Abstract
Objective:
We studied the prognosis of olfactory and gustatory dysfunctions in patients with long COVID (Coronavirus Disease 2019) after treatment with oral zinc and steroids.
Methods:
We measured olfactory and gustatory functions of long COVID patients at their first visits, and after 2–4 months of treatment with oral zinc and steroids using the traditional Chinese version of the University of Pennsylvania Smell Identification Test and the Waterless Empirical Taste Test. We also assessed by phone the recovery of olfactory and gustatory functions at a mean of about 10 months of follow-up.
Results:
Among our 71 long COVID patients, 34 complained of loss of smell and taste. Their objective test results showed 88.2% hyposmic, 23.5% hypogeusic at the first visit. After treatment, 77.8% of the patients were hyposmic, and 16.7% were hypogeusic. After a mean follow-up of 10.35 months, 91.2% of the patients reported improvement in their olfactory function. Among the 36 patients who had complained only of smell loss, the objective test results showed 75% hyposmic at their first visit. After treatment, 71.4% of the patients were hyposmic. After a mean of 10.42 months of follow-up, 77.8% of the patients reported improvement in their olfactory function. Only one patient complained of taste loss.
Conclusions:
We found that olfactory dysfunction in most long COVID patients persisted for more than 10 months.
Keywords: COVID-19, gustatory dysfunction, long COVID, olfactory dysfunction, prognosis, smell test, taste test
Introduction
Coronavirus Disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a major global health issue.1,2 The World Health Organization has designated five variants of SARS-CoV-2 of concern: Alpha, Beta, Gamma, Delta, and Omicron. The variant Omicron was first reported in South Africa in November 2021. 3 By April 20, 2022, the Omicron was the predominant variant in the world. 2
Smell and taste losses are common symptoms in patients with COVID-19. 4 Hyposmia, with or without hypogeusia, is considered a potential indicator of mild COVID-19 infection.5,6 Although smell and taste dysfunctions in 89% of COVID-19 patients typically resolves within 4 weeks, 4 many patients have residual smell loss. 7 Patients with COVID-19 who experience prolonged symptoms that last from 4 to 24 weeks are considered to have long COVID. 8
In this study, we investigated details of both the olfactory and gustatory recovery of long COVID patients who were infected with SARS-CoV-2 during the Omicron prevalent period in Taiwan. Olfaction and taste were measured with the traditional Chinese version of the University of Pennsylvania Smell Identification Test (UPSIT-TC, Sensonics International, Haddon Heights, NJ, USA) and the Waterless Empirical Taste Test (WETT, Sensonics International, Haddon Heights, NJ, USA). UPSIT-TC is a validated test for olfaction and WETT is a validated test for gustation. These tests are commercially available.9,10 The tests are self-administered and can be performed by patients with COVID-19 themselves.
Materials and methods
Study subjects
This was a retrospective study. We enrolled COVID-19 patients who were infected by SARS-CoV-2 during the Omicron variant pandemic in Taiwan and who had persistent loss of smell and/or taste for >1 month after infection between May 2022 and December 2023. We carefully collected the history of these long COVID patients. We included only those who had reported normal olfactory and gustatory functions preinfection and who had noticed losses of olfactory and/or gustatory functions postinfection. These eligible patients did not have a recent history of head trauma or diagnosis of autoimmune diseases. Patients received nasal endoscopy to examine the nasal cavity and olfactory clefts. If abnormal signs were detected, such as discharge, polypoid mucosa, or polyps suggesting sinonasal diseases, the patient was excluded from the study. They also received UPSIT-TC and WETT to evaluate their olfactory and gustatory functions. Then oral prednisolone (10 mg bid) and a zinc gluconate tablet (10 mg tid) were prescribed for a month. Any patient who had reported complete recovery from olfactory or gustatory function after treatment was also excluded from the study.
All eligible patients took another 1–3 months course of oral prednisolone and zinc gluconate, and received a second set of tests with UPSIT-TC and WETT for follow-up. At the end of December 2023, patients checked on the phone about their recovery of smell and/or taste functions. Our study was approved by the Institutional Review Board (I) of Taichung Veterans General Hospital (code: CE24036A). Written informed consent was exempted by the Institutional Review Board because this was a retrospective study.
