Abstract
Background and objectives:
Atypical thymic carcinoids (ATCs) are rare mediastinal malignancies that lack established treatment guidelines. Capecitabine and temozolomide (CapTem) has demonstrated significant efficacy in pancreatic neuroendocrine neoplasms (NENs), while its applicability and effectiveness in ATCs remain underexplored. This study seeks to investigate the efficacy, safety, and prognostic factors associated with CapTem in ATC patients.
Design and methods:
Thirty-eight ATC patients treated with CapTem at our center were analyzed. We assessed the objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and treatment-related adverse effects. We also examined patients’ clinicopathological characteristics and their correlations with CapTem efficacy.
Results:
The cohort achieved a 15.8% ORR and 89.5% DCR, with a median PFS of 13.0 months. Multivariate analysis identified the platelet-to-lymphocyte ratio (PLR) as a significant independent prognostic factor for PFS, with a PLR ⩾ 235 associated with shorter PFS (7 months vs. undefined, p = 0.0004). Age was an independent prognostic factor for OS, with patients over 50 years experiencing shorter OS (36 months vs. undefined, p = 0.015). Safety analysis showed rare severe toxicities and no treatment-related fatalities.
Conclusion:
CapTem is an effective and well-tolerated treatment for ATC patients. Pretreatment PLR and age appear to be potential prognostic markers for CapTem therapy; however, these results warrant validation in larger patient cohorts.
Keywords: atypical thymic carcinoids, capecitabine and temozolomide, platelet-to-lymphocyte ratio, prognosis
Plain language summary
Efficacy, safety and prognostic factors of CapTem in ATCs
Previous studies have demonstrated the effectiveness of the Capecitabine plus Temozolomide regimen in patients with pancreatic neuroendocrine neoplasms (NENs), but its potential effectiveness in atypical thymic carcinoids (ATCs) remains underexplored. The cases presented in our study complement previous retrospective series, reinforcing the notion that the CapTem regimen can be a rational and well-tolerated treatment option with favorable responses for patients with ATCs. Importantly, our study is the first to identify pretreatment platelet-to-lymphocyte ratio (PLR) as an independent prognostic biomarker associated with progression-free survival (PFS) in patients with ATCs treated with CapTem. We established the optimal cutoff for PLR as 235. Additionally, our findings revealed that age over 50 years is an independent adverse prognostic factor for overall survival (OS). These findings provide valuable insights for clinicians when selecting patients who are more likely to benefit from the CapTem regimen.
Introduction
Thymic neuroendocrine neoplasms (T-NENs) are uncommon malignancies primarily located in the anterior mediastinal compartment, constituting only about 5% of all thymic neoplasms and less than 0.5% of all neuroendocrine neoplasms (NENs).1 –3 T-NENs encompass a spectrum of tumors, ranging from well-differentiated neuroendocrine tumors (NETs) with low-grade typical carcinoids (TCs) and intermediate-grade atypical thymic carcinoids (ATCs) to the more aggressive poorly differentiated small cell neuroendocrine carcinomas (SCNECs) and large cell neuroendocrine carcinomas (LCNECs). 1
According to data from the SEER database, the annual incidence of T-NENs is approximately 0.04 per 100,000 individuals among Asian/Pacific Islanders. This incidence is notably lower than that of broncho-pulmonary NENs and gastroenteropancreatic NENs. 2 The rarity of T-NENs has resulted in limited large-scale clinical series and clinical trials, contributing to a scarcity of consensus statements and treatment guidelines. 3 Furthermore, there is a lack of specific phase III trials exclusively dedicated to T-NENs, with available evidence primarily derived from retrospective analyses or extrapolated from the management of broncho-pulmonary carcinoids. 3 It is crucial to note that despite their relative rarity, T-NENs often display a more aggressive nature and pose greater treatment challenges. 4 Approximately 25% of T-NENs are associated with multiple endocrine neoplasia type 1 (MEN-1), while a significant proportion may exhibit ectopic Cushing’s syndrome or both. Consequently, the management of T-NENs can be especially complex and multifaceted. 4
Surgical resection is widely recognized as the primary curative method for resectable T-NENs, offering the best chance of long-term remission. In contrast, for advanced patients with unresectable or metastatic disease, chemotherapy, with or without the addition of radiotherapy, stands as the cornerstone of therapeutic strategies. 3 Historically, streptozocin-based regimens have been the primary approach for treating advanced NETs, while platinum-based chemotherapy regimens remain pivotal in managing poorly differentiated neuroendocrine carcinomas.5,6 However, it is worth noting that there is no compelling evidence supporting the efficacy of either regimen in low- or intermediate-grade T-NENs. Furthermore, both these treatment approaches are associated with significant toxicity and potential adverse effects. Consequently, there exists a pressing clinical demand for the development of new chemotherapy regimens that not only demonstrate a favorable treatment response but also exhibit a reduced burden of toxicity, especially in patients with advanced T-NENs.
Temozolomide, an oral alkylating agent, operates with reduced toxicity by inducing DNA methylation, ultimately triggering apoptosis. This unique mechanism is not limited to specific phases of the cell cycle, allowing it to inhibit tumor cell growth at all stages, even in tumors with a low proliferation rate, such as well-differentiated NETs. 7 Capecitabine, on the other hand, serves as an oral prodrug of 5-fluorouracil, a thymidylate synthase inhibitor. Prior treatment with capecitabine depletes the DNA repair enzyme O6-methylguanine DNA methyltransferase (MGMT), thereby amplifying the impact of temozolomide. The combination of capecitabine with temozolomide, often referred to as capecitabine and temozolomide (CapTem), is a well-established regimen known for its synergistic effects. It has consistently shown superior results in terms of progression-free survival (PFS) and overall survival (OS) compared to temozolomide monotherapy. 8 In patients with metastatic NENs, the CapTem regimen has exhibited significant activity, with an objective response rate (ORR) typically ranging from 30% to 70% and median PFS extending from 12 months to 22.7 months.9 –12 However, due to the rarity of T-NENs, there remains a scarcity of data regarding the efficacy and safety of CapTem in treating this specific condition. Numerous critical questions pertaining to the optimal treatment schedule, timing, duration, factors associated with improved responses, prolonged PFS and OS, and potential long-term side effects remain unanswered. Hence, we conducted a retrospective analysis to assess the efficacy and safety of the CapTem regimen in ATC patients at our center, and to explore clinicopathological factors that may hold prognostic significance.
Materials and methods
Study design and patients
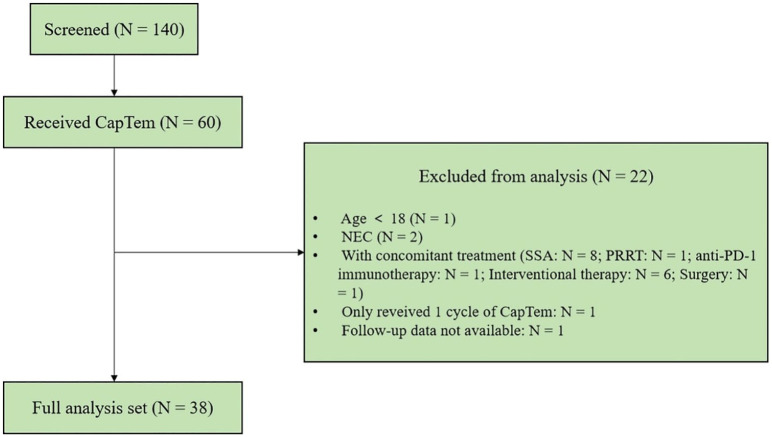
This retrospective study examined records of all consecutive patients with T-NENs who underwent >1 cycle of the CapTem regimen between October 2014 and June 2023 at the First Affiliated Hospital of Sun Yat-sen University. Eligible participants were aged ⩾18 and had histologically confirmed T-NENs with measurable disease as defined by RECIST 1.1 criteria. 13 The tumor-node-metastasis (TNM) stage was determined according to the 8th edition of the AJCC Cancer Staging Manual. 14 The tumor pathology for each case was reviewed by Dr. Yuan Lin, following the 5th edition of the World Health Organization (WHO) Classification of Thoracic Tumors. 1 T-NENs were classified into four categories: TC, ATC, SCNEC, and LCNEC. Notably, after an extensive review of our in-house database, it was found that nearly all T-NEN patients who received CapTem at our center were diagnosed with ATCs, with no instances of TC, and only two cases of LCNEC that received CapTem. To minimize confounding factors, this study exclusively included patients diagnosed with ATCs. Patients concurrently receiving other anticancer therapies such as somatostatin analogs (SSAs), targeted agents, additional cytotoxic chemotherapy, or radionuclide therapy during the CapTem treatment period were excluded. Following the outlined inclusion and exclusion criteria, a total of 38 eligible patients with ATCs were included in the subsequent analysis (see Figure 1).
