Abstract
Background:
The arrhythmic burden and cardiovascular risks of cardiac amyloidosis compared with other types of restrictive cardiomyopathies (RCM), such as hemochromatosis and cardiac sarcoid, have not been well characterized in the literature. An increase in emphasis on screening has resulted in more diagnoses of cardiac amyloidosis and a larger data pool to analyze the cardiovascular outcomes of this cardiomyopathy.
Methods and results:
We queried the National Inpatient Sample (NIS) database to identify all adult patients diagnosed with cardiac amyloidosis or other RCM between the years 2016 and 2019. Discharge-weighted analysis using survey regressions accounts for discharge weights and characteristics found to be significantly different between groups. A total sample size of 13 345 patients was obtained, including cardiac amyloidosis (N = 8365; 62.7%) and other RCM (N = 4980; 37.3%). Cardiac amyloidosis was associated with a significantly increased risk of stroke (Odds ratio = 3.91: 95% confidence interval = [2.15, 7.11], P < .001) and ventricular tachycardia (1.98 [1.35-2.91], P < .001). Cardiac amyloidosis had a decreased risk of atrial fibrillation (0.56 [0.47-0.68], P < .001). Significant differences in risk were not observed among the different types of heart block and supraventricular arrhythmias. In-hospital mortality was similar between the 2 groups (P = .72).
Conclusions:
Cardiac amyloidosis was associated with an increased risk of stroke and ventricular tachycardia compared to other types of RCM. Significant differences in in-hospital mortality, bundle branch blocks, and supraventricular arrhythmias were not appreciated. A subgroup analysis comparing light chain (AL) and wild-type transthyretin (ATTR) amyloidosis outcomes would further delineate the cardiovascular risks of cardiac amyloidosis.
Keywords: Cardiac amyloidosis, restrictive cardiomyopathy, arrhythmia, national inpatient sample, outcomes analysis
Introduction
Amyloidosis is caused by the accumulation of abnormal proteins in the organs and tissues. In cardiac amyloidosis, the amyloid fibrils get deposited in the myocardium, which impairs cardiac contractility and relaxation of the ventricles and leads to congestive heart failure. 1 Cardiac amyloidosis is a common form of RCM, which refers to diastolic dysfunction of a non-dilated ventricle whose etiology includes infiltration of abnormal substances such as transthyretin or the creation of scar tissue from radiation. 2 Restrictive cardiomyopathies account for approximately 2% to 5% of cardiomyopathy diagnoses, with a differential including but not limited to hemochromatosis, scleroderma, carcinoid syndrome, inherited metabolic disorders, and radiation-induced fibrosis. 3 Genetic causes of RCM involve mutations in specific genes such as FLNC and CRYAB. 4 Mutations in the FLNC and CRYAB genes, which encode filamin C and alpha-b-crystallin respectively, disrupt the structural integrity of cardiac muscle fibers. The malfunction of proteins critical for maintaining the stability and function of the cardiac muscle contributes to the development of RCM. 4 This study on the genetic causes of RCM occurred after the Declaration of Helsinki, which laid the framework for responsible and ethical research in medical genetics.
With the growing emphasis on cardiac amyloidosis screening, it is imperative to understand how health outcomes associated with cardiac amyloidosis compare to other forms of RCM. This will help clinicians and researchers identify arrhythmic and other cardiovascular complications of RCM. By highlighting an increasingly diagnosed RCM such as amyloidosis, knowledge of disease burden can become more apparent across all subtypes of the disease. We hypothesize arrhythmic and cardiovascular complications will be comparable between amyloidosis and the other RCM due to similarities in their respective pathophysiologies.
Furthermore, a cognizance of cerebrovascular, thrombotic, and other complications would aid in patient discussions stressing the importance of early treatment after diagnosis. Multiple clinical studies have demonstrated an association between atrial and ventricular arrhythmias in light chain and wild-type transthyretin cardiac amyloidosis.5,6 Jamal et al 7 conducted an NIS study of amyloidosis in patients with and without atrial fibrillation, demonstrating increased mortality and other cardiovascular complications in the former. However, all these studies excluded other RCM in their analyses. Therefore, limited data and comparison trials exist when comprehensively analyzing RCM.