Smell test
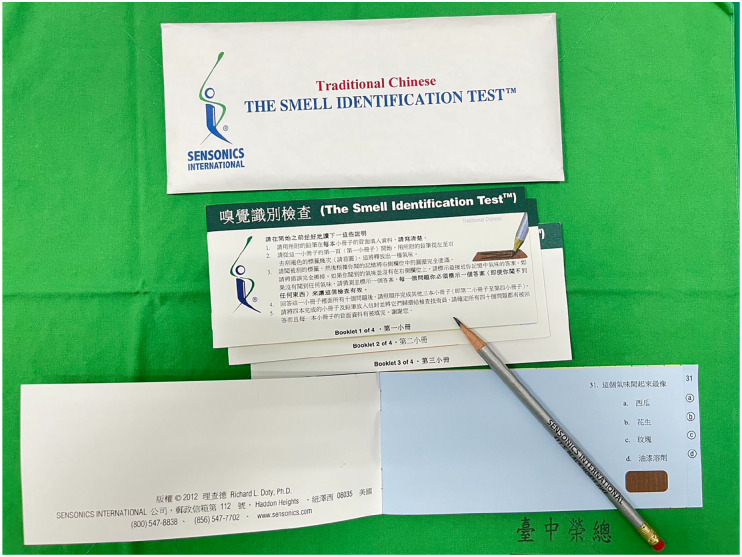
UPSIT-TC was used to assess olfactory function in this study. It is a modified version of the original American version of the University of Pennsylvania Smell Identification Test. It has 10-odorant booklets that can be self-administered and completed in 10–15 min (Figure 1). 11 Each booklet contains 10 “scratch & sniff” odorants that are embedded in microcapsules of 10–50 µm microcapsules fixed in a proprietary binder, and placed on brown strips at the bottom of the pages of each booklet. 12 Of the 40 odorants in the American version of the University of Pennsylvania Smell Identification Test, eight of them were replaced by other odorants in UPSIT-TC. Specifically, odorant “clove” was replaced by “sandalwood” (item 8), “cheddar cheese” by “fish” (item 14), “cinnamon” by “coffee” (item 15), “gingerbread” by “rubber tire” (item 20), “dill pickle” by “jasmine” (item 25), “lime” by “grapefruit” (item 27), “wintergreen” by “magnolia” (item 29) and “grass” by “body powder” (item 32).
Figure 1.
Traditional Chinese version of the University of Pennsylvania Smell Identification Test.
When performing UPSIT-TC, the patient was required to release each of the 40 odorants one by one, by scratching a brown strip with a pencil tip, and smell the odorant. The patient was required to identify each released odorant in a multiple choice question by selecting one of four odor choices. The final test score was the number of correctly identified odors.
The diagnosis of olfactory dysfunction was based on the criteria established in our previous study. 11 For male subjects aged 20–59 years, UPSIT-TC cut-off scores were the following: (a) 29.5 between normosmic and mildly hyposmic patients, (b) 26.5 between mildly hyposmic and moderately hyposmic patients, and (c) 16.5 between moderately hyposmic and anosmic patients. For female subjects of similar ages, UPSIT-TC cut-off scores were as follows: (a) 30.5 between normosmic and mildly hyposmic patients, (b) 27.5 between mildly hyposmic and moderately hyposmic patients, and (c) 17.5 between moderately hyposmic and anosmic patients. For older male subjects aged >60 years old, the cut-off scores were as follows: (a) 23.5 between normosmic and mildly hyposmic subjects, (b) 20.5 between mildly hyposmic and moderately hyposmic subjects, and (c) 13.5 between moderately hyposmic and anosmic subjects. For elderly female subjects aged >60 years old, UPSIT-TC cut-off scores were as follows: (a) 20.5 between normosmic and mildly hyposmic subjects, (b) 24.5 between mildly hyposmic and moderately hyposmic subjects, and (c) 15.5 between moderately hyposmic and anosmic subjects. In summary, male subjects aged 20–59 years with UPSIT-TC scores >30, were defined as having normal olfactory function. Female subjects of similar ages with UPSIT-TC scores >31 were defined as having normal olfactory function. Male subjects aged >60 years with UPSIT-TC scores >24 were defined as having normal olfactory function. Female subjects aged >60 years old with UPSIT-TC scores >25 were defined as having normal olfactory function.
For UPSIT-TC, a change between individual test scores and retest scores was defined as a value that fell outside the 95% confidence interval. It was calculated based on the change of four or more points in the American version of the University of Pennsylvania Smell Identification Test. 13 Therefore, we defined an increase of four or more points in the UPSIT-TC as an improvement in olfactory function.
Taste test
WETT was used to assess the gustatory function in this study. It consists of 53 disposable plastic strips. 14 The tip of each 1 × 6 cm2 strip has a 1 × 2.5 cm2 pad, which is made of monomer cellulose (Figure 2). On the pads of these strips, 40 are loaded with tastant and 13 are not. The pad of each strip of tastant is loaded with one of the following five tastants: sucrose, citric acid, sodium chloride, caffeine, and monosodium glutamate. Each tastant has four different concentrations. These concentrations vary according to the tastants: 0.20, 0.10, 0.05, and 0.025 g/ml for dried sucrose; 0.025, 0.05, 0.10, and 0.20 g/ml for citric acid; 0.0313, 0.0625, 0.125, and 0.25 g/ml for sodium chloride; 0.011, 0.022, 0.044, and 0.088 g/ml for caffeine; and 0.017, 0.034, 0.068, and 0.135 g/ml for monosodium glutamate. In total, there are 20 different types of tastant strip in the WETT. In a single WETT test, 20 different types of tastant strip were presented, each repeated twice, in counterbalanced order. Therefore, a total of 53 strips (that is, 40 with and 13 without tastants) are used in each taste test. The 13 strips without tastant are interspersed among the tastant strips in a specific sequence to avoid the need to rinse the mouth.
Figure 2.
Waterless Empirical Taste Test.
When performing the WETT, all patients were handed a strip. Patients placed the pad at the tip of the strip in the middle of the tongue, closed the mouth, and moved the strip slightly around. When patients thought they had tasted the strip, they were asked to select one of the six answers (sweet, sour, salty, bitter, brothy, or no taste at all). A point was scored if a correct answer was made, the scores from the 13 blank strips were not used for analysis of the scoring of the test, thus generating a 40 score maximum for completely correct quality identification. Based on our previous study, the normative WETT values were established at 16 for male adults and 23 for female adults. 15 The minimal detectable change is the minimal amount of change that a measurement must show to be greater than the variability and measurement error within the subject. 16 The minimal detectable change in the was set at eight for male adults and six for female adults.
Statistical analyses
Data were presented as mean ± standard deviation (SD). The gender of the patients was compared using the Pearson’s Chi-square test, between patients complaining of smell and taste loss and those who complained only of smell loss. The age of the patients was compared using the Mann–Whitney U test, between patients complaining of smell and taste loss and those who complained only of smell loss. The interval between SARS-CoV-2 infection and the date of the first outpatient visit was compared using the Mann–Whitney U test, between patients complaining of smell and taste loss and those who complained only of smell loss. The UPSIT-TC and WETT scores before treatment and treatment were compared in patients complaining of loss of smell and taste using the Wilcoxon Signed Ranks test. The UPSIT-TC scores before treatment and treatment were compared in patients who only complained of smell loss using the Wilcoxon Signed Ranks test. The analyzes were all performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Two-tailed p-values <0.05 were considered statistically significant.
Results
Study subjects
In this study, we enrolled 71 patients with long COVID with complaints of persistent loss of smell and/or taste functions for at least 1 month. They were infected with SARS-CoV-2 during the period from March 2022 to August 2023. They had then visited our outpatient clinic for help for periods from 1 to 11 months (mean + SD: 2.48 + 1.95 months). Among these patients, 34 complained of smell and taste loss, 36 complained only of smell loss, and one complained only of taste loss. For the 34 patients who complained of smell and taste loss, 11 were male and 23 were female. For the 36 patients who complained only of smell loss, 15 were male and 21 were female. The patient who complained only of taste loss was a woman. There was no significant gender difference between patients who complained of smell and taste loss and patients who complained only of smell loss (p = 0.576). For those who complained of smell and taste loss, their ages ranged from 20 to 73 years, with a mean of 38.35 and a SD of 15.44 years. For those complaining only of smell loss, their ages ranged from 22 to 69 years, with a mean of 44.92 and a SD of 14.20 years. The age of the patient who only complained of taste loss was 71 years. There was no significant age difference between those who complained of smell and taste loss and those who complained only of smell loss (p = 0.054). For patients who complained of both smell and taste loss, the interval between SARS-CoV-2 infection and the date of the first outpatient visit ranged from 1 to 11 months (mean + SD: 2.53 + 2.21 months). For patients complaining only of smell loss, the interval between SARS-CoV-2 infection and the date of the first outpatient visit ranged from 1 to 7 months (mean + SD: 2.47 + 2.72 months). For the patient who complained only of taste loss, the interval between SARS-CoV-2 infection and the date of the first outpatient visit was 1 month. We found no significant differences in these intervals between patients complaining of smell and taste loss and patients complaining only of smell loss (p = 0.873).
Patients complaining of loss of smell and taste
Among the 34 patients who complained of loss of smell and taste, their UPSIT-TC results at the first outpatient visit showed olfactory dysfunction in 30 patients (88.2%), but normal olfactory function in the remaining 4 patients (Table 1). Their UPSIT-TC scores ranged from 6 to 32 (mean + SD: 23.50 + 7.02). Similarly, their WETT results showed gustatory dysfunction in 8 patients (23.5%), but normal gustatory function in the remaining 26 patients (Table 1). Their WETT scores ranged from 13 to 37 (mean + SD: 25.82 + 6.96).
Table 1.
Patients complaining of smell and taste loss.
| Olfactory and gustatory function | First outpatient visit | Second outpatient visit a | Phone review |
|---|---|---|---|
| Olfactory dysfunction | 30/34 (88.2%) b | 14/18 (77.8%) b | 22/34 (64.7%) |
| Improvement rate | 11/17 (64.7%) b | 31/34 (91.2%) | |
| Gustatory dysfunction | 8/34 (23.5%) c | 3/18 (16.7%) c | 16/34 (47.1%) |
| Improvement rate | 0/3 (0%) c | 30/34 (88.2%) |
The outpatient visit after 2–4 month treatment.
Smell test using the traditional Chinese version of the University of Pennsylvania Smell Identification Test.
Taste test using the Waterless Empirical Taste Test.
After 2–4 months of treatment, 18 patients who still complained of loss of smell and taste function returned to receive another set of tests with UPSIT-TC and WETT (Table 2). Among these 18 patients, only one patient had a normal UPSIT-TC score at the first outpatient visit and continued to be normosmic with the second UPSIT-TC test. For the remaining 17 patients, 11 (64.7%) had their second UPSIT-TC scores improved after treatment. Their UPSIT-TC scores ranged from 6 to 31 (mean + SD: 20.06 + 7.72) before treatment and from 14 to 34 (mean + SD: 25.28 + 6.23) after treatment. The UPSIT-TC scores increased significantly after treatment (p = 0.001). In total, 14 of these 18 patients (77.8%) remained hyposmic. Regarding gustatory function, WETT results showed gustatory dysfunction in 3 patients (16.7%) and normal gustatory function in the remaining 15 patients. Among those three hypogeusic patients, none showed improvement in the WETT score. Their WETT scores ranged from 14 to 37 (mean + SD: 26.28 + 7.13) before treatment and from 10 to 39 (mean + SD: 28.61 + 8.38) after treatment. The WETT scores increased significantly after treatment (p = 0.019). In total, 3 of these 18 patients (16.7%) remained hypogeusic.
Table 2.
Patients complaining of smell and taste loss.
| Patient | Sex | Age | First outpatient visit | Second outpatient visit † | ||
|---|---|---|---|---|---|---|
| UPSIT ‡ | WETT § | UPSIT | WETT | |||
| 1 | M | 21 | 26 | 23 | 28 | 27 |
| 2 | M | 20 | 27 | 33 | 25 | 39 |
| 3 | M | 37 | 24 | 33 | 27 | 33 |
| 4 | F | 39 | 11 | 14 b | 18 | 10 b |
| 5 | F | 41 | 25 | 25 | 27 | 35 |
| 6 | F | 33 | 31 a | 34 | 34 a | 39 |
| 7 | M | 24 | 19 | 27 | 25 | 32 |
| 8 | F | 36 | 10 | 37 | 30 | 37 |
| 9 | M | 33 | 10 | 28 | 14 | 30 |
| 10 | F | 66 | 6 | 15 b | 17 | 15 b |
| 11 | F | 23 | 10 | 20 b | 19 | 17 b |
| 12 | F | 73 | 19 | 37 | 15 | 35 |
| 13 | F | 49 | 29 | 28 | 33 a | 30 |
| 14 | F | 23 | 16 | 27 | 28 | 29 |
| 15 | M | 30 | 23 | 19 | 31 a | 25 |
| 16 | M | 30 | 24 | 17 | 24 | 19 |
| 17 | F | 56 | 27 | 27 | 32 a | 32 |
| 18 | F | 43 | 24 | 29 | 28 | 31 |
Sex: M indicating male, F indicating female; Age: standing for years old.
UPSIT: University of Pennsylvania Smell Identification Test; WETT: Waterless Empirical Taste Test.
Smell function being normal.
Impaired gustatory function.
The outpatient visit after 2–4 month treatment.
Smell test using the traditional Chinese version of the University of Pennsylvania Smell Identification Test.
Taste test using the Waterless Empirical Taste Test.
At the end of December 2023, these 34 patients were notified by phone about recovery of their smell and taste function. We found that 12 patients had complete recovery of their olfactory function, 19 patients had partial recovery, and 3 patients did not improve. The improved olfactory function was 91.2%, with complete recovery in 35.3% of patients (Table 1). Gustatory function was completely restored in 18 patients, partially restored in 12 patients, and without improvement in 4 patients. The improvement rate for gustatory function was 88.2%, with complete recovery in 52.9% of the patients (Table 1). The interval between the date of the first outpatient visit and the date of phone follow-up ranged from 2 to 19 months (mean + SD: 10.35 + 5.50 months).
Patients complaining only of smell loss
For the 36 patients who complained only of smell loss, their UPSIT-TC results at the first outpatient visit were olfactory dysfunction in 27 patients (75%), and normal in the remaining 9 patients (Table 3). Their UPSIT-TC scores ranged from 4 to 35 (mean + SD: 22.19 + 8.10). Similarly, their WETT results were gustatory dysfunction in 6 patients (16.7%) and normal in the remaining 30 patients. Their WETT scores ranged from 3 to 39 (mean + SD: 26.08 + 7.68).
Table 3.
Patients complaining of only smell loss.
| Olfactory function | First outpatient visit | Second outpatient visit a | Phone review |
|---|---|---|---|
| Olfactory dysfunction | 27/36 (75%) b | 15/21 (71.4%) b | 25/36 (69.4%) |
| Improvement rate | 11/18 (61.1%) b | 28/36 (77.8%) |
The outpatient visit after 2–4 month treatment.
Smell test using the traditional Chinese version of the University of Pennsylvania Smell Identification Test.
After 2–4 months of treatment, 21 patients who still complained of smell loss returned to receive another UPSIT-TC (Table 4). Their UPSIT-TC scores at the first outpatient visit showed normal olfactory function in three patients. Two patients remained normosmic on the second UPSIT-TC test and one became hyposmic. For the remaining 18 patients, UPSIT-TC results showed improvement in olfactory function in 11 patients (61.1%). Their UPSIT-TC scores ranged from 4 to 32 (mean + SD: 20.14 + 7.71) before treatment and from 4 to 35 (mean + SD: 23.81 + 8.10) after treatment. The UPSIT-TC scores increased significantly after treatment (p = 0.008). In total, 15 of these 21 patients (71.4%) still remained hyposmic. Only six patients were normosmic.
Table 4.
Patients complaining of only smell loss.
| Patient | Sex | Age | First outpatient visit | Second outpatient visit † | |
|---|---|---|---|---|---|
| UPSIT ‡ | WETT § | UPSIT | |||
| 1 | M | 42 | 32 a | 28 | 35 a |
| 2 | M | 50 | 20 | 22 | 19 |
| 3 | F | 51 | 22 | 20 b | 26 |
| 4 | F | 40 | 30 | 24 | 30 |
| 5 | M | 32 | 10 | 30 | 14 |
| 6 | F | 62 | 20 | 23 | 28 a |
| 7 | F | 31 | 30 | 35 | 30 |
| 8 | M | 38 | 11 | 24 | 11 |
| 9 | F | 67 | 30 a | 31 | 31 a |
| 10 | F | 32 | 16 | 33 | 30 |
| 11 | F | 25 | 19 | 27 | 27 |
| 12 | M | 41 | 4 | 3 b | 4 |
| 13 | M | 31 | 20 | 20 | 24 |
| 14 | F | 64 | 27 a | 24 | 21 |
| 15 | M | 66 | 14 | 18 | 26 a |
| 16 | M | 22 | 26 | 27 | 32 a |
| 17 | M | 56 | 25 | 23 | 27 |
| 18 | M | 53 | 24 | 15 b | 29 |
| 19 | M | 37 | 17 | 24 | 12 |
| 20 | F | 64 | 10 | 30 | 19 |
| 21 | F | 38 | 16 | 36 | 25 |
Sex: M indicating male, F indicating female; Age: standing for years old.
UPSIT: University of Pennsylvania Smell Identification Test; WETT: Waterless Empirical Taste Test.
Smell function being normal.
Impaired gustatory function.
The outpatient visit after 2–4 month treatment.
Smell test using the traditional Chinese version of the University of Pennsylvania Smell Identification Test.
Taste test using the Waterless Empirical Taste Test.
At the end of December 2023, these 36 patients were checked by phone calls about recovery from their smell loss. Olfactory function completely recovered in 11 patients, partially recovered in 17 patients, and no improvement in 8 patients. The rate of improvement in olfactory function was 77.8%, with 30.6% of patients showing complete recovery in olfactory function. The interval between the date of their first outpatient visit and phone follow-up ranged from 2 to 18 months (mean + SD: 10.42 + 5.35 months).
Patients complaining of only taste loss
Only one patient complained of taste loss. She was a 71-year-old woman. She came to our outpatient clinic in July 2023 after a taste loss for 1 month. At the first outpatient visit, her UPSIT-TC score was 23, and the WETT score was 1. After 2 months of treatment with prednisolone and zinc, her UPSIT-TC score was 19 and her WETT score increased to 31. Five months after the first outpatient visit, she reported on telephone follow-up a partial recovery of her taste function.
