Abstract
The nuclear vitamin D receptor (VDR) is a member of a nuclear receptor superfamily and acts as a ligand-dependent transcription factor. A family of cotranscriptional activators (SRC-1, TIF2, and AIB-1) interacts with and activates the transactivation function of nuclear receptors in a ligand-dependent way. We examined interaction of VDR with these coactivators that was induced by several vitamin D analogs, since they exert differential subsets of the biological action of vitamin D through unknown mechanisms. Unlike other vitamin D analogs tested, OCT (22-oxa-1α,25-dihydroxyvitamin D3) induced interaction of VDR with TIF2 but not with SRC-1 or AIB-1. Consistent with these interactions, only TIF2 was able to potentiate the transactivation function of VDR bound to OCT. Thus, the present findings suggest that the structure of VDR is altered in a vitamin D analog-specific way, resulting in selective interactions of VDR with coactivators. Such selective interaction of coactivators with VDR may specify the array of biological actions of a vitamin D analog like OCT, possibly through activating a particular set of target gene promoters.
The calciotropic hormone 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] regulates calcium homeostasis as well as cell proliferation and differentiation (5, 6, 21, 53). Most of the biological actions of 1α,25(OH)2D3 are thought to be mediated by the vitamin D receptor (VDR), which is a member of a nuclear receptor superfamily acting as a ligand-inducible transcription factor (4, 32). VDR binds as a heterodimer with one of three retinoid X receptors (RXR) to vitamin D-responsive elements (VDRE) in the promoters of vitamin D target genes (12, 22). For the transactivation function of VDR, only the ligand-binding domain (E region) is thought to be responsible in a ligand-binding-dependent way (27), although two transactivation domains, one at the N terminus (AF-1) and one at the C terminus (AF-2), are present in most nuclear receptors. To achieve ligand-induced transactivation, the nuclear receptors recruit several nuclear receptor coactivators. They include members of the SRC-1/TIF2 family (38, 48), CBP/p300 (9, 29), and RIP140 (8). Members of the SRC-1/TIF2 family [SRC-1 (p160, ERAP160) (18, 23), TIF2 (Grip-1) (10), and AIB-1 (ACTR) (3)] mediate the function of the AF-2 of the nuclear receptors, and the interaction site has been mapped to the minimal activation domain (AD) of AF-2 (13, 23, 50). Interestingly, it was recently shown that the interactions of estrogen receptor with SRC-1 or TIF2 are induced by estrogen (E2) but not by its antagonists, tamoxifen and ICI164,384. These findings indicate that the structure of the ligand-bound E region recruiting coactivators is ligand specific (18). This idea is further supported by recent findings from crystallographic analysis that the position of the AF-2 AD (helix 12) in the estrogen receptor E region which binds the E2 antagonists clearly differs from the one which binds E2 (17). From the structural similarity of the ligand-binding domains of nuclear receptors, ligand type-specific alterations in their structures, at least in the helix 12 positions, are postulated (7).
Several synthetic 1α,25(OH)2D3 derivatives, such as F6-1α,25-(OH)2D3 [26,26,26,27,27,27-hexafluoro-1α,25(OH)2D3] (45), ED-71 [2β-(3-hydroxypropoxy)-1α,25(OH)2D3] (37), and OCT [22-oxa-1α,25(OH)2D3] (35), have been developed as vitamin D analogs, and their biological actions have been shown to be not identical. In particular, OCT is prominent as a potent vitamin D agent with reduced calcemic activity (1, 2), while F6-1α,25-(OH)2D3 and ED-71 generally mimic 1α,25(OH)2D3 actions to a greater extent than does OCT (25, 40, 44). Taking these facts together, it is reasonable to speculate that the selective interactions of VDR with coactivators induced by vitamin D analogs specify the biological activities of the vitamin D analogs. Such differential combinations of transcription factors and coactivators are believed to activate only particular sets of target gene promoters (46).
To test this possibility, we studied the interaction of vitamin D analog-bound VDR with nuclear receptor coactivators and interacting factors. We found that although the in vivo and in vitro interactions of VDR with SRC-1, TIF2, and AIB-1 were induced by F6-1α,25(OH)2D3 and ED-71 as well as by 1α,25(OH)2D3, OCT induced interaction only with TIF2. Such interactions were also observed in the VDR-RXR heterodimer bound to DNA. Consistent with the interactions, only TIF2 potentiated the transactivation function of VDR bound to OCT. Thus, the present findings suggest that the VDR structure is altered in a vitamin D analog-specific way, resulting in selective interaction of VDR with coactivators. Such selective coactivator interaction with VDR may specify the array of biological actions of a vitamin D analog such as OCT, possibly through activating a particular set of target gene promoters.
MATERIALS AND METHODS
Yeast two-hybrid system and β-galactosidase assay.
