Abstract
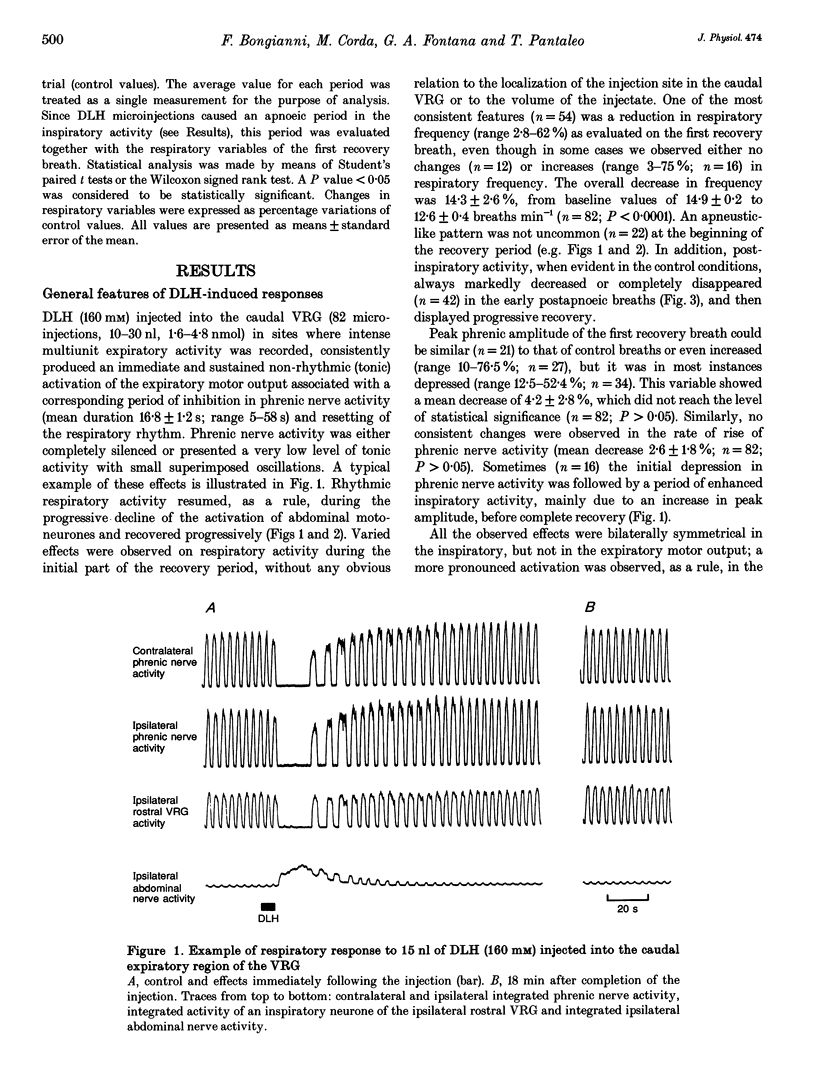
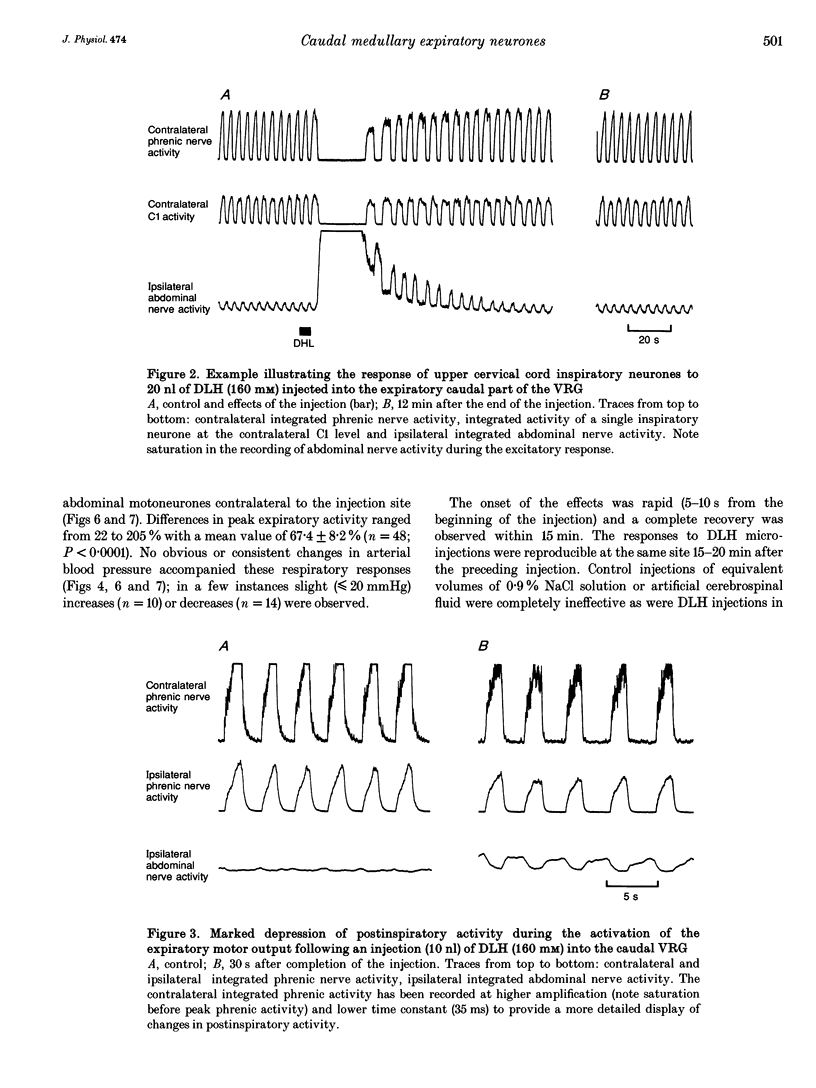
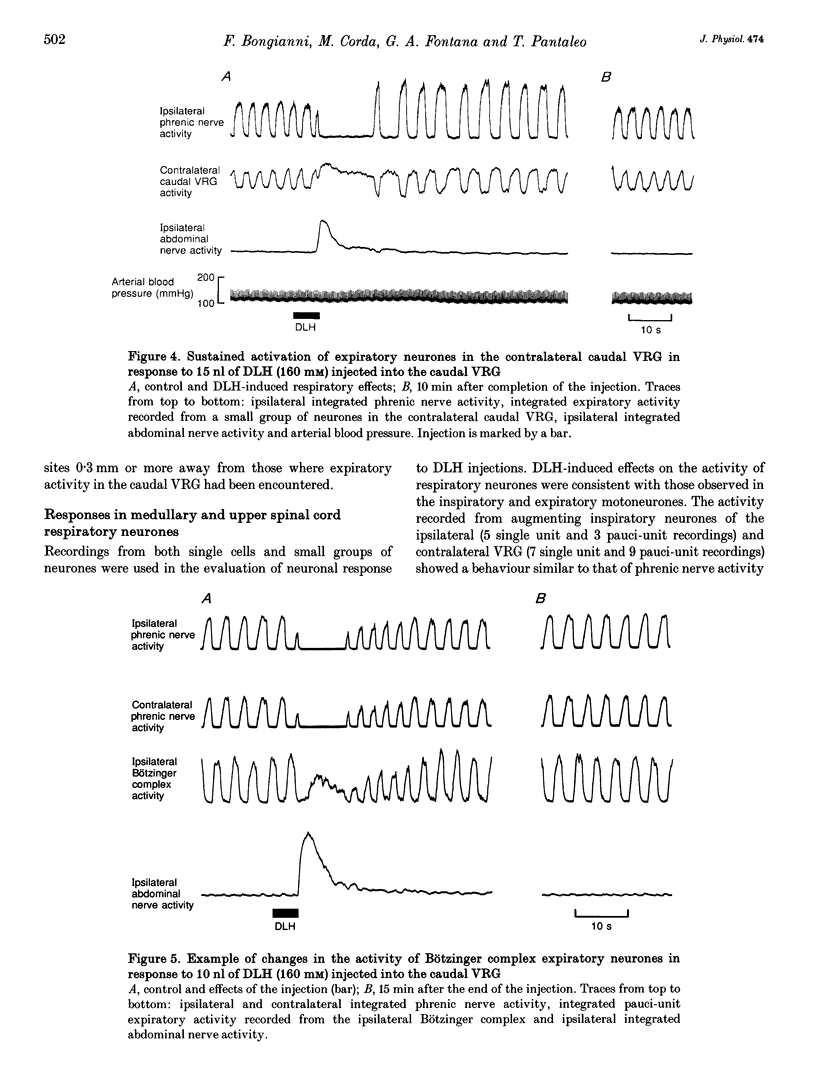
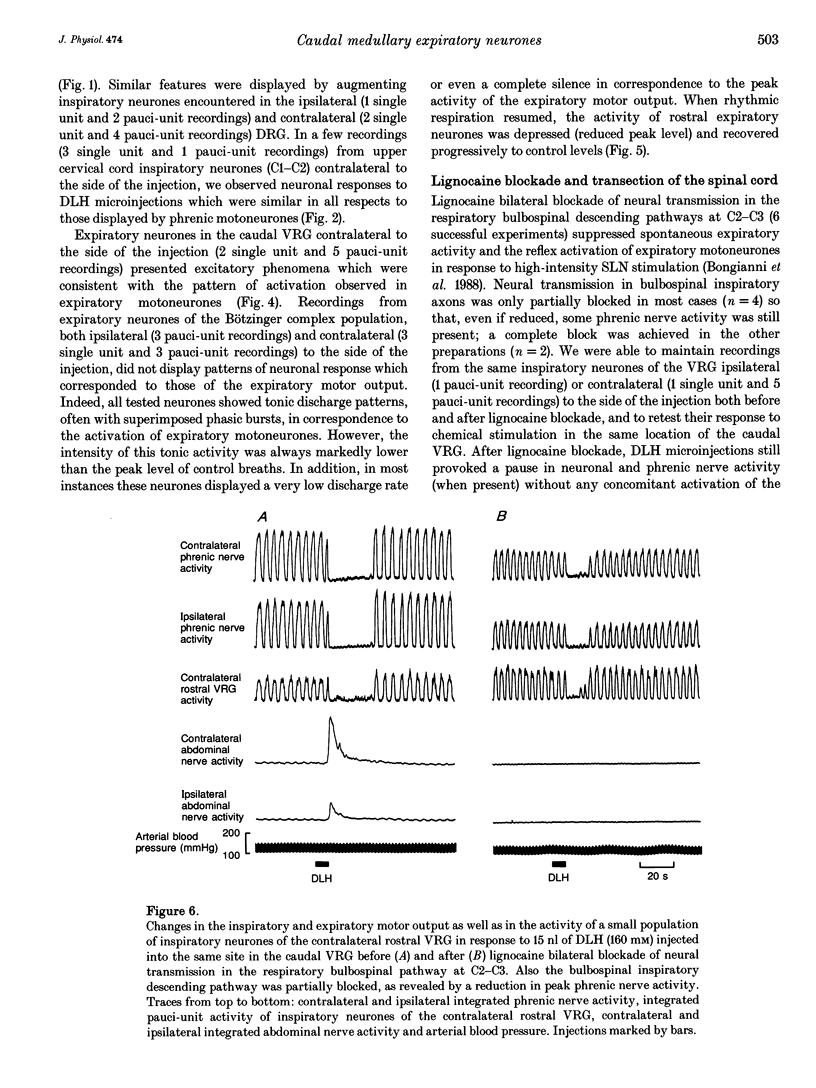
1. The purpose of this work was to ascertain whether the activation of caudal expiratory neurones located in the caudal part of the ventral respiratory group (VRG) may affect the pattern of breathing via medullary axon collaterals. 2. We used microinjections of DL-homocysteic acid (DLH) to activate this population of neurones in pentobarbitone-anaesthetized, vagotomized, paralysed and artificially ventilated cats. Both phrenic and abdominal nerve activities were monitored; extracellular recordings from medullary and upper cervical cord respiratory neurones were performed. 3. DLH (160 mM) microinjected (10-30 nl for a total of 1.6-4.8 nmol) into the caudal VRG, into sites where expiratory activity was encountered, provoked an intense and sustained activation of the expiratory motor output associated with a corresponding period of silence in phrenic nerve activity. During the progressive decline of the activation of abdominal motoneurones, rhythmic inspiratory activity resumed, displaying a decrease in frequency and a marked reduction or the complete suppression of postinspiratory activity as its most consistent features. 4. Medullary and upper cervical cord inspiratory neurones exhibited inhibitory responses consistent with those observed in phrenic nerve activity, while expiratory neurones in the caudal VRG on the side contralateral to the injection showed excitation patterns similar to those of abdominal motoneurones. On the other hand, in correspondence to expiratory motor output activation, expiratory neurones of the Bötzinger complex displayed tonic discharges whose intensity was markedly lower than the peak level of control breaths. 