Discussion
Smell and taste impairments are common symptoms in patients with COVID-19. 17 Their frequency of olfactory dysfunction ranges from 22% to 68%, and their frequency of gustatory dysfunction ranges from 20% to 33%. 18 The exact pathogenesis of SARS-CoV-2 infection to olfactory and gustatory dysfunctions remains unclear.19,20 There are some hypotheses of the pathogenesis of their olfactory dysfunction.2,4,8,21 One likely mechanism involves altered functioning of sensory olfactory neurons, which are associated with infection, and the death of olfactory supporting cells, microvillar cells, and vascular pericytes. 4 Other mechanisms include edema of the olfactory bulb with impaired transmission, and injury to the OB. 7 Magnetic resonance spectroscopy is a noninvasive quantitative imaging technique that has a significant impact on the diagnosis and treatment of disorders of the central nervous system. It has been used to indicate a possible association between central nervous system impairment and persistent COVID19-related anosmia. 22 However, some radiological examinations suggest that local edema in olfactory clefts is another possible etiology. 23
In comparison, relatively little is known about the pathogenesis of their taste dysfunction. 24 It has been hypothesized that gustatory dysfunction may be related to indirect damage to taste receptors due to infected epithelial cells and the local inflammation that follows.25,26 Another possible etiology involves the expression of angiotensin-converting enzyme-2 in taste organs after viral infection of the salivary gland. 27
Olfactory and gustatory dysfunctions in most patients with COVID-19 recover within the first week of infection. 28 Recovery rates 1 month after SARS-CoV-2 infection are reported to be 87% for olfaction and 82% for gustation. 29 For patients without early recovery within the first week, little or no recovery is expected in the following 3 weeks.30,31 Therefore, we used the definition of Nalbandian et al. to define long COVID as persistent symptoms of SARS-CoV-2 infection beyond 4 weeks from the onset of symptoms. 32
Although olfactory and gustatory dysfunctions in most COVID 19 patients have been subjectively evaluated in most reports, objective measurements of the function of smell and taste remain limited. This objective assessment is fundamental to the quantification of the severity of dysfunctions and the monitoring of recovery. 33 In this study, we enrolled 34 long COVID patients who complained of loss of smell and taste function for at least 1 month, and we applied the objective smell test (UPSIT-TC) to show impaired olfactory function in 88.2% of patients, while the objective taste test (WETT) showed impaired gustatory function in only 23.5% of patients. Our results were similar to those of a nationwide study in Japan. 34 In that study, COVID-19 patients who were aware of their hyposmia had low scores on the olfactory test in 83.1% of patients, and similarly, those who were aware of their hypogeusia had low scores on the taste test in only 26.7% of patients. This indicates that most COVID-19 patients who had complained of gustatory dysfunction did not really have a taste problem, but rather had developed a flavor disorder associated with olfactory dysfunction. 34 On the contrary, our 36 long COVID patients had complained of only smell loss, but felt that their taste function was normal. Their objective smell test (UPSIT-TC) showed impaired olfactory function in 75% of patients, and the objective taste test (WETT) showed impaired gustatory function in 16.7% of the patients. Only one long COVID patient complained of loss of taste and felt that her smell function was normal. The objective smell test of this patient showed impaired olfactory function (UPSIT-TC score: 23), and the objective taste test also showed impaired gustatory function (WETT score: 1). Our results suggested that the smell and taste functions should best be tested simultaneously in patients with long COVID who complained of persistent loss of smell and/or taste function.
After 2–4 months of treatment with prednisolone and zinc, these long COVID patients received objective taste and smell tests again. Among the 34 long COVID patients who complained of loss of both smell and taste functions, 18 received objective smell and taste tests again. The second UPSIT-TC test showed impaired olfactory function in 77.8% of the patients, and the WETT test showed impaired gustatory function in 16.7% of patients. Although most patients were still hyposmic, their UPSIT-TC scores showed improved olfactory function in 64.7% of the patients. Among 36 long COVID patients who complained only of loss of smell function, 21 patients received the second objective smell test. Their UPSIT-TC scores showed impaired olfactory function in 71.4% of the patients, but olfactory function improved in 61.1% of the patients. It appeared that if your olfactory dysfunction did not recover rapidly within the first month, the olfactory function recovered gradually in about half of the patients. We should point out that about half of our patients did not return to receive the second objective test, and therefore, our results should be interpreted with caution. One possible explanation for the high dropout rate in this study was that our patient did not care much about the loss of their olfactory and/or gustatory function. The optimal treatment modality for olfactory and gustatory dysfunctions in patients with COVID-19 has not been established. 35 Several medications have been used to treat COVID-19-related smell and taste losses. 36 Currently, steroids and olfactory training are among the most common modalities for treating such smell and taste losses. 37 Somewhat related to this, we have previously reported that zinc is beneficial in treating traumatic anosmia. 38 In addition to a high dropout rate, we did not include control patients who did not receive medical treatment in this study. Therefore, the effect of oral zinc and prednisolone on long COVID hyposmia and hypogeusia with should be further investigated with an adequate control group.
After a mean follow-up of 10.35 months, 31 (91.2%) of the 34 long COVID patients who complained of loss of smell and taste reported improvements in their olfactory function when compared to their olfactory function tested in their first outpatient visit, with no complete recovery in 22 patients (64.7%). On the other hand, 30 patients (88.2%) reported improved gustatory function compared to their gustatory function at the first outpatient visit, with no complete recovery in 16 patients (47.1%). After a mean follow-up of 10.42 months, 28 (77.8%) of 36 long COVID patients who had complained only of loss of smell reported improved olfactory function compared with their olfactory function tested at the first outpatient visit, with no complete recovery in 25 patients (69.4%). Our results showed a minimal correlation between the results of subjective and objective assessments in olfactory and gustatory functions. Therefore, results derived from our phone call follow-ups should also be interpreted with caution.
There are several other limitations to this study. There were several factors that could affect the outcomes of our treatment, including young women, variations in ethnicity, virus variants, higher body mass index, absence of allergic rhinitis, normal taste perception, absence of upper respiratory infection symptoms, and the presence of COVID-19 pneumonia. 39 Most of the studies were conducted in Caucasians. Our study was the first study in Asians to report the prognosis of long COVID hyposmia and hypogeusia. The second is that our patients were only infected with the Omicron variant of SARS-CoV-2, which probably caused the most infrequent smell loss of all variants. In contrast, Miwa et al. 34 conducted a Japanese nationwide study during the Alpha variant epidemic. Another limitation of our study is that the calculation and justification of the sample size was not done. More patients might be needed to confirm our results.
Conclusion
Among our long COVID patients who complained of smell and taste loss, objective tests showed hyposmic in 88.2% of the patients, while hypogeusic in only 23.5% of the patients. After 2–4 months of treatment with oral zinc and steroids, UPSIT-TC scores were hyposmic in 77.8% of the patients, with an improvement in olfaction in 64.7% of the patients. On the other hand, 16.7% of the patients were hypogeusic. After a follow-up of an average of 10.35 months, 91.2% of the patients reported improvements in their olfactory function, with no complete recovery in 64.7% of the patients. In addition, 47.1% of the patients reported no complete recovery in their gustation. Among our long COVID patients who complained only of smell loss, their UPSIT-TC scores showed hyposmic in 75% of the patients. After 2–4 months of treatment with oral zinc and steroids, their UPSIT-TC scores showed hyposmic in 71.4% of the patients, with improved olfaction in 61.1% of patients. After an average of 10.42 months of follow-up, 77.8% of the patients reported improved olfaction, with no complete recovery in 69.4% of the patients. Our results showed that olfactory dysfunction in many long COVID patients persisted for >10 months, despite gradual improvements in their olfactory function. In comparison, gustatory dysfunction was not as prevalent in our long COVID patients.
Acknowledgments
The authors thank the Taichung Veterans General Hospital Biostatistics Task Force, Taichung, Taiwan, for assistance with the statistical analysis and are grateful to all research participants and staff involved in the study.
Footnotes
Authors’ contributions: Yi-Fang Chiang drafted the manuscript. Yi-Fang Chiang performed the literature review. Rong-San Jiang performed the procedures. Rong-San Jiang collected and analyzed the data. All authors read and approved the final manuscript.
Data availability statement: The data presented in this study are available on request from the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Institutional Review Board (I) of the Taichung Veterans General Hospital (Protocol code: CE24036A).
Patient consent: Written informed consent was exempted by the Institutional Review Board because this was a retrospective study.
Consent for publication: Not applicable.
Trial registration: Not applicable because this was not a clinical trial.