The pGBT9(GAL4-DBD)-VDR(DEF) fusion plasmid was constructed by inserting rat VDR-DEF regions (encoding amino acids 89 to 424) (43) into the pGBT9 vector (Clontech). Each coactivator cDNA was inserted into pGAD10 or pGAD424 (Clontech), which included a GAL4 transactivation domain, to construct pGAD-SRC1, pGAD-TIF2, pGAD-AIB1, pGAD-RIP140, pGAD-SUG1 (49), and pGAD-VAF-1 (26). The coactivator cDNA was cotransformed with pGBT9(GAL4-DBD)-VDR(DEF) into Saccharomyces cerevisiae Y153 (MATa gal4 gal80 his3 trp1-901 ade2-101 ura3-52 leu2-3 leu2-112 URA3::GAL HIS3) by the lithium acetate method. Transformants were plated on medium lacking leucine and tryptophan and were grown overnight in 2 ml of SD medium lacking leucine and tryptophan. These samples, diluted to an optical density at 600 nm of 0.02, were cultured overnight with or without 1α,25(OH)2D3, F6-1α,25-(OH)2D3, ED-71, OCT, or 24R,25(OH)2D3 (24R,25-dihydroxyvitamin D3) (24). Cells were then harvested and assayed for β-galactosidase activity as described previously (15, 26).
Mammalian two-hybrid assay.
COS-1 cells were maintained in Dulbecco’s modified Eagle’s medium without phenol red, supplemented with 5% fetal calf serum which had been stripped with dextran-coated charcoal. Cells were transfected by means of calcium phosphate coprecipitation, as previously described (31). A reporter plasmid (2 μg) containing the GAL4 upstream activation sequence (UAS) (17-mer [×2], β-globin promoter, and chloramphenicol acetyltransferase gene [CAT]) was cotransfected with 0.5 μg of pVP-VDR(DEF) (43) plus 0.5 μg of pM-SRC-1, pM-TIF2, or pM-AIB-1. As a reference for normalization, 2 μg of plasmid pCH110 was used (Pharmacia). Bluescribe M13+ (Stratagene) was used as the carrier to adjust the total amount of DNA to 5 μg. Either 1α,25(OH)2D3 or OCT was added to the medium at 12 h after transfection and every 8 h thereafter at each exchange of the medium. After 48 h, CAT activity was assayed and the transfection efficiency was normalized to β-galactosidase activity, as previously described (31).
GST pull-down assay.
Full-length rat VDR was expressed as a glutathione S-transferase (GST) fusion protein (GST-VDR) in Escherichia coli, as described previously (14). The expression of a protein of the predicted size was then monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For GST pull-down assays, bacterially expressed GST or GST-VDR was bound to glutathione-Sepharose 4B beads (Pharmacia Biotech) (26). Human SRC-1 (hSRC-1) and hTIF2 cDNA in pBluescript vector (Stratagene) were used to generate [35S]methionine (Amersham International)-labeled protein by using a TNT-coupled in vitro translation system (Promega). The 35S-labeled SRC-1 and TIF2 were incubated with beads containing either GST or GST-VDR in the absence or presence of either 10−8 M 1α,25(OH)2D3 or the indicated analog in NET-N buffer (0.5% Nonidet P-40, 20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1 mM EDTA) with 1 mM phenylmethylsulfonyl fluoride. After 3 h of incubation, free proteins were washed away from the beads with NET-N buffer. Bound proteins were extracted into loading buffer, separated by SDS–7.5% PAGE, and visualized by autoradiography (41). Before the polyacrylamide gels were dried and exposed to autoradiography, they were lightly stained with Coomassie brilliant blue to verify that equal quantities of fusion proteins had been used in each reaction.
EMSA.
The interaction of SRC-1 with ligand-bound VDR-RXR was determined by electrophoretic mobility shift assay (EMSA) with DNA probes, as described previously (14). The rat VDR (rVDR) and mouse RXRα (mRXRα) cDNAs were cotransfected in COS-1 cells in the presence of 10−7 M 1α,25(OH)2D3, OCT, or vehicle (ethanol), and the nuclear extracts were prepared with hypotonic buffer. hSRC-1 and hTIF2 proteins were expressed in E. coli as a GST fusion protein and purified by digestion with thrombin followed by affinity column chromatography (30). Digested samples were applied to Sephadex G-100 to further purify the hSRC-1 and hTIF2 proteins. The purified proteins were monitored by SDS-PAGE in fixed quantities. In a typical assay, 10 μg of total protein of nuclear extracts containing rVDR and mRXRα with or without 10 ng of hSRC-1 and hTIF2 proteins were incubated for 30 min on ice in binding buffer (5 mM Tris [pH 8.0], 40 mM KCl, 6% glycerol, 1 mM dithiothreitol, 0.05% Nonidet P-40), 2 μg of poly(deoxyinosinic-deoxycytidylic) acid, 0.1 μg of denatured salmon sperm DNA, and 10 μg of bovine serum albumin in a final volume of 20 μl. For use as probes, double-stranded consensus VDRE (DR3, 5′-AGCTTCAGTTCAGGAAGTTCAGT-3′) and mouse osteopontin-VDRE (opn-VDRE, 5′-AGCTTGCTCGGGTAGGGTTCACGAGGTTCACTCGACTCG T-3′) DNA fragments were end labeled with [γ-32P]ATP and T4 polynucleotide kinase (14). VDRE DNA fragments were added to the binding mixtures, and the mixtures were incubated for a further 20 min at room temperature. The entire reaction mixture (20 μl) was loaded onto 4.5% polyacrylamide gels with 0.5× Tris-acetate-EDTA buffer and electrophoresed at 4°C. The gels were dried on filter paper and exposed to X-ray film.