Figure 1.
Flow diagram of study population.
The decision to initiate the CapTem regimen was made by our NEN-dedicated multidisciplinary tumor boards during our weekly meetings. This retrospective investigation received approval from the Institutional Ethics Committee for Clinical Research of the First Affiliated Hospital of Sun Yat-sen University, with approval number 2023[561]. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement 15 (Supplemental Table 2).
Chemotherapy administration and patient management
The CapTem regimen was administered as follows: patients received oral capecitabine at a dosage of 750 mg/m2 twice daily during days 1–14 of a 28-day cycle. Simultaneously, temozolomide was administered at a dosage of 200 mg/m2 once daily during days 10–14 within the same 28-day cycle. This treatment schedule was continued until disease progression or the emergence of unacceptable toxicity. 16 In cases where necessary, dosages were adjusted to mitigate the potential for high-risk patient toxicity. To minimize the risk of digestive tract reactions, patients took antiemetic tablets (such as aprepitant, ondansetron, etc.) before combining temozolomide. Patients underwent complete blood count assessments, as well as liver and renal function tests before the initiation of each chemotherapy cycle. Additionally, they were subjected to regular follow-up examinations at 3-month intervals, which included imaging modalities with a contrast-enhanced thoracoabdominopelvic computed tomography (CT) scan to evaluate the effectiveness of the CapTem regimen.
Data collection
For this study, we retrospectively collected an array of patient baseline demographics, tumor characteristics, functional imaging properties from 18F-fluorodeoxyglucose positron emission tomography ( 18 FDG-PET) and somatostatin receptor imaging (68Ga-DOTATOC PET/CT), history of prior treatments, time to initiate the CapTem regimen, duration of CapTem administration, time to treatment cessation, time to disease progression, and side effects. These data were obtained from the Electronic Medical Record and our telephone follow-up records. Tumor-to-Liver Uptake Ratio from 18FDG-PET and 68Ga-DOTATOC PET/CT was assessed by a professional nuclear medicine specialist (Dr. Qiao He). MGMT score, which is the sum of both intensity and percentage scores, was evaluated by a professional pathologist (Dr. Yuan Lin). Intensity of staining was rated as 1 (weak), 2 (moderate), or 3 (intense). The percentage of MGMT positive tumor cells was semi-quantitatively scored on a six-tiered scale: 0 (0%), 1 (⩽1%), 2 (1%–10%), 3 (11%–33%), 4 (34%–66%), and 5 (>66%). The sum of both intensity and percentage scores defined the final score, categorized as negative (score 0), weak (scores 1–3), moderate (scores 4–6), or strong (scores 7–8). 11 To investigate prognostic clinical parameters, baseline results of blood routine tests and a panel of serum tumor markers were collected, including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), squamous cell carcinoma antigen (SCC), carbohydrate antigen 199 (CA199), protein induced by vitamin K absence or antagonist II (PIVKA-II), neuron-specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1), pro-gastrin-releasing peptide (ProGRP), lactate dehydrogenase (LDH). Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) were calculated from blood routine tests. Baseline blood counts data and serum tumor markers were defined as the most recent blood count within 1 week before CapTem initiation and excluded recent infection or immunomodulation and connective tissue disease. Evaluation of tumor responses was conducted according to RECIST 1.1 by an experienced radiologist (Dr. Yanji Luo) from our NEN-dedicated Multidisciplinary Tumor Boards. Clinically favorable responses included stable disease (SD), partial response (PR), and complete response (CR), in contrast to disease progression (PD). The ORR was defined as the sum of CR and PR rates, while the disease control rate (DCR) was defined as the percentage of patients achieving CR, PR, or SD. PFS was defined as the duration from the initiation of CapTem treatment to either disease progression or death, whichever occurred first. OS was defined as the time between the initiation of CapTem treatment and death from any cause. Tolerance to treatment was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 17
Statistical analysis
Statistical analyses, including descriptive statistics, frequencies, log-rank tests, Kaplan–Meier curves, and Cox regression, were conducted using the SPSS 25.0 software package (IBM SPSS Statistics, Armonk, NY, USA) or GraphPad Prism version 8 software (GraphPad, Inc., La Jolla, CA, USA), as appropriate. Qualitative variables were presented as frequencies (percentages) and compared using Fisher’s exact test. The association of each factor with PFS or OS was assessed using the univariable Cox proportional hazards model. Factors for which the hazard ratios (HRs) were significant at a level of significance of 0.1 were subsequently included in a multivariable Cox proportional hazard model to identify independent factors influencing PFS or OS. For independent prognostic factors, a Classification and Regression Tree (CART) analysis was performed using the rpart package of R software (version 4.3.1, The R Foundation for Statistical Computing, Vienna, Austria) to determine the optimal cutoff for the factor, enabling the stratification of patients with favorable or unfavorable outcomes. PFS and OS curves were generated using the Kaplan–Meier method and compared using log-rank tests. Differences with p < 0.05 were considered statistically significant.
Results
Demographics and clinicopathological variables
Table 1 summarizes the demographics and clinicopathological characteristics of the patients under investigation. The CapTem regimen was administered to a total of 38 ATC patients, comprising 5 women and 33 men, with a median age of 48 years (range 23–66). Of these 38 patients, 36 had sporadic tumors, while 2 presented with familial NENs as part of the MEN-1 syndrome. Notably, four patients had functional tumors associated with ectopic Cushing’s syndrome. The most prevalent sites of ATC metastases included peripheral lymph nodes (30 out of 38, 78.9%), bone (27 out of 38, 71.1%), and pleura (16 out of 38, 42.1%). Liver metastases were less common, occurring in only 3 out of 38 patients (7.9%). In terms of prior surgery history, 17 patients underwent surgery with curative or debulking intent, while 21 patients were considered to have unresectable disease at the time of diagnosis, leading them to receive systemic medical treatment. All patients initiated CapTem treatment with metastatic or locally advanced (inoperable) disease, with 3 patients categorized as stage III and 35 as stage IV. Notably, CapTem was administered as the first-line regimen in 18 patients, while pretreated patients had undergone various prior therapies, including SSAs in one case, molecular-targeted agents in four cases, and other cytotoxic chemotherapy in 18 cases.
Table 1.
Baseline patient and tumor characteristics of the study cohort.
| Characteristics | N | % |
|---|---|---|
| Total | 38 | 100 |
| Gender | ||
| Male | 33 | 86.8 |
| Female | 5 | 13.2 |
| Age, median (range), year | ||
| At diagnosis | 47 (20–66) | |
| At CapTem initiation | 48 (23–66) | |
| Inheritance | ||
| Sporadic | 36 | 94.7 |
| Familial (MEN1) | 2 | 5.3 |
| Functional status | ||
| Nonfunctioning | 34 | 89.5 |
| Functioning | 4 | 10.5 |
| TNM | ||
| III | 3 | 7.9 |
| IV | 35 | 92.1 |
| Ki67 index | ||
| <3% | 0 | 0 |
| 3%–20% | 29 | 76.3 |
| >20% | 9 | 23.7 |
| Mitosis (2 mm2) | ||
| <2 | 2 | 5.3 |
| 2–20 | 29 | 76.3 |
| >20 | 1 | 2.6 |
| NA | 6 | 15.8 |
| MGMT expression | ||
| Negative | 1 | 2.6 |
| Week | 1 | 2.6 |
| Moderate | 11 | 28.9 |
| Strong | 16 | 42.1 |
| NA | 9 | 23.7 |
| Metastatic sites | ||
| None | 1 | 2.6 |
| Lymph nodes | 30 | 78.9 |
| Pleura | 16 | 42.1 |
| Pericardium | 8 | 21.1 |
| Lung | 7 | 18.4 |
| Pancreas | 7 | 18.4 |
| Liver | 3 | 7.9 |
| Bone | 27 | 71.1 |
| Prior resective surgery | ||
| Yes | 17 | 44.7 |
| No | 21 | 55.3 |
| Prior radiotherapy | ||
| Yes | 14 | 36.8 |
| No | 24 | 63.2 |
| Previous systemic treatments | ||
| No prior treatment | 18 | 47.4 |
| SSA | 1 | 2.6 |
| Molecular-targeted agents | 4 | 10.5 |
| Everolimus | 2 | 5.3 |
| Surufatinib | 2 | 5.3 |
| Chemotherapy | 18 | 47.4 |
| Taxel & Cisplatin | 1 | 2.6 |
| Cyclophosphamide & Pirarubicin & Cisplatin | 1 | 2.6 |
| Docetaxel & Cisplatin | 1 | 2.6 |
| Etoposide & Cisplatin | 14 | 36.8 |
| Paclitaxel & Carboplatin & Bevacizumab | 1 | 2.6 |
| CapTem line of treatment | ||
| First line (naïve) | 18 | 47.4 |
| ⩾Second line (pretreated) | 20 | 52.6 |
CapTem, capecitabine and temozolomide; MGMT, O6-methylguanine DNA methyltransferase; SSA, somatostatin analog; TNM, tumor-node-metastasis.
Duration of treatment
CapTem treatment continued until one of the following conditions occurred: disease progression, unacceptable toxicity, or the consideration of long-term administration-related toxicity despite a favorable response. For the entire cohort, the median duration of CapTem treatment was 9 months (range 2–15). Notably, in 14 patients, the CapTem regimen was administered until PD or SD with slow progression. In two patients, intolerable digestive tract side effects led to the discontinuation of treatment after 7 and 5 months, respectively. Given the absence of evidence-based recommendations regarding the standard duration of CapTem treatment, the decision to extend treatment cycles beyond 12 months was made on a case-by-case basis by our NEN-dedicated Multidisciplinary Tumor Boards. Among these patients, 13 have received long-term CapTem administration for ⩾12 months. In this subgroup, three patients experienced disease progression, while the remaining patients demonstrated favorable SD without significant toxicity.
Overall efficacy
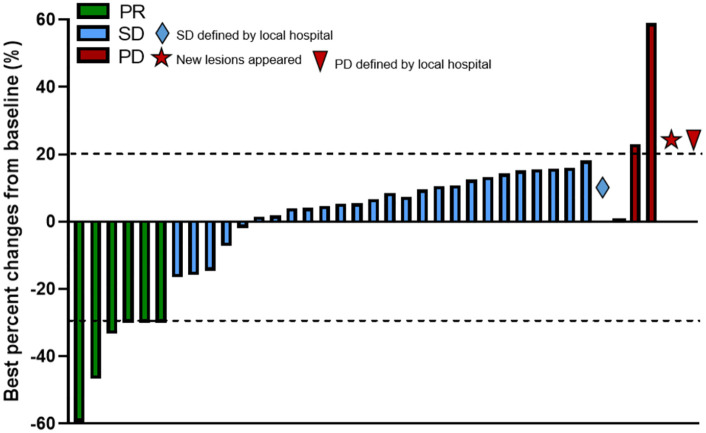
The best treatment response during the administration of CapTem was assessed according to RECIST version 1.1 based on the patients’ imaging studies during the follow-up visits. In total, 6 patients (15.8%) exhibited PR, 28 patients showed SD (73.7%), and 4 patients had radiological PD (10.5%) (see Figure 2). No patients died during the treatment period, and there were no cases of CR to CapTem in our cohort. However, two patients did show favorable responses that allowed for the surgical removal of previously unresectable lesions.
Figure 2.
Waterfall plot showing the best percentage changes in the sum of target lesions from baseline.
Favorable responses (PR + SD) showed no statistically significant differences between different subgroups (see Table 2). After stratifying patients based on their history of prior surgery, it was found that favorable response rates were better for the group without prior surgery (100%) compared to the group with prior surgery (76.5%), with a statistically significant difference (p = 0.0322). When stratified by the line of treatment, CapTem administered as first-line treatment yielded a relatively higher ORR than second-or later-line treatment (27.8% and 5%, respectively, p = 0.083) (see Table 3).
Table 2.
Responses with CapTem chemotherapy regimen in different subgroups.
| Characteristic | PR, n (%) | SD, n (%) | PD, n (%) | Favorable outcome (PR + SD) | |
|---|---|---|---|---|---|
| n (%) | p Value | ||||
| Overall | 6/38 (15.8) | 28/38 (73.7) | 4/38 (10.5) | 34/38 (89.5) | |
| Gender | |||||
| Male | 6/33 (18.2) | 24/33 (72.7) | 3/33 (9.1) | 30/33 (90.9) | 0.4456 |
| Female | 0/5 (0) | 4/5 (80) | 1/5 (20) | 4/5 (80) | |
| Age | |||||
| <50 | 4/22 (18.2) | 15/22 (68.2) | 3/22 (13.6) | 19/22 (86.4) | 0.6245 |
| ⩾50 | 2/16 (12.5) | 13/16 (81.25) | 1/16 (6.2) | 15/16 (93.8) | |
| Inheritance | |||||
| Sporadic | 6/36 (16.7) | 27/36 (75) | 3/36 (8.3) | 33/36 (91.7) | 0.2020 |
| Familial (MEN1) | 0/2 (0) | 1/2 (50) | 1/2 (50) | 1/2 (50) | |
| Functional status | |||||
| Nonfunctioning | 4/34 (11.8) | 26/34 (76.5) | 4/34 (11.8) | 30/34 (88.2) | >0.9999 |
| Functioning | 2/4 (50) | 2/4 (50) | 0/4 (0) | 4/4 (100) | |
| TNM | |||||
| III | 0 | 3/3 (100) | 0 | 3/3 (100) | 1.0000 |
| IV | 6/35 (17.14) | 25/35 (71.43) | 4/35 (11.43) | 31/35 (88.57) | |
| Ki67 index | |||||
| ⩽10% | 2/15 (13.3) | 12/15 (80) | 1/15 (6.7) | 14/15 (93.3) | >0.9999 |
| >10% | 4/23 (17.4) | 16/23 (69.6) | 3/23 (13.0) | 20/23 (87.0) | |
| Mitosis | |||||
| ⩽10 | 3/28 (10.7) | 24/28 (85.7) | 1/28 (3.6) | 27/28 (96.4) | 0.2379 |
| >10 | 1/4 (25) | 2/4 (50) | 1/4 (25) | 3/4 (75) | |
| FDG-SUVmax (tumor/liver) | |||||
| ⩽2 | 0/9 (0) | 8/9 (88.9) | 1/9 (11.1) | 8/9 (88.9) | >0.9999 |
| >2 | 4/24 (16.7) | 18/24 (75) | 2/24 (8.3) | 22/24 (91.7) | |
| SSA-SUVmax (tumor/liver) | |||||
| ⩽1 | 4/26 (15.4) | 20/26 (76.9) | 2/26 (7.7) | 24/26 (92.3) | >0.9999 |
| >1 | 1/9 (11.1) | 7/9 (77.8) | 1/9 (11.1) | 8/9 (88.9) | |
| MGMT expression | |||||
| Negative to moderate | 4/13 (30.8) | 8/13 (61.5) | 1/13 (7.7) | 12/13 (92.3) | >0.9999 |
| Strong | 1/16 (6.25) | 13/16 (81.25) | 2/16 (12.5) | 14/16 (87.5) | |
| Prior surgery | |||||
| No | 2/21 (9.5) | 19/21 (90.5) | 0/21 (0) | 21/21 (100) | 0.0322* |
| Yes | 4/17 (23.5) | 9/17 (52.9) | 4/17 (23.5) | 13/17 (76.5) | |
| Prior radiotherapy | |||||
| No | 5/24 (20.8) | 17/24 (70.8) | 2/24 (8.3) | 22/24 (91.6) | 0.6161 |
| Yes | 1/14 (7.1) | 11/14 (78.6) | 2/14 (14.3) | 12/14 (85.7) | |
| CapTem line of treatment | |||||
| First line (naïve) | 5/18 (27.8) | 12/18 (66.7) | 1/18 (5.6) | 17/18 (94.4) | 0.6062 |
| ⩾Second line (pretreated) | 1/20 (5) | 16/20 (80) | 3/20 (15) | 17/20 (85) | |
| Lymph nodes metastasis | |||||
