Abstract
Background
The pRESET stent retriever is a self-expanding nitinol stent designed for mechanical thrombectomy in cases of large vessel occlusion during acute ischemic stroke. This systematic review and meta-analysis synthesize the available evidence on the safety and efficacy of the pRESET device.
Methods
This is a systematic review and meta-analysis study conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The electronic databases of PubMed, Embase, WoS, and Scopus were systematically reviewed from inception to 8 July 2024.
Results
A total of eight studies involving 1163 patients were included. The pooled mortality rate was 18% with a 95% CI of [12%, 25%]. The rates of any hemorrhagic complication, parenchymal hemorrhage, and subarachnoid hemorrhage were 22% with a 95% CI of [12%, 36%], 7% with a 95% CI of [4%, 13%], and 10% with a 95% CI of [5%, 17%], respectively. The rate of favorable functional outcome (modified Rankin Scale 0-2) at 90 days was 43% with a 95% CI of [34%, 52%]. Successful recanalization rates were 60% with a 95% CI of [52%, 67%] after the first pass and 90% with a 95% CI of [83%, 95%] after the final pass. Rescue devices were used in 13% with a 95% CI of [7%, 24%] of cases.
Conclusions
The pRESET stent retriever demonstrates high recanalization rates and reasonable safety outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke due to large vessel occlusion. Further randomized trials directly comparing pRESET to other stent retrievers are warranted.
Keywords: Endovascular, mechanical thrombectomy, emboli, thromotic, cerebrovascular
Introduction
Mechanical thrombectomy has become the standard of care for acute ischemic stroke caused by large vessel occlusion, dramatically improving patient outcomes compared to intravenous thrombolysis alone.1,2 The pRESET stent retriever (Phenox GmbH, Bochum, Germany) is a self-expanding nitinol stent designed for mechanical thrombectomy in cases of large vessel occlusions in the anterior circulation. 3 Since its introduction, studies have examined its performance compared to other stent retrievers such as the Solitaire device.
Multiple studies have reported high rates of successful recanalization using the pRESET device, ranging from 86% to 96% in cases of distal occlusions and 77-100% overall. 3-5.3,4 The pRESET achieves comparable or superior revascularization rates compared to the Solitaire device. 1, 6. These rates are also comparable to those reported for the Solitaire and Trevo devices in a systematic review and meta-analysis, which found no significant differences in weighted mean recanalization rates between these two popular retriever designs. Successful recanalization with the pRESET is associated with good functional outcomes, with reported rates of functional independence at 90 days ranging from 40 to 68%. This compares favorably with the functional independence rates reported for Solitaire (52.1% [95% CI: 46.3%, 57.8%]) and Trevo (47.6% [95% CI: 36.7%, 58.8%]) at 3 months post-procedure.2,5,6
In addition to efficacy, the pRESET demonstrates a favorable safety profile. Reported rates of symptomatic intracranial hemorrhage range from 0 to 11%, while mortality ranges from 16 to 28%.2–4,6,7 Comparative studies report no significant differences in complication rates between the pRESET and other stent retrievers.1,2,7 This review synthesizes all available evidence on the use of the pRESET device for mechanical thrombectomy in acute ischemic stroke caused by large vessel occlusion.
Despite the promising evidence on safety and efficacy, further research is needed to optimize patient selection and technique for pRESET thrombectomy. Future randomized controlled trials directly comparing the pRESET to next-generation stent retrievers would provide valuable data on comparative effectiveness. Cost-effectiveness analyses are also warranted, given the high device costs associated with mechanical thrombectomy. This review synthesizes evidence on the safety and efficacy of the pRESET, device for thrombectomy in acute ischemic stroke.
Method
This research has been recorded in the International Prospective Register of Systematic Reviews (PROSPERO) under the code CRD42024518940. The preparation of this study strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. 8
Search strategy
The search strategy employed keywords such as “Ischemic Stroke,” “Brain Infarction,” “stroke,” “Thrombectomy,” and “Brain Ischemia” To define search criteria for an electronic database. PubMed, Embase, WoS, and Scopus were systematically searched from their inception to 8 July 2024, without imposing restrictions on publication date, type, or language. The complete search syntax is provided in the supplemental Table S1.
Study selection process
The data from each electronic database were input into EndNote v.20. Following the removal of duplicate articles, two reviewers (S.S and AR.B) independently conducted a two-step title/abstract screening to identify pertinent studies. Subsequently, a full-text assessment was carried out to ascertain the studies that met the eligibility criteria. The confirmation of the study selection process was overseen by a third senior reviewer (MA.H).
Eligibility criteria
Inclusion criteria
1- English studies
2- Clinical studies
3- Studies on patients with Ischemic Stroke
4- Original studies of randomized and non-randomized clinical trials, cohorts, cross-sectional, case-control, case series
Data extraction
Two reviewers independently conducted the data extraction of the included studies. The information of studies was extracted, including the name of the author, year of publication, country, number of patients, mean age, gender of patients, follow-up time, Location of stroke lesion, MCA M1, Stroke etiology (Large or small artery atherosclerosis), Stroke etiology (Cardio embolism), Risk factors (Dyslipidemia, Diabetes mellitus, Atrial fibrillation, Previous stroke, Coronary artery disease, and Smoke), pRESET characteristics, NIHSS score base, All Hemorrhagic complications rate, Symptomatic intracranial hemorrhage (sICH) Rate, Favorable functional outcome rate (mRS 0–2) at 90 days, Modified Rankin Scale (mRS 3–6) (each score for 3,4,5,6) at 90 days, mortality (%), Post-operative Overall Recanalization Rate (First pass), Latest follow-up Overall Recanalization Rate (final pass), number of passes, Time from symptoms to puncture (min), Procedure time (min), duration of exposure.
Quality assessment
All included papers underwent methodological assessment using the Joanna Briggs Institute (JBI) quality rating checklist for non-randomized experimental studies. This comprehensive tool evaluates crucial criteria, including the presence of a control group, use of multiple outcome measurement methods before and after intervention, completion of follow-up, description and analysis of group differences at follow-up, clarity of cause and effect, similarity of participants across comparison groups, consistency in treatment or care (except for the intervention of interest), consistent order of outcome measurement, reliability of measurements, and appropriateness of statistical analysis. Studies scoring 50% or above on these quality evaluation metrics were categorized as good quality. To ensure rigor, two writers conducted independent quality control checks. Any disagreements arising during the data abstraction process were resolved through consensus, maintaining the integrity of the quality assessment procedure (Supplemental Table S2).
Data synthesis and meta-analysis
Cochrane Handbook was used to select the proper effect size for pooling the outcomes. 9 The diversity among studies was quantified, and a high level of heterogeneity was deemed to exist if the Q test p-value was <0.001 and I2 exceeded 40%. The robustness of outcomes was investigated by a sensitivity analysis using a leave-one-out meta-analysis. Given the limited number of included studies, we approached the interpretation of our findings with caution. Meta-regression was performed to find the factors that significantly impact outcomes and determine the source of heterogeneity (Supplemental Table S3). The variable with R^2 metrics >0% was considered a source of heterogeneity. The variables with a p-value <.05 significantly impact their metrics. The meta-analysis was conducted using the “meta” package of “R.”