Transactivation assays.
COS-1 cells were maintained as described above for the mammalian two-hybrid system. The following plasmids were used for transfection: a reporter plasmid (2 μg) containing the GAL4 UAS (17-mer [×2], β-globin promoter, and CAT) was cotransfected with 0.5 μg of pM(GAL4-DBD)-VDR(DEF) (43), pM-VDR(L417S), or pM-VDR(E420Q) (33) with or without 2 μg of the expression vector for either hSRC-1 or hTIF2. As a reference plasmid for normalization, 2 μg of pCH110 plasmid was used. Bluescribe M13+ (Stratagene) was used as a carrier to adjust the total amount of DNA to 5 μg. 1α,25(OH)2D3 or vitamin D analogs were added to the medium at 12 h after transfection and every 8 h thereafter at each exchange of the medium. After 48 h, CAT activity was measured as previously described (31).
RESULTS
Differential interactions between the nuclear receptor coactivators and 1α,25(OH)2D3-bound VDR or analog-bound VDR in the yeast two-hybrid system.
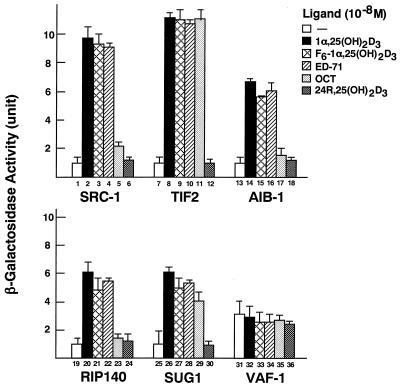
The transactivation function (AF-2) of VDR is activated by binding of 1α,25(OH)2D3 or its analogs (27). It is known that AF-2 of VDR requires nuclear receptor coactivators, such as the SRC-1/TIF2 family, which directly interact with the AF-2 AD in the VDR ligand-binding domain in a ligand-dependent way (33, 38, 48). Although it has been shown that 1α,25(OH)2D3 induces binding of VDR to these coactivators (17), it is still unknown whether vitamin D analogs can induce interactions between VDR and such coactivators. Thus, we first examined the analog-induced interactions of VDR with distinct classes of coactivators in a yeast two-hybrid system. For this assay, the ligand-binding domain of VDR, which harbors the AF-2 AD, was fused to the GAL4 DNA-binding domain in the pGBT-9 vector [pGBT9(GAL4-DBD)-VDR(DEF)], and several coactivators (SRC-1, TIF2, AIB-1, and p300) and interacting proteins (RIP140, SUG1, ARA70, and VAF-1) (26, 49, 52) were fused to the GAL4 activation domain in pGAD10 or pGAD424 vectors. 1α,25(OH)2D3 (10−8 M) induced interactions of VDR with SRC-1, TIF2, AIB-1, RIP140, and SUG1 (Fig. 1), but not with p300 or ARA70 (data not shown). 24R,25(OH)2D3, which is known not to induce the AF-2 function of VDR, did not induce any interaction. The interactions between VDR and coactivators induced by F6-1α,25(OH)2D3 and ED-71 were comparable to those induced by 1α,25(OH)2D3; however, OCT had different effects on the VDR-coactivator interactions; i.e., in the presence of OCT, a significant interaction between VDR and TIF2 was detected (Fig. 1, column 11), but no interaction between VDR and SRC-1, AIB-1, or RIP140 was detected (Fig. 1, columns 5, 17, and 23).
FIG. 1.
Differential interactions between VDR and coactivators induced by vitamin D analogs in the yeast two-hybrid system. pGBT9(GAL4-DBD)-VDR(DEF) fusion protein and one of the indicated coactivators (SRC-1, TIF2, or AIB-1) or interacting proteins (RIP140, SUG1, or VAF-1) were expressed in yeast containing the lacZ gene controlled by the GAL4 enhancer. After incubation for 16 h in the presence of the 1α,25(OH)2D3 (10−8 M), 24R,25(OH)2D3 (10−8 M), or vitamin D analogs (10−8 M), as indicated, interaction of VDR with the coactivator or interacting protein was assessed by measuring β-galactosidase activity. Results are means ± standard deviations of three independent experiments.
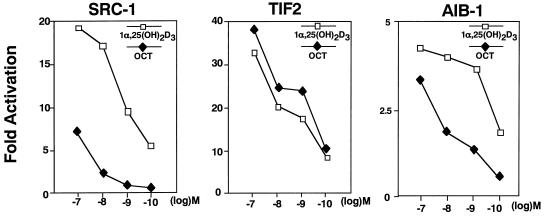
Differential interactions between the SRC-1/TIF2 proteins and OCT-bound VDR in the mammalian two-hybrid system.
