Abstract
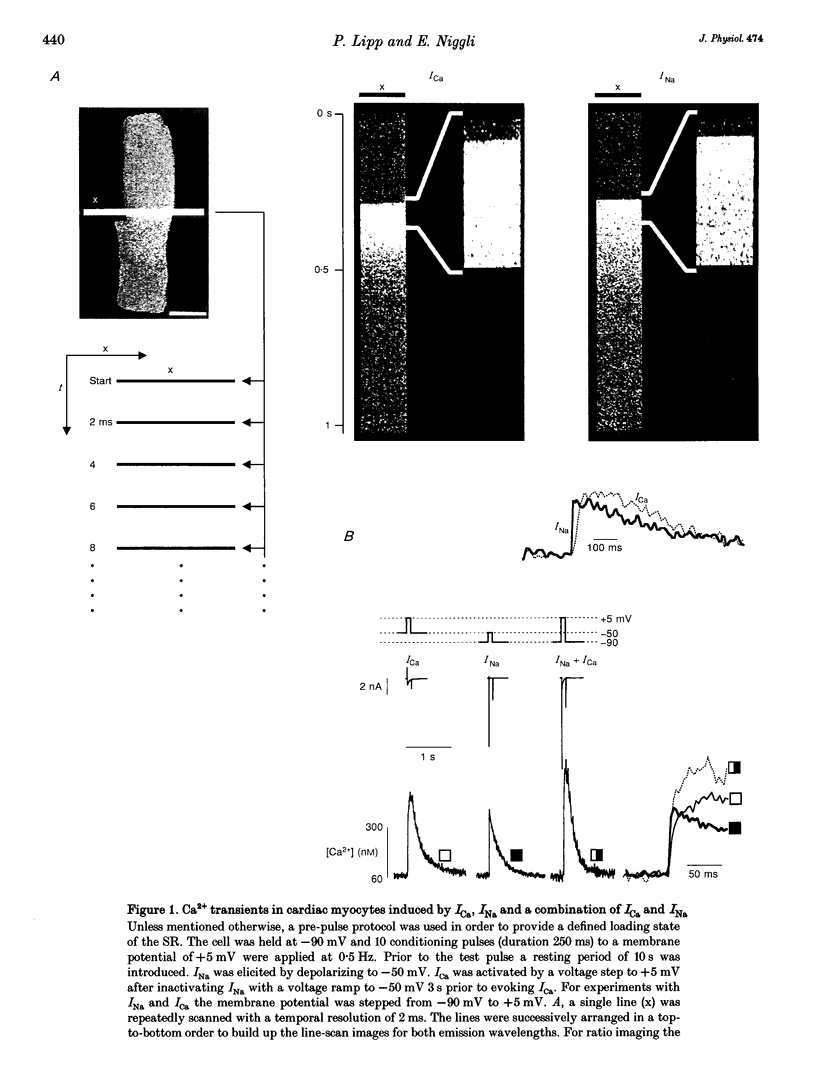
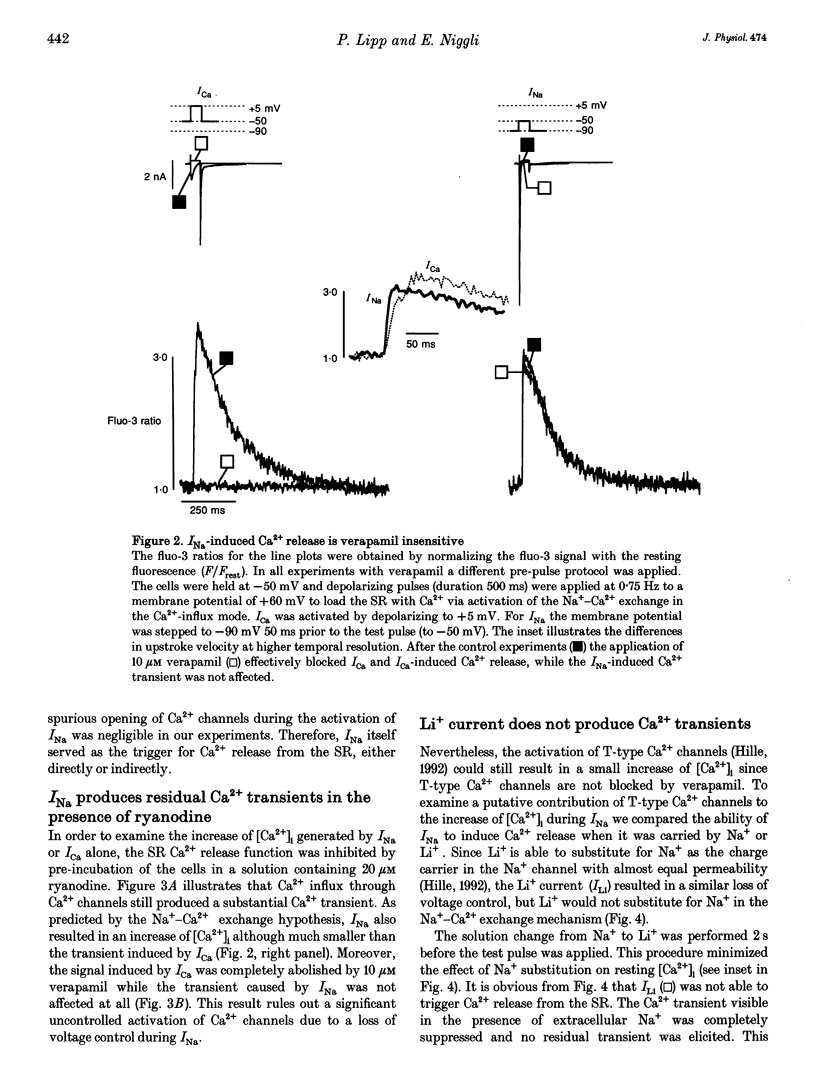
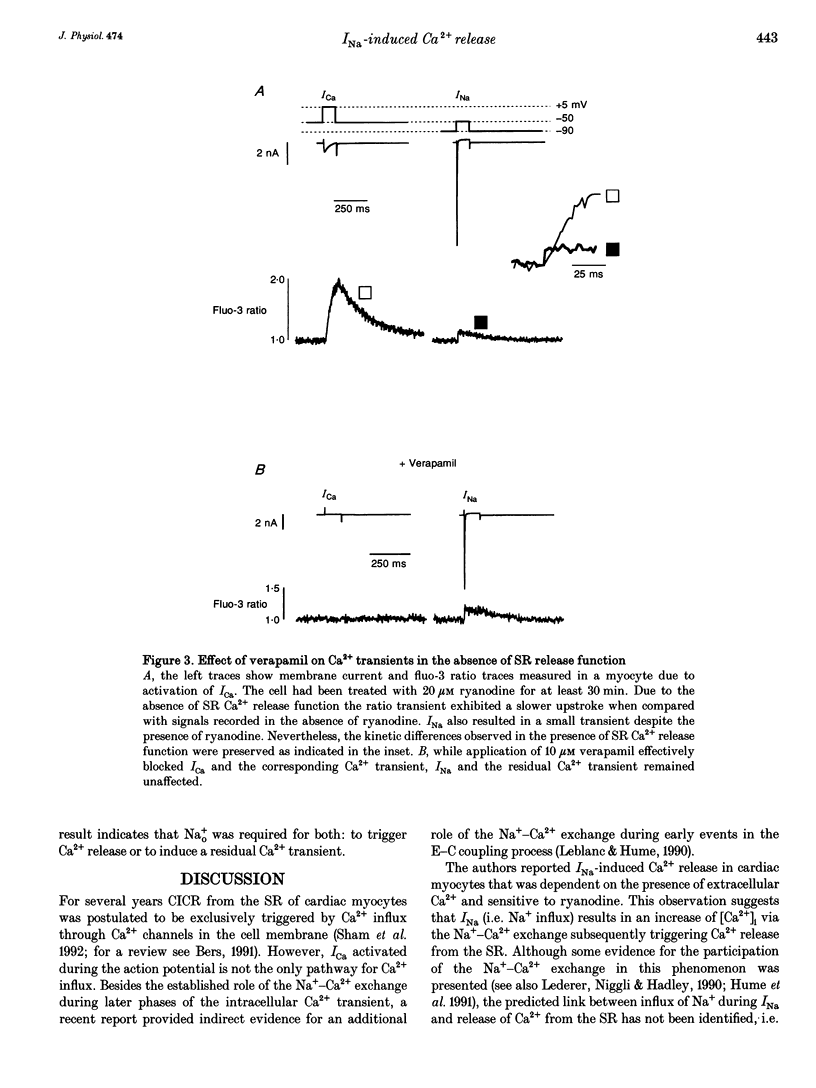
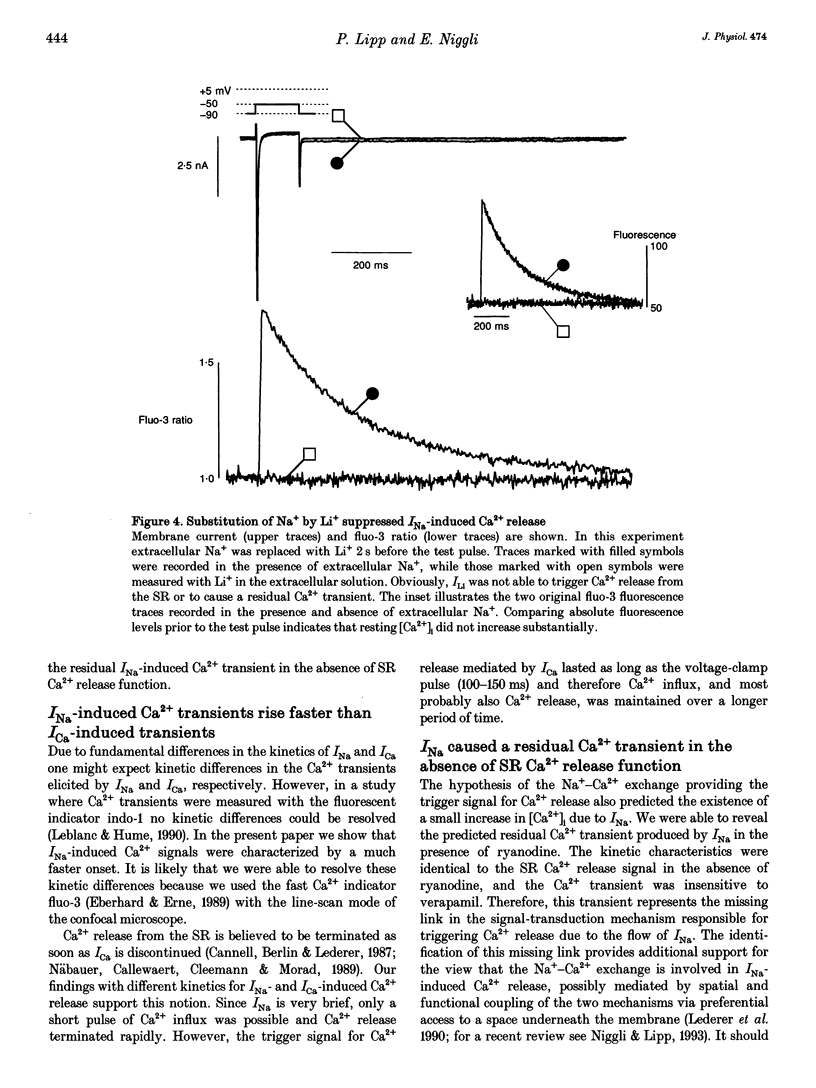
1. Na+ current (INa)-induced Ca2+ transients were studied in ventricular myocytes isolated from adult guinea-pig hearts. The fluorescent Ca2+ indicator fluo-3 or a mixture of fluo-3 and fura-red were used in conjunction with confocal microscopy to follow the intracellular Ca2+ concentration while membrane currents were measured simultaneously with the whole-cell configuration of the patch-clamp technique. 2. Ca2+ release from the sarcoplasmic reticulum (SR) could be triggered either by Ca2+ current (ICa) or Na+ current (INa). Analysis of INa-induced Ca2+ signals at higher temporal resolution revealed a faster upstroke of these transients when compared with those triggered by ICa. 3. In the presence of 20 microM ryanodine to block SR Ca2+ release ICa elicited a verapamil-sensitive Ca2+ transient with a slow upstroke. INa also induced a residual Ca2+ transient that was insensitive to 10 microM verapamil and characterized by a rapid upstroke. 