Results
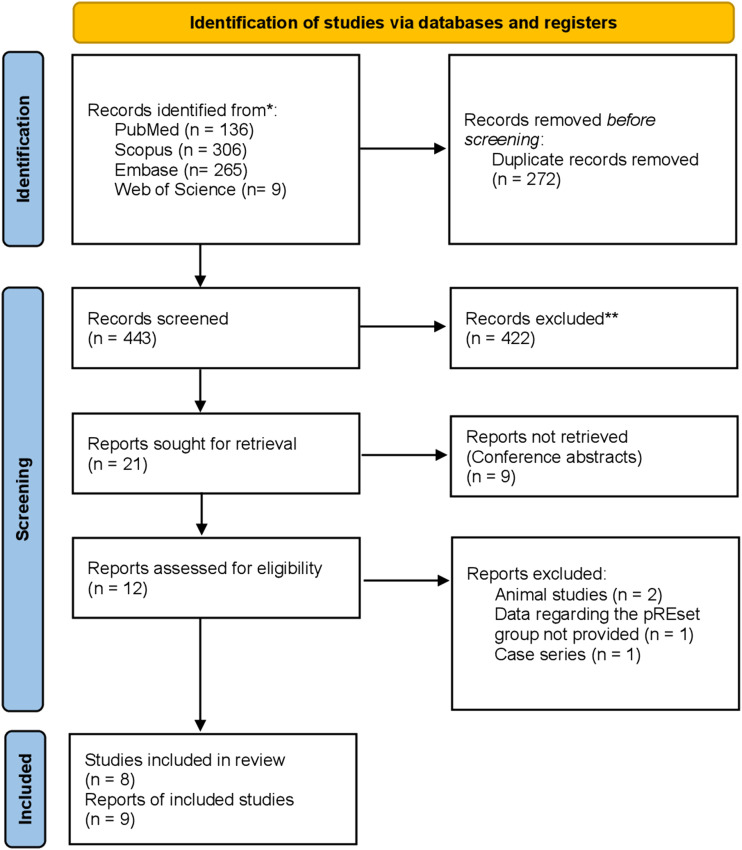
Overall, 693 articles were identified through database searches. After removing 262 duplicates, the titles and abstracts of the remaining articles were reviewed. After this stage, 20 articles were selected for full-text review. In the full-text review, 13 articles were removed due to conference abstracts (n = 9), animal studies (n = 2), not reporting the desired outcome (n = 1), and case series (n = 1). Finally, eight articles were chosen for quality assessment and meta-analysis. One study (Candel et al. 2021 10 ) used two different devices and provided data regarding each device separately. To account for the potential differences and avoid biasing the results, we treated these datasets as separate studies within our meta-analysis. Figure 1 illustrates the PRISMA flowchart of the study selection process.
Figure 1.
PRISMA flowchart of the study selection process.
Table 1 demonstrates the descriptive characteristics of the studies. There were four prospective studies, four retrospective studies, and one randomized clinical trial in which the pRESET stent retriever was compared to the solitaire stent retriever. Since our study focuses on the pRESET device, we only collected the data regarding the pRESET group in this article. Overall, the studies included 1163 patients (572 male/591 female) with acute ischemic stroke. All studies were performed in Germany, except for Nogueira et al. 2024, 11 which was performed in both Germany and the United States.
Table 1.
Demographic characteristics.
| Author/Year | Country | Type of study | Number of patients | Male/Female | Mean age | Follow-up | NIHSS score at baseline | Patients received tPA |
|---|---|---|---|---|---|---|---|---|
| Schwaiger 2014 15 | Germany | Retrospective study | 48 | 24/24 | 71 ± 11.9 | 3 months | 15 | 65.4% |
| Candel 2021 10 | Germany | Prospective study | 57 | 27/30 | 77.2 ± 11.6 | 3 months | 16 | 47.4% |
| Candel 2021 10 | Germany | Prospective study | 113 | 46/67 | 77.6 ± 13 | 3 months | 14 | 35.4% |
| Prothmann 2017 14 | Germany | Prospective study | 100 | 45/55 | 68.3 ± 13.8 | 3 months | 15 | 63% |
| Nogueira 2024 7 | USA & Germany | RCT | 173 | 90/83 | 73 | 3 months | 16 | 32.9% |
| Kurre 2014 13 | Germany | Retrospective study | 271 | 132/139 | 71 (19-93) | 3 months | 15 | _ |
| Kurre 2017 12 | Germany | Prospective study | 76 | 42/34 | 71 (36-93) | NA | 14 | _ |
| Kraejling 2023 2 | Germany | Retrospective study | 98 | 52/46 | 71.4 ± 15.5 | NA | 17 | 48% |
| Wang 2024 16 | Germany | Retrospective study | 227 | 114/113 | 69.5±4 | 3 months | 10 | 45% |
Table 2 demonstrates the procedure characteristics in each study. Four different sizes of pRESET stent-like stent retrievers were used: (4 × 20), (5 × 40), (6 × 30), and (6 × 50).
Table 2.
Procedure characteristics.
| Author/Year | Device used | Stroke locations | Number of passes | Time from symptoms to puncture (min) | Time from puncture to revascularization (min) | General anesthesia (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCA | ACA | PCA | ICA | BA | Other arteries | |||||||
| M1 (%) | M2 | |||||||||||
| Schwaiger 2014 15 | pREset 4 × 20 | 100 | _ | _ | _ | _ | _ | _ | Median: 2 (1-8) | 246 ± 88 | 85.40 | |
| Candel 2021 10 | pREset 4 × 20 | 100 | _ | _ | _ | _ | _ | _ | 1.6 ± 1.1 (1.6) | 246 IQR: (180 - 432) | 100 | |
| Candel 2021 10 | pREset 5 × 40 | _ | _ | _ | _ | _ | _ | Mean: 2 ± 1.4 (1-9) | 252 IQR: (186 - 444) | 100 | ||
| Prothmann 2017 14 | pREset 4 × 20 and 6 × 30 | 0.9% | 3.7% | 13.8% | 7.3% | 8% multiple strokes | Mean: 1.9 ± 1.31 | 207 (106 - 310) | 88 | |||
| Nogueira 2024 7 | pREset 4 × 20 and 5 × 40 | 51.5 | 24.9% | _ | _ | 13.3% | 5.2% | NA | 183 (133-252) | 27 (19-38) | 37 | |
| Kurre 2014 13 | pREset 4 × 20 and 6 × 30 | 51.7 | 11.1% | _ | 22.5% | 11.7% | _ | Median: 2 (1-10) | 265 (85 - 639) | 100 | ||
| Kurre 2017 12 | pREset Lite (3 × 20 and 4 × 20) | 1.1 | 67.8% | 4.4% | _ | 1.1% | 1.3 (0-4) | 255 (112-486) | 100 | |||
| Kraejling 2023 2 | pREset 6 × 50 | 71 | 6% | _ | _ | 17% | 5% | _ | 1.5 (1-6) | 344 ± 250 | 46 ± 28 | 100 |
| Wang 2024 16 | 2(1-3) | 250 (161–477) | 6 | |||||||||