To examine the effects of OCT on the interaction of VDR with the SRC-1/TIF2 family of proteins, we used a mammalian two-hybrid system. For this assay, the ligand-binding domain of VDR (DEF) was fused to the VP16 domain in the pVP vector [pVP-VDR(DEF)], and SRC-1, TIF2, and AIB-1 were fused to the GAL4 DNA-binding domain in pM vectors. A pattern of selective interactions of the SRC-1/TIF2 proteins with vitamin D analog-bound VDR similar to that in the yeast two-hybrid system was seen at 10−8 M concentrations of 1α,25(OH)2D3 and OCT in the mammalian two-hybrid system (Fig. 1 and 2). We then examined the dose dependencies of the interactions. As shown in Fig. 2, while the effects on the interactions of VDR and SRC-1 or AIB-1 were maximally induced by 10−10 M 1α,25(OH)2D3, no effect of OCT was detected even when the cells were treated at a 100-fold higher concentration (10−8 M). However, at 10−7 M, weak interactions of VDR with either SRC-1 or AIB-1 were induced by OCT. In sharp contrast to the effects on the binding of SRC-1 and AIB-1, TIF2 was induced to interact with VDR more strongly by OCT than by 1α,25(OH)2D3 at all concentrations tested.
FIG. 2.
Selective interaction of VDR with coactivators induced by OCT in the mammalian two-hybrid system. COS-1 cells were transiently transfected with a reporter plasmid (17M2-G-CAT) and pVP(VP16)-VDR(DEF) with or without pM(GAL4-DBD)-SRC-1, pM(GAL4-DBD)-TIF2, or pM(GAL4-DBD)-AIB-1 expression plasmid. Twelve hours after transfection, the transfected cells were treated with the indicated analogs at 10−7 to 10−10 M concentrations and harvested for CAT assays at 48 h posttransfection. Results are means ± standard deviations of three independent experiments.
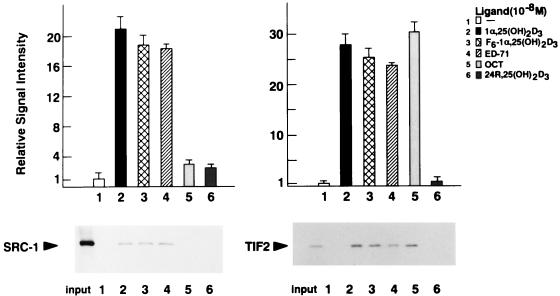
OCT-induced selective binding of SRC-1/TIF2 family proteins with VDR in vitro.
In order to confirm the interactions of VDR with coactivators implied by the effects seen in vivo, GST pull-down assays were performed. [35S]methionine-labeled SRC-1 and TIF2 which had been translated in vitro were precipitated with the GST-fused VDR protein attached to glutathione beads in the presence of ligands. Consistent with the results from the yeast and mammalian two-hybrid systems, 1α,25(OH)2D3, F6-1α,25-(OH)2D3, and ED-71 induced interaction of VDR with SRC-1, TIF2 (Fig. 3), and AIB-1 (data not shown). However, OCT induced interaction of VDR with only TIF2, not with SRC-1 (Fig. 3).
FIG. 3.
Vitamin D analog-induced interactions between GST-VDR and SRC-1/TIF2 family proteins in a GST pull-down assay. GST-VDR was expressed in E. coli and immobilized on glutathione-Sepharose beads. In vitro-translated SRC-1 and TIF2 labeled with [35S]methionine were incubated with the beads in the absence of added ligand (−) or in the presence of 1α,25(OH)2D3, F6-1α,25-(OH)2D3, ED-71, OCT, or 24R,25(OH)2D3 at a concentration of 10−8 M. The bound proteins were analyzed by SDS–7.5% PAGE and visualized by autoradiography. As a control, 1/10 of the amount of labeled SRC-1 (left panel) and TIF2 (right panel) included in the binding reactions are shown in the first lane. Representative GST pull-down assays and graphs corresponding to means ± standard deviations for triplicate independent experiments are shown. Note that any interaction of GST with these coactivators was not induced in the presence of ligands (data not shown).
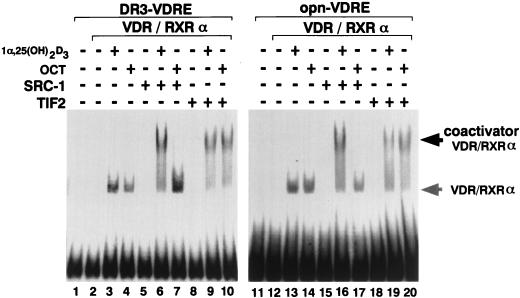
OCT-induced selective binding of SRC-1/TIF2 family proteins with VDR-RXR heterodimer bound to DNA.