5. Bilateral lignocaine blockades of neural transmission at C2-C3 affecting the expiratory and, to a varying extent, the inspiratory bulbospinal pathways as well as spinal cord transections at C2-C3 or C1-C2, did not suppress the inhibitory effect on inspiratory neurones of either the ipsi- or contralateral VRG in response to DLH microinjections into the caudal VRG. 6. The results show that neurones within the column of caudal VRG expiratory neurones promote inhibitory effects on phrenic nerve activity and resetting of the respiratory rhythm. We suggest that these effects are mediated by medullary bulbospinal expiratory neurones, which may, therefore, have a function in the control of breathing through medullary axon collaterals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders K., Ballantyne D., Bischoff A. M., Lalley P. M., Richter D. W. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. J Physiol. 1991 Jun;437:1–25. doi: 10.1113/jphysiol.1991.sp018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M., Mori S., Kawahara K., Watanabe H., Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980 Nov 24;202(1):51–63. [PubMed] [Google Scholar]
- Arita H., Kogo N., Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987 Jan 20;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham M. C., Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990 Nov 12;533(1):141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- Bongianni F., Corda M., Fontana G., Pantaleo T. Expiration-related neurons in the caudal ventral respiratory group of the cat: influences of the activation of Bötzinger complex neurons. Brain Res. 1990 Sep 3;526(2):299–302. doi: 10.1016/0006-8993(90)91235-9. [DOI] [PubMed] [Google Scholar]
- Bongianni F., Corda M., Fontana G., Pantaleo T. Influences of superior laryngeal afferent stimulation on expiratory activity in cats. J Appl Physiol (1985) 1988 Jul;65(1):385–392. doi: 10.1152/jappl.1988.65.1.385. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L., Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985 Nov;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Plum F. Separation of descending spinal pathways to respiratory motoneurons. Exp Neurol. 1972 Jan;34(1):78–94. doi: 10.1016/0014-4886(72)90189-6. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. J Physiol. 1984 May;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild A. K., Dampney R. A., Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods. 1982 Nov;6(4):351–363. doi: 10.1016/0165-0270(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Huang Q., St John W. M. Respiratory neural activities after caudal-to-rostral ablation of medullary regions. J Appl Physiol (1985) 1988 Apr;64(4):1405–1411. doi: 10.1152/jappl.1988.64.4.1405. [DOI] [PubMed] [Google Scholar]
- Kalia M. P. Anatomical organization of central respiratory neurons. Annu Rev Physiol. 1981;43:105–120. doi: 10.1146/annurev.ph.43.030181.000541. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Munson J. B., Sears T. A., Westgaard R. H. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988 Jan;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter F., Richter D. W., Camerer H., Senekowitsch R. Morphological and electrical description of medullary respiratory neurons of the cat. Pflugers Arch. 1977 Nov 25;372(1):7–16. doi: 10.1007/BF00582200. [DOI] [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988 Dec;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Lipski J., Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61(3):625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Lipski J., Trzebski A., Chodobska J., Kruk P. Effects of carotid chemoreceptor excitation on medullary expiratory neurons in cats. Respir Physiol. 1984 Sep;57(3):279–291. doi: 10.1016/0034-5687(84)90077-x. [DOI] [PubMed] [Google Scholar]
- Long S., Duffin J. The neuronal determinants of respiratory rhythm. Prog Neurobiol. 1986;27(2):101–182. doi: 10.1016/0301-0082(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970 Nov 11;24(1):11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Tan L. K., Lakos S. F. Brainstem projections to cats' upper lumbar spinal cord: implications for abdominal muscle control. Brain Res. 1989 Jul 31;493(2):348–356. doi: 10.1016/0006-8993(89)91169-4. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):350–355. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- Mulloney B., Selverston A. Antidromic action potentials fail to demonstrate known interactions between neurons. Science. 1972 Jul 7;177(4043):69–72. doi: 10.1126/science.177.4043.69. [DOI] [PubMed] [Google Scholar]
