Abstract
Rabies remains a global health threat despite being preventable with post-exposure prophylaxis (PEP). This study assessed one-year humoral and T cell immunity in PEP recipients of the Insitut Pasteur du Cambodge (IPC) regimen, recommended by WHO. We analyzed rabies virus (RABV) neutralizing antibodies (nAbs) and T cell responses at baseline, 7 and 14 days, 6 and 12 months after PEP. A total of 148 patients were included, with 78 bitten by confirmed RABV-positive dogs receiving PEP and equine rabies immunoglobulins (eRIG), and 70 bitten by RABV-negative dogs receiving only PEP. Fourteen days after PEP, all but two individuals seroconverted for nAbs ( ≥ 0.5 IU/mL) with 87% maintaining this response even after 12 months. Interleukin-4 (IL-4) and interferon-gamma (IFN-γ)-secreting T cells were significantly elevated after 14 days and sustained for one year. No differences were observed between the RABV-exposed and -unexposed groups. This study demonstrates robust one-year immunity after IPC PEP.
Subject terms: Inactivated vaccines, Immunological memory, Antibodies
Introduction
Rabies causes approximately 59,000 deaths annually, which primarily affects poor rural communities in Asia and Africa, with 40% of the cases occurring in children under the age of 151–3. In Cambodia, rabies is endemic, with a high incidence in humans as estimated 425,000 dog bite injuries occur per year leading to around 800 annual deaths4,5. Rabies infection is almost always fatal once symptoms appear, with no effective treatment available6. However, timely and adequate post-exposure prophylaxis (PEP) can prevent rabies if given before symptoms develop. A post-bite vaccination is administered to more than 29 million individuals yearly, preventing tens of thousands of rabies-related fatalities2. The additional administration of rabies immunoglobulin (RIG) can be required, depending on the severity of the exposure and the rabies status of the involved animal7. WHO recommends receiving both the rabies vaccine and RIG for previously unvaccinated individuals with rabies category III exposure7, severely immunocompromised individuals with category II and III exposure, or those bitten by confirmed RABV-positive animals1,7.
Currently WHO recommends three PEP vaccination protocols, including the so-called Essen and Zagreb regimens, which involve intramuscular (IM) injection over two to four weeks (usually utilizing 3–5 mL of vaccine), and the Institut Pasteur du Cambodge (IPC) regimen, which is a shortened version of the modified Thai Red Cross (TRC) protocol of four two-site intradermal (ID) injections of 0.1 mL vaccine carried out over 28 days1,7,8. The IPC regimen involves three sessions of two-site ID injections of 0.1 mL/site within one week, thereby utilizing only 0.6 ml of vaccine1,7,8. To date, the IPC regimen is the shortest and most vaccine-sparing rabies PEP protocol.
The induction of anti-rabies antibodies is considered the main mechanism of protection after PEP. Indeed, RABV neutralizing antibodies (nAbs) are considered a correlate of protection, and vaccination efficacy is determined by the development of nAbs (seroconversion)9. The WHO has designated that a titer of at least 0.5 international units per milliliter (IU/mL) of RABV nAbs measured 14 days after starting the vaccination course is considered to be protective10. However, the Advisory Committee on Immunization Practices (ACIP) recommends a minimum acceptable RABV nAb titer of 0.1–0.3 IU/mL11. It has been suggested that nAbs play an important role in RABV elimination. An experimental study of intranasal attenuated RABV infection in gene-knockout mice showed those that lacked the capacity to produce antibodies could not clear the virus from the central nervous system (CNS) and died within 21–24 days after virus infection12. Furthermore, nAbs are lacking in animals that die from non-lethal infection with wild-type RABV, highlighting the importance of antibody-mediated immunity in the post-infection elimination of RABV13.
Studies have demonstrated a long-term nAb response up to 14 years after pre-exposure prophylaxis (PrEP) and PEP using various vaccination protocols14–17. However, how long the positive nAb titers will last after PEP using the WHO 2018 recommended IPC regimen is unclear.
Besides nAbs, T cells might have a protective role in controlling RABV replication in CNS through the production of interferon-gamma (IFN-γ) by infiltrating T cells, at least in mice models18–20. Additionally, infiltrating CD4+ T cells are needed to enhance the blood-brain barrier (BBB) permeability in response to neurotropic infection21–23. In experimental studies on mice models, the enhancement of the BBB permeability led to greater clearance of RABV from the CNS24–26. The protective role of CD8+ T cells in RABV infection is even less understood. After rabies PrEP, mainly CD4+ T cells are activated27. It has been suggested that immunodeficient patients with low CD4+ T cell count have a poor or no nAb response to rabies vaccination (both PrEP and PEP)28–30. Consequently, CD4+ T cells may play a crucial role in the development of RABV nAbs. Unlike RABV nAbs, a threshold for T cell-specific immune responses linked to protection against rabies and to vaccination efficacy remains to be established. One study reported T cell proliferation at different timepoints during PEP using the Zagreb regimen. They demonstrated that 5/21 people exhibited a T cell proliferative response to European bat lyssavirus (a close relative of RABV within the same genus) stimulation as early as 7 days following the first PEP session, and all participants had a T cell response 5 days after the completion of the PEP regimen31. Post rabies immunization, the presence of pluripotent RABV-specific T lymphocytes secreting various cytokines has been demonstrated by us and others32–34. Information on the long-term T cell memory response to rabies vaccination is scarce. One study employing the modified TRC PEP regimen showed that six months post-vaccination, the levels of rabies antigen-specific T cells are comparable to baseline34. Additionally, CD8+ T cells do not seem to be particularly important in the cellular immune response to rabies PrEP or PEP since their frequency decreases after vaccination15.
Therefore, to enhance the understanding of long-term immunity following rabies PEP, we aimed to investigate the sustained humoral and T cell immune responses elicited by the IPC PEP regimen one year after vaccination. We analyzed the development of RABV nAbs and T cell responses in individuals bitten by rabid or non-rabid dogs, measured at baseline and at intervals of 7 days, 14 days, 6 months, and 12 months after immunization.