As OCT induced selective interaction of VDR with SRC-1/TIF2 family proteins, we wanted to know whether vitamin D analog-induced interaction of VDR with SRC-1/TIF2 family proteins occurs on a VDR-RXR heterodimer bound to DNA. Therefore, we tested this in an EMSA. We used as DNA probes a consensus VDRE (DR3) and a VDRE from the osteopontin (opn) gene promoter, which is one of the well-characterized VDREs (14). As shown in Fig. 4, 1α,25(OH)2D3 induced binding of the VDR-RXR dimer to each of these VDREs (lanes 3 and 13), and a larger complex was formed in the presence of either SRC-1 (lanes 6 and 16) or TIF2 (lanes 9 and 19). However, OCT induced formation of the larger complex containing the VDR-RXR dimer with only TIF2 (lanes 10 and 20), not with SRC-1 (lanes 7 and 17). These results clearly demonstrate that although the ligand-induced DNA binding of VDR-RXR is promoted by all vitamin D analogs, the interactions of VDR with SRC-1/TIF2 family proteins are vitamin D analog specific.
FIG. 4.
Selective interaction of the OCT-VDR-RXR heterodimer bound to VDREs with SRC-1/TIF2 family proteins. EMSA analysis was performed with [γ-32P]ATP-labeled VDREs (DR3, and opn-VDRE). rVDR and mRXRα were expressed in transfected COS-1 cells, and the nuclear extracts therefrom were used for this assay. Bacterially expressed SRC-1 and TIF2 fused to GST were purified with glutathione-Sepharose beads, and SRC-1 and TIF2 were excised from the fusion protein. These proteins were incubated at room temperature with approximately 10 ng of labeled DR3 or opn-VDRE in the absence or presence of 10−7 M 1α,25(OH)2D3 (lanes 3, 6, 9, 13, 16, and 19) or OCT (lanes 4, 7, 10, 14, 17, and 20).
The transactivation function of OCT-bound VDR is potentiated by TIF2 but not by SRC-1.
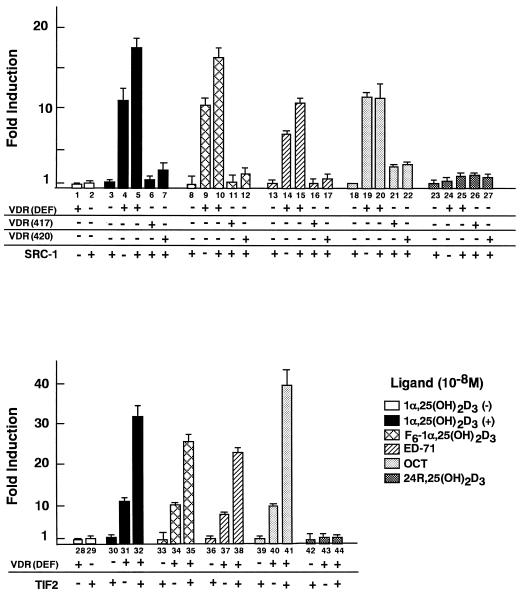
The observation that OCT induces selective interaction of VDR with SRC-1/TIF2 family proteins on the VDRE suggested that the transactivation function of OCT-bound VDR would be differentially potentiated by SRC-1/TIF2 family proteins. We therefore examined whether SRC-1 and TIF2 enhance the VDR-mediated transactivation induced by OCT. We also examined two VDR mutants [VDR(L417S) and VDR(E420Q)] which are point mutated in the AF-2 AD and have thereby lost the ability to bind to the SRC-1/TIF2 proteins (33). A transient expression assay was performed in COS-1 cells by using pM(GAL4-DBD)-VDR(DEF), pM-VDR(L417S), or pM-VDR(E420Q) and a reporter plasmid (17M2-G-CAT) containing the CAT gene and the consensus GAL4 UAS. As shown in Fig. 5, the transactivation induced by OCT (column 19) was comparable to that induced by 1α,25(OH)2D3, F6-1α,25(OH)2D3, and ED-71 (columns 4, 9, and 14). SRC-1 and TIF2 significantly enhanced the transactivation function of VDR induced by 1α,25(OH)2D3 (columns 5 and 32), F6-1α,25(OH)2D3 (columns 10 and 35), and ED-71 (columns 19 and 38). However, the OCT-induced transactivation function of VDR was potentiated by only TIF2 (column 41), not by SRC-1 (column 20). The finding that OCT induces selective interaction of VDR only with TIF2, not with SRC-1 or AIB-1, suggests that TIF2 primarily mediates the function of the AF-2 AD of OCT-bound VDR. Moreover, we found that, unlike the other ligands, only OCT could slightly induce the transactivation of the mutated VDRs [VDR(L417S) and VDR(E420Q)] (Fig. 5, columns 21 and 22), raising the possibility that OCT induces the AF-2 function of VDR through a domain other than the AF-2 AD, possibly with other coactivators (26).
FIG. 5.
VDR-mediated transactivation stimulated by OCT is potentiated by TIF2 but not by SRC-1. The effects of SRC-1 (upper panel) and TIF2 (lower panel) on the AF-2 of VDR bound to vitamin D analog were examined in COS-1 cells. The CAT assay was performed with a reporter plasmid (17M2-G-CAT) and pM(GAL4-DBD)-VDR(DEF), pM(GAL4-DBD)-VDR(L417S), or pM(GAL4-DBD)-VDR(E420Q) mutant expression vector with or without an expression plasmid of the SRC-1/TIF2 protein family, as described in the legend for Fig. 1. Values are expressed as means ± standard deviations of triplicate transfections.