4. The existence of a residual Ca2+ transient in the absence of SR Ca2+ release and L-type ICa indicates that INa is indeed able to evoke an increase in [Ca2+]i without uncontrolled activation of Ca2+ channels. 5. Substitution of extracellular Na+ by Li+ suppressed INa-induced Ca2+ transients, suggesting that the Ca2+ release and the residual Ca2+ transient can only be elicited by influx of Na+ and not by Li+. This result supports the notion that both the residual Ca2+ transient as well as the INa-induced Ca2+ release are mediated by the Na(+)-Ca2+ exchange.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc Res. 1992 May;26(5):433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Eberhard M., Erne P. Kinetics of calcium binding to fluo-3 determined by stopped-flow fluorescence. Biochem Biophys Res Commun. 1989 Aug 30;163(1):309–314. doi: 10.1016/0006-291x(89)92136-0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Response. Science. 1991 Mar 15;251(4999):1371–1371. doi: 10.1126/science.251.4999.1371. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Calcium transients caused by calcium entry are influenced by the sarcoplasmic reticulum in guinea-pig atrial myocytes. J Physiol. 1992 Aug;454:321–338. doi: 10.1113/jphysiol.1992.sp019266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular restricted spaces: significance for cell signalling and excitation-contraction coupling. J Muscle Res Cell Motil. 1993 Jun;14(3):288–291. doi: 10.1007/BF00123093. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Sham J. S., Cleemann L., Morad M. Gating of the cardiac Ca2+ release channel: the role of Na+ current and Na(+)-Ca2+ exchange. Science. 1992 Feb 14;255(5046):850–853. doi: 10.1126/science.1311127. [DOI] [PubMed] [Google Scholar]
- Sodium-calcium exchange. Science. 1991 Mar 15;251(4999):1370–1371. [PubMed] [Google Scholar]