Results
Study Cohort
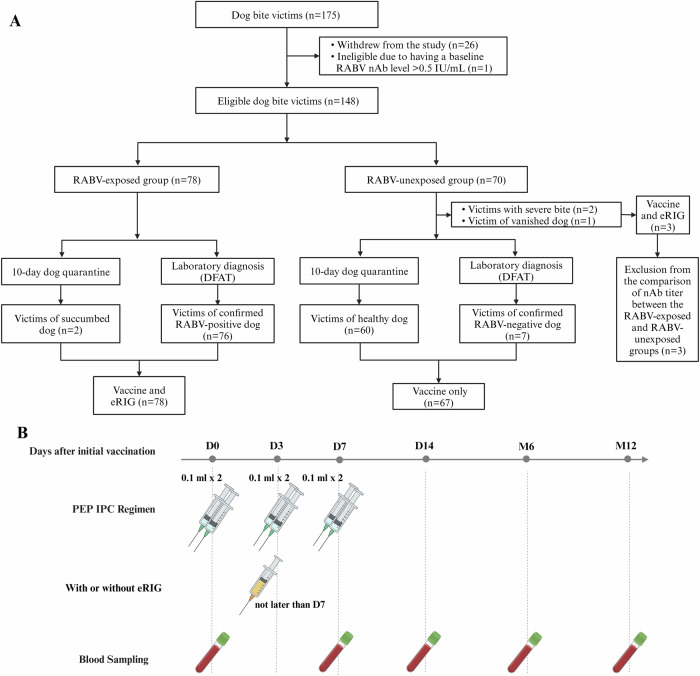
In total, 175 subjects were initially included (Table 1). All of them completed the full vaccination schedule and 96% of them were recruited within less than 4 days of potential exposure. However, 26 participants withdrew from the study or were excluded from analysis, unrelated to the vaccination, due to the absence of blood sampling at one or more of the study’s timepoints. Reasons include lack of available transportation during the COVID-19 lockdown, discomfort from blood withdrawal, or other personal reasons (Table 1). One person was positive for RABV nAbs ( > 0.5 IU/mL) at inclusion (D0) and was therefore subsequently excluded from data analysis. RABV infection of the dogs was either confirmed by Direct Fluorescent Antibody Test (DFAT)35 on brain necropsy samples of culled dogs, or based on the outcome of a 10-day quarantine of the dogs, where dogs that succumbed during this period were assumed to be RABV-positive, and dogs that survived were considered RABV-negative (Fig. 1A). Overall, sequential biological samples (D0, D7, D14, M6, and M12) from 148 individuals were included for analysis (Table 2). Among the 148 subjects, 78 were categorized as RABV-exposed either by laboratory-confirmed positive rabies diagnostic of the biting dog (97.4%) or due to appearance of rabies symptoms in the dog during its 10-day quarantine (2.6%). The other 70 study participants were categorized as RABV-unexposed due to the negative laboratory diagnosis (10.0%) of the dog, its survival of the 10-day quarantine (85.7%), disappearance of the biting dog (1.4%), or the patient suffering severe bite (2.9%). The mean age of study subjects was 25 years old (range 2–69 years old) and the male:female ratio was 1:1. Additionally to PEP, eRIG was given to all participants in the RABV-exposed group due to the RABV status of the biting dog. Three individuals (4.3%) in the RABV-unexposed group also received eRIG in addition to the PEP because they had serious bite wounds, or the biting dog had vanished.
Table 1.
Subject inclusions
| Timepoints | Action | RABV-Exposed Group n (%) | RABV-Unexposed Group n (%) | Total n (%) |
|---|---|---|---|---|
| D0 | Blood sampling and vaccination | 86 (100.0) | 89 (100.0) | 175 (100.0) |
| eRIG administration | 86 (100.0) | 3 (3.4) (*) | 89 (50.9) | |
| D3 | Vaccination | 86 (100.0) | 89 (100.0) | 175 (100.0) |
| D7 | Blood sampling | 84 (97.7) | 88 (98.9) | 172 (98.3) |
| Vaccination | 86 (100.0) | 89 (100.0) | 175 (100.0) | |
| D14 | Blood sampling | 84 (97.7) | 85 (95.5) | 169 (96.6) |
| M6 | Blood sampling | 79 (91.9) | 78 (87.6) | 157 (89.7) |
| M12 | Blood sampling (**) | 78 (90.7) | 71 (79.8) | 149 (85.1) |
| Individuals included in analysis (#) | 78 (90.7) | 70 (78.6) | 148 (84.6) |
(*) These three individuals in rabies virus (RABV)-unexposed group who received eRIG were not included in the analysis comparing the neutralizing antibody (nAb) and T cell responses between RABV-exposed versus RABV-unexposed group. (**) Individuals present throughout the entire study timepoints. In total, 26 participants withdrew from the study for various causes not related to vaccination, including personal matters, discomfort during blood collection, and COVID-19 lockdown. None was excluded due to PEP side effects. (#) One subject in RABV-unexposed group had a RABV nAb > 0.5 IU/mL at D0, before receiving PEP, so we excluded this patient from all data analysis. eRIG equine rabies immunoglobulin.
Fig. 1. Schematic presentation of study design.
Overall, 175 individuals with no history of previous rabies vaccination were initially included. All individuals received post-exposure prophylaxis (PEP) using the Institut Pasteur du Cambodge (IPC) regimen. A Participants were divided into two groups, RABV-exposed group and RABV-unexposed group. Groups were based on the result of RABV diagnostic or observation of the dog involved in bite incident. Laboratory diagnosis was performed using Direct Fluorescent Antibody Test (DFAT) on brain necropsy samples, and observation for clinical rabies signs was done for 10 days quarantine. Equine rabies immunoglobulin (eRIG) was provided in addition to vaccine to RABV-exposed group and RABV-unexposed group with severe bite, dead or disappeared dog. B PEP was administered at day 0 (D0), day 3 (D3) and day 7 (D7) with or without additional eRIG on D0. Blood samples were collected at baseline (D0; before vaccine administration), at day 7 (D7; last day of vaccination session), day 14 (D14; one week after PEP completion), month 6 (M6), and month 12 (M12) after the initial PEP vaccination. This figure was created using BioRender.com.
Table 2.