| No | 1/8 (12.5) | 6/8 (75) | 1/8 (12.5) | 7/8 (87.5) | >0.9999 |
| Yes | 5/30 (16.7) | 22/30 (73.3) | 3/30 (10) | 27/30 (90) | |
| Pleura metastasis | |||||
| No | 3/22 (13.6) | 17/22 (77.3) | 2/22 (9.1) | 20/22 (90.9) | >0.9999 |
| Yes | 3/16 (18.75) | 11/16 (68.75) | 2/16 (12.5) | 14/16 (87.5) | |
| Pericardium metastasis | |||||
| No | 5/30 (16.7) | 22/30 (73.3) | 3/30 (10) | 27/30 (90) | >0.9999 |
| Yes | 1/8 (12.5) | 6/8 (75) | 1/8 (12.5) | 7/8 (87.5) | |
| Lung metastasis | |||||
| No | 6/31 (19.4) | 23/31 (74.2) | 2/31 (6.5) | 29/31 (93.5) | 0.1475 |
| Yes | 0/7 (0) | 5/7 (71.4) | 2/7 (28.6) | 5/7 (71.4) | |
| Pancreas metastasis | |||||
| No | 5/31 (16.1) | 23/31 (74.2) | 3/31 (9.7) | 28/31 (90.3) | >0.9999 |
| Yes | 1/7 (14.3) | 5/7 (71.4) | 1/7 (14.3) | 6/7 (85.7) | |
| Liver metastasis | |||||
| No | 6/35 (17.1) | 25/35 (71.4) | 4/35 (11.4) | 31/35 (88.6) | >0.9999 |
| Yes | 0/3 (0) | 3/3 (100) | 0/3 (0) | 3/3 (100) | |
| Bone metastasis | |||||
| No | 1/11 (9.1) | 9/11 (81.8) | 1/11 (9.1) | 10/11 (90.9) | >0.9999 |
| Yes | 5/27 (18.5) | 19/27 (70.4) | 3/27 (11.1) | 24/27 (88.9) | |
CapTem, capecitabine and temozolomide; MGMT, O6-methylguanine DNA methyltransferase; PD, progressive disease; PR, partial response; SD, stable disease; TNM, tumor-node-metastasis.
The bold values indicate significant values.
Table 3.
Treatment responses according to the line of treatment.
| Response category, n (%) | All patients (n = 38); n (%) | First line therapy, (n = 18); n (%) | ⩾Second line therapy, (n = 20); n (%) | p Value |
|---|---|---|---|---|
| CR | 0 | 0 | 0 | — |
| PR | 6 (15.8) | 5 (27.8) | 1 (5.0) | 0.083 |
| SD | 28 (73.7) | 12 (66.7) | 16 (80.0) | 0.468 |
| PD | 4 (10.5) | 1 (5.6) | 3 (15.0) | 0.606 |
| Disease control rate (DCR = CR + PR + SD) | 34 (89.5) | 17 (94.5) | 17 (85.0) | 0.606 |
| mPFS | 13.0 | Not reached | 13.0 | |
| mOS | Not reached | Not reached | Not reached |
CR, complete response; mOS, median overall survival; mPFS, median progression-free survival; PD, progressive disease; PR, partial response; SD, stable disease; TNM, tumor-node-metastasis.
PFS and OS analysis of clinicopathological prognostic factors at baseline
The median PFS from the initiation of the CapTem regimen in the entire cohort was 13 months (95% CI, 11.2–14.8 months). We assessed the associations of various clinicopathological factors at baseline (such as age, gender, inheritance, functional status, TNM stage, Ki67 index, mitosis, MGMT expression, fluorodeoxyglucose standardized uptake value maximum (FDG-SUVmax) of tumor/liver, somatostatin analogues standardized uptake value maximum (SSA-SUVmax) of tumor/liver, prior surgery, line of treatment, metastatic sites, and blood parameters) with PFS using univariable Cox proportional hazards models. Factors for which the hazard ratios and odds ratios were statistically significant at a significance level of 0.1 were subsequently included in a multivariable Cox proportional hazard model (see Table 4). Ultimately, our analysis revealed that PLR is an independent prognostic factor that correlates with PFS in patients with T-NEN receiving CapTem treatment. An elevated PLR was associated with a significant increase in the risk of disease progression (HR 3.707, 95% CI: 1.086–12.654, p = 0.036).
Table 4.
Univariate and multivariate Cox analysis of PFS for patients receiving the CapTem regimen from baseline (CapTem initiation).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender | 0.904 | |||
| Female | 1 | |||
| Male | 0.879 (0.109–7.084) | |||
| Age | 0.593 | |||
| <50 | 1 | |||
| ⩾50 | 1.408 (0.401–4.942) | |||
| Inheritance | 0.337 | |||
| Sporadic | 1 | |||
| Familial (MEN1) | 2.773 (0.345–22.270) | |||
| Functional status | 0.668 | |||
| Nonfunctioning | 1 | |||
| Functioning | 0.639 (0.083–4.930) | |||
| TNM | 0.469 | |||
| III | 1 | |||
| IV | 23.190 (0.005–114483.176) | |||
| Ki67 index | 0.601 | |||
| ⩽10% | 1 | |||
| >10% | 0.737 (0.235–2.312) | |||
| Mitosis | 0.199 | |||
| ⩽10 | 1 | |||
| >10 | 2.439 (0.626–9.498) | |||
| MGMT expression | 0.758 | |||
| Negative to moderate | 1 | |||
| High | 1.207 (0.365–3.996) | |||
| FDG-SUVmax (tumor/liver) | 0.139 | |||
| ⩽2 | 1 | |||
| >2 | 0.416 (0.130–1.331) | |||
| SSA-SUVmax (tumor/liver) | 0.894 | |||
| ⩽1 | 1 | |||
| >1 | 0.914 (0.246–3.393) | |||
| Prior surgery | 0.052 | 0.732 | ||
| No | 1 | 1 | ||
| Yes | 3.225 (0.990–10.509) | 1.299 (0.291–5.799) | ||
| CapTem line of treatment | 0.148 | |||
| First line (naïve) | 1 | |||
| ⩾Second line (pretreated) | 2.591 (0.712–9.424) | |||
| Lymph node metastasis | 0.602 | |||
| No | 1 | |||
| Yes | 0.708 (0.193–2.594) | |||
| Pleura metastasis | 0.858 | |||
| No | 1 | |||
| Yes | 1.105 (0.369–3.308) | |||
| Pericardium metastasis | 0.281 | |||
| No | 1 | |||
| Yes | 2.090 (0.548–7.976) | |||
| Lung metastasis | 0.678 | |||
| No | 1 | |||
| Yes | 1.315 (0.361–4.785) | |||
| Pancreas metastasis | 0.483 | |||
| No | 1 | |||
| Yes | 0.481 (0.062–3.727) | |||
| Liver metastasis | 0.626 | |||
| No | 1 | |||
| Yes | 0.044 (0–12175.405) | |||
| Bone metastasis | 0.074 | 0.964 | ||
| No | 1 | 1 | ||
| Yes | 6.431 (0.833–49.664) | 190985.623 (0–9.473E + 232) | ||
| Blood parameters | ||||
| NLR | 0.960 (0.811–1.136) | 0.636 | ||
| LMR | 1.320 (0.988–1.764) | 0.06 | 1.285 (0.632–2.613) | 0.489 |
| PLR | 2.676 (1.509–4.745) | 0.001** | 3.707 (1.086–12.654) | 0.036* |
| AFP | 9.213 (0–547076.711) | 0.692 | ||
| CEA | 1.829 (0.463–7.215) | 0.389 | ||
| CA125 | 0.977 (0.841–1.135) | 0.761 | ||
| SCC | 0.727 (0.023–22.535) | 0.856 | ||
| CA199 | 0.937 (0.661–1.327) | 0.714 | ||
| PIVKA-II | 0.063 (0.000–10.598) | 0.290 | ||
| NSE | 1.081 (0.881–1.326) | 0.456 | ||
| CFRA21-1 | 1.695 (0.913–3.147) | 0.095 | 1.018 (0.306–3.387) | 0.977 |
| ProGRP | 1.005 (0.965–1.047) | 0.805 | ||
| LDH | 0.480 (0.086–2.689) | 0.404 | ||
AFP, alpha-fetoprotein; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199; CapTem, capecitabine and temozolomide; CEA, carcinoembryonic antigen; CFRA21-1, cytokeratin 19 fragment; FDG-SUVmax, fluorodeoxyglucose standardized uptake value maximum; LDH, lactate dehydrogenase; LMR, lymphocyte-to-monocyte ratio; MGMT, O6-methylguanine DNA methyltransferase; NLR, neutrophil-to-lymphocyte ratio; NSE, protein induced by vitamin K absence or antagonist II; PFS, progression-free survival; PIVKA-II, protein induced by vitamin K absence or antagonist II; PLR, platelet-to-lymphocyte ratio; ProGRP, pro-gastrin-releasing peptide; SCC, squamous cell carcinoma antigen; SSA-SUVmax, somatostatin analogues standardized uptake value maximum; TNM, tumor-node-metastasis.