Hemorrhagic complications
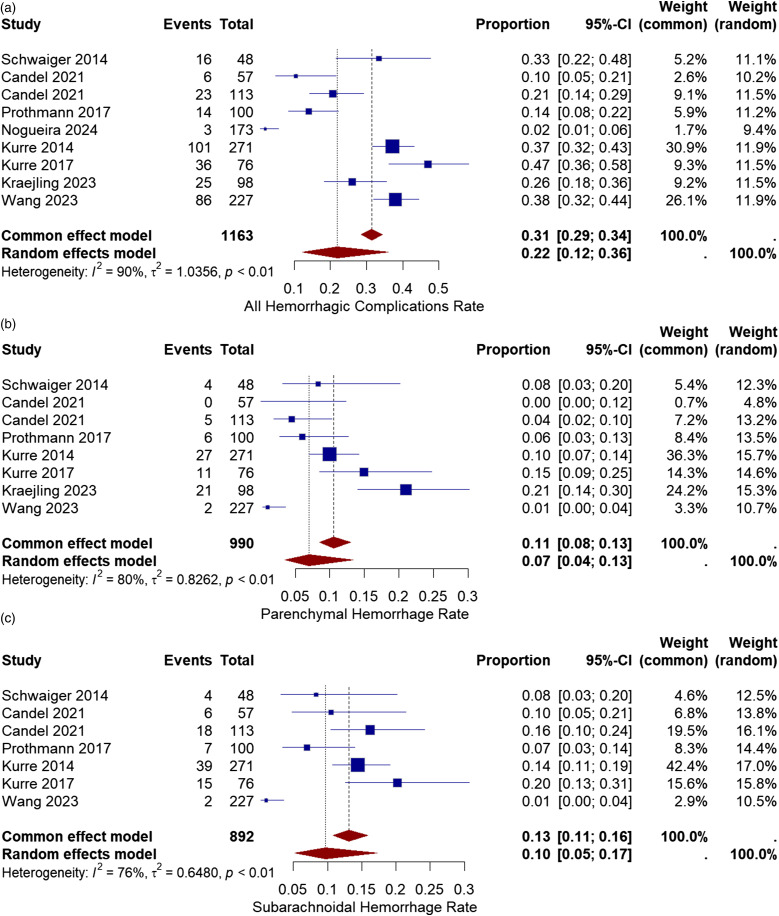
Hemorrhagic complications after thrombectomy include subarachnoidal hemorrhage, parenchymal hemorrhage, petechial hemorrhage, and intracranial hemorrhage. All eight studies reported the rate of hemorrhage after the procedure.2,10–16 The hemorrhagic complication rate varied from 2% (only ICH) to 47% (any hemorrhage detected). The pooled proportion of all hemorrhagic complications was 22% with a 95% CI of [12%, 36%]. Statistical analysis showed high heterogeneity among the studies (I2 = 90%, chi-square p-value<.01) (Figure 2(a)).
Figure 2.
(a) Forest plot for all hemorrhagic complications after treatment with the pRESET device. (b) Forest plot for parenchymal hemorrhage rate after treatment with pRESET device. (c) Forest plot for subarachnoidal hemorrhage rate after treatment with the pRESET device.
To identify the source of heterogeneity observed in all hemorrhagic complication rate and factors associated with it, meta-regression was performed. Only, ICA with (p-value<.01) has a meaningful association.
Seven studies reported the rate of parenchymal hemorrhage (PH) in their articles.2,10,12–16 The PH incidence in the studies varied from 0% to 21%. The Overall rate of parenchymal hemorrhage was 7% with a 95% CI of [4%, 13%]. The meta-analysis showed high heterogeneity among studies (I2 = 80%, chi-square p-value<.01) (Figure 2(b)).
The source of heterogeneity in PH and associated variables was examined using meta-regression. Coronary artery disease with (p-value = .023), and time from symptoms to puncture with (p-value = .025) show significant association.
Six studies reported the rate of subarachnoid hemorrhage (SAH) after the procedure based on the imaging.10,12,13,17,18 The reported SAH rate varied from 7% to 20%. The pooled proportion of subarachnoid hemorrhage was 10% with a 95% CI of [5%, 17%]. The studies showed moderate heterogeneity, which was not significant (I2 = 76%, chi-square p-value<.001) (Figure 2(c)).
Meta-regression was employed to investigate the sources of heterogeneity in SAH and the related variables. Consequently, significant correlations were found with atrial fibrillation with (p-value = .005), and diabetes mellitus with (p-value < .001).
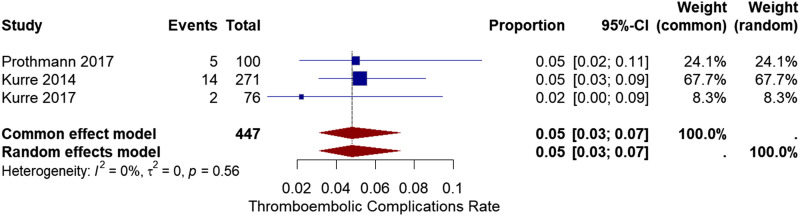
Thromboembolic complications
Three studies12,13,17 reported device-related embolic complications in the patients after the procedure. In the study by Prothmann et al. 2017, 17 and Kurre et al. 2017, 12 neither of these emboli cause any symptoms or deterioration. In the Kurre et al. 2014 13 study, these emboli cause three (5%) new infarcts. The pooled ratio of embolic complications was 5% with a 95% CI of [3%, 7%]. The heterogeneity analysis showed no significant heterogeneity (I2 = 0%, chi-square p-value = .56) (Figure 3).
Figure 3.
Forest plot for thromboembolic complications after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in thromboembolic complications and related variables. However, none of them exhibit a significant correlation.
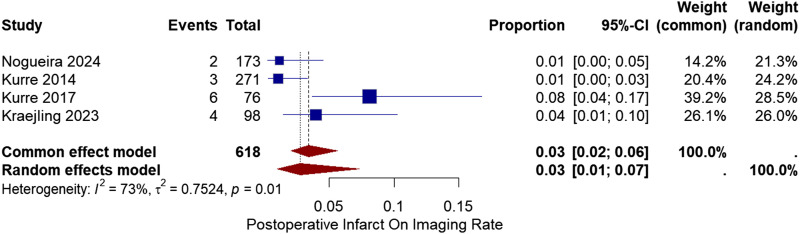
Post-operative infarction
Post-operative infarction was determined based on the imaging. Four studies reported the infarction rate on the imaging after the procedure.2,7,12,13 The pooled proportion of post-operative infarct was 3% with a 95% CI of [1%, 7%]. No significant heterogeneity was found between the studies (I2 = 73%, chi-square p-value = .01) (Figure 4).
Figure 4.
Forest plot for post-operative infarction rate on imaging after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in post-operative infarction and related variables with it. Only number of passes with (p-value = .015) exhibit a significant correlation.
NIHSS score at discharge
While all studies calculated the pre-operative NIHSS score, only three reported the NIHSS score at discharge.2,17,18 In the study by Schwaiger et al. 2014 18, the median NIHSS score was reduced to 7 at discharge. Prothmann et al. in 2017 17 showed an improvement in NIHSS scores reporting from 0 to 27. Also, Kraejling et al. 2023 2 reported a reduction in the median NIHSS score reporting 7.5.