Cohort characteristics
| Groups | RABV-Exposed Group (n = 78) | RABV-Unexposed Group (n = 70) | Total (n = 148) |
|---|---|---|---|
| Laboratory confirmed n (%) | 76 (97.4) | 7 (10.0) | 83 (56.1) |
| Dog and severity of bite observation (*) n (%) | |||
| Healthy dog after 10-day quarantine | 0 (0.0) | 60 (85.7) | 60 (40.4) |
| Succumbed dog after 10-day quarantine | 2 (2.6) | 0 (0.0) | 2 (1.4) |
| Severe bite | 0 (0.0) | 2 (2.9) | 2 (1.4) |
| Disappearance of the dog | 0 (0.0) | 1 (1.4) | 1 (0.7) |
| Age range, year (mean) | 2–69 (26) | 5–68 (25) | 2–69 (25) |
| Sex n (%) | |||
| Male | 40 (51.3) | 35 (50.0) | 75 (50.7) |
| Female | 38 (48.7) | 35 (50.0) | 73 (49.3) |
| Province of bite incident n (%) | |||
| Phnom Penh | 11 (14.1) | 48 (68.6) | 59 (39.9) |
| Prey Veng | 25 (32.1) | 3 (4.3) | 28 (19.0) |
| Kandal | 5 (6.4) | 12 (17.1) | 17 (11.5) |
| Takeo | 11 (14.1) | 1 (1.4) | 12 (8.1) |
| Kampong Cham | 6 (7.7) | 3 (4.3) | 9 (6.1) |
| Kampong Speu | 7 (9.0) | 1 (1.4) | 8 (5.4) |
| Kampot | 6 (7.7) | 1 (1.4) | 7 (4.7) |
| Kampong Thom | 4 (5.1) | 0 (0.0) | 4 (2.7) |
| Svay Rieng | 2 (2.6) | 1 (1.4) | 3 (2.0) |
| Kampong Chnnang | 1 (1.3) | 0 (0.0) | 1 (0.7) |
| eRIG treatment n (%) | |||
| With eRIG (#) | 78 (100.0) | 3 (4.3) | 81 (54.7) |
| Without eRIG | 0 (0.0) | 67 (95.7) | 67 (45.3) |
The analysis included only paired samples. (*) Classification due to observation: rabies virus (RABV)-positive dogs were identified due to the appearance of typical rabies symptoms during the 10-day quarantine of the dog; RABV-negative dogs were identified due to survival of the quarantine. (#) Three individuals in RABV-unexposed group received eRIG prior to the RABV-negative diagnosis due to severe bite incidents or the biting dog vanished. These three individuals were not included in the analysis for the neutralizing antibody (nAb) titer comparison between RABV-exposed versus RABV-unexposed group. eRIG, equine rabies immunoglobulin.
Neutralizing Antibodies Persist Up to One Year after PEP
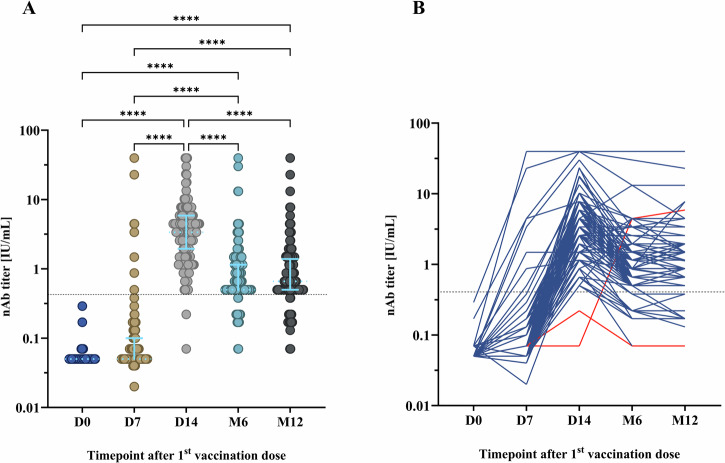
The RABV nAbs response at D0, D7, D14, M6 and M12 after PEP using the IPC regimen is shown in Fig. 2. As expected, all individuals had negative nAb titers ( ≤ 0.5 IU/mL) before receiving PEP. The median RABV nAb titer was 0.05 IU/mL (IQR 0.05–0.1) at D7, 3.38 IU/mL (IQR 1.95–5.87) at D14, 0.5 IU/mL (IQR 0.5–1.15) at M6 and 0.66 IU/mL (IQR 0.5–1.2) at M12. One week after completion of the PEP (D14), all except two individuals seroconverted for RABV nAbs measured by FAVNT (Fig. 2A). The response of RABV nAbs was significantly higher at D14 compared to D7 (p < 0.0001) and D0 (p < 0.0001). In comparison to D14, the RABV nAbs response at M6 (p < 0.0001) and M12 (p < 0.0001) significantly declined; yet these responses remained at a significantly higher level compared to D7 (p < 0.0001) and D0 (p < 0.0001). Among the recipients who seroconverted for RABV nAbs at D14, the majority (87.7%) remained seropositive 6 months after PEP, and 87.0% still had protective levels of RABV nAbs 12 months after PEP (Fig. 2B). Among the two individuals whose RABV nAb titer at D14 was below the threshold that is considered protective (0.5 IU/mL), one individual had a protective nAb titer at M6 and remained positive up to1 year after PEP, while the other individual did not develop a protective RABV nAb level throughout the whole study (Fig. 2B).
Fig. 2. The development of rabies virus (RABV) neutralizing antibody (nAb) titers at different timepoints after post-exposure prophylaxis (PEP) using the Institut Pasteur du Cambodge (IPC) regimen.
Overall, 148 participants were included for nAb analysis. The fluorescent antibody virus neutralization test (FAVNT) was used to measured RABV nAb titers at baseline (D0), day 7 (D7), day 14 (D14), month 6 (M6), and month 12 (M12) after PEP. A Comparison of RABV nAb titer at various timepoints. Each dot represents a single individual. Light blue lines represent the median and interquartile range. Dashed line indicates the threshold of protective RABV nAb titer at 0.5 international unit per milliliter (IU/mL). Statistics: Friedman test, followed by Dunn’s multiple comparisons test. Asterisks represent significance levels as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. B Individual evolution of RABV nAb titer. Red lines represent the RABV nAb titer evolution of individuals who did not develop protective threshold at D14. For visualization purposes, data was transformed into logarithmic scales before being used in statistical analysis.