The bold values indicate significant values.
We then applied the CART model to determine the optimal cutoff value for PLR, which would allow us to classify patients into groups with either favorable or unfavorable outcomes. Through this analysis, a cutoff value of 235 was identified by the CART method to distinguish patients with high PLR (PLRhigh) from those with low PLR (PLRlow). Subsequent Kaplan–Meier analysis demonstrated that patients with PLRhigh (⩾235) exhibited a significantly shorter median PFS compared to patients with PLRlow (<235) (7 months vs. undefined, p = 0.0004) (see Figure 3). These findings underscore the prognostic significance of PLR in ATC patients receiving CapTem treatment.
Figure 3.
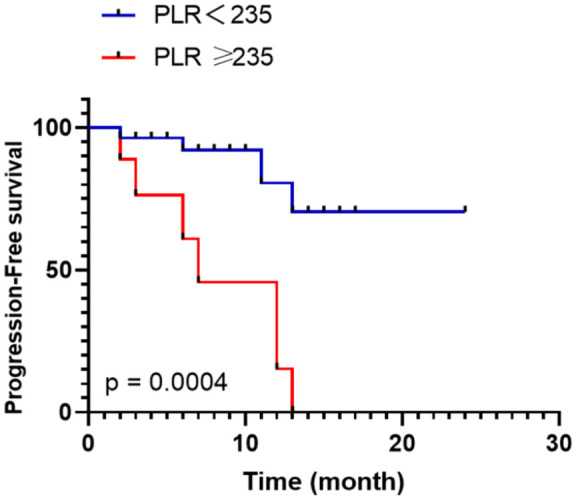
Kaplan–Meier estimates of PFS between the PLRhigh (⩾235) and PLRlow (<235) groups.
PFS, progression-free survival; PLRhigh, high platelet-to-lymphocyte ratio; PLRlow, low platelet-to-lymphocyte ratio; OS, overall survival.
The median OS was not reached in this cohort, but there were observed deaths in seven cases (18.4%) before the study closeout date. In univariable Cox analysis, it was found that age over 50 years (HR 15.142, 95% CI: 1.779–128.878, p = 0.013), an increased level of CA125 (HR 1.027, 95% CI: 1.005–1.049, p = 0.015), and elevated CA199 (HR 1.112, 95% CI: 1.108–1.215, p = 0.018) were all statistically significantly associated with shorter OS (refer to Table 5). Subsequent multivariable Cox analysis, which included the factors identified as significant by univariable Cox analysis, revealed that only age over 50 years (HR 11.214, 95% CI: 1.189–105.796, p = 0.035) remained statistically significantly associated with shorter OS. Kaplan–Meier analysis confirmed these findings by showing that patients aged 50 years or older had a significantly shorter median OS compared to patients under 50 years (36 months vs. undefined, p = 0.015) (see Figure 4). Therefore, we identify older age (⩾50 years) as an independent prognostic factor correlated with poorer OS in ATC patients receiving CapTem treatment.
Table 5.
Univariate and multivariate Cox analysis of OS for patients treated with CapTem regimen from baseline (since CapTem initiation).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender | 0.396 | |||
| Female | 1 | |||
| Male | 31.571 (0.011–91454.19) | |||
| Age | 0.013* | 0.035* | ||
| <50 | 1 | 1 | ||
| ⩾50 | 15.142 (1.779–128.878) | 11.214 (1.189–105.796) | ||
| Inheritance | 0.308 | |||
| Sporadic | 1 | |||
| Familial (MEN1) | 3.062 (0.356–26.371) | |||
| Functional status | 0.161 | |||
| Nonfunctioning | 1 | |||
| Functioning | 3.262 (0.625–17.034) | |||
| TNM | 0.855 | |||
| III | 1 | |||
| IV | 0.820 (0.098–6.885) | |||
| Ki67 index | 0.077 | |||
| ⩽10% | 1 | |||
| >10% | 0.144 (0.017–1.229) | |||
| Mitosis | 0.661 | |||
| ⩽10 | 1 | |||
| >10 | 1.620 (0.188–13.956) | |||
| MGMT expression | 0.314 | |||
| Negative to moderate | 1 | |||
| High | 3.216 (0.331–31.233) | |||
| FDG-SUVmax (tumor/liver) | 0.332 | |||
| ⩽2 | 1 | |||
| >2 | 33.178 (0.028–39022.579) | |||
| SSA-SUVmax (tumor/liver) | 0.340 | |||
| ⩽1 | 1 | |||
| >1 | 2.080 (0.463–9.354) | |||
| Prior surgery | 0.380 | |||
| No | 1 | |||
| Yes | 0.479 (0.093–2.475) | |||
| CapTem line of treatment | 0.897 | |||
| First line (naïve) | 1 | |||
| ⩾Second line (pretreated) | 0.905 (0.200–4.101) | |||
| Lymph node metastasis | 0.611 | |||
| No | 1 | |||
| Yes | 1.733 (0.208–14.416) | |||
| Pleura metastasis | 0.212 | |||
| No | 1 | |||
| Yes | 0.260 (0.031–2.161) | |||
| Pericardium metastasis | 0.784 | |||
| No | 1 | |||
| Yes | 0.743 (0.089–6.191) | |||
| Lung metastasis | 0.859 | |||
| No | 1 | |||
| Yes | 0.825 (0.098–6.940) | |||
| Pancreas metastasis | 0.679 | |||
| No | 1 | |||
| Yes | 1.616 (0.166–15.681) | |||
| Liver metastasis | 0.097 | |||
| No | 1 | |||
| Yes | 6.840 (0.704–66.471) | |||
| Bone metastasis | 0.412 | |||
| No | 1 | |||
| Yes | 0.527 (0.114–2.436) | |||
| Blood parameters | ||||
| NLR | 0.991 (0.819–1.200) | 0.927 | ||
| LMR | 1.051 (0.646–1.712) | 0.840 | ||
| PLR | 0.393 (0.089–1.730) | 0.217 | ||
| AFP | 57664.187 (0.041–8094E+10) | 0.129 | ||
| CEA | 3.105 (0.556–17.345) | 0.197 | ||
| CA125 | 1.027 (1.005–1.049) | 0.015* | 1.059 (0.746–1.505) | 0.748 |
| SCC | 0.627 (0.005–73.402) | 0.848 | ||
| CA199 | 1.112 (1.108–1.215) | 0.018* | 0.857 (0.205–3.588) | 0.833 |
| PIVKA-II | 1.840 (0.007–513.518) | 0.832 | ||
| NSE | 1.382 (0.984–1.943) | 0.062 | ||
| CFRA21-1 | 1.384 (0.584–3.279) | 0.460 | ||
| ProGRP | 1.012 (0.966–1.059) | 0.620 | ||
| LDH | 0.424 (0.033–5.397) | 0.508 | ||
AFP, alpha-fetoprotein; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199; CapTem, capecitabine and temozolomide; CEA, carcinoembryonic antigen; CFRA21-1, cytokeratin 19 fragment; FDG-SUVmax, fluorodeoxyglucose standardized uptake value maximum; LDH, lactate dehydrogenase; LMR, lymphocyte-to-monocyte ratio; MGMT, O6-methylguanine DNA methyltransferase; NLR, neutrophil-to-lymphocyte ratio; NSE, protein induced by vitamin K absence or antagonist II; OS, overall survival; PIVKA-II, protein induced by vitamin K absence or antagonist II; PLR, platelet-to-lymphocyte ratio; ProGRP, pro-gastrin-releasing peptide; SCC, squamous cell carcinoma antigen; SSA-SUVmax, somatostatin analogues standardized uptake value maximum; TNM, tumor-node-metastasis.
The bold values indicate significant values.
Figure 4.
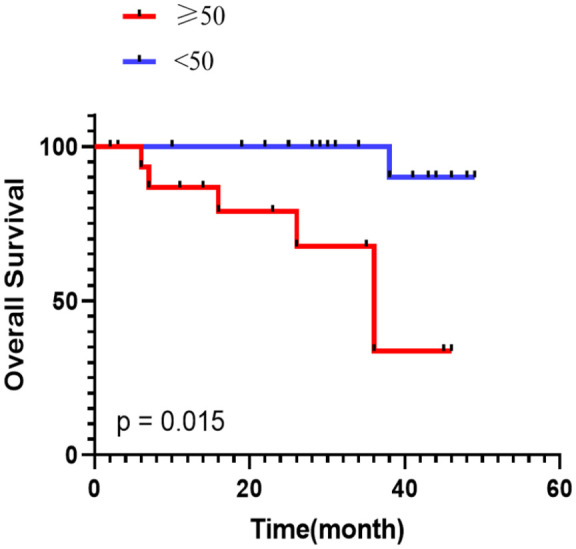
Kaplan–Meier estimates of OS between the older (⩾50) and younger (<50) groups.
OS, overall survival.
Toxicity
Table 6 presented the treatment-related adverse events that patients experienced during the treatment, graded according to the Common Terminology Criteria for Adverse Events, version 5.0. In the entire cohort of 38 patients, 16 (42.1%) experienced some grade of side effects, primarily grades 1 and 2. The most common grades 1–2 side effects included fatigue, gastrointestinal symptoms (anorexia, nausea, vomiting, constipation), hematological complications (neutropenia), and mucocutaneous complications (pigmentation).
Table 6.
Type, frequency, and severity (grade) of adverse effects noticed in patients treated with CapTem, according to CTCAE version 5.0.
| Toxicity | Maximum toxicity grade | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| n | % | n | % | n | % | n | % | |
| Hematologic | ||||||||
| Anemia | 1 | 2.70 | — | — | — | — | — | — |
| Neutropenia | 4 | 10.81 | — | — | — | — | — | — |
| Thrombocytopenia | 1 | 2.70 | — | — | — | — | — | — |
| Gastrointestinal | ||||||||
| Abdominal pain | 1 | 2.63 | — | — | — | — | — | — |
| Nausea | 6 | 15.79 | 9 | 23.68 | 1 | 2.63 | — | — |
| Vomiting | 5 | 13.16 | 10 | 26.32 | 1 | 2.63 | — | — |
| Constipation | 4 | 10.53 | — | — | — | — | — | — |
| Diarrhea | 1 | 2.63 | 1 | 2.63 | — | — | — | — |
| Anorexia | 10 | 26.32 | 1 | 2.63 | — | — | — | — |
| Mucocutaneous | ||||||||
| Pruritus | 1 | 2.63 | 2 | 5.26 | — | — | — | — |
| Pigmentation | 4 | 10.53 | — | — | — | — | — | — |
| Rash maculo-papular | — | — | 2 | 5.26 | — | — | — | — |
| Palmar-plantar erythrodysesthesia syndrome | 2 | 5.26 | — | — | — | — | — | — |
| Musculoskeletal and connective tissue disorders | ||||||||
| Arthralgia | 1 | 2.63 | — | — | — | — | — | — |
| Other | ||||||||
| Fatigue | 12 | 31.58 | — | — | — | — | — | — |
| Insomnia | 2 | 5.26 | — | — | — | — | — | — |
| Headache | 2 | 5.26 | — | — | — | — | — | — |
| Anaphylaxis | — | — | 1 | 2.63 | — | — | — | — |
| Peripheral sensory neuropathy | 1 | 2.63 | — | — | — | — | — | — |
Only one patient experienced serious (grade 3) toxicities related to nausea and vomiting, which necessitated treatment discontinuation. No grade 4 toxicities were observed. Notably, we did not identify any treatment-related hepatobiliary or renal disorders in our cohort. Overall, the CapTem regimen was well-tolerated by the patients. Most treatment-related adverse events were mild and successfully resolved with appropriate clinical care. Importantly, no treatment-related deaths occurred.
A typical case who achieved a favorable PR response on CapTem regimen
A 62-year-old male initially presented with symptoms of chest tightness, shortness of breath, chest and back pain. A PET-CT revealed a 7.5-cm anterior mediastinal mass, multiple lymph node metastases, bilateral lung and pleural metastases, pancreatic metastases, and iliac metastases. Pathological analysis of the left supraclavicular lymph node confirmed a diagnosis of NET with three mitoses per 10 high-power fields and a Ki-67 index of 20%, which was considered ATC.
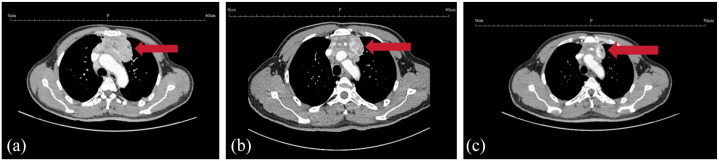
Following the diagnosis, the patient was initiated on treatment with CapTem, and tumor efficacy assessments were conducted after every three cycles of treatment. Remarkably, after completing six cycles of treatment, there was a significant reduction in tumor burden, as evident in the Chest CT images shown in Figure 5.
Figure 5.
Contrast-enhanced CT scans of the typical case who achieved favorable PR response on CapTem regimen. (a) 2022-5-18 Baseline (78 mm*40 mm). (b) 2022-8-17 After three cycles of CapTem (64 mm*30 mm). (c) 2022-11-9 After six cycles of CapTem (32 mm*20 mm).
CapTem, capecitabine and temozolomide; PR, partial response.
Throughout the treatment, the patient experienced manageable grade 1–2 side effects, including gastrointestinal symptoms (grade 2 anorexia, grade 1 nausea), hematological complications (grade 1 neutropenia), mucocutaneous complications (grade 1 palmar-plantar erythrodysesthesia syndrome), and other systemic reactions (grade 1 fatigue, grade 1 insomnia). These side effects were effectively managed and resolved with appropriate clinical care. The patient commenced the CapTem regimen on May 26, 2022, and as of his most recent follow-up visit, he continues to receive treatment with no evidence of tumor progression.
Discussion
In this study, we have summarized the clinical characteristics, treatment activity, and safety of the CapTem regimen in a cohort of ATC patients. Additionally, we conducted an analysis of various clinicopathological factors to identify independent prognostic factors for PFS and OS.