ORCID iD: Rong-San Jiang  https://orcid.org/0000-0002-8280-6029
https://orcid.org/0000-0002-8280-6029
References
- 1. Allhaiby NM, Allihybi SM, Almhmadi AH, et al. Prevalence of long-lasting loss of smell and taste after coronavirus disease 2019 infection in Saudi Arabia. J Family Community Med 2023; 30(4): 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang J, Chen Y, Huang J, et al. Prevalence of taste and smell dysfunction in mild and asymptomatic COVID-19 patients during Omicron prevalent period in Shanghai, China: a cross-sectional survey study. BMJ Open 2023; 13(3): e067065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021; 600(7887): 21. [DOI] [PubMed] [Google Scholar]
- 4. Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett 2021; 748: 135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altin F, Cingi C, Uzun T, et al. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol 2020; 277(10): 2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burges Watson DL, Campbell M, Hopkins C, et al. Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PLoS One 2021; 16(9): e0256998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klimek L, Hagemann J, Döge J, et al. Olfactory and gustatory disorders in COVID-19. Allergo J Int 2022; 31(7): 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021; 53(10): 737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang RS, Liang KL. Establishment of olfactory diagnosis for the traditional Chinese version of the University of Pennsylvania Smell Identification Test. Int Forum Allergy Rhinol 2016; 6(12): 1308–1314. [DOI] [PubMed] [Google Scholar]
- 10. Doty RL, Wylie C, Potter M. Validation of the Waterless Empirical Taste Test (WETT®). Behav Res Methods 2021; 53(2): 864–873. [DOI] [PubMed] [Google Scholar]
- 11. Jiang RS, Su MC, Liang KL, et al. A pilot study of a traditional Chinese version of the University of Pennsylvania Smell Identification Test for application in Taiwan. Am J Rhinol Allergy 2010; 24(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 12. Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol 2007; 21(4): 460–473. [DOI] [PubMed] [Google Scholar]
- 13. Doty RL, Yousem DM, Pham LT, et al. Olfactory dysfunction in patients with head trauma. Arch Neurol 1997; 54(9): 1131–1140. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Ren X, Yan H, et al. Comparison of Chinese and American subjects on the self-administered Waterless Empirical Taste Test. J Sens Stud 2022; 26: e12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang RS, Wang JJ. Validation of the clinical applicability of the brief self-administered waterless empirical taste test during the era of COVID-19. J Chin Med Assoc 2022; 85(12): 1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dontje ML, Dall PM, Skelton DA, et al. Reliability, minimal detectable change and responsiveness to change: Indicators to select the best method to measure sedentary behaviour in older adults in different study designs. PLoS One 2018; 13(4): e0195424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meunier N, Briand L, Jacquin-Piques A, et al. COVID 19-induced smell and taste impairments: putative impact on physiology. Front Physiol 2021; 11: 625110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carrillo-Larco RM, Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: a systematic review. Wellcome Open Res 2020; 5: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-Induced anosmia: the first report. Acad Radiol 2020; 27(6): 892–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandemirli SG, Altundag A, Yildirim D, et al. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol 2021; 28(1): 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehraeen E, Behnezhad F, Salehi MA, et al. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence. Eur Arch Otorhinolaryngol 2021; 278(2): 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemati S, Haghani Dogahe M, Saberi A, et al. Magnetic resonance spectroscopy findings of brain olfactory areas in patients with COVID-19-related anosmia: a preliminary comparative study. World J Otorhinolaryngol Head Neck Surg 2023; 10(2): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eliezer M, Hamel AL, Houdart E, et al. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology 2020; 95(23):e3145-e3152. [DOI] [PubMed] [Google Scholar]
- 24. Cooper KW, Brann DH, Farruggia MC, et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron 2020; 107(2): 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Zhou J, Marshall B, et al. SARS-CoV-2 receptor ACE2 is enriched in a subpopulation of mouse tongue epithelial cells in nongustatory papillae but not in taste buds or embryonic oral epithelium. ACS Pharmacol Transl Sci 2020; 3(4): 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva Pedrosa M, Sipert CR, Nogueira FN. Altered taste in patients with COVID-19: the potential role of salivary glands. Oral Dis 2021; 27(Suppl 3): 798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hintschich CA, Fischer R, Hummel T, et al. Persisting olfactory dysfunction in post-COVID-19 is associated with gustatory impairment: Results from chemosensitive testing eight months after the acute infection. PLoS One 2022; 17(3):e0265686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg 2020; 163(6): 1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect 2021, 27, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrocelli M, Cutrupi S, Salzano G, et al. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol 2021; 135(5): 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27(4): 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 2020; 42(6): 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miwa T, Mori E, Sekine R, et al. Olfactory and taste dysfunctions caused by COVID-19: a nationwide study. Rhinology 2023; 61(6): 552–560. [DOI] [PubMed] [Google Scholar]
- 35. Seo MY, Lee SH. Treatment and prognosis of COVID-19 associated olfactory and gustatory dysfunctions. J Pers Med 2021; 11(10): 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khani E, Khiali S, Beheshtirouy S, et al. Potential pharmacologic treatments for COVID-19 smell and taste loss: a comprehensive review. Eur J Pharmacol 2021; 912: 174582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Bon SD, Konopnicki D, Pisarski N, et al. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol 2021; 278(8): 3113–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang RS, Twu CW, Liang KL. Medical treatment of traumatic anosmia. Otolaryngol Head Neck Surg 2015; 152(5): 954–958. [DOI] [PubMed] [Google Scholar]
- 39. Kreesaeng P, Tangbumrungtham N, Rachapattayakhom R, et al. Prevalence and prognostic factors associated with early recovery of olfactory dysfunction in COVID-19 patients. Ear Nose Throat J 2024; 103(1_suppl): 68S–75S. [DOI] [PubMed] [Google Scholar]