RABV-Specific Cytokine-Secreting T Cells Are Present Up to One Year after PEP
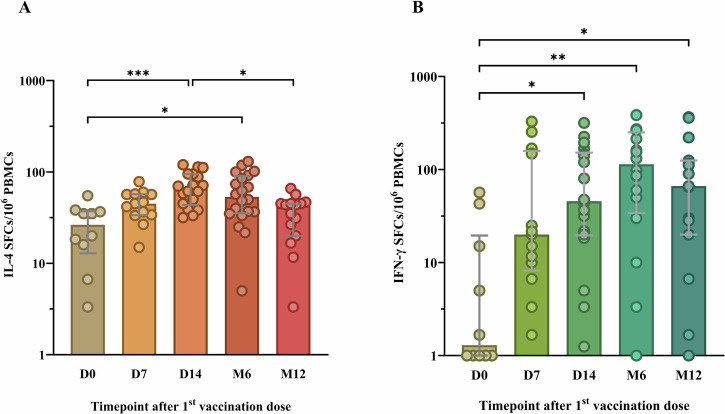
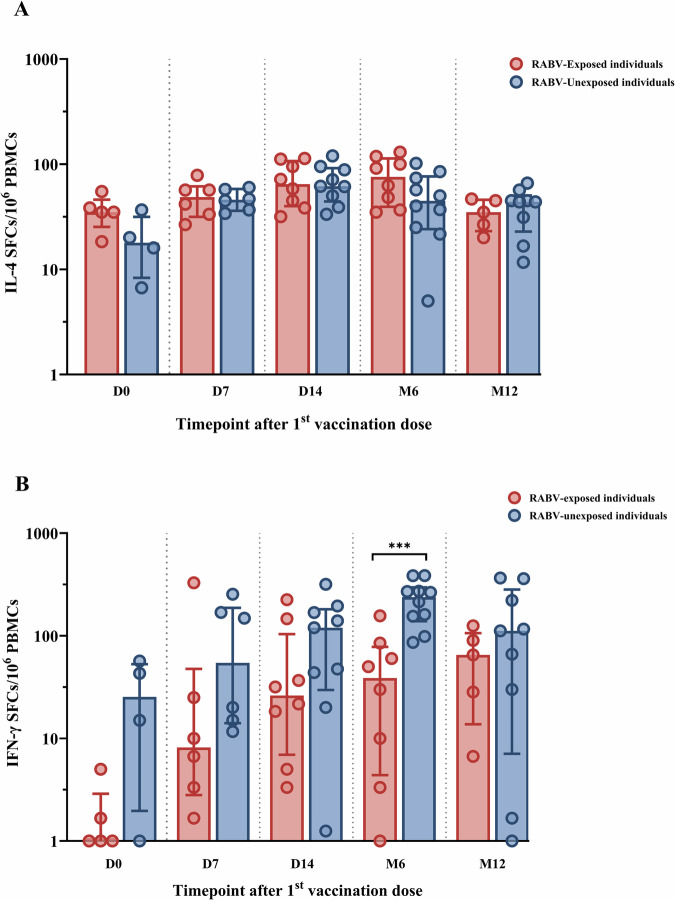
Besides nAbs, RABV-specific T cells are reported by our team and others to develop up to six months after PEP vaccination31,33,34. Overall, 76 samples covering all five study timepoint across 38 participants were included for the analysis of their T cell response (Supplementary Table 1, Supplementary Table 2) based on sufficient cell viability. Among these 38 patients, 17 were bitten by a RABV-positive dog and 21 were bitten by a RABV-negative dog. Following the stimulation of patients’ PBMCs with the RABV peptide pool, the frequency of RABV-specific IL-4-secreting T cells was significantly increased one week after PEP completion (D14) compared to before the vaccine administration (D0; p = 0.0007) and this increase was sustained even after six months (p = 0.0291, Fig. 3A). One year after PEP, the frequencies of RABV-specific IL-4-secreting T cells significantly decreased compared to their frequency at D14 (p = 0.0114, Fig. 3A).
Fig. 3. The development of rabies virus (RABV)-specific interleukin-4 (IL-4) and interferon-gamma (IFN-γ) producing T cells at various timepoints after post-exposure prophylaxis (PEP) using the Institut Pasteur du Cambodge (IPC) regimen.
The fluorospot assay was used to determine IL-4 (A) and IFN-γ (B) secreting cells expressed as spot-forming cells (SFCs) at baseline (D0), day 7 (D7), day 14 (D14), month 6 (M6), and month 12 (M12) after PEP. For visualization purposes, data was transformed into logarithmic scales before being used in statistical analysis. Each dot represents a single individual. Grey lines represent the median and interquartile range. PBMCs, human peripheral blood mononuclear cells. Statistics: Kruskal-Wallis test, followed by Dunn’s multiple comparisons test. Asterisks represent significance levels as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
In addition to the RABV-specific IL-4-secreting T cell response, the RABV-specific IFN-γ-secreting T cell response was also analyzed. Similar to the RABV-specific IL-4 response, there was an increase in the frequencies of RABV-specific IFN-γ-secreting T cell observed at D7 (Fig. 3B), however non-significant. At D14 and M6, the frequencies of IFN-γ-secreting T cells was found to be significantly increased compared to D0 (p = 0.0284 and p = 0.0016, respectively; Fig. 3B). Interestingly, one year after PEP, the frequencies of RABV-specific IFN-γ-producing T cells remained high and significantly higher than they were prior PEP administration (D0; p = 0.0297).
Next, we assessed if there was a correlation between RABV nAbs titer with IL-4 or IFN-γ-secreting T cells at different timepoints. Solely at D7, we observed a moderate negative correlation between RABV nAbs titer and IL-4-secreting T cells (r = − p = 0.036) and a moderate positive correlation between RABV nAbs titer and IFN-γ-secreting T cells (r = 0.560, p = 0.049), whereas at the other study timepoint no correlation between humoral and T cell response was found (Supplementary Fig. 1).
RABV-Exposed Individuals and RABV-Unexposed Individuals Develop Similar Antibody and T cell Immunity after PEP
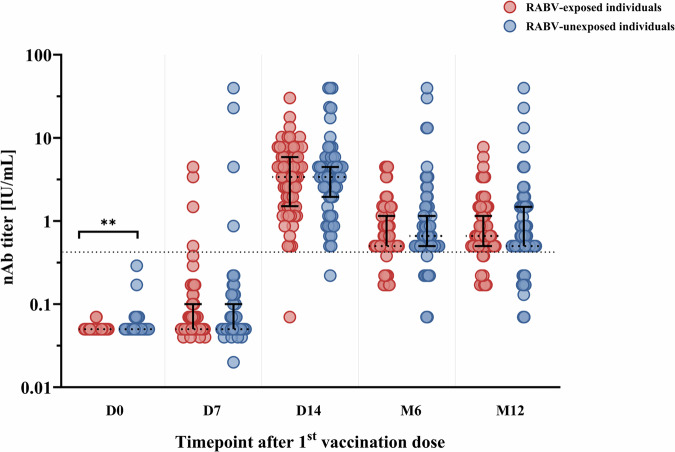
In order to understand if there are differences in the kinetics or magnitude of the antibody or the T cell responses after exposure to RABV or eRIG administration, we analyzed the nAb and T cell response in patients bitten by RABV-positive confirmed dogs, who received eRIG, compared to patients bitten by RABV-negative confirmed dogs, who did not receive eRIG. This analysis excluded the three individuals in the RABV-unexposed group who received eRIG due to severe bite incidents or the disappearance of the biting dog during the 10-day quarantine. In our results we found induction of both nAb and T cell responses against RABV (Supplementary Fig. 2). However, for all study timepoints except D0, the RABV nAbs titer were not significant different for individuals bitten by RABV-positive dogs that received PEP and eRIG compared to the individuals bitten by RABV-negative dogs that received PEP without eRIG (Fig. 4, Supplementary Fig. 2).
Fig. 4. The comparison of rabies virus (RABV) neutralizing antibody (nAb) titers between RABV-exposed versus RABV-unexposed individuals at different timepoints after post-exposure prophylaxis (PEP) using the Institut Pasteur du Cambodge (IPC) regimen.
The RABV nAb titers of individuals bitten by RABV-positive dogs that received PEP with eRIG (red, n = 78) and individuals bitten by RABV-negative dogs that received PEP without eRIG (blue, n = 67) were measured by fluorescent antibody virus neutralization test (FAVNT) at baseline (D0), day 7 (D7), day 14 (D14), month 6 (M6), and month 12 (M12) after PEP. For visualization purposes, data was transformed into logarithmic scales before being used in statistical analysis. Each dot represents a single individual. Black lines represent the median and interquartile range. Dashed line indicates the threshold of protective RABV nAb titer at 0.5 international unit per milliliter (IU/mL). Statistics: Mann-Whitney test. Asterisks represent significance levels as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
The comparison of the T cell specific response to the RABV peptide pool between the RABV-exposed group (PEP with eRIG) and the RABV-unexposed group (PEP without eRIG) is shown in Fig. 5. Following RABV-specific stimulation, we found no significant difference in the production of IL-4-secreting T cells at various timepoints between the two groups (Fig. 5A). In contrast, the RABV-exposed group showed a trend towards lower frequencies of RABV-specific IFN-γ-secreting T cells at different timepoints with a significant decrease at M6 (p = 0.0012) compared to the RABV-unexposed group (Fig. 5B).
Fig. 5. The comparison of rabies virus (RABV)-specific interleukin-4 (IL-4) and interferon-gamma (IFN-γ) producing T cells between RABV-exposed versus RABV-unexposed individuals at various timepoints after post-exposure prophylaxis (PEP) using the Institut Pasteur du Cambodge (IPC) regimen.
The fluorospot assay was used to determine IL-4 (A) and IFN-γ (B) secreting cells expressed as spot-forming cells (SFCs) at baseline (D0), day 7 (D7), day 14 (D14), month 6 (M6), and month 12 (M12) after PEP in RABV-exposed group (red, n = 17) and RABV-unexposed group (blue, n = 19). Each dot represents a single individual. Data was transformed into logarithmic scales before being used in statistical analysis for visualization purposes. Red and blue lines represent the median and interquartile range. PBMCs, human peripheral blood mononuclear cells. Statistics: Mann-Whitney test. Asterisks represent significance levels as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Discussion
Our study demonstrates that the IPC regimen, despite being a shortened and vaccine-sparing protocol, elicits a durable rabies-specific immune response, with nAbs persisting above protective levels in 87% of individuals one year post-PEP. This robust humoral response indicates that the IPC regimen is highly effective in sustaining immunity and minimizing the need for additional doses. The production of nAbs, a primary mechanism of protection, correlates with survival in rabies-exposed individuals and serves as a key indicator of vaccine efficacy10. These findings underscore the potential of the IPC regimen as a practical, protective approach for rabies post-exposure prophylaxis, particularly in resource-limited settings8. To our knowledge, this is the first study on the assessment of the one year humoral and T cell responses in PEP recipients who were actual victims of rabid dogs that either were tested positive for RABV or succumbed during quarantine likely due to RABV infection. Despite being the shortest PEP regimen, our study shows that the IPC PEP regimen using the Vero cell-based rabies vaccine (Verorab) induces humoral immunity 14 days after vaccination that lasts at least one year, as we found that RABV nAbs remained above a protective threshold in 87% of individuals. This result is in agreement with previous studies for rabies PrEP or PEP using either modified ID TRC regimen or IM Essen regimen, and with other vaccines, such as the purified chick embryo cell vaccine (Rabipur) and the purified duck embryo vaccine (Vaxirab)34,36–41.
The progression of rabies from exposure to active disease can vary significantly, with a mean incubation period of one month42, but occasionally extending beyond one year43. This variability in the incubation period highlights the critical nature of the one-year protection observed in our study. The persistent immune responses up to and beyond one-year post-exposure are particularly vital, potentially offering essential protection during these variable and sometimes extended incubation periods.
While RABV nAbs have been previously known to be the hallmark of protective immunity against RABV infection, a broader understanding of cellular immunity following rabies vaccination is warranted. Several studies have suggested that the cellular immune response may be crucial in eradicating RABV from the central nervous system44. We observed that IFN-γ, mainly produced by T helper 1 (Th1) cells, and IL-4, mainly produced by T helper 2 (Th2) cells, increased significantly 14 days after the first dose of PEP. This finding is consistent with previous literature where a RABV-specific T cell response can be detected 1-2 weeks after completion of different vaccination regimen, including both IM and ID administrations31,32,34. Indeed, we have shown before by monitoring activation-induced markers (AIM) and intracellular cytokine production that both IM and ID regimens induce a polyfunctional T cell response where RABV-specific T cells produced IFN-γ, interleukin-2 (IL-2) and tumor necrosis factor α (TNF-α)33. Despite these studies, the maintenance of long-term cellular immunity following rabies vaccination has not been well investigated. Here, we observed that RABV-specific IL-4-producing T cells and IFN-γ-producing T cells remained above baseline even six months and up to a year after PEP, respectively. This was not observed in earlier studies using the modified TRC regimen, which reported baseline-like levels of RABV-specific IFN-γ and IL-4-secreting T cells six months after PEP34.
Interestingly, after receiving PEP using the IPC protocol, the T cell response remains high even after one year, demonstrating that this PEP regimen can induce a long-lasting T cell immunity against RABV. Importantly, unlike for RABV nAbs, there is no threshold to define protective immunity.
Our real-life cohort allowed the comparison of the immune response between RABV-exposed individuals receiving eRIG and non-exposed individuals not receiving eRIG. Indeed, it has been shown before that eRIG administration can dampen the immune response to rabies PEP45. Immune complexes can influence the production of high affinity antibodies via interaction with type II Fc-gamma receptor (FcγRII), even though binding of equine IgG to different human FcγRII remains to be fully investigated46,47. The development of protective nAb titers or the seroconversion rate were not significantly impacted in the RABV-exposed group compared to the RABV-unexposed group. In contrast, frequencies of RABV-specific IFN-γ-secreting T cells were decreased in patients who were exposed to RABV. The presence of RABV might evade or suppress the vaccine-induced immune response48, as RABV infection can lead to a downregulation of the major histocompatibility complex (MHC-I), which may lead to impaired induction of adaptive immunity21,49. Moreover, RABV infection induces a Th2 skewing of the T cell response21,48,50,51.
Correlations between the magnitude of the T cell response and the magnitude of nAb titers after PEP remains to be further investigated. One study found a correlation of nAb titers with IL-4-secreting T cells and with IFN-γ-secreting T cells, while no correlation with IL-2 produced by peripheral blood lymphocytes was indicated in other studies34,52. In our cohort, we found that a higher Th1 T cell response at D7, measured as frequency of RABV-specific IFN-γ-secreting T cells, positively correlated with higher nAb titers. In contrast, a higher Th2 T cell response at D7, measured as frequency of RABV-specific IL-4-secreting T cells, was negatively correlated with higher nAb titers. Indeed, vaccine formulation used and the route of administration can influence the Th1/Th2 balance34,53. These data indicate that an early and robust Th1 type response to PEP contributes to a protective immune response following PEP. It would be beneficial to examine additional cytokines in further studies to obtain a more comprehensive understanding of the T cell responses. For example, interleukin-17 (IL-17) produced by T helper 17 (Th17) cells have also been reported to be a crucial part of the immune response against RABV infection due to the ability of IL-17 to induce a blood-brain barrier disruption21.
However, one RABV-unexposed individual did not develop RABV nAbs that exceeded the protective threshold as designated by the WHO at any timepoint during the study. However, on day 14, the nAb titer of this individual increased to above 0.1 IU/mL, which is higher than the lowest acceptable RABV nAb titer advised by ACIP11. The reason for this inability to develop sufficiently high RABV nAb titers is unclear, especially since the patient had no known underlying health conditions or medication use during the study. However, this individual was an active smoker, consuming on average seven cigarettes daily. Indeed, recent studies indicate that smokers could have a reduced nAb response to vaccination, exemplified by their reduced responses to the COVID-19 mRNA vaccine and the inactivated whole-virion SARS-CoV-2 vaccine compared to non-smokers54,55. It is also possible that this individual might have experienced seroconversion followed by subsequent sero-reversion between the D14 and M6 sampling points and that this potential change in antibody status might not have been detected due to the timing of our sample collection.
In this study, we were unable to conduct a longitudinal analysis of the RABV-specific T cell response in the same individuals as not all samples obtained from all individuals at all timepoints passed quality control. This prevents the T cell response from being studied using a paired analysis. Additionally, as we follow the WHO guidelines regarding eRIG administration, where all individuals bitten by RABV-positive dogs received eRIG1. Hence, we cannot distinguish between the possible side effects from eRIG and the presence of RABV on the immune response following PEP.
While our study provides significant insights into the IPC regimen’s sustained antibody response, future research should explore the longevity of this immune response beyond one year and assess the factors influencing variability in antibody and T cell responses across diverse populations. Investigating the complex dynamics of long-term immunity will aid in refining rabies PEP protocols and understanding how individual differences, including genetic and lifestyle factors, may affect immunogenicity and duration of protection. This ongoing study is focused on the persistence of nAbs and T cell responses, aiming to provide vital data on long-term immunity. Such information could significantly influence the development of future PEP protocols. Moreover, we aim to explore the variability in immune responses across different populations, emphasizing the potential effects of genetic or environmental factors, such as smoking on PEP response. These studies are crucial for tailoring PEP more effectively to diverse global demographics and enhancing our understanding of long-term immunological landscapes post-vaccination.
Overall, in this study we show in real-life PEP practice that T cell responses and protective RABV nAbs of dog bite victims receiving IPC PEP can be maintained at least up to one year after PEP, regardless of whether they were bitten by a RABV-positive dog and receiving additional eRIG or if the bite incident was from a RABV-negative dog. Further long-term follow-up of the patients is needed to determine if nAb and T cell responses may wane slowly or persist over a longer time. These results will provide a better understanding of the long-term immunity conferred by rabies PEP, which might enable fewer frequent PEP administrations in endemic countries like Cambodia or direct the development of treatments that enhance long-term protection. Our findings highlight the effectiveness of this IPC regimen in eliciting a sustained antibody response, supporting its broader implementation in rabies prevention.
Methods
Study Design and Participants
Patient inclusion started in October 2019 and continued until April 2022. Ethical approval for the study was obtained from the National Ethics Committee of Health Research of Cambodia (No 222-2019 NECHR). Individuals with category II or III exposure and no previous history of rabies vaccination were included among the people seeking rabies PEP at the IPC Rabies Prevention Center in Phnom Penh. Exposure category was defined according to the WHO guidelines where exposure category II refers to minor scratches or abrasions without bleeding, and category III includes single or multiple transdermal bites or scratches1. Prior to enrollment, written informed consent was obtained from all participants or the guardians of participants under the age of 18. A total of 175 participants were initially included. The study design is outlined in Fig. 1. PEP comprised a Vero cell-based rabies vaccine (Verorab; Sanofi, Lyon, France) that was administered ID according to the IPC PEP regimen7 (day 0, 3, and 7 following the date of inclusion; Fig. 1B). The criteria for additional administration of equine rabies immunoglobulin (eRIG) EQUIRAB (Bharat Serum and Vaccines Limited, India; vials of 5 mL with 1,000 IU) were (i) severe bite, (ii) disappearance or death of the dog or clinical diagnosis of rabid dog during 10-day quarantine, or (iii) laboratory diagnosis of RABV infection in the dog via positive DFAT result (Fig. 1A).
Samples Collection and Processing
Before the initial PEP vaccination, blood samples were collected from participants at day of enrollment (D0; baseline), and then subsequential at 7 days (D7; the last day of vaccination schedule), 14 days (D14), 6 months (M6), and 12 months (M12) after the vaccination using dry tubes for serum collection and heparin tubes for acquisition of peripheral blood mononuclear cells (PBMCs; Fig. 1B) as described in33.
Fluorescent Antibody Virus Neutralization Test (FAVNT)
The FAVNT is recommended by the WHO for determining the titer of RABV nAbs1 and performed as described earlier56. The threshold for positivity is ≥ 0.5 IU/mL and the immune status of study participants was considered as seroconverted when they were negative for nAb at D0 ( < 0.5 IU/mL) and subsequently became RABV nAb positive by reaching this threshold.
FluoroSpot IFN-γ /IL-4 Assay
The cryopreserved PBMCs were thawed and rested at 4˚C for at least 2 hours in RPMI-1640 medium (SIGMA Life Sciences) supplemented with 10% FBS, 2 mM of L-glutamine (Invitrogen), and 100 units/ml of penicillin-streptomycin (Gibco). The PBMCs were assessed for their viability as described previously33. Only PBMC samples with a viability >70% were included in the analysis. RABV-specific T cell responses were evaluated by the FluoroSpot Plus Human IFN-γ/IL-4 kit (Mabtech) following manufacturer’s instructions. Briefly, the fluorospot plates (Mabtech) pre-coated with anti-IFN-γ (1-D1K; Mabtech) and anti-IL-4 (IL4-I; Mabtech) were washed three times with sterile 1x phosphate buffered saline (PBS) and blocked with supplemented RPMI-1640 medium for at least 30 minutes at room temperature (RT). Then, PBMCs were plated with 300,000 cells/well in duplicate and stimulated with GlyRab peptide pool (Mimotopes, Victoria, Australia)33 at a concentration of 10 μg/mL. Plates were incubated for 45 hours at 37˚C in a humidified incubator with 5% CO2, washed with PBS, and incubation with primary and secondary antibodies according to manufacturer’s procedure. Next, plates were washed with PBS and incubated for 10 minutes with fluorophore enhancer in the dark at RT. Afterwards, the fluorophore enhancer was discarded and the plates were air dried in the dark. The plates were read using the AID EliSpot/FluoroSpot Reader (Advanced Imaging Devices GmbH, Strassberg, Germany), and spots were analyzed using AID EliSpot/FluoroSpot software version 7.0.
To obtain the count of RABV-specific spot-forming cells (SFCs), the mean of non-specific spots in the negative control wells was subtracted from the mean spots in the GlyRab-stimulated wells and expressed as mean SFCs per 106 input cells.
Statistical Analysis
The data was analyzed using GraphPad Prism software version 9.5.0 (GraphPad, San Diego, CA, USA). The data was tested for a normal distribution and found to be non-parametric. The RABV nAbs titer data was compared in paired analysis at various timepoints using Friedman test, followed by Dunn’s multiple comparisons test. Due to the loss of longitudinal samples due to the exclusion of PBMC samples that did not meet the quality control, the T cell responses was compared in unpaired analysis at the various timepoints using Kruskal-Wallis statistical test, followed by Dunn’s multiple comparisons test.
The RABV nAbs and T cell responses between the groups of individuals bitten by RABV-positive dog and individuals bitten by RABV-negative dog were compared using Mann-Whitney test (non-paired data). The correlation between RABV-specific T cell response and RABV nAbs titer were analyzed by Spearman’s correlation coefficient test. In all cases, statistical significance was considered at p < 0.05. Asterisks indicating significance levels were denoted as follows: (*) if p < 0.05, (**) if p < 0.01, (***) if p < 0.001 and (****) if p < 0.0001.
Supplementary information
Acknowledgements
We thank all study participants for their participation in the study. The study was funded by Institut Pasteur du Cambodge seed funds. H.A. is supported by the German Centre for International Migration and Development as part of national support provided by the German cooperation (GIZ).
Author contributions
The conceptualization and design of the study and laboratory investigations: H.A., S.L., T.C. Subjects’ inclusion, sample collection and epidemiology analysis: S.L., S.T., P.T., C.L., Y.P. Laboratory performance: N.Y., S.I., C.Y., S.S., B.H. Data analysis and figures making: N.Y. Supervision: S.L., V.D., T.C., H.A. Paper writing—original draft: N.Y. Paper writing—review & editing: T.C., H.A., S.L., Y.P., V.D. Funding acquisition: T.C., S.L.
Data availability
All data supporting the findings of this study are included within this manuscript and its supplementary information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nisa Ya, Heidi Auerswald.
These authors jointly supervised this work: Sowath Ly, Tineke Cantaert.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-01030-8.
References
- 1.World Health Organization. WHO Expert Consultation on Rabies: Third Report. (World Health Organization, Geneva, 2018).
- 2.Rabies. https://www.who.int/news-room/fact-sheets/detail/rabies.
- 3.Gilbert, A. T. Rabies virus vectors and reservoir species. Rev. Sci. Tech. Int. Off. Epizoot37, 371–384 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ly, S. et al. Rabies situation in Cambodia. PLoS Negl. Trop. Dis.3, e511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalier, V. et al. Large scale dog population demography, dog management and bite risk factors analysis: a crucial step towards rabies control in Cambodia. PLoS ONE16, e0254192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan, H. et al. Global burden of rabies in 204 countries and territories, from 1990 to 2019: results from the Global Burden of Disease Study 2019. Int. J. Infect. Dis.126, 136–144 (2023). [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization null. Rabies vaccines: WHO position paper, April 2018 - Recommendations. Vaccine36, 5500–5503 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Cantaert, T. et al. A 1-week intradermal dose-sparing regimen for rabies post-exposure prophylaxis (RESIST-2): an observational cohort study. Lancet Infect. Dis.19, 1355–1362 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Gholami, A. et al. Seroconversion after three doses of intramuscular rabies vaccine as a post-exposure treatment. Virus Res.278, 197883 (2020). [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Expert Committee on Biological Standardization: fifty-sixth report. (2007).
- 11.Rao, A. K. et al. Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: recommendations of the advisory committee on immunization practices—United States, 2022. Morb. Mortal. Wkly. Rep.71, 619–627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper, D. C. et al. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol.72, 3711–3719 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnanadurai, C. W. et al. Presence of virus neutralizing antibodies in cerebral spinal fluid correlates with non-lethal rabies in dogs. PLoS Negl. Trop. Dis.7, e2375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khawplod, P. et al. Revision of the Thai Red Cross intradermal rabies post-exposure regimen by eliminating the 90-day booster injection. Vaccine24, 3084–3086 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Thraenhart, O. et al. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin. Immunol. Immunopathol.71, 287–292 (1994). [DOI] [PubMed] [Google Scholar]
- 16.De Pijper, C. A. et al. Long-term memory response after a single intramuscular rabies booster vaccination 10-24 years after primary immunization. J. Infect. Dis.226, 1052–1056 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, J. et al. Long-term immunity and the effect of one or two booster doses with a lyophilized human rabies vaccine (human diploid cells) at 10 years post primary vaccination in China. Hum. Vaccines Immunother.17, 3162–3168 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkhouse, D. A., Faber, M. & Hooper, D. C. Pre- and post-exposure safety and efficacy of attenuated rabies virus vaccines are enhanced by their expression of IFNγ. Virology0, 174–180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkhouse, D. A. et al. Expression of interferon gamma by a recombinant rabies virus strongly attenuates the pathogenicity of the virus via induction of type I interferon. J. Virol.89, 312–322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafon, M. Modulation of the immune response in the nervous system by rabies virus. Curr. Top. Microbiol. Immunol.289, 239–258 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Katz, I. S. S., Guedes, F., Fernandes, E. R. & Dos Ramos Silva, S. Immunological aspects of rabies: a literature review. Arch. Virol.162, 3251–3268 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Phares, T. W., Kean, R. B., Mikheeva, T. & Hooper, D. C. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. Baltim. Md.176, 7666–7675 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Phares, T. W., Fabis, M. J., Brimer, C. M., Kean, R. B. & Hooper, D. C. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J. Immunol. Baltim. Md.178, 7334–7343 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Gnanadurai, C. W. & Fu, Z. F. CXCL10 and blood-brain barrier modulation in rabies virus infection. Oncotarget7, 10694–10695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, H. et al. Intracerebral administration of recombinant rabies virus expressing GM-CSF prevents the development of rabies after infection with street virus. PLOS ONE6, e25414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper, D. C. et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol.14, 691–698 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Overduin, L. A., van Dongen, J. J. M. & Visser, L. G. The cellular immune response to rabies vaccination: a systematic review. Vaccines7, 110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilde, H., Khawplod, P. & Hemachudha, T. Post-exposure rabies prophylaxis in patients with AIDS. Vaccine27, 5726–5727 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Tantawichien, T., Jaijaroensup, W., Khawplod, P. & Sitprija, V. Failure of multiple-site intradermal postexposure rabies vaccination in patients with human immunodeficiency virus with low CD4+ T lymphocyte counts. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.33, E122–E124 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Pancharoen, C. et al. Failure of pre- and postexposure rabies vaccinations in a child infected with HIV. Scand. J. Infect. Dis.33, 390–391 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Herzog, M. et al. T and B cell human responses to European bat lyssavirus after post-exposure rabies vaccination. Clin. Exp. Immunol.85, 224–230 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijaya, L. et al. An accelerated rabies vaccine schedule based on toll-like receptor 3 (TLR3) agonist PIKA adjuvant augments rabies virus specific antibody and T cell response in healthy adult volunteers. Vaccine35, 1175–1183 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Auerswald, H. et al. Side-by-side comparative study of the immunogenicity of the intramuscular and intradermal rabies post-exposure prophylaxis regimens in a cohort of suspected RABV exposed individuals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. ciad304 10.1093/cid/ciad304 (2023). [DOI] [PMC free article] [PubMed]
- 34.Venkataswamy, M. M. et al. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin. Exp. Vaccine Res.4, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. in Chapter 3.1.18. Rabies (Infection with Rabies Virus and Other Lyssaviruses) (WOAH, 2023).
- 36.Tantawichien, T. et al. Safety and immunogenicity of chromatographically purified Vero cell rabies vaccine for intradermal pre- and post-exposure rabies prophylaxis. Expert Rev. Vaccines13, 1593–1601 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Ashwathnarayana, D. H. et al. A comparative study on the safety and immunogenicity of Purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purifed vero cell rabies vaccine (PVRV, Verorab). Vaccine28, 148–151 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Janewongwirot, P. et al. A randomized open-label trial of 2-dose or 3-dose pre-exposure rabies prophylaxis among Thai children. Vaccine37, 5307–5313 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Bijok, U. et al. Clinical trials in healthy volunteers with the new purified chick embryo cell rabies vaccine for man. J. Commun. Dis.16, 61–69 (1984). [PubMed] [Google Scholar]
- 40.Pengsaa, K. et al. A three-year clinical study on immunogenicity, safety, and booster response of purified chick embryo cell rabies vaccine administered intramuscularly or intradermally to 12- to 18-month-old Thai children, concomitantly with Japanese encephalitis vaccine. Pediatr. Infect. Dis. J.28, 335–337 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Liu, H. et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum. Vaccin.7, 220–224 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Tojinbara, K. et al. Estimating the probability distribution of the incubation period for rabies using data from the 1948-1954 rabies epidemic in Tokyo. Prev. Vet. Med.123, 102–105 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Shengli, M. et al. A case of human rabies with a long incubation period in Wuhan. IDCases23, e00998 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooper, D. C., Roy, A., Barkhouse, D. A., Li, J. & Kean, R. B. Rabies virus clearance from the central nervous system. Adv. Virus Res.79, 55–71 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Lang, J., Simanjuntak, G. H., Soerjosembodo, S. & Koesharyono, C. Suppressant effect of human or equine rabies immunoglobulins on the immunogenicity of post-exposure rabies vaccination under the 2-1-1 regimen: a field trial in Indonesia. MAS054 Clinical Investigator Group. Bull. World Health Organ.76, 491–495 (1998). [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Y. et al. Specificity of mouse and human Fcgamma receptors and their polymorphic variants for IgG subclasses of different species. Eur. J. Immunol.52, 753–759 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Wang, T. T. et al. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell162, 160–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lafon, M. Evasive strategies in rabies virus infection. Adv. Virus Res.79, 33–53 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Senba, K. et al. Passive carriage of rabies virus by dendritic cells. SpringerPlus2, 419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carosella, E. D., Moreau, P., Aractingi, S. & Rouas-Freiss, N. HLA-G: a shield against inflammatory aggression. Trends Immunol.22, 553–555 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Mégret, F. et al. Modulation of HLA-G and HLA-E expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum. Immunol.68, 294–302 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Perrin, P. et al. Rabies-specific production of interleukin-2 by peripheral blood lymphocytes from human rabies vaccinees. Vaccine9, 549–558 (1991). [DOI] [PubMed] [Google Scholar]
- 53.Zhao, H. et al. Immune response of inactivated rabies vaccine inoculated via intraperitoneal, intramuscular, subcutaneous and needle-free injection technology-based intradermal routes in mice. Int. J. Mol. Sci.24, 13587 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, J. et al. Down-regulation of SARS-CoV-2 neutralizing antibodies in vaccinated smokers. MedComm.3, e166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moncunill, G. et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers. EBioMedicine75, 103805 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cliquet, F., Aubert, M. & Sagné, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods212, 79–87 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are included within this manuscript and its supplementary information file.