ATCs exhibit distinct demographic and clinical characteristics when compared to NENs from other origins. In this cohort, the male-to-female ratio was 6.6 (33 males and 5 females), consistent with previous literature suggesting a male predilection for ATCs,4,18 unlike NENs from other origins, such as pancreatic NENs and gastrointestinal NENs, where no such gender tendency is observed. 19 Furthermore, unlike NENs originating in the pancreas or digestive tract, which frequently metastasize to the liver, ATCs preferentially metastasize to lymph nodes (30/38, 78.9%), bone (27/38, 71.1%), and pleura (16/38, 42.1%). Liver metastases in ATCs are relatively uncommon (3/38, 7.9%). This unique metastasis pattern of T-NENs has also been noted in previous case series. 20 Considering these distinctive characteristics, it becomes evident that clinical management strategies for ATCs should not be simply extrapolated from experiences with NENs from other origins. Instead, further investigations tailored to ATC cohorts are warranted to draw evidence-based conclusions.
The CapTem regimen has demonstrated notable efficacy in treating diverse populations of patients with NETs. It typically achieves the highest ORRs in pancreatic NETs (30%–70%),8 –12 intermediate responses in lung NETs (30%), 21 and lower responses in gastrointestinal NETs (19%). 22 While substantial data exist for these NET types, information on the efficacy of CapTem in ATCs remains limited. Currently, only two retrospective studies in T-NET cohorts, involving 3 and 9 patients, have been reported.23,24 Saranga-Perry et al. assessed three T-NET patients treated with CapTem, reporting one PR, one minor response, and one SD. 23 Wang et al. found that eight T-NET patients achieved SD, while one patient showed PD. 24
However, these limited case series do not provide conclusive data on the efficacy and prognostic factors for T-NETs. In our study, we report, for the first time, the ORR of CapTem in a more substantial and homogeneous group of ATC patients, with an ORR of 15.8% (6/38), a DCR of 89.5%, and a median PFS of 13 months.
Our findings, aligned with existing literature, demonstrate that first-line CapTem treatment is more effective than subsequent lines. A multicenter retrospective study of 308 patients with metastatic NETs treated with CapTem showed that first-line therapy yielded superior ORR, PFS, and OS outcomes compared to later-line treatments, regardless of tumor location. 25 Similar results have been reported in multiple studies across various centers.9,26 –29 The greater efficacy of CapTem as a first-line treatment may be due to several factors: (i) Lack of prior treatment resistance—tumors can develop resistance mechanisms over the course of therapy, reducing CapTem’s effectiveness when used in later lines; (ii) Optimal dosing and lower cumulative toxicity in treatment-naïve patients—first-line therapy allows for optimal dosing and scheduling, which may not be achievable in later treatments due to the accumulated toxicity from previous therapies or the need for dose adjustments in patients with declining health. However, the exact mechanisms remain unclear. Further rigorous research is necessary to investigate the molecular and genetic/epigenetic factors contributing to these observations and their broader clinical implications.
In our exploration of prognostic factors in ATC patients undergoing CapTem treatment, we found that neither Ki-67 nor MGMT status was associated with treatment response or PFS. While Ki-67 and MGMT have been established as prognostic indicators in patients with NETs originating from the pancreas or gastrointestinal tract,11,30 the inconsistent findings in ATCs underscore their unique characteristics. This highlights the need for the development of novel biomarkers tailored to this specific patient population.
Notably, our study identified, for the first time, the prognostic value of PLR in ATC patients receiving the CapTem regimen. Elevated PLR was significantly associated with an increased risk of disease progression. Accumulating clinical observations suggest that elevated PLR is linked to poorer outcomes in various malignancies, although the precise mechanisms by which PLR influences clinical outcomes remain largely unknown.31 –33
Previous research has proposed that platelet aggregation can expedite tumor progression by facilitating new blood vessel formation and adhesion molecule production, thus enhancing the migration and invasion of tumor cells. 34 Conversely, lymphocytes play a crucial role in defending against cancer by mounting an anti-tumor immune response. 35 Elevated peripheral lymphocytes are typically regarded as a favorable prognostic factor, while lymphocytopenia reflects impaired lymphocyte-mediated antitumor responses. PLR, being a combination of circulating platelet and lymphocyte counts, represents both thrombocytosis and lymphocytopenia, potentially contributing to aggressive cancer progression and poorer outcomes. However, the precise mechanisms through which PLR influences PFS in ATC patients receiving CapTem warrant further investigation.
When examining clinicopathological factors associated with the OS of ATC patients receiving the CapTem regimen, we identified age as an independent factor linked to OS. Notably, patients aged 50 and above demonstrated significantly shorter OS compared to patients under 50, suggesting that younger age may serve as an indicator of potential benefits from this treatment. This finding aligns with a previous multicenter retrospective study that analyzed data from 308 patients with metastatic NETs treated with CapTem in 34 hospitals across different regions of Turkey, revealing distinct outcomes for patients categorized by age of 50. 25
The CapTem regimen in our study exhibited a safety profile consistent with previous reports,8 –12,16,21 –24 and no new safety concerns emerged. Most of the treatment-related adverse events were of grade 1–2 severity and were effectively managed with appropriate clinical care. There was only one instance of grade 3 toxicities related to nausea and vomiting. Notably, no grade 4 toxicities or treatment-related deaths occurred, indicating that this regimen can be considered a viable and well-tolerated option for patients with ATC.
One of the primary debates surrounding the CapTem regimen pertains to the ideal treatment duration and the safety of long-term administration. Temozolomide, being an alkylating agent that can induce DNA damage, raises concerns about the potential development of secondary malignancies, such as myelodysplastic syndrome, leukemia, or aplastic anemia, after prolonged use.36 –38 The randomized ECOG 2211 study suggested a treatment duration of 1 year with the CapTem regimen, while allowing additional cycles on a case-by-case basis. 8 However, currently, no evidence-based recommendations exist regarding the optimal treatment duration for T-NET patients. Our study is the first to present real-world practice data and report on a considerable number of patients who underwent CapTem treatment for 12 months or longer. Encouragingly, among the 13 cases in our study with treatment durations of ⩾12 months, no secondary malignancies were observed during a median follow-up of 31 months (range 12–46). Nevertheless, it is essential to underscore that further clinical testing involving a larger number of cases and more extended follow-up periods is necessary to comprehensively evaluate the safety of prolonged CapTem administration.
While our study provides valuable clinical insights into the administration of CapTem regimen for metastatic ATCs, several limitations must be acknowledged. First, the retrospective nature of the study introduces potential biases, as patient data were collected and analyzed after treatment, limiting the ability to control for confounding factors. Second, this is a single-center study, which may affect the generalizability of the results to broader patient populations. Third, the limited sample size, due to the rarity of T-NEN, restricts the statistical power of our findings, which may result in an underestimation or overestimation of certain outcomes. Despite these limitations, we believe that our research will serve as a foundation for future, larger-scale studies.
Conclusion
In summary, the cases presented in our study complement previous retrospective series, reinforcing the notion that the CapTem regimen can be a rational and well-tolerated treatment option with favorable responses for patients with ATCs. Importantly, our study is the first to identify pretreatment PLR as an independent prognostic biomarker associated with PFS in patients with ATCs treated with CapTem. We established the optimal cutoff for PLR as 235. Additionally, our findings revealed that age greater than 50 years is an independent adverse prognostic factor for OS. These findings may provide potential insights for clinicians in identifying patients most likely to benefit from the CapTem regimen. However, the small sample size limits the strength of our statistical conclusions, and as such, these results could not be used for immediate clinical decision-making at this time. To validate these findings and gain a deeper understanding, further mechanistic investigations, prospective and multicenter studies focusing on this particularly rare patient population, will be crucial in confirming the robustness and broader applicability of our results.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241297578 for Efficacy, safety, and prognostic factors of capecitabine plus temozolomide regimen in patients with atypical thymic carcinoids by Man Liu, Xu Yan, Xiaoxuan Lin, Luohai Chen, Yu Wang, Yanji Luo, Yuan Lin, Qiao He, Jie Chen and Ning Zhang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241297578 for Efficacy, safety, and prognostic factors of capecitabine plus temozolomide regimen in patients with atypical thymic carcinoids by Man Liu, Xu Yan, Xiaoxuan Lin, Luohai Chen, Yu Wang, Yanji Luo, Yuan Lin, Qiao He, Jie Chen and Ning Zhang in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank the patients and their families, the nurses, and the pathologists who participated in this study.
Footnotes
ORCID iD: Xu Yan  https://orcid.org/0009-0009-1445-252X
https://orcid.org/0009-0009-1445-252X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Man Liu, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Xu Yan, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Xiaoxuan Lin, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Luohai Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Yu Wang, Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Yanji Luo, Department of Radiology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Yuan Lin, Department of Pathology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Qiao He, Department of Nuclear Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Jie Chen, Center for Neuroendocrine Tumors, Fudan University Shanghai Cancer Center, No.270 Dongan Road, Xuhui District, Shanghai, China.
Ning Zhang, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, No.58 Zhongshan Er Road, Guangdong Province, Guangzhou, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Institutional Ethics Committee (IEC) for Clinical Research of the First Affiliated Hospital of Sun Yat-sen University (approval No. 2023[561]). The requirement for formal consent was waived by the approving ethics committee due to the retrospective nature of this study.
Consent for publication: Not applicable.
Author contributions: Man Liu: Data curation; Formal analysis; Methodology; Writing – original draft.
Xu Yan: Data curation; Formal analysis; Methodology; Writing – original draft.
Xiaoxuan Lin: Writing – review & editing.
Luohai Chen: Writing – review & editing.
Yu Wang: Project administration.
Yanji Luo: Project administration.
Yuan Lin: Project administration.
Qiao He: Project administration.
Jie Chen: Supervision.
Ning Zhang: Supervision.
The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (grant numbers: 82002502 and 82141104), and the Natural Science Foundation of Guangdong Province (grant numbers: 2019A1515012027 and 2019A1515011719).
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. WHO Classification of Tumours Editorial Board. WHO classification Thoracic tumours. Lyon: IARC Press, 2021. [Google Scholar]
- 2. Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010; 251: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 3. Baudin E, Caplin M, Garcia-Carbonero R, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol 2021; 32: 439–451. [DOI] [PubMed] [Google Scholar]
- 4. Jia R, Sulentic P, Xu J-M, et al. Thymic neuroendocrine neoplasms: biological behaviour and therapy. Neuroendocrinology 2017; 105: 105–114. [DOI] [PubMed] [Google Scholar]
- 5. Turner N, Strauss S, Sarker D, et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer 2010; 102: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Carbonero R, Rinke A, Valle JW, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms. Systemic therapy 2: chemotherapy. Neuroendocrinology 2017; 105: 281–294. [DOI] [PubMed] [Google Scholar]
- 7. Koumarianou A, Kaltsas G, Kulke MH, et al. Temozolomide in advanced neuroendocrine neoplasms: pharmacological and clinical aspects. Neuroendocrinology 2015; 101: 274–288. [DOI] [PubMed] [Google Scholar]
- 8. Kunz PL, Graham NT, Catalano PJ, et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol 2023; 41: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011; 117: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the pancreas center at Columbia University experience. Cancer Chemother Pharmacol 2013; 71: 663–670. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Zhang Y, Peng Y, et al. A Ki-67 index to predict treatment response to the capecitabine/temozolomide regimen in neuroendocrine neoplasms: a retrospective multicenter study. Neuroendocrinology 2021; 111: 752–763. [DOI] [PubMed] [Google Scholar]
- 12. Ramirez RA, Beyer DT, Chauhan A, et al. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist 2016; 21: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93–99. [DOI] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 16. Jeong H, Shin J, Jeong JH, et al. Capecitabine plus temozolomide in patients with grade 3 unresectable or metastatic gastroenteropancreatic neuroendocrine neoplasms with Ki-67 index < 55%: single-arm phase II study. ESMO Open 2021; 6: 100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf(2017)
- 18. Soga J, Yakuwa Y, Osaka M. Evaluation of 342 cases of mediastinal/thymic carcinoids collected from literature: a comparative study between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg 1999; 5: 285–292. [PubMed] [Google Scholar]
- 19. Liu M, Hu W, Zhang Y, et al. Clinical implications of immune checkpoint markers and immune infiltrates in patients with thymic neuroendocrine neoplasms. Front Oncol 2022; 12: 917743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000; 114: 100–110. [DOI] [PubMed] [Google Scholar]
- 21. Al-Toubah T, Morse B, Strosberg J. Capecitabine and Temozolomide in advanced lung neuroendocrine neoplasms. Oncologist 2020; 25: e48-e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Toubah T, Morse B, Strosberg J. Efficacy of capecitabine and temozolomide in small bowel (Midgut) neuroendocrine tumors. Curr Oncol 2022; 29: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saranga-Perry V, Morse B, Centeno B, et al. Treatment of metastatic neuroendocrine tumors of the thymus with capecitabine and temozolomide: a case series. Neuroendocrinology 2013; 97: 318–321. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Li Y, Duan J, et al. Capecitabine and temozolomide as a promising therapy for advanced thymic atypical carcinoid. Oncologist 2019; 24: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ünal Ç, Azizy A, Karabulut S, et al. Efficacy of capecitabine and temozolomide regimen in neuroendocrine tumors: data from the Turkish Oncology Group. Oncologist 2023; 28: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu AJ, Ueberroth BE, McGarrah PW, et al. Treatment outcomes of well-differentiated high-grade neuroendocrine tumors. Oncologist 2021; 26: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crespo G, Jiménez-Fonseca P, Custodio A, et al. Capecitabine and temozolomide in grade 1/2 neuroendocrine tumors: a Spanish multicenter experience. Future Oncol 2017; 13: 615–624. [DOI] [PubMed] [Google Scholar]
- 28. Peixoto RD, Noonan KL, Pavlovich P, et al. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J Gastrointest Oncol 2014; 5: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahu A, Jefford M, Lai-Kwon J, et al. CAPTEM in metastatic well-differentiated intermediate to high grade neuroendocrine tumors: a single centre experience. J Oncol 2019; 2019: 9032753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cros J, Hentic O, Rebours V, et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016; 23: 625–633. [DOI] [PubMed] [Google Scholar]
- 31. Liu D, Czigany Z, Heij LR, et al. The value of platelet-to-lymphocyte ratio as a prognostic marker in cholangiocarcinoma: a systematic review and meta-analysis. Cancers (Basel) 2022; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schobert IT, Savic LJ, Chapiro J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol 2020; 30: 5663–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–181. [DOI] [PubMed] [Google Scholar]
- 34. Jiang L, Luan Y, Miao X, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer 2017; 117: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei J, Zheng W, Chapman NM, et al. T cell metabolism in homeostasis and cancer immunity. Curr Opin Biotechnol 2021; 68: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jalali R, Singh P, Menon H, et al. Unexpected case of aplastic anemia in a patient with glioblastoma multiforme treated with Temozolomide. J Neurooncol 2007; 85: 105–107. [DOI] [PubMed] [Google Scholar]
- 37. Villano JL, Collins CA, Manasanch EE, et al. Aplastic anaemia in patient with glioblastoma multiforme treated with temozolomide. Lancet Oncol 2006; 7: 436–438. [DOI] [PubMed] [Google Scholar]
- 38. Noronha V, Berliner N, Ballen KK, et al. Treatment-related myelodysplasia/AML in a patient with a history of breast cancer and an oligodendroglioma treated with temozolomide: case study and review of the literature. Neuro Oncol 2006; 8: 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241297578 for Efficacy, safety, and prognostic factors of capecitabine plus temozolomide regimen in patients with atypical thymic carcinoids by Man Liu, Xu Yan, Xiaoxuan Lin, Luohai Chen, Yu Wang, Yanji Luo, Yuan Lin, Qiao He, Jie Chen and Ning Zhang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241297578 for Efficacy, safety, and prognostic factors of capecitabine plus temozolomide regimen in patients with atypical thymic carcinoids by Man Liu, Xu Yan, Xiaoxuan Lin, Luohai Chen, Yu Wang, Yanji Luo, Yuan Lin, Qiao He, Jie Chen and Ning Zhang in Therapeutic Advances in Medical Oncology