Neurological outcome
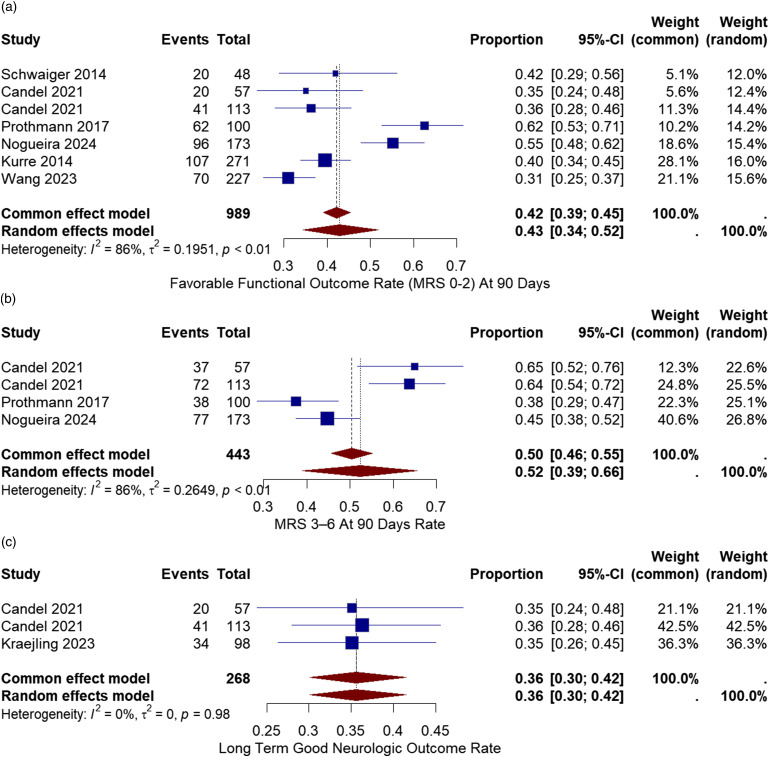
The neurological outcome was measured after 3 months using the modified Ranking Scale (mRS). A good neurological outcome was defined as an mRS score of (0-2). All six studies reported good neurological outcomes after 90 days.10,11,13–16 The good neurologic outcome varied from 31% to 62%. The overall rate of favorable neurological outcome was 43% with a 95% CI of [34%, 52%]. There was considerable heterogeneity between the studies (I2 = 86%, chi-square p-value<.01) (Figure 5(a)).
Figure 5.
(a) Forest plot for Good neurological outcome (mRS 0-2) after 3 months rate after treatment with the pRESET device. (b) Forest plot for neurological outcome (mRS 3-6) after 3 months rate after treatment with the pRESET device. (c) Forest plot for Good neurological outcome (mRS 0-2) after 3 months rate after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in good neurological outcomes after 90 days and related variables. Therefore, coronary artery disease with (p-value < .001), smoke with (p-value < .001), cardioembolic stroke with (p-value = .025), time from symptoms to puncture with (p-value = .002), dyslipidemia with (p-value = .033), ICA with (p-value = .004) and MCA with (p-value = .03) exhibit a significant correlation.
Three studies reported mRS score (3-6) outcomes after 90 days.10,11,13–16 The neurologic outcome varied from 38% to 64%. The overall rate of neurological outcome was 52% with a 95% CI of [39%, 66%]. There was considerable heterogeneity between the studies (I2 = 86%, chi-square p-value<.01) (Figure 5(b)).
Meta-regression was employed to investigate the sources of heterogeneity in mRS score (3-6) outcomes after 90 days and the related variables. Consequently, significant correlations were found with procedure time with (p-value < .001), coronary artery disease with (p-value < .001), smoke with (p-value < .001), cardioembolic stroke with (p-value < .001), time from symptoms to puncture with (p-value = .023), dyslipidemia with (p-value < .001) and mean age with (p-value < .001).
Two studies reported long-term good neurologic outcome.2,10 The overall rate of long-term good neurologic outcome was 36% with a 95% CI of [30%, 42%]. There was no considerable heterogeneity between the studies (I2 = 0%, chi-square p-value = .98) (Figure 5(c)).
Meta-regression was used to analyze the source of heterogeneity in long-term good neurologic outcome and related variables. However, none of them exhibit a significant correlation.
Recanalization rate (first pass and final pass)
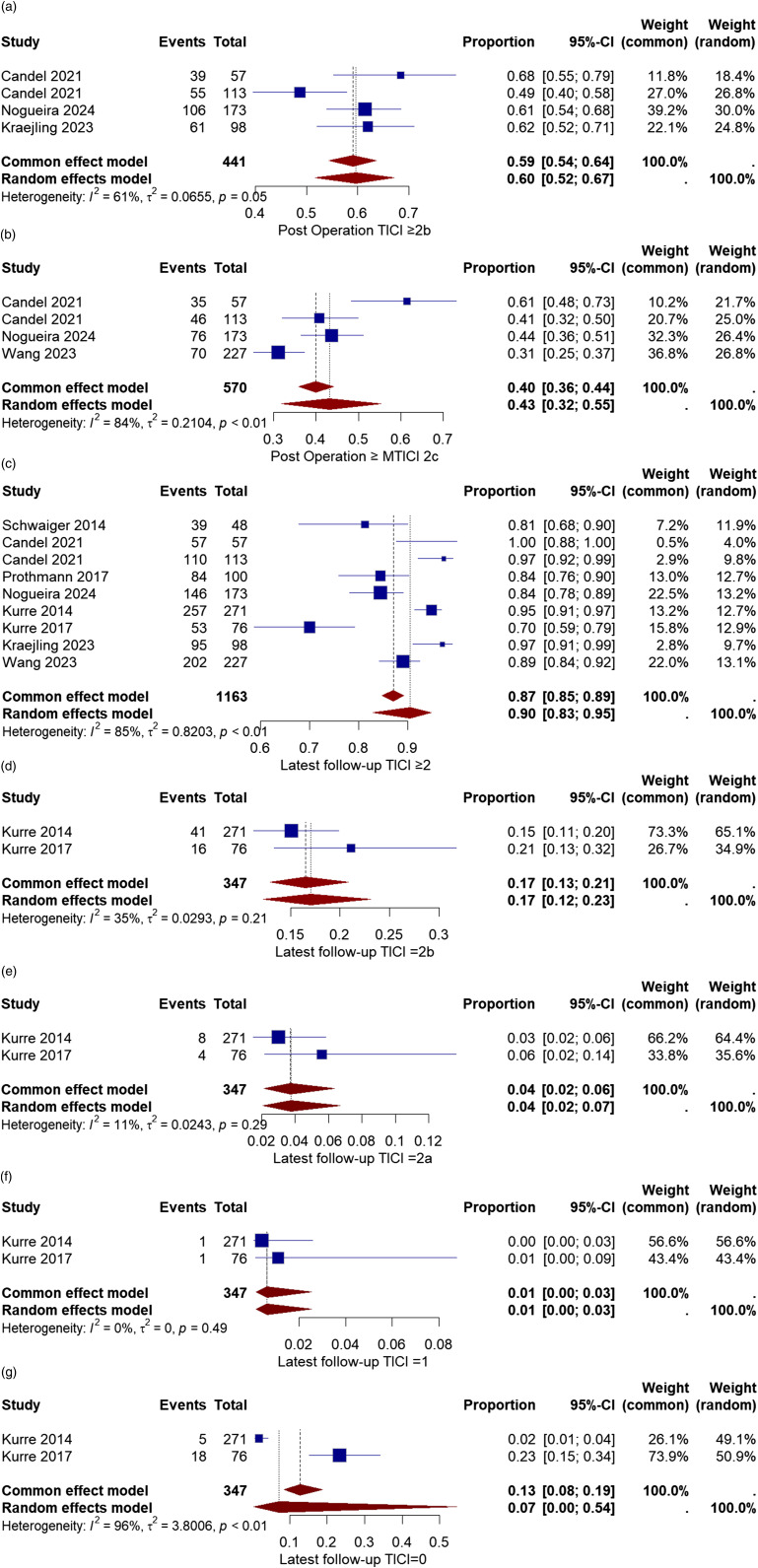
The modified thrombolysis in cerebral infarction score (mTICI) determined the recanalization success. A successful recanalization was defined as a TICI≥2b, and a complete recanalization was described as a TICI≥2c. Three studies provided the successful recanalization rate after the first pass.,2,10,11 and three also provided the complete recanalization rate after the first pass.10,11,16
The rate of successful recanalization (TICI≥2b) in the first pass varied from 49% to 68%. The pooled ratio was 60% with a 95% CI of [52%, 67%]. The overall heterogeneity between the studies was moderate (I2 = 61%, chi-square p-value = .05) (Figure 6(a)).
