Abstract
1. In Rana oocytes, measurements with chloride-sensitive microelectrodes show that the mean intracellular chloride activity (34.8 +/- 6.3 mM, n = 79) is three times higher than that expected for the passive distribution of chloride ions across the outer membrane (12.4 mM, mean membrane potential -43 +/- 8.8 mV, n = 79). 2. Reuptake of chloride into oocytