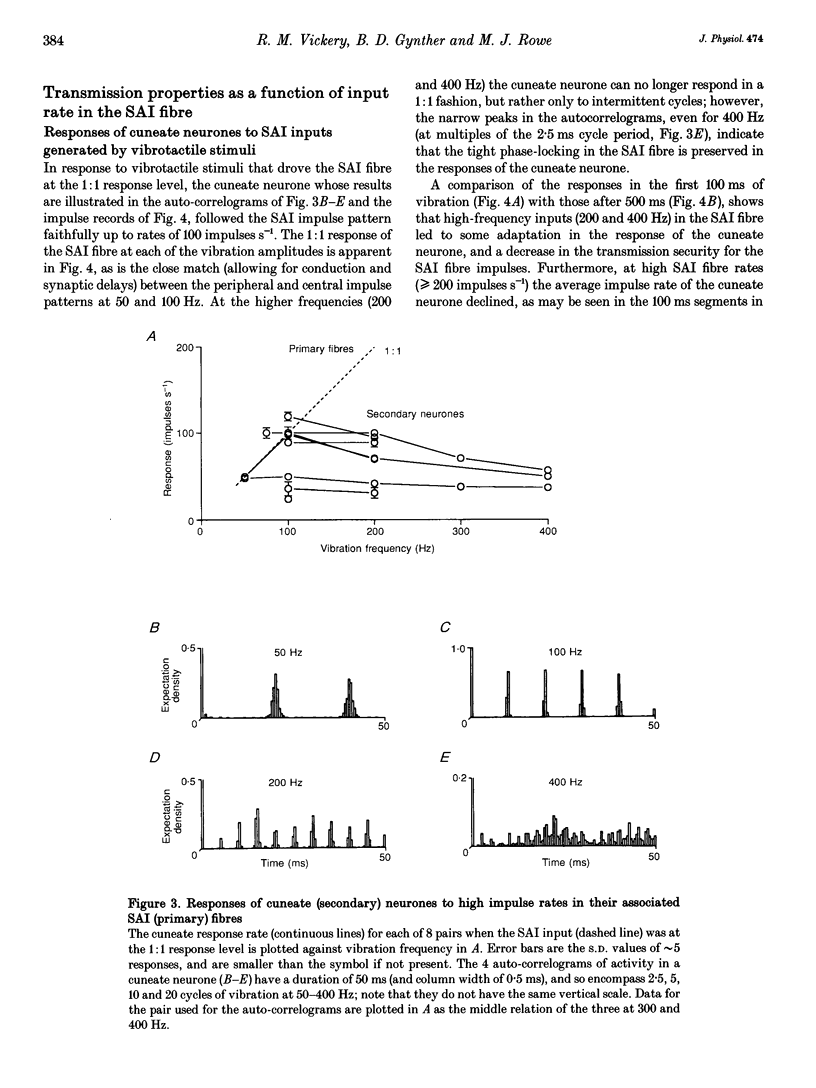
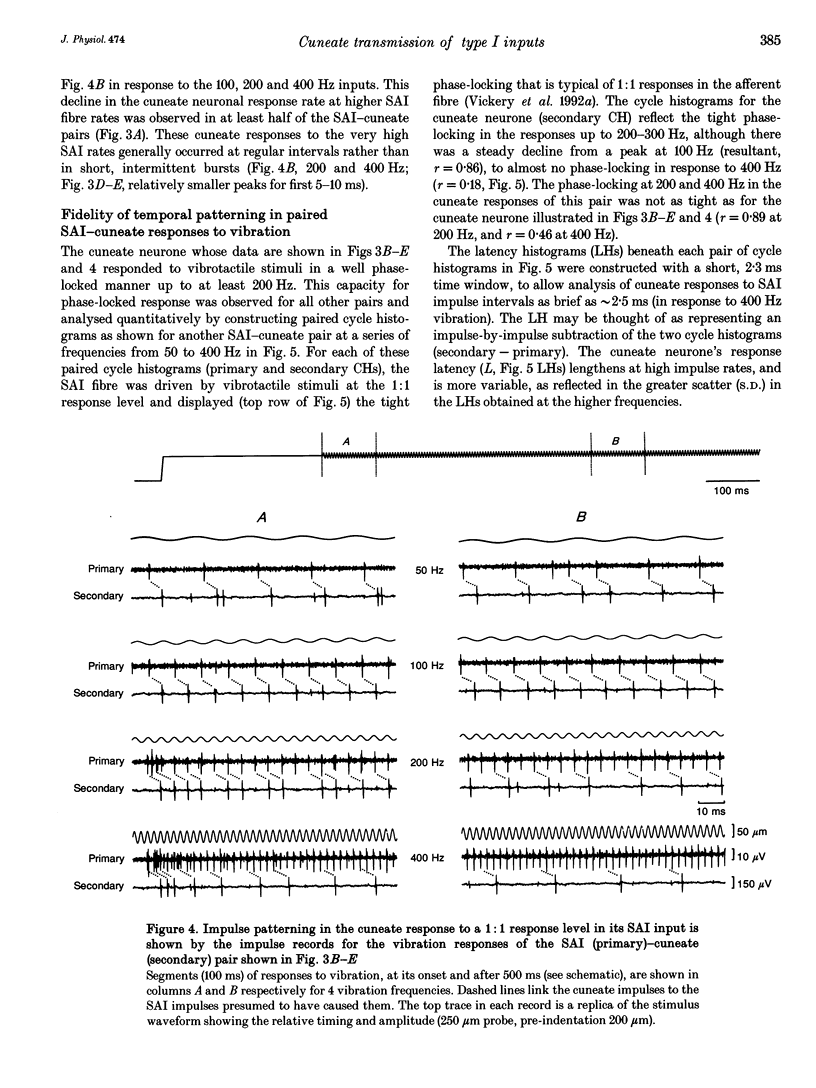
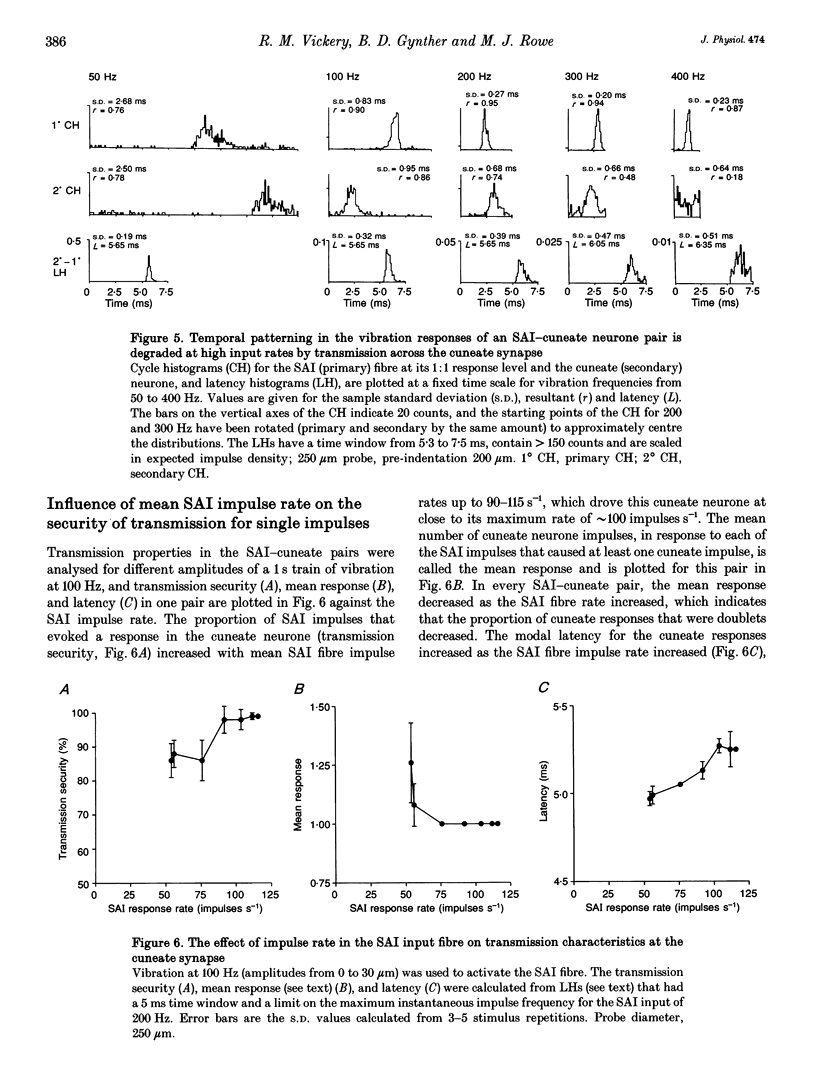
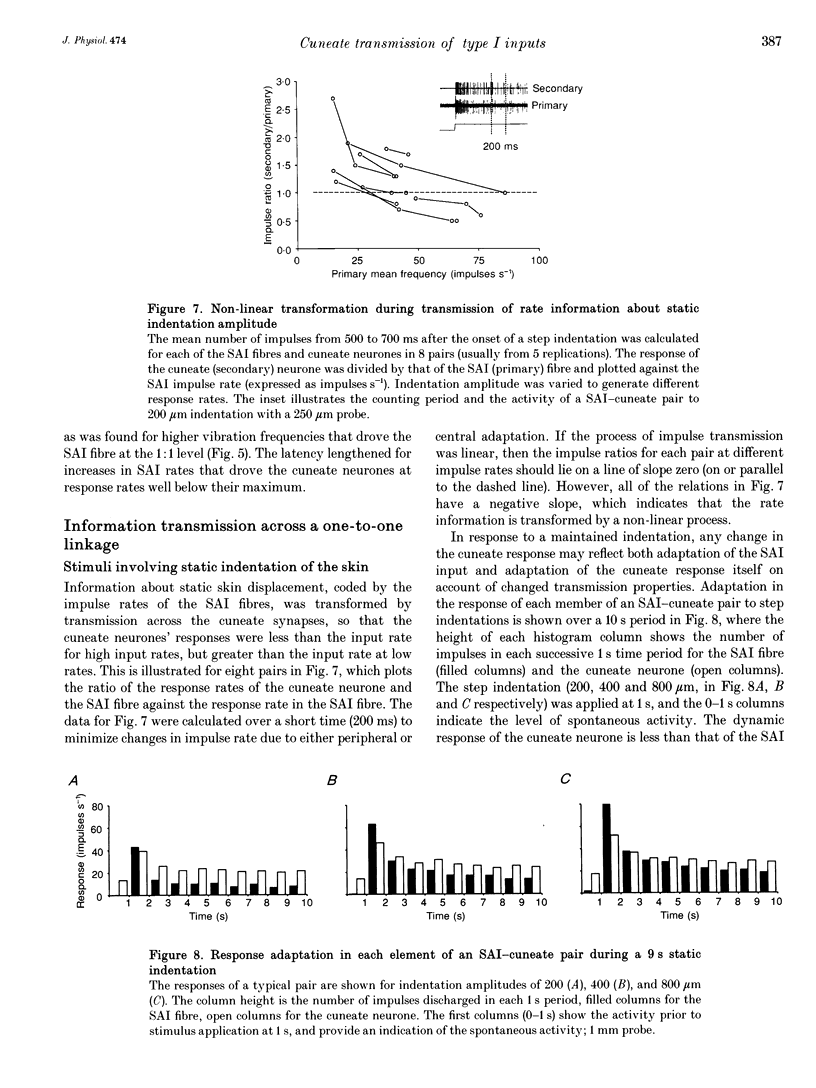
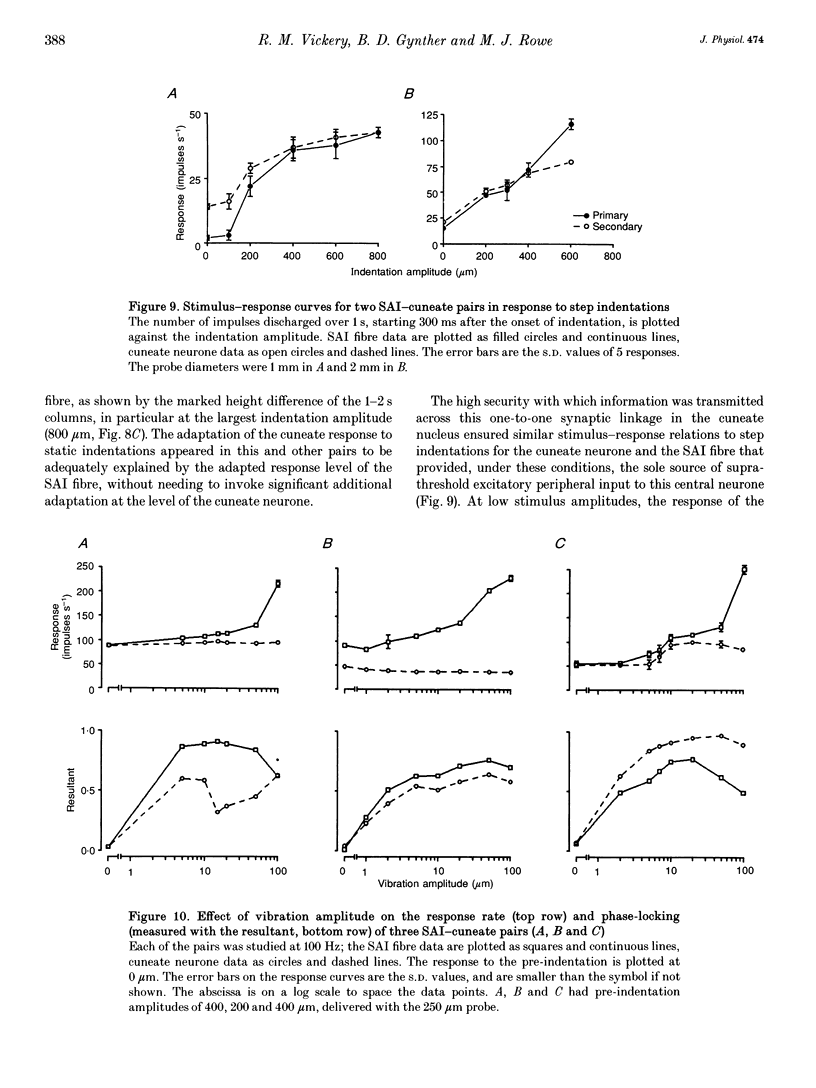
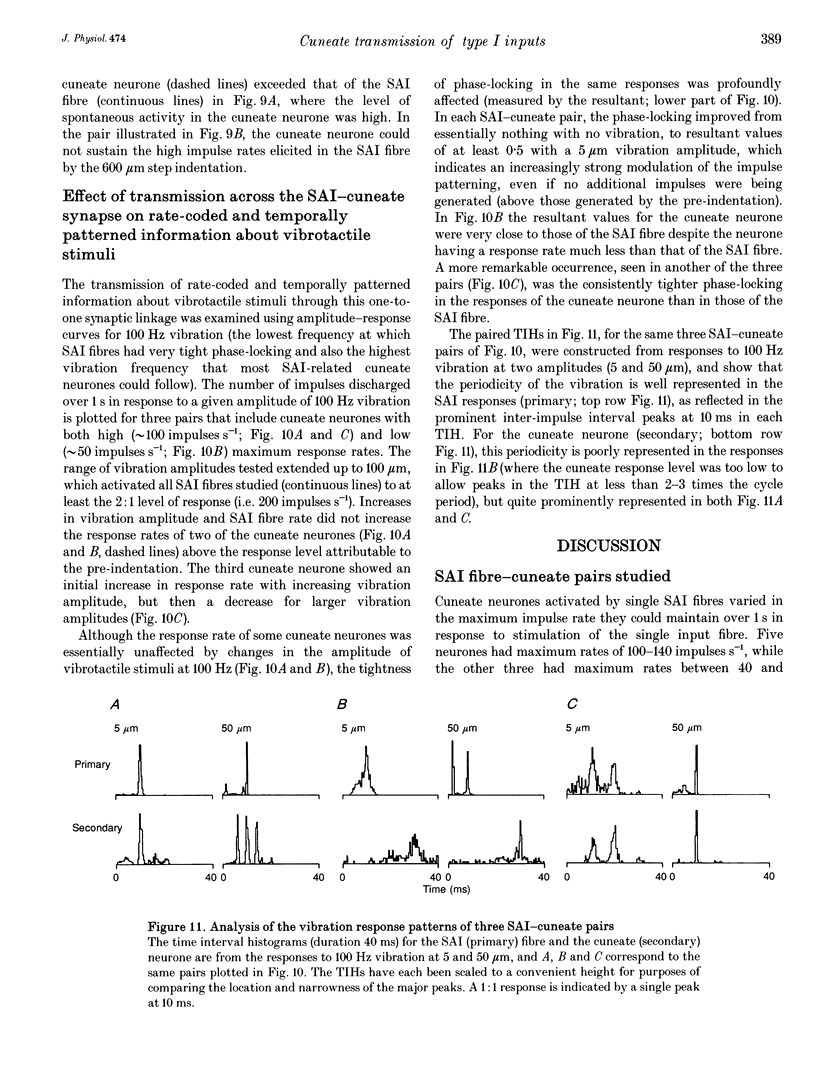
1. The synaptic linkage between single, identified slowly adapting type I (SAI) fibres and their central target neurones of the cuneate nucleus was examined in pentobarbitone-anaesthetized cats. Simultaneous extracellular recordings were made from individual cuneate neurones and from fine, intact fascicles of the lateral branch of the superficial radial nerve in which it was possible to identify and monitor the activity of each group II fibre. Individual SAI fibres were activated by static displacement and by vibration delivered with a fine probe (0.25-2 mm diameter) to their associated touch domes in the hairy skin of the forelimb. 2. Transmission properties across the synapse were analysed for nine SAI-cuneate pairs in which the single SAI fibre of each pair provided a suprathreshold input to the cuneate neurone. Neither spatial nor temporal summation was required for effective impulse transmission, and often more than 80% of SAI impulses led to a response in the cuneate neurone. Responses of the cuneate neurones to single SAI impulses occurred at a short, fixed latency (S.D. often < 0.1 ms), and frequently consisted of a burst of two or three impulses, at low SAI input rates in particular. 3. The tight phase-locking in the responses to vibration of single SAI fibres was preserved in the cuneate responses for frequencies up to approximately 400 Hz. However, as the impulse rates of the cuneate neurones were less than 150 impulses s-1, their impulse patterns could not directly signal the vibration periodicity at frequencies > 100-150 Hz despite 1:1 responses in their single SAI input fibres up to approximately 500 Hz. 4. The reliable transmission of touch dome-associated SAI input across the cuneate nucleus indicates that transmission failure at this first relay is unlikely to be responsible for the reported failure of touch dome-SAI inputs to contribute to tactile perception. 5. The transmission characteristics for the SAI fibres were very similar to those demonstrated previously for fibres associated with Pacinian corpuscles, which argues against any marked differential specialization in transmission characteristics for dorsal column nuclei neurones that receive input from different tactile fibre classes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Gordon G., Kay R. H. A study of single axons in the cat's medial lemniscus. J Physiol. 1974 Jan;236(1):225–246. doi: 10.1113/jphysiol.1974.sp010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Actions of trains and pairs of impulses from single primary afferent fibres on single spinocervical tract cells in cat. J Physiol. 1987 Jan;382:313–329. doi: 10.1113/jphysiol.1987.sp016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987 Jan;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. B., Moraff H., Tapper D. N. Functional organization of the cat's dorsal horn: spontaneous activity and central cell response to single impulses in single type I fibers. J Neurophysiol. 1973 Sep;36(5):827–839. doi: 10.1152/jn.1973.36.5.827. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987 May;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. High gain transmission of single impulses through dorsal column nuclei of the cat. Neurosci Lett. 1986 Apr 24;65(3):277–282. doi: 10.1016/0304-3940(86)90274-0. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Integrative processing of vibratory information in cat dorsal column nuclei neurones driven by identified sensory fibres. J Physiol. 1987 May;386:311–331. doi: 10.1113/jphysiol.1987.sp016536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovchinsky V. Patterns of responses of neurons in cuneate nucleus to controlled mechanical stimulation of cutaneous velocity receptors in the cat. J Neurophysiol. 1980 Jun;43(6):1673–1699. doi: 10.1152/jn.1980.43.6.1673. [DOI] [PubMed] [Google Scholar]
- Greenstein J., Kavanagh P., Rowe M. J. Phase coherence in vibration-induced responses of tactile fibres associated with Pacinian corpuscle receptors in the cat. J Physiol. 1987 May;386:263–275. doi: 10.1113/jphysiol.1987.sp016533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T., Merzenich M. M. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10(3):251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ramsey R. L. Cortical progection of the Type I slowly adapting cutaneous afferent units. J Physiol. 1971;217 (Suppl):46P–47P. [PubMed] [Google Scholar]
- Johnson K. O. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974 Jan;37(1):48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Mountcastle V. B. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975 May;38(3):539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Lindblom U., Tapper D. N. Terminal properties of a vibro-tactile sensor. Exp Neurol. 1967 Jan;17(1):1–15. doi: 10.1016/0014-4886(67)90117-3. [DOI] [PubMed] [Google Scholar]
- Macefield G., Gandevia S. C., Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990 Oct;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. D., Kasprzak H., Hiltz F. L., Tapper D. N. Activity in single cutaneous afferents: spinal pathways and cortical evoked potentials. Brain Res. 1972 Apr 14;39(1):61–70. doi: 10.1016/0006-8993(72)90785-8. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Torebjörk E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983 Sep;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGGIO G. F., VIERNSTEIN L. J. TIME SERIES ANALYSIS OF IMPULSE SEQUENCES OF THALAMIC SOMATIC SENSORY NEURONS. J Neurophysiol. 1964 Jul;27:517–545. doi: 10.1152/jn.1964.27.4.517. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr, Haring J. H., Rowinski M. J. Patterns of resting discharge in neurons of the raccoon main cuneate nucleus. J Neurophysiol. 1989 Jun;61(6):1131–1141. doi: 10.1152/jn.1989.61.6.1131. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Brown P. B., Moraff H. Functional organization of the cat's dorsal horn: connectivity of myelinated fiber systems of hairy skin. J Neurophysiol. 1973 Sep;36(5):817–826. doi: 10.1152/jn.1973.36.5.817. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Wiesenfeld Z. A dorsal spinal neural network in cat. I. Responses to single impulses in single type I cutaneous input fibers. J Neurophysiol. 1980 Dec;44(6):1190–1213. doi: 10.1152/jn.1980.44.6.1190. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Olsson K. A., Westberg K. G., Clark F. J. Microstimulation of single tactile afferents from the human hand. Sensory attributes related to unit type and properties of receptive fields. Brain. 1984 Sep;107(Pt 3):727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Vickery R. M., Gynther B. D., Rowe M. J. Vibrotactile sensitivity of slowly adapting type I sensory fibres associated with touch domes in cat hairy skin. J Physiol. 1992;453:609–626. doi: 10.1113/jphysiol.1992.sp019247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery R. M., Morley J. W., Rowe M. J. The role of single touch domes in tactile perception. Exp Brain Res. 1993;93(2):332–334. doi: 10.1007/BF00228402. [DOI] [PubMed] [Google Scholar]
- Vierck C. J., Jr Comparisons of punctate, edge and surface stimulation of peripheral, slowly-adapting, cutaneous, afferent units of cats. Brain Res. 1979 Oct 12;175(1):155–159. doi: 10.1016/0006-8993(79)90524-9. [DOI] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]