DISCUSSION
In this study, we explored the biological potencies of 1α,25(OH)2D3 and four 1α,25(OH)2D3 analogs in terms of some of the essential steps in the hormone action pathway. We hypothesized that by studying 1α,25(OH)2D3 analog action directly at the level of ligand-induced interaction between coactivators and nuclear receptors, insight might be gained into the mechanisms by which the 1α,25(OH)2D3 analogs differ in functional potency with regard to transactivation of target genes. The results of the present study show that the ligand-induced interactions of VDR with the coactivators are analog type specific. Binding analyses in vivo and in vitro demonstrated that OCT induces selective interaction of VDR with SRC-1/TIF2 family proteins, whereas other analogs induce interactions with all of these coactivators. Ligand-dependent interactions with VDR were also observed in RIP140 and SUG1. Interestingly, OCT induced interaction of VDR with SUG1 but not with RIP140, though the physiological roles of RIP140 and SUG1 in the transactivation function of VDR are unknown. As previous studies showed that SRC-1/TIF2 family proteins have similar structures and transactivation functions (17, 28, 47), it is of interest to know whether each of them has a distinct role in the multitude of vitamin D actions in intact animals. In this respect, the reported production of SRC-1 knockout mice (51) will provide a good model to test OCT-specific actions.
In a previous study, we investigated the molecular mechanism by which one vitamin D analog, F6-1α,25(OH)2D3, exhibits strong potency in the biological action of vitamin D (40). We found that F6-1α,25(OH)2D3 stabilizes DNA binding of the VDR-RXR heterodimer more than does 1α,25(OH)2D3 in experiments measuring the dissociation time of the VDR-RXR heterodimer from the consensus VDRE in the presence of VDR ligands. Stabilization of the VDR-RXR-DNA complex by F6-1α,25(OH)2D3 seemed to be due to the specific VDR structure caused by ligand binding. We tested whether OCT or ED71 could cause such a stabilization compared with 1α,25(OH)2D3 and F6-1α,25(OH)2D3. We did not detect a significant difference among the dissociation times in the presence of 1α,25(OH)2D3, OCT, or ED71 (data not shown). Thus, it appears that, unlike F6-1α,25(OH)2D3, OCT induces an alteration only in the ligand-binding domain, and this structural alteration results in selective interaction of VDR with some coactivators.
The SRC-1/TIF2 family of proteins contacts the minimal activation domain (AF-2 AD) at the C terminus of the ligand-binding domain (E domain) in a ligand-dependent way. More recent reports show that the SRC-1/TIF2 proteins also contact a hydrophobic cleft in the ligand-binding pocket of thyroid hormone receptors (16). This interaction further recruits other factors to form a larger complex for initiating transcription with modulation of chromatin structure (11, 36, 42). For this ligand transactivation of nuclear receptors, the other coactivators and/or interacting factors appear to act by interacting with regions other than the AF-2 AD and the surrounding surface of the ligand-binding pocket. Indeed, the VDR mutants L417S and E420A, in which the AF-2 AD is not able to interact with SRC-1/TIF2 family proteins (33), were not potentiated by SRC-1/TIF2 family proteins. However, OCT, but not the other vitamin D analogs, activated these VDR mutants to some extent. These findings raise the possibility that OCT-bound VDR recruits other coactivators or/and stabilizes a weak interaction(s) with a coactivator(s) interacting with VDR, although such a coactivator(s) remains to be identified.
It is generally known that different combinations of transcriptional factors and coactivators have different promoter selectivities for transactivation (46). Recently, it was reported that the tissue distribution of TAF, one of the basal transcriptional factors, determines the expression of target genes (19, 20). Nuclear receptor coactivators may mediate the function of the basal transcriptional machinery, and some coactivators are known to be expressed in different target tissues at variable levels (34). Therefore, differential coactivator binding to nuclear receptors may target a particular set of gene promoters and then promote following activation of the selected target genes. OCT is shown to induce approximately 10-fold-greater cytodifferentiation than 1α,25(OH)2D3 in HL60 cells, with less calcemic activity (1, 2). Taken together with the results of the present study, such differential biological actions of OCT may be achieved through a particular set of target genes activated by OCT-bound VDR interacting with only selected coactivators. To test this hypothesis, a more biological approach with a reconstituted in vitro transcription assay (39) will be useful.
In summary, we have shown that several analogs of 1α,25(OH)2D3 induce differential binding between coactivators and VDR and that the type of coactivator necessary for ligand-bound VDR varies depending on the ligand. Therefore, the differential biological actions of the vitamin D analogs may be partly explained by the expression levels and the tissue-specific distributions of coactivators. We think that similar studies may be useful in the evaluation of new analogs and in designing structural changes in future analogs based on the ability of the agonistic or antagonistic compounds to induce this critical step in vitamin D action.