- NELSON J. R. Single unit activity in medullary respiratory centers of cat. J Neurophysiol. 1959 Sep;22:590–598. doi: 10.1152/jn.1959.22.5.590. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985 May 6;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Oliven A., Deal E. C., Jr, Kelsen S. G., Cherniack N. S. Effects of hypercapnia on inspiratory and expiratory muscle activity during expiration. J Appl Physiol (1985) 1985 Nov;59(5):1560–1565. doi: 10.1152/jappl.1985.59.5.1560. [DOI] [PubMed] [Google Scholar]
- Orem J., Brooks E. G. The activity of retrofacial expiratory cells during behavioral respiratory responses and active expiration. Brain Res. 1986 May 28;374(2):409–412. doi: 10.1016/0006-8993(86)90440-3. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Mitra J., Overholt J. L., Cherniack N. S. Analysis of postinspiratory activity of phrenic motoneurons with chemical and vagal reflexes. J Appl Physiol (1985) 1986 Oct;61(4):1499–1509. doi: 10.1152/jappl.1986.61.4.1499. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Morrison D. E., Ellenberger H. H., Otto M. R., Feldman J. L. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989 Mar 1;281(1):69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Speck D. F., Beck E. R. Respiratory rhythmicity after extensive lesions of the dorsal and ventral respiratory groups in the decerebrate cat. Brain Res. 1989 Mar 20;482(2):387–392. doi: 10.1016/0006-8993(89)91206-7. [DOI] [PubMed] [Google Scholar]
- St John W. M., Bartlett D., Jr, Knuth K. V., Hwang J. C. Brain stem genesis of automatic ventilatory patterns independent of spinal mechanisms. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jul;51(1):204–210. doi: 10.1152/jappl.1981.51.1.204. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