This study compares cardiovascular outcomes and common arrhythmias in cardiac amyloidosis against other RCM such as cardiac sarcoid, carcinoid, and hemochromatosis. To do this, we used the NIS database from 2016 to 2019, which contains information on an approximate 20% sample of United States inpatient admissions. Given the relative rarity of RCM, NIS provides an ideal source in which to study inpatient outcomes, including stroke, in-hospital mortality, and conduction disorders.
Methods
Data source
Data was obtained from the Healthcare Cost and Utilization Project (HCUP) NIS files between 2016 and 2019. The NIS is a public database containing an extensive collection of all-payer inpatient care and discharge-level data provided by the states participating in HCUP. Criteria to aggregate hospital data include geographic region, rural or urban location, teaching status, patient volume, hospital bed size, primary payer, and patient median household income.
This study was exempt from the Southern Illinois University College of Medicine Institutional Review Board because the HCUP-NIS is a publicly available database and contains deidentified patient information.
Study population
We used the 10th edition of the International Classification of Diseases Clinical Modification (ICD-10-CM) diagnosis codes E85.4 and I50 to identify all adult patients diagnosed with cardiac amyloidosis. Patients who had RCM without amyloidosis had the code I42.5 with E85 excluded. Exclusion criteria involved patients with a history of hyperthyroidism (E05), alcohol abuse (F10.10), and cardiac bypass (Z95.1, I25.810).
Outcome measures
Our primary outcome of interest included arrhythmic burdens such as atrial arrhythmias, ventricular arrhythmias, bundle branch blocks, and atrioventricular blocks. Secondary outcomes were in-patient mortality, stroke, cost of stay, and length of stay. ICD-10-CM codes for primary outcomes are provided in Table 1.
Table 1.
Unadjusted outcomes of cardiac amyloidosis versus restrictive cardiomyopathy without cardiac amyloidosis. Numbers, odds ratios, and comparisons account for discharge weights only.
| Outcomes | Cardiac amyloidosis (N = 8365) | Restrictive cardiomyopathy without CA (N = 4980) | OR/Mean difference (95% CI) | P-value |
|---|---|---|---|---|
| In hospital mortality, n (%) | 515 (6.2) | 310 (6.2) | 0.99 (0.71, 1.37) | .94 |
| Length of stay, days, median (IQR) | 5 (3, 10) | 6 (3, 10) | −1.09 (−1.97, −0.22) | .01 |
| Cost of stay, thousands of dollars, median (IQR) | 46.2 (24.0, 95.4) | 50.4 (27.2, 106.5) | −17.0 (−35.9, 1.7) | .08 |
| Atrial fibrillation, n (%) | 4820 (57.6) | 3025 (60.7) | 0.88 (0.75, 1.03) | .11 |
| Ventricular fibrillation, n (%) | 35 (0.4) | 15 (0.3) | 1.39 (0.36, 5.40) | .63 |
| Ventricular tachycardia, n (%) | 750 (9.0) | 215 (4.3) | 2.18 (1.54, 3.09) | <.001 |
| Supraventricular tachycardia, n (%) | 320 (3.8) | 200 (4.0) | 0.95 (0.64, 1.42) | .81 |
| First degree heart block, n (%) | 225 (2.7) | 70 (1.4) | 1.94 (1.06, 3.55) | .03 |
| Second degree heart block, n (%) | 90 (1.1) | 35 (0.7) | 1.54 (0.64, 3.69) | .33 |
| Complete heart block, n (%) | 90 (1.1) | 35 (0.7) | 1.54 (0.64, 3.69) | .33 |
| Left bundle branch block, n (%) | 120 (1.4) | 80 (1.6) | 0.89 (0.47, 1.69) | .72 |
| Right bundle branch block, n (%) | 270 (3.2) | 135 (2.7) | 1.20 (0.75, 1.91) | .45 |
| Stroke, n (%) | 405 (4.8) | 70 (1.4) | 3.57 (2.01, 6.33) | <.001 |
Patient and hospital characteristics
Baseline patient characteristics used included demographics (age, sex, primary payer, median household income) and common comorbidities known to be risk factors for cardiovascular disease (cerebrovascular disease, coronary artery disease, peripheral artery disease, obstructive sleep apnea, atrial fibrillation, obesity, hypertension, type 2 diabetes mellitus, chronic kidney disease, and tobacco use). A list of ICD-10-CM codes used to identify baseline comorbidities is provided in the first column of Table 2. We also included hospital variables such as weekend admission percentage, hospital bed size (small, medium, large), hospital location, and teaching status (rural, urban non-teaching, urban teaching).