ACKNOWLEDGMENTS
We thank T. Kitamoto, H. Tai, Y. Kodera, K. Sekine, T. Hashimoto, Y. Yanagi, T. Toriyabe, and H. Harada for helpful technical advice; Chugai Pharmaceuticals and Sumitomo Pharmaceuticals for vitamin D-related compounds; and P. Chambon for the generous gift of mRXRα cDNA.
This work was supported in part by a grant-in-aid for priority areas from the Ministry of Education, Science, Sports and Culture of Japan (to S.K.).
REFERENCES
- 1.Abe J, Moriya Y, Saito M, Sugawara Y, Suda T, Nishii Y. Modulation of cell growth, differentiation, and production of interleukin-3 by 1 alpha, 25-dihydroxyvitamin D3 in the murine myelomonocytic leukemia cell line WEHI-3. Cancer Res. 1986;53:2534–2537. [PubMed] [Google Scholar]
- 2.Abe J, Nakano T, Nishii Y, Matsumoto T, Ogata E, Ikeda K. A novel vitamin D3 analog, 22-oxa-1,25-dihydroxyvitamin D3, inhibits the growth of human breast cancer in vitro and in vivo without causing hypercalcemia. Endocrinology. 1991;129:832–837. doi: 10.1210/endo-129-2-832. [DOI] [PubMed] [Google Scholar]
- 3.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 4.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 5.Bikle D D, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr Rev. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- 6.Bouillon R, Okamura W H, Norman A W. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 7.Brzozowski A M, Pike A C W, Dauter Z, Hubbard R E, Bonn T, Engstrom O, Ohman L, Greene G L, Gustafsson J A, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 8.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner P J, Parker M G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montiminy M, Evans R M. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptor. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histidine acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 12.Darwish H, DeLuca H F. Vitamin D-regulated gene expression. Crit Rev Eukaryot Gene Expr. 1993;3:89–116. [PubMed] [Google Scholar]
- 13.Durand B, Saunders M, Gaudon C, Roy B, Losson R, Chambon P. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994;13:5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebihara K, Masuhiro Y, Kitamoto T, Suzawa M, Uematsu Y, Yoshizawa T, Ono T, Harada H, Matsuda K, Hasegawa T, Masushige S, Kato S. Intron retention generates a novel isoform of the murine vitamin D receptor that acts in a dominant negative way on the vitamin D signaling pathway. Mol Cell Biol. 1996;16:3393–3400. doi: 10.1128/mcb.16.7.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estojak J, Brent R, Golemis E A. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W, Ribeiro R C J, Wagner R L, Nguyen H, Apriletti J W, Fletterick R J, Baxter J D, Kushner P J, West B L. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science. 1998;280:1747–1749. doi: 10.1126/science.280.5370.1747. [DOI] [PubMed] [Google Scholar]
- 17.Gill R K, Atkins L M, Hollis B W, Bell N H. Mapping the domains of the interaction of the vitamin D receptor and steroid receptor coactivator-1. Mol Endocrinol. 1998;12:57–65. doi: 10.1210/mend.12.1.0048. [DOI] [PubMed] [Google Scholar]
- 18.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 19.Hansen S K, Takada S, Jacobson R H, Lis J T, Tjian R. Transcription properties of a cell type-specific TATA-binding protein, TRF. Cell. 1997;91:71–83. doi: 10.1016/s0092-8674(01)80010-6. [DOI] [PubMed] [Google Scholar]
- 20.Hansen S K, Tjian R. TAFs and TFIIA mediate differential utilization of the tandem Adh promoters. Cell. 1995;82:565–575. doi: 10.1016/0092-8674(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 21.Haussler M R, Mangelsdorf D J, Komm B S, Terpening C M, Yamaoka K, Allegretto E A, Baker A R, Shine J, McDonnell D P, Hughes M, et al. Molecular biology of the vitamin D hormone. Recent Prog Horm Res. 1988;44:263–305. doi: 10.1016/b978-0-12-571144-9.50013-2. [DOI] [PubMed] [Google Scholar]
- 22.Haussler M R, Whitfield G K, Haussler C A, Hsieh J C, Thompson P D, Selznick S H, Dominguez C E, Jurutka P W. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 23.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 24.Henry H L, Norman A W. Vitamin D: two dihydroxylated metabolites are required for normal chicken egg hatchability. Science. 1978;201:835–837. doi: 10.1126/science.684411. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Kohno H, Yamamuro T, Kasai R, Ohta S, Okumura H, Konishi J, Kikuchi H, Shigeno C. The effect of active vitamin D3 analogs and dexamethasone on the expression of osteocalcin gene in rat tibiae in vivo. Biochem Biophys Res Commun. 1992;189:1231–1235. doi: 10.1016/0006-291x(92)92336-v. [DOI] [PubMed] [Google Scholar]