Figure 6.
(a) Forest plot for successful recanalization (mTICI≥2b) after first-pass rate after treatment with the pRESET device. (b) Forest plot for complete recanalization (mTICI≥2c) after first-pass rate after treatment with the pRESET device. (c) Forest plot for successful recanalization (mTICI≥2b) after the final pass rate after treatment with the pRESET device. (d) Forest plot for TICI = 2b after the final pass rate after treatment with the pRESET device. (e) Forest plot for TICI = 2a after the final pass rate after treatment with the pRESET device. (f) Forest plot for TICI = 1 after the final pass rate after treatment with the pRESET device. (g) Forest plot for TICI = 0 after the final pass rate after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in successful recanalization (TICI≥2b) in the first pass and related variables with it. Only the NIHSS score base with (p-value = .018) exhibits a significant correlation.
The complete recanalization (TICI≥2c) rate in the first pass varied from 31% to 61%. The overall rate was 43% with a 95% CI of [32%, 55%]. The calculated heterogeneity was substantial (I2 = 84%, chi-square p-value<.01) (Figure 6(b)).
The source of heterogeneity in complete recanalization (TICI≥2c) rate in the first pass and associated variables were examined using meta-regression. NIHSS score base with (p-value = .044), and number of passes with (p-value = .008) show significant association.
The rate of successful recanalization after the final pass was calculated in all eight studies.2,10–16 In addition, two studies provided the rate of TICI = 2b, TICI = 2a, TICI = 1, and TICI = 0 after the final pass.12,13 The rate of success varied from 70% to 100%. The pooled rate for successful recanalization (mTICI≥2b) was 90% with a 95% CI of [83%, 95%]. The heterogeneity among the studies was considered substantial (I2 = 85%, chi-square p-value<.01) (Figure 6(c)).
The overall rate of TICI = 2b, TICI = 2a, TICI = 1, and TICI = 0 after the final pass were 17% with a 95%CI [12%, 23%], 4% with a 95%CI [2%, 7%], 1% with a 95%CI [0%, 3%] and 7% with a 95%CI [0%, 54%], respectively (Figure 6(d)–(g)).
Meta-regression was used to analyze the source of heterogeneity in successful recanalization after the final pass and related variables. Therefore, coronary artery disease with (p-value = .013), and PCA with (p-value < .001) exhibit a significant correlation.
Rescue devices
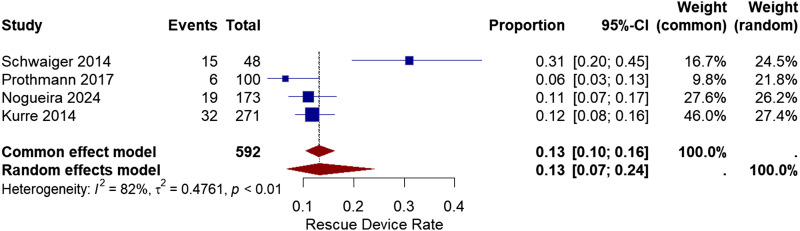
In cases of treatment failure, rescue devices were used to achieve successful recanalization. Rescue devices include aspiration catheters, intracranial stenting, and stent-like stent retrievers from other manufacturers. Four studies reported the use of different devices.11,13–15 The rate of rescue device use reported among the studies varied from 6% to 31%. The overall use of supplementary devices was 13% with a 95% CI of [7%, 24%]. The overall heterogeneity between the studies was (I2 = 82%, chi-square p-value<.01) (Figure 7).
Figure 7.
Forest plot for rescue devices used after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in rescue devices and related variables. However, none of them exhibit a significant correlation.
Mortality
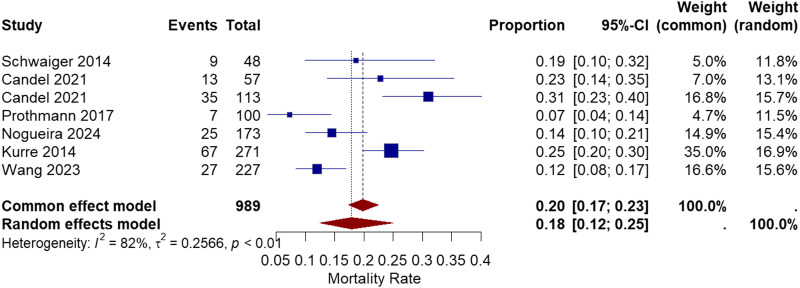
Six studies reported the mortality rate from the stroke onset until 3 months follow-up.10,11,13–16 The range of mortality rate was between 7% and 31%. The pooled proportion of mortality rate was 18% with a 95% CI of [12%, 25%]. The heterogeneity among the studies was (I2 = 82%, chi-square p-value<.01) (Figure 8).
Figure 8.
Forest plot for 3 months mortality rate after treatment with the pRESET device.
Meta-regression was used to analyze the source of heterogeneity in mortality rate and related variables. Coronary artery disease with (p-value = .014), smoke with (p-value = .001), and mean age with (p-value = .008) exhibit a significant correlation.
Publication bias
The Egger test evaluated the publication bias. The funnel plots are provided in the supplementary file. No significant publication bias was found for mortality (t = −1.16, p-value: 0.2992), post-operative infarct (t = −3.39, p-value: 0.0772), Thromboembolic complications (t = −1.92, p-value: 0.3058), subarachnoid hemorrhage (t = −2.41, p-value: 0.0606), long term good neurologic outcome (t = −0.83, p-value: 0.5604), mRS 3–6 At 90 days (t = 0.79, p-value: 0.5105), Favorable functional outcome rate (t = 0.31, p-value: 0.7662), successful recanalization in first-pass (t = 0.55, p-value: 0.6384), complete recanalization in first-pass (t = 2.15, p-value: 0.1645), successful recanalization in the final pass (t = 1.64, p-value: 0.1454), and use of rescue devices (t = 0.11, p-value: 0.9224). The publication bias for all hemorrhagic complication rates (t = −3.37, p-value: 0.0119) and parenchymal hemorrhage (t = −2.40, p-value: 0.0535 was significant.