Table 2.
Patient characteristics for those with cardiac amyloidosis versus restrictive cardiomyopathy with amyloidosis excluded. Numbers and comparisons incorporate the discharge weights provided in the NIS. Data is from 2016 to 2019.
| Cardiac amyloidosis w/HF (N = 8365) | Restrictive cardiomyopathy w/o CA (N = 4980) | P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 74.8 (11.6) | 67.9 (16.9) | <.001 |
| Female, n (%) | 3380 (40.4) | 2840 (57.0) | <.001 |
| Weekend admission, n (%) | 1660 (19.8) | 1035 (20.8) | .56 |
| Primary payer | <.001 | ||
| Medicare | 6635 (79.4) | 3615 (72.7) | |
| Medicaid | 385 (4.6) | 445 (9.0) | |
| Private insurance | 1180 (14.1) | 795 (16.0) | |
| Others/self-pay | 155 (1.9) | 115 (2.3) | |
| Median household income (quartile) | <.001 | ||
| 0-25th percentile | 1795 (21.8) | 1260 (25.9) | |
| 25-50th percentile | 1775 (21.5) | 1230 (25.3) | |
| 50-75th percentile | 2070 (25.1) | 1260 (25.9) | |
| 75-100th percentile | 2610 (31.6) | 1115 (22.9) | |
| Hospital bed-size | .03 | ||
| Small | 1190 (14.2) | 850 (17.1) | |
| Medium | 2220 (26.5) | 1125 (22.6) | |
| Large | 4955 (59.2) | 3005 (60.3) | |
| Hospital location and teaching status | <.001 | ||
| Rural | 235 (2.8) | 230 (4.6) | |
| Urban non-teaching | 740 (8.8) | 730 (14.7) | |
| Urban teaching | 7390 (88.3) | 4020 (80.7) | |
| Comorbidities, n (%) | |||
| Cerebrovascular disease I6X | 1630 (19.5) | 245 (4.9) | <.001 |
| CAD I25 | 2900 (34.7) | 1685 (33.8) | .66 |
| Peripheral artery disease I73.9 | 255 (3.0) | 185 (3.7) | .35 |
| Obstructive sleep apnea G47.33 | 1190 (14.2) | 1135 (22.8) | <.001 |
| Obesity E66 | 1015 (12.1) | 1200 (24.1) | <.001 |
| Hypertension I10 | 4490 (59.7) | 2540 (51.0) | <.001 |
| Type 2 diabetes E11.9 | 2440 (29.2) | 1835 (36.8) | <.001 |
| Chronic kidney disease N18 | 4850 (58.0) | 2665 (53.5) | .02 |
| Tobacco use F17 | 410 (4.9) | 370 (7.4) | .007 |
Statistical analysis
Individual admissions were weighted per recommendations from the NIS. 8 Applying discharge weights allows for a nationally representative sample that reduces bias in inferences made to the overall US population. 9 Detailed information on the design of the NIS is available at www.hcup-us.ahrq.gov.
Univariate comparisons of patient characteristics between those with cardiac amyloidosis and those with other types of RCM were done using weighted t-tests and chi-squared tests for continuous and categorical variables, respectively. Unadjusted comparisons of outcomes were made using weighted logistic and linear regressions for binary and continuous outcomes, respectively. The only variable included in unadjusted models was RCM type with other types of RCM as the reference group. Adjusted comparisons were made using multivariate weighted logistic and linear regressions as in unadjusted models. Adjusted models further accounted for discharge characteristics found to be significantly different between groups (i.e. age, gender, primary payer, household income, hospital size, hospital location, cardiovascular disease, obstructive sleep apnea, obesity, hypertension, diabetes, chronic kidney disease, and tobacco use).