- 26.Imai T, Matsuda K, Shimojima T, Hashimoto T, Masuhiro Y, Kitamoto T, Sugita A, Suzuki K, Matsumoto H, Masushige S, Nogi Y, Muramatsu M, Handa H, Kato S. ERC-55, a binding protein for the papilloma virus E5 oncoprotein, specifically interacts with vitamin D receptor among nuclear receptors Biochem. Biophys Res Commun. 1997;233:765–769. doi: 10.1006/bbrc.1997.6531. [DOI] [PubMed] [Google Scholar]
- 27.Jurutka P W, Hsieh J C, Remus L S, Whitefield G K, Thompson P D, Haussler C A, Blanco J C, Ozato K, Haussler M R. Mutations in the 1,25-dihydroxyvitamin D3 receptor identifying C-terminal amino acids required for transcriptional activation that are functionally dissociated from hormone binding, heterodimeric DNA binding, and interaction with basal transcription factor IIB, in vitro. J Biol Chem. 1997;272:14592–14599. doi: 10.1074/jbc.272.23.14592. [DOI] [PubMed] [Google Scholar]
- 28.Kalkhoven E, Valentine J E, Heery D M, Parker M G. Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 1998;17:232–243. doi: 10.1093/emboj/17.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 31.Kato S, Sasaki H, Suzawa M, Masushige S, Tora L, Chambon P, Gronemeyer H. Widely spaced, directly repeated PuGGTCA elements act as promiscuous enhancers for different classes of nuclear receptors. Mol Cell Biol. 1995;15:5858–5867. doi: 10.1128/mcb.15.11.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. The nuclear receptor superfamily. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuyama H, Brownfield C M, St-Arnaud R, MacDonald P N. Evidence for ligand-dependent intramolecular folding of the AF-2 domain in vitamin D receptor-activated transcription and coactivator interaction. Mol Endocrinol. 1997;11:1507–1517. doi: 10.1210/mend.11.10.9990. [DOI] [PubMed] [Google Scholar]
- 34.May M, Mengus G, Lavigne A C, Chambon P, Davidson I. Human TAF(II28) promotes transcriptional stimulation by activation function 2 of the retinoid X receptors. EMBO J. 1996;15:3093–3104. [PMC free article] [PubMed] [Google Scholar]
- 35.Murayama E, Miyamoto K, Kubodera N, Mori T, Matsunaga I. Synthetic studies of vitamin D3 analogues. Synthesis of 22-oxavitamin D3 analogues. Chem Pharm Bull (Tokyo) 1986;34:4410–4413. doi: 10.1248/cpb.34.4410. [DOI] [PubMed] [Google Scholar]
- 36.Ogryzko V V, Schlitz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 37.Okano T, Tsugawa N, Masuda S, Takeuchi A, Kobayashi T, Takita Y, Nishii Y. Regulatory activities of 2 beta-(3-hydroxypropoxy)-1 alpha, 25-dihydroxyvitamin D3, a novel synthetic vitamin D3 derivative, on calcium metabolism. Biochem Biophys Res Commun. 1989;163:1444–1449. doi: 10.1016/0006-291x(89)91140-6. [DOI] [PubMed] [Google Scholar]
- 38.Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 39.Rachez C, Suldan Z, Ward J, Chang C P, Burakov D, Erdjument B H, Tempst P, Freedman L P. A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev. 1998;12:1787–1800. doi: 10.1101/gad.12.12.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki H, Harada H, Handa Y, Morino H, Suzawa M, Shimpo E, Katsumata T, Masuhiro Y, Matsuda K, Ebihara K, Ono T, Masushige S, Kato S. Transcriptional activity of a fluorinated vitamin D analog on VDR-RXR-mediated gene expression. Biochemistry. 1995;34:370–377. doi: 10.1021/bi00001a045. [DOI] [PubMed] [Google Scholar]
- 41.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH3 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 42.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 43.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1 alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka Y, DeLuca H F, Kobayashi Y, Ikekawa N. 26,26,26,27,27,27-Hexafluoro-1,25-dihydroxyvitamin D3: a highly potent, long-lasting analog of 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1984;229:348–354. doi: 10.1016/0003-9861(84)90161-9. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Pahuja D N, Wichmann J K, DeLuca L H, Kobayashi Y, Taguchi T, Ikekawa N. 25-Hydroxy-26,26,26,27,27,27-hexafluorovitamin D3: biological activity in the rat. Arch Biochem Biophys. 1982;218:134–141. doi: 10.1016/0003-9861(82)90328-9. [DOI] [PubMed] [Google Scholar]
- 46.Tansey W P, Herr W. TAFs: guilt by association: Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 47.Voegel J J, Heine M J, Tini M, Vitat V, Chambon P, Gronemeyer H. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 1998;17:507–519. doi: 10.1093/emboj/17.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voegel J J, Heine M J, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 49.vom B E, Zechel C, Heery D, Heine M J, Garnier J M, Vivat V, Le D B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- 50.Wurtz J M, Bourguet W, Renaud J P, Vivat V, Chambon P, Moras D, Gronemeyer H. A canonical strucuture for the ligand-binding domain of nuclear receptors. Natl Struct Biol. 1996;3:87–94. doi: 10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M J, O’Malley B W. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 52.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]