Sensitivity analysis
A sensitivity analysis was conducted using a leave-one-out analysis to assess the robustness of outcomes. The study revealed the robustness of outcomes for all hemorrhagic complication rate (all p-value<.001), thromboembolic complications (all p-value<.001), favorable functional outcome rate (all p-value<.001), mRS 3–6 At 90 days (all p-value<.001), long term good neurologic outcome (all p-value<.001), post-operative infarct (all p-value<.001), mortality (all p-value<.001), use of rescue devices (all p-value<.001), parenchymal hemorrhage (all p-value<.001), subarachnoid hemorrhage (all p-value<.001), complete recanalization in first-pass (all p-value<.001), successful recanalization in first-pass (all p-value<.001), TICI = 1 in final-pass (all p-value<.001) and TICI = 0 in final-pass (all p-value<.001) (supplementary materials).
Discussion
This systematic review analyzed the studies in which the pRESET stent-like stent retriever was used to treat acute ischemic stroke. There have been several systematic reviews on different stent retrievers, but to our knowledge, this is the first meta-analysis to evaluate pRESET stent retrievers. The pRESET stent retriever is utilized in the treatment of thromboembolic stroke by effectively recanalizing intracranial vessel occlusions.19,20 This device operates by engaging in thrombectomy passes to restore blood flow through the occluded vessel, achieving successful recanalization in a significant percentage of cases. 19 The safety and efficacy of the pRESET stent retriever have been demonstrated in real-world clinical settings, with favorable clinical outcomes observed in a considerable proportion of patients.19,20 Additionally, the device’s mechanism involves minimizing device-related complications, such as parenchymal hematomas and subarachnoid hemorrhages, to ensure optimal patient outcomes. 19 The pRESET stent retriever’s ability to swiftly and effectively restore blood flow in thromboembolic stroke cases underscores its importance in acute stroke interventions. 20
Stent retrievers are currently considered safe and effective devices for mechanical thrombectomy in patients with acute ischemic stroke. Different manufacturers have developed various stent retrievers, which vary in size, diameter, and mechanical properties. 21 There have not been many studies that directly compare stent retriever devices. Most research on mechanical thrombectomy has focused on comparing different techniques and approaches, like using direct aspiration first versus stent retriever, instead of looking at the pros and cons of various devices. 22 The PROST trial performed by Nogueira et al., 7 compared pRESET to Solitaire stent retriever. There was no significant difference in the number of passes, recanalization success, procedure time, mortality, complications, and favorable outcomes between groups, and thus concluded that the pRESET stent retriever was a safe and effective option for patients. Mechanical thrombectomy is currently the treatment of choice for patients with large vessel occlusions, but its efficacy and safety in patients with distal and small vessel occlusions are still not thoroughly investigated. 23 Two small case series studies, one by Cerejo et al., 24 which used a Mindframe device, and one by Haussen et al., 25 which used the Baby Trevo stent retriever, suggested that stent retrievers can result in good recanalization in small vessels with reasonable safety margins. A clinical trial by Kühn et al., 26 examined Baby Trevo in a larger population, which showed a successful recanalization of 85.7% of patients. In another trial by Hofmeister et al., 23 which used the Cath Mini stent retriever, 78% of patients achieved successful recanalization. In the study by Kurre et al. 2017, 12 the stent retriever pRESET Lite was used, which exhibited a recanalization rate of 70%. These results suggest that stent retrievers can also be used in small vessel strokes, and the pRESET device could be an acceptable choice. However, more clinical trials are needed to determine its efficacy compared to other devices.
Meta-regression analysis revealed that heterogeneity in the study outcomes was significantly influenced by various risk factors. Notably, coronary artery disease and smoking were found to be major contributors to this heterogeneity. These findings suggest that individuals with these risk factors may experience different outcomes, thereby increasing variability in the data. Additionally, the location of the stroke emerged as another important factor affecting heterogeneity. This indicates that the impact of a stroke can vary considerably depending on which part of the brain is affected, further contributing to the observed variability. These insights underscore the importance of considering these variables when analyzing stroke outcomes and tailoring interventions accordingly.
Recanalization is a primary endpoint in evaluating the efficacy of stent retrievers. The pRESET device demonstrates a 90% successful recanalization rate (mTICI≥2b) after the final pass, indicating a robust performance in restoring blood flow. This compares favorably to the Solitaire and Trevo devices, which collectively achieve an 84.5% recanalization rate. 27 Zaidat et al. reported the first-pass and final-pass recanalization rates at 50.8% and 86.6% for EmboTrap, 42.1% and 82.8% for Trevo, 46.5% and 81.7% for Solitaire, without any meaningful difference among the groups. 22 The slightly higher recanalization rate of pRESET suggests it may be more effective in achieving the primary goal of mechanical thrombectomy, which is the reopening of occluded vessels.
Functional outcomes are assessed using the mRS, with a score of 0-2 indicating a favorable neurological outcome. The pRESET device reports that 43% of patients achieve a favorable outcome at 3 months. In contrast, the Solitaire and Trevo devices report a higher rate of 51.2% and 47.6% for achieving independent functional outcomes.5,27 Zaidat et al. reported 57.4% for EmboTrap, 50% for Trevo, and 45.3% for Solitaire. 22 Saber et al. also reported 2.55 OR for Solitaire and 4.14 for Trevo. 28 The lower rate of favorable outcomes with pRESET could be influenced by various factors including patient selection and procedural differences, but it highlights a potential area for further investigation and improvement.
The mortality rate at 3 months for the pRESET device is 18%, which is comparable to 16.2% with Solitaire, 5 22.2% 5 with Trevo and 16.8% 27 both. Zaidat et al. reported the mortality rate for EmboTrap at 11.2%, Trevor at 14.5%, and Solitaire at 20.4%. 22 This similarity suggests that the pRESET device does not present additional mortality risk compared to these other devices.
The efficiency of a stent retriever can also be measured by the number of passes required to achieve successful recanalization. Both pRESET and the combined results for Solitaire and Trevo report a mean of 2.0 passes. 27
The pRESET device has a 22% rate of all hemorrhagic complications and a 7% rate of parenchymal hemorrhage. The rate of hemorrhagic complications with pRESET warrants careful consideration, as it suggests a potential safety concern that could impact its overall clinical utility.
In the context of stent placement for thromboembolic stroke, studies have shown that trans-radial access (TRA) is a feasible and safe alternative to transfemoral access, with comparable outcomes in terms of procedural success and major bleeding rates. 29 Research indicates that TRA may offer advantages such as reduced hospital stay, shorter procedural times, and lower rates of major bleeding compared to femoral access. 30 While femoral access has historically been the standard for mechanical thrombectomy (MT), recent evidence supports the efficacy and safety of radial access for neuro-interventional procedures, including in posterior circulation strokes. 30 Tailoring vascular access to individual patient characteristics may further optimize outcomes in stent placement for thromboembolic stroke, highlighting the importance of considering the benefits of TRA in this context. 31
Several factors can impact the efficacy of mechanical thrombectomy as well as short and long-term outcomes. The size of the retriever can significantly affect the procedure outcomes. In the study by Candel et al., 10 the 5 × 40 device achieved higher successful perfusion in a lower number of passes. The article by Haussen et al., 32 which used Solitare and Trevo stent retrievers, also showed that the length of the device was independently associated with a successful first-pass reperfusion rate. The baseline NIHSS score can affect the outcomes of mechanical thrombectomy. Patients with lower NIHSS scores at admission had significantly better outcomes.33–37 In addition, lower NIHSS after 24 h was associated with favorable functional outcomes.35,37 Other predictors of favorable outcomes after a mechanical thrombectomy include a higher DWI-ASPECT score34–36,38 and successful recanalization.34–36 The use of intravenous thrombolysis (IV tpa) can also affect perfusion rates and neurological outcomes, but its role remains controversial. A meta-analysis by Kaesmacher et al., 39 which included 20 studies, found that although the use of IV tpa was associated with better neurological function and lower mortality, the quality of evidence is low, and more controlled trials are needed. Longer onset-to-recanalization time, 40 a higher number of passes (more than 3),41,42 and use of rescue devices 42 are associated with worse outcomes in mechanical thrombectomy with stent retrievers. The anatomy of the vessels also plays a role in the outcome, as curved and tortuous arteries have lower chances of recanalization. 43
Limitations
This study is limited by the heterogeneity in the procedure and the devices used that could affect the results. Also, all the studies were conducted in Germany, except for one study that was conducted in both Germany and the USA. So, these results could not be attributed to different populations. Furthermore, the clinical significance of complications was not fully explained in all of the studies and, therefore, could not be analyzed.
Conclusion
Treatment devices for acute ischemic stroke continue to evolve. The pRESET stent retriever is a new device for mechanical thrombectomy, and it is a safe and effective option. This systematic review shows that it has high recanalization rates with low clinically significant adverse events and is comparable to other stent retrievers such as Solitaire, Trevo, and EmboTrap. However, more randomized controlled trials are needed to directly compare its effectiveness and safety against other stent retrievers.
Supplemental Material
Supplemental Material for The safety and efficacy of pRESET stent retriever for treatment of thrombo-embolic stroke; a systematic review and meta-analysis by Mohammad Amin Habibi, Muhammad Hussain Ahmadvand, Pouria Delbari, Saba Sabet, Amir Hessam Zare, Mohammad Sina Mirjani, Amir Reza Boskabadi, Zahra Aslani Kolur and Maryam Bozorgi in The Neuroradiology Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethical approval
The study is deemed to exempt to received ethical approval
ORCID iD
Mohammad Amin Habibi https://orcid.org/0000-0001-7600-6925
References
- 1.Piasecki P, Wierzbicki M, Narloch J, et al. Mechanical thrombectomy of large vessel occlusion using adjustable vs. self-expanding stent-retriever-Comparison of Tigertriever device with stent-like stent-retrievers: a propensity score analysis. Front Neurol 2022; 13: 1032307. 20230118. DOI: 10.3389/fneur.2022.1032307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraehling H, Akkurt BH, Elsharkawy M, et al. Evaluation of effectiveness and safety of the large-format pRESET 6-50 thrombectomy stent-retriever in the endovascular treatment of ischemic stroke: real-world experiences from two tertiary comprehensive stroke centers. Front Neurol 2023; 14: 1256365. 20231117. DOI: 10.3389/fneur.2023.1256365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prothmann S, Kober F, Wunderlich S, et al. Mechanical recanalization ofacutem1 occlusions with the preset® stentretriever in 48 patients-technical success and clinical outcome. Neuroradiology 2013; 55: S26–S27. Conference Abstract. DOI: 10.1007/s00234-013-1236-8. [DOI] [Google Scholar]
- 4.Fusaro F, Corraine S, Erta M, et al. Acute ischemic stroke due to distal vessel occlusion treated with preset lite stent retriever. Single center experience. J Neurointerventional Surg 2023; 15: A71–A72. Conference Abstract. DOI: 10.1136/jnis-2023-ESMINT.178. [DOI] [Google Scholar]
- 5.Grech R, Pullicino R, Thornton J, et al. An efficacy and safety comparison between different stentriever designs in acute ischaemic stroke: a systematic review and meta-analysis. Clin Radiol 2016; 71: 48–57: 20151117. DOI: 10.1016/j.crad.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Comelli S, Fusaro F, Erta M, et al. Treatment of distal intranial occlusion with pREset LITE and LUX stent retriever. A single center experience. Intervent Neuroradiol 2022; 28: 194. Conference Abstract. DOI: 10.1177/15910199221142495. [DOI] [Google Scholar]
- 7.Nogueira RG, Lobsien D, Klisch J, et al. Thrombectomy with the pRESET vs solitaire stent retrievers as first-line large vessel occlusion stroke treatment. JAMA Neurol. 2024; 81(2): 170–178. DOI: 10.1001/jamaneurol.2023.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthew JP, Joanne EM, Patrick MB, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. DOI: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks JJ, Higgins JP, Altman DG, et al. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A.). 2019: 241–284. 10.1002/9781119536604 [DOI] [Google Scholar]
- 10.Serna CC, Aguilar Pérez M, Bäzner H, et al. First-pass reperfusion by mechanical thrombectomy in acute M1 occlusion: the size of retriever matters. Front Neurol 2021; 12: 679402. 20210622. DOI: 10.3389/fneur.2021.679402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira RG, Lobsien D, Klisch J, et al. Thrombectomy with the pRESET vs solitaire stent retrievers as first-line large vessel occlusion stroke treatment: a randomized clinical trial. JAMA Neurol 2024; 81: 170–178. DOI: 10.1001/jamaneurol.2023.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurre W, Aguilar-Pérez M, Martinez-Moreno R, et al. Stent retriever thrombectomy of small caliber intracranial vessels using pREset LITE: safety and efficacy. Clin Neuroradiol 2017; 27: 351–360: 20160121. DOI: 10.1007/s00062-016-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurre W, Aguilar-Pérez M, Schmid E, et al. Clinical experience with the pREset stent retriever for the treatment of acute ischemic stroke--a review of 271 consecutive cases. Neuroradiology 2014; 56: 397–403: 20140312. DOI: 10.1007/s00234-014-1346-y. [DOI] [PubMed] [Google Scholar]
- 14.Prothmann S, Schwaiger BJ, Gersing AS, et al. Acute Recanalization of Thrombo-Embolic Ischemic Stroke with pREset (ARTESp): the impact of occlusion time on clinical outcome of directly admitted and transferred patients. J Neurointerv Surg 2017; 9: 817–822: 20160816. DOI: 10.1136/neurintsurg-2016-012556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwaiger BJ, Kober F, Gersing AS, et al. The pREset stent retriever for endovascular treatment of stroke caused by MCA occlusion: safety and clinical outcome. Clin Neuroradiol 2016; 26: 47–55: 20140812. DOI: 10.1007/s00062-014-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Henkes H, Ghozy S, et al. Use of the pRESET LITE thrombectomy device in combined approach for medium vessel occlusions: a multicenter evaluation. Neuroradiology 2024; 66: 631–641: 20240221. DOI: 10.1007/s00234-024-03302-5. [DOI] [PubMed] [Google Scholar]
- 17.Prothmann S, Schwaiger BJ, Gersing AS, et al. Acute Recanalization of Thrombo-Embolic Ischemic Stroke with pREset (ARTESp): the impact of occlusion time on clinical outcome of directly admitted and transferred patients. J Neurointerventional Surg 2017; 9: 817. DOI: 10.1136/neurintsurg-2016-012556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwaiger BJ, Kober F, Gersing AS, et al. The pREset stent retriever for endovascular treatment of stroke caused by MCA occlusion: safety and clinical outcome. Clin Neuroradiol 2016; 26: 47–55. DOI: 10.1007/s00062-014-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurre W, Aguilar-Pérez M, Schmid E, et al. Clinical experience with the pREset stent retriever for the treatment of acute ischemic stroke—a review of 271 consecutive cases. Neuroradiology 2014; 56: 397–403. DOI: 10.1007/s00234-014-1346-y. [DOI] [PubMed] [Google Scholar]
- 20.Prothmann S, Schwaiger BJ, Gersing AS, et al. Acute Recanalization of Thrombo-Embolic Ischemic Stroke with pREset (ARTESp): the impact of occlusion time on clinical outcome of directly admitted and transferred patients. J Neurointerventional Surg 2017; 9: 817–822. DOI: 10.1136/neurintsurg-2016-012556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen N, Oberbeck K, de Miranda RL, et al. Comparison of efficacy, embolism rate and safety of thrombectomy with stent retrievers in an anterior circulation stroke model. In RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 2018. © Georg Thieme Verlag KG, pp. 1053–1058. [DOI] [PubMed] [Google Scholar]
- 22.Zaidat OO, Ikeme S, Sheth SA, et al. Mastro I: meta-analysis and systematic review of thrombectomy stent retriever outcomes: comparing functional, safety and recanalization outcomes between EmboTrap, Solitaire and Trevo in acute ischemic stroke. J Comp Eff Res 2023; 12: e230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmeister J, Kulcsar Z, Bernava G, et al. The Catch Mini stent retriever for mechanical thrombectomy in distal intracranial occlusions. J Neuroradiol 2018; 45: 305–309. DOI: 10.1016/j.neurad.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 24.Cerejo R, John S, Bauer A, et al. Emergent mechanical thrombectomy for acute stroke using the Mindframe Capture LP system: initial single-center experience. J Neurointerventional Surg 2016; 8: 1178–1180. [DOI] [PubMed] [Google Scholar]
- 25.Haussen DC, Lima A, Nogueira RG. The Trevo XP 3× 20 mm retriever (‘Baby Trevo’) for the treatment of distal intracranial occlusions. J Neurointerventional Surg 2016; 8: 295–299. [DOI] [PubMed] [Google Scholar]
- 26.Kühn AL, Wakhloo AK, Lozano JD, et al. Two-year single-center experience with the ‘Baby Trevo’stent retriever for mechanical thrombectomy in acute ischemic stroke. J Neurointerventional Surg 2017; 9: 541–546. [DOI] [PubMed] [Google Scholar]
- 27.Grech R, Mizzi A, Pullicino R, et al. Functional outcomes and recanalization rates of stent retrievers in acute ischaemic stroke: a systematic review and meta-analysis. NeuroRadiol J 2015; 28: 152–171: 20150430. DOI: 10.1177/1971400915576678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saber H, Rajah GB, Kherallah RY, et al. Comparison of the efficacy and safety of thrombectomy devices in acute stroke : a network meta-analysis of randomized trials. J Neurointerv Surg 2018; 10: 729–734: 20171215. DOI: 10.1136/neurintsurg-2017-013544. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez D, Requena M, Olivé-Gadea M, et al. Radial versus femoral access for mechanical thrombectomy in patients with stroke: a noninferiority randomized clinical trial. Stroke 2024; 55: 840–848: 20240201. DOI: 10.1161/strokeaha.124.046360. [DOI] [PubMed] [Google Scholar]
- 30.Catapano J, Naik A, Smoot M, et al. E-204 Outcomes of radial and femoral access in mechanical thrombectomy for posterior circulation occlusions: a multicenter propensity-adjusted analysis. J Neurointerventional Surg 2022; 14: A189. DOI: 10.1136/neurintsurg-2022-SNIS.315. [DOI] [Google Scholar]
- 31.Barranco-Pons R, Caamaño IR, Guillen AN, et al. Transradial versus transfemoral access for acute stroke endovascular thrombectomy: a 4-year experience in a high-volume center. Neuroradiology 2022; 64: 999–1009: 20211112. DOI: 10.1007/s00234-021-02850-4. [DOI] [PubMed] [Google Scholar]
- 32.Haussen DC, Al-Bayati AR, Grossberg JA, et al. Longer stent retrievers enhance thrombectomy performance in acute stroke. J Neurointerventional Surg 2019; 11: 6–8. [DOI] [PubMed] [Google Scholar]
- 33.Haranhalli N, Javed K, Boyke A, et al. A predictive model for functional outcome in patients with acute ischemic stroke undergoing endovascular Thrombectomy. J Stroke Cerebrovasc Dis 2021; 30: 106054. [DOI] [PubMed] [Google Scholar]
- 34.Panni P, Gory B, Xie Y, et al. Acute stroke with large ischemic core treated by thrombectomy: predictors of good outcome and mortality. Stroke 2019; 50: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 35.Soize S, Barbe C, Kadziolka K, et al. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology 2013; 55: 977–987. [DOI] [PubMed] [Google Scholar]
- 36.Tajima Y, Hayasaka M, Ebihara K, et al. Predictors of poor outcome after successful mechanical thrombectomy in patients with acute anterior circulation stroke. J Clin Interv Radiol ISVIR 2017; 1: 139–143. [Google Scholar]
- 37.Yildirim S. Predictors of good clinical outcome in acute ischemic stroke patients after mechanical thrombectomy. Turk J Cerebrovasc Dis 2023; 29: 00. [Google Scholar]
- 38.Cagnazzo F, Derraz I, Dargazanli C, et al. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS≤ 6: a meta-analysis. J Neurointerventional Surg 2020; 12: 350–355. [DOI] [PubMed] [Google Scholar]
- 39.Kaesmacher J, Mordasini P, Arnold M, et al. Direct mechanical thrombectomy in tPA-ineligible and-eligible patients versus the bridging approach: a meta-analysis. J Neurointerventional Surg 2019; 11: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozdemir O, Giray S, Arlier Z, et al. Predictors of a good outcome after endovascular stroke treatment with stent retrievers. Sci World J 2015;2015:403726. DOI: 10.1155/2015/403726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Maria F, Kyheng M, Consoli A, et al. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 2021; 16: 20–28. DOI: 10.1177/1747493020923051. [DOI] [PubMed] [Google Scholar]
- 42.Linfante I, Walker GR, Castonguay AC, et al. Predictors of mortality in acute ischemic stroke intervention. Stroke 2015; 46: 2305-2308. DOI: 10.1161/STROKEAHA.115.009530. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko N, Komuro Y, Yokota H, et al. Stent retrievers with segmented design improve the efficacy of thrombectomy in tortuous vessels. J Neurointerventional Surg 2019; 11: 119–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The safety and efficacy of pRESET stent retriever for treatment of thrombo-embolic stroke; a systematic review and meta-analysis by Mohammad Amin Habibi, Muhammad Hussain Ahmadvand, Pouria Delbari, Saba Sabet, Amir Hessam Zare, Mohammad Sina Mirjani, Amir Reza Boskabadi, Zahra Aslani Kolur and Maryam Bozorgi in The Neuroradiology Journal