For all analyses, statistical significance was set at P < .05. All analyses were performed using the “survey” package in R statistical software.10,11
Results
Patient and hospital characteristics
The final discharge-weighted analysis included 8365 and 4980 cardiac amyloidosis and RCM patients. Females accounted for a minority of the amyloidosis group, which was significantly lower than that observed in the RCM cohort. Average age was notably higher in the amyloidosis group, 74.8 versus 67.9. Medicare and Medicaid were the predominant primary payers, accounting for over 80% in both groups. The weekend admission percentage was insignificant, totaling approximately 20% in each group. Large and medium hospitals categorized by bed-size were responsible for holding more than 80% of patients in both groups. A relatively even distribution by quartile was seen when calculating patients’ median household incomes in both cohorts.
In-hospital outcomes
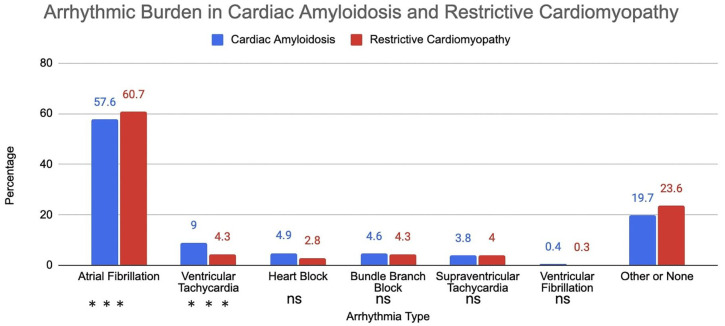
Unadjusted analysis showed a statistically significant increase in the risk of ventricular tachycardia in the cardiac amyloidosis group (OR = 2.18: 95% CI = [1.54-3.09], P < .001). Very few cases of ventricular fibrillation were identified in either group, and no significant change was observed in either cohort. Atrial fibrillation was observed in a majority of patients in both groups (57.6% vs 60.7%); however, no statistically significant change was detected (OR = 0.88: 95% CI [0.75-1.03], P = .63). None of the heart blocks (first degree, second degree, complete block) were highly associated in either group or did not account for more than 3% of the sample size. A similar trend was recorded in both right and left bundle branch blocks. Supraventricular tachycardia was diagnosed in approximately 4% of patients in both groups, thus ruling out a significant association in risk. The percentage of each arrhythmia observed in both groups is depicted in a bar graph (Figure 1). Patients with cardiac amyloidosis had higher rates of stroke compared to other RCM (OR = 3.57: 95% CI [2.01-6.33], P < .001). There was no statistically significant change in in-hospital mortality between the 2 groups (OR = 0.99: 95% CI [0.71-1.37], P = .94). The cost of stay was similar in both groups, while cardiac amyloidosis patients spent one less day in the hospital per interquartile range analysis.
Figure 1.
Arrhythmic burden in patients with cardiac amyloidosis and restrictive cardiomyopathy.
Adjusted analysis showed patients with cardiac amyloidosis had a significantly increased risk of stroke (OR = 3.91: 95% CI = [2.15, 7.11], P < .001) and ventricular tachycardia (1.98 [1.35-2.91], P < .001) (Table 3). Cardiac amyloidosis subjects had a decreased risk of atrial fibrillation (0.56 [0.47-0.68], P < .001). In-hospital mortality was similar between the 2 groups (OR = 0.94: 95% CI [0.66-1.33], P = .72). There was no statistically significant association in the risk of development of supraventricular tachycardia, heart block, or bundle branch block in either group. A substantial difference in cost of stay and length of stay was not appreciated.
Table 3.
Adjusted outcomes of patients with cardiac amyloidosis versus restrictive cardiomyopathy without cardiac amyloidosis. Survey regressions (linear or logistic) account for discharge weights and characteristics found to be significantly different between groups (i.e. age, gender, primary payer, household income, hospital size, and hospital location).
| Outcomes | OR/Mean difference (95% CI) | P-value |
|---|---|---|
| In hospital mortality | 0.94 (0.66, 1.33) | .72 |
| Length of stay, days | −0.78 (−1.61, 0.06) | .07 |
| Cost of stay, thousands of dollars | −8.4 (−22.7, 5.9) | .25 |
| Atrial fibrillation | 0.56 (0.47, 0.68) | <.001 |
| Ventricular fibrillation | 1.77 (0.34, 9.2) | .50 |
| Ventricular tachycardia | 1.98 (1.35, 2.91) | <.001 |
| Supraventricular tachycardia | 1.18 (0.76, 1.83) | .47 |
| First degree heart block | 1.81 (0.99, 3.31) | .06 |
| Second degree heart block | 0.95 (0.38, 2.39) | .91 |
| Complete heart block | 0.95 (0.38, 2.39) | .91 |
| Left bundle branch block | 0.77 (0.39, 1.55) | .47 |
| Right bundle branch block | 0.99 (0.62, 1.60) | .98 |
| Stroke | 3.91 (2.15, 7.11) | <.001 |
Discussion
In our study, cardiac amyloidosis was associated with an increased risk of stroke and ventricular tachycardia based on the adjusted outcomes analysis. Despite an increase in cerebrovascular accidents, the cardiac amyloidosis group had a paradoxical decrease in the risk of atrial fibrillation events. Conversely, no statistically significant increase in in-hospital mortality, heart blocks, supraventricular arrhythmias, bundle branch blocks, and hospital cost metrics was observed in either cohort. The unadjusted outcomes analysis was consistent with the adjusted outcomes data, except atrial fibrillation showed no proportional risk increase in either group. This likely occurred due to large differences in sample size between the amyloid and restrictive cardiomyopathy groups in addition to large within-group variance. Furthermore, interaction data or the presence of a third variable, as is the case when exclusion criteria are not exhaustive, can be seen in this scenario.
Prior retrospective studies have also reported an increased risk of ventricular arrhythmias in patients with cardiac amyloidosis.12,13 Routine 24-hour Holter monitoring of AL amyloid patients determined a prevalence of non-sustained ventricular tachycardia ranging from 5% to 27%. Fewer studies have documented ventricular arrhythmia burden in ATTR amyloidosis; however, a pre-operative analysis of Portuguese-type ATTR amyloidosis found a prevalence of 18.5% of ventricular tachycardia. 14 There are multiple theories behind the pathophysiology of ventricular events in cardiac amyloidosis. Brenner et al 15 suggested AL amyloidosis can disrupt myocyte function by increasing oxidative stress, contributing to conduction abnormalities from the accumulation of cell damage and separation of myocytes by the amyloid fibrils. It has been well established that patients with underlying structural heart disease are predisposed to the development of ventricular arrhythmias. Infarcted myocardium after an acute coronary syndrome can be susceptible to early after/depolarization, leading to triggered activity and cardiac fibrosis. 16 Differences in cardiomyocyte properties in healthy myocardium and fibrotic areas from scarring are more predisposed to reentry mechanisms, creating an ideal environment for developing arrhythmias. 17 We postulate that endomyocardial fibrosis precipitated by radiation-induced scarring in the setting of RCM also creates a conducive environment for arrhythmias.
Furthermore, we suspect all RCM predispose patients to atrial and ventricular arrhythmias because of a common restrictive pathophysiology, irrespective of etiology. Whether it is amyloid or a different infiltrative substance inducing myocardial damage, we expect researchers to extrapolate amyloid data to other RCM. This could be particularly useful in determining when primary prevention of sudden cardiac death is indicated depending on ventricular arrhythmia burden. Unadjusted outcomes in Table 1 showed a majority of patients in both subgroups developed atrial fibrillation and a relatively high percentage of ventricular tachycardia. The mechanism may be due to inflammation and scarring from the infiltrative substrate. In cardiac sarcoid, the accumulation of granulomas in the atria leads to increased end-diastolic pressures from sarcoid infiltration of the left ventricle. 18 Autopsy analysis of 113 hearts with cardiac sarcoid found 97% and 22% of granulomas in the left ventricle and atria, respectively. 19 In the same study, 17% of patients had atrial fibrillation. 18 In scleroderma with cardiac involvement, fibrosis and ischemia of the conduction system are hypothesized to predispose affected patients to ventricular arrhythmias. 20 The extent of myocardial infiltration may be predictive of premature ventricular ectopy burden and subsequent development of ventricular tachycardia. Paradiso et al 21 discovered that 46% of scleroderma patients had late ventricular potentials, which can serve as substrates for reentry ventricular tachycardia. The increased risk of ventricular arrhythmias warrants electrophysiologic (EP) studies and implantable cardioverter-defibrillators (ICDs) with inducible ventricular tachycardia. Characterizing the incidence of ventricular arrhythmic burden was one of the reasons we sought to compare amyloidosis and other RCM. Sudden cardiac death is a known complication of AL amyloidosis due to ventricular tachycardia or fibrillation. 22 However, there needs to be more data on the prevalence of this issue in the other RCM, likely due to missed out-of-hospital events and a small case number overall. If a link were established, we suspect more patients would be evaluated with EP studies to determine candidacy for ICD placement.
The differential diagnosis for other RCM is broad, with common pathologies, including hemochromatosis, carcinoid, and scarring from radiation therapy. Hemochromatosis refers to systemic iron accumulation due to decreased hepcidin concentration, a regulatory hormone responsible for iron homeostasis. 23 Multiple mutations in the human homeostatic iron regulator protein (HFE) gene have been implicated in the pathogenesis of hemochromatosis; however, non-hereditary mutations have been identified in hepcidin, ferroportin, transferrin receptor 2, and hemojuvelin genes. 23 Organ involvement primarily extends to the liver and pancreas, as affected patients are at high risk of diabetes and cirrhosis. Cardiac manifestations occur in approximately 15% of patients with echocardiography showing a non-dilated left ventricle (LV) and a restrictive LV filling pattern.24,25 An analysis by Shizukuda et al 26 studied 42 patients with asymptomatic hereditary hemochromatosis and could not find a statistically significant increase in the risk of ventricular ectopy or heart block. No large trials that could provide a more definitive conclusion were identified. Carcinoid syndrome is a constellation of symptoms caused by the release of peptides, prostaglandins, and biogenic amines from a neuroendocrine tumor. 27 It primarily affects the gastrointestinal and bronchopulmonary systems but can extend to the heart in approximately 40% of patients. 28 This subgroup with carcinoid heart disease is at risk for tricuspid valvular disease, pulmonary stenosis, and eventually pulmonary hypertension in advanced disease. 29 Ventricular tachycardia has been reported in a patient with carcinoid without underlying ischemic heart disease, while associations have been made with atrial arrhythmias in 2 case reports.30 -32 The full extent of arrhythmic burden in carcinoid heart disease has yet to be explored in a large trial from our literature review. Cardiac scarring from radiation therapy in treating thoracic malignancies leads to RCM through adverse cardiac remodeling due to the proliferation of fibroblasts, transforming growth factor beta, and peroxisome proliferator-activated receptor alpha. 33 Manifestations of arrhythmias occur in about 5% of patients who underwent radiation therapy and involve QTc prolongation, ventricular tachycardia, supraventricular arrhythmias, and pathological sinus node syndrome.34,35 The small degree of events mirrors what our retrospective study identified in the RCM group. When combined with other causes of RCM, tachyarrhythmias, and bundle branch blocks did not have a statistically significant increased risk of occurring when compared to cardiac amyloidosis.
Study limitations
Our study has certain limitations. Data obtained from publicly available databases are at risk from errors in procedure coding or incorrect diagnostic labeling. Even though in-hospital mortality was able to be measured, other causes of death could not be appropriately differentiated. Since there is no present-on-admission filter, the database cannot differentiate between conditions present before admission and those diagnosed during the hospitalization. 36 Hence, it burdens the researcher to develop a comprehensive list of comorbidities and complications unlikely to overlap during hospitalization. 36 Although the NIS data is a large, nationally representative sample, the potential for selection bias in comparing across cardiomyopathy types carries a risk for confounding even with the adjustment for baseline characteristics, sex, and age. Three exclusion criteria were utilized to eliminate other potential causes of cardiomyopathy; however, this list was not comprehensive, and other factors, such as illicit drug use, may have skewed the data. Since the NIS only includes data from United States hospital admissions, it would be difficult to extrapolate our results to international populations where demographics and comorbidity burden differ. The sample did not differentiate between ATTR and AL subtypes of amyloidosis, which may skew some of the arrhythmic data given complications that develop during chemotherapy for patients with AL amyloidosis. Since chemotherapy can induce a hypercoagulable state and increase atrial clot burden, this could affect mortality and arrhythmic burden, given differences in stroke risk. Lastly, our outcomes analysis was limited to in-hospital events. Thus, we were unable to comment on follow-up data.
Conclusions
In our retrospective study, we observed cardiac amyloidosis had a statistically significant increased risk of ventricular tachycardia and stroke compared to other RCM. Conversely, the risk of atrial fibrillation was lower in the cardiac amyloidosis group. Neither group was found to have a statistically significant increased risk of bundle branch block, supraventricular arrhythmia, atrioventricular block, or in-hospital mortality. To evaluate outcomes comprehensively, further research should compare arrhythmic burden and cardiovascular outcomes between ATTR and AL amyloidosis. The expanded use of cardiac magnetic resonance imaging and technetium pyrophosphate scintigraphy has increased the diagnostic yield of amyloidosis, which would allow for a more extensive, high-powered study to be conducted in this field.
Acknowledgments
None.
Footnotes
Author Contributions: Andrew Sagalov: Conceptualization, Writing – original draft, Writing – review & editing. Waqas Ullah: Methodology. Yevgeniy Brailovsky: Methodology. Michael Buhnerkempe: Formal Analysis, Writing – review & editing. Steve Scaife: Data Curation. Abhishek Kulkarni: Supervision, Validation. Mohamed Labedi: Supervision, Validation. Shruti Hegde: Supervision, Validation.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Note: With the exception of age and sex, no identifying information was used in the manuscript.
Informed Consent: Informed consent was obtained from each patient for using their hospital course and imaging to create a manuscript to be published in an academic journal.
ORCID iD: Andrew Sagalov  https://orcid.org/0000-0002-8324-6537
https://orcid.org/0000-0002-8324-6537
References
- 1. Kittleson M, Maurer M, Ambardekar A, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American heart association [published correction appears in circulation. 2021 Jul 6;144(1):e10] [published correction appears in circulation. 2021 Jul 6;144(1):e11]. Circulation. 2020;142:e7-e22. [DOI] [PubMed] [Google Scholar]
- 2. Muchtar E, Blauwet LA, Gertz MA. Restrictive Cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:819-837. [DOI] [PubMed] [Google Scholar]
- 3. Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies. J Am Coll Cardiol. 2018;71:1149-1166. [DOI] [PubMed] [Google Scholar]
- 4. Brodehl A, Gerull B. Genetic insights into primary restrictive cardiomyopathy. J Clin Med. 2022;11:2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol. 2020;6:351-361. [DOI] [PubMed] [Google Scholar]
- 6. Khanna S, Lo P, Cho K, Subbiah R. Ventricular arrhythmias in cardiac amyloidosis: a review of current literature. Clin Med Insights Cardiol. 2020;14:1179546820963055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamal S, Kichloo A, Bailey B, et al. Clinical outcomes and disease burden in amyloidosis patients with and without atrial fibrillation-insight from the national inpatient sample database. J Innov Card Rhythm Manag. 2021;12:4542-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. 1993-2002 Nationwide Inpatient Sample (NIS) Supplemental Discharge-Level Files and 1993-2011 NIS Trend Weights Files. https://hcup-us.ahrq.gov. [Google Scholar]
- 9. Solon G, Haider SJ, Wooldridge JM. What are we weighting for? J Hum Resour. 2015;50:301-316. [Google Scholar]
- 10. Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1-19. [Google Scholar]
- 11. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 12. Palladini G, Malamani G, Cò F, et al. Holter monitoring in AL amyloidosis: prognostic implications. Pacing Clin Electrophysiol. 2001;24:1228-1233. [DOI] [PubMed] [Google Scholar]
- 13. Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535-537. [DOI] [PubMed] [Google Scholar]
- 14. Hörnsten R, Wiklund U, Olofsson B-O, Jensen SM, Suhr OB. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation. 2004;78:112-116. [DOI] [PubMed] [Google Scholar]
- 15. Brenner DA, Jain M, Pimentel DR, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008-1010. [DOI] [PubMed] [Google Scholar]
- 16. Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm. 2014;30:389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verma A, Marrouche NF, Schweikert RA, et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:465-471. [DOI] [PubMed] [Google Scholar]
- 18. Mehta D, Willner JM, Akhrass PR. Atrial fibrillation in cardiac sarcoidosis. J Atr Fibrillation. 2015;8:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleming HA. Sarcoidosis of the heart. Am J Med. 1978;64:915-916. [DOI] [PubMed] [Google Scholar]
- 20. Steen V. The heart in systemic sclerosis. Curr Rheumatol Rep. 2004;6:137-140. [DOI] [PubMed] [Google Scholar]
- 21. Paradiso M, Di Franco M, Musca A, et al. Ventricular late potentials in systemic sclerosis: relationship with skin involvement. J Rheumatol. 2002;29:1388-1392. [PubMed] [Google Scholar]
- 22. D'Errico S, Mazzanti A, Baldari B, et al. Sudden death in lambda light chain AL cardiac amyloidosis: a review of literature and update for clinicians and pathologists. Int J Clin Exp Pathol. 2020;13:1474-1482. [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace DF, Subramaniam VN. Non-HFE haemochromatosis. World J Gastroenterol. 2007;13:4690-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulati V, Harikrishnan P, Palaniswamy C, et al. Cardiac involvement in hemochromatosis. Cardiol Rev. 2014;22:56-68. [DOI] [PubMed] [Google Scholar]
- 25. Aronow WS. Management of cardiac hemochromatosis. Arch Med Sci. 2018;14:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shizukuda Y, Tripodi DJ, Zalos G, et al. Incidence of cardiac arrhythmias in asymptomatic hereditary hemochromatosis subjects with C282Y homozygosity. Am J Cardiol. 2012;109:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM. Carcinoid heart disease. Int J Cardiol. 2008;129:318-324. [DOI] [PubMed] [Google Scholar]
- 29. Westberg G, Wängberg B, Ahlman H, et al. Prediction of prognosis by echocardiography in patients with midgut carcinoid syndrome. Br J Surg. 2001;88:865-872. [DOI] [PubMed] [Google Scholar]
- 30. Rupp AB, Ahmadjee A, Morshedzadeh JH, Ranjan R. Carcinoid syndrome-induced ventricular tachycardia. Case Rep Cardiol. 2016;2016:9142598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuczma JA, Grzywa M. [Broncho-pulmonary carcinoid with the cardiac arrhythmia manifestation]. Pol Arch Med Wewn. 2006;116:971-973. [PubMed] [Google Scholar]
- 32. Langer C, Piper C, Vogt J, et al. Atrial fibrillation in carcinoid heart disease: the role of serotonin. A review of the literature. Clin Res Cardiol. 2007;96:114-118. [DOI] [PubMed] [Google Scholar]
- 33. Ma CX, Zhao XK, Li YD. New therapeutic insights into radiation-induced myocardial fibrosis. Ther Adv Chronic Dis. 2019;10:2040622319868383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Wei J, Zheng Q, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finch W, Shamsa K, Lee MS. Cardiovascular complications of radiation exposure. Rev Cardiovasc Med. 2014;15:232-244. [DOI] [PubMed] [Google Scholar]
- 36. Mori M, Brown KJ, Geirsson A. Understanding limitations of the national inpatient sample to facilitate its proper use. JAMA Surg. 2019;154:881-882. [DOI] [PubMed] [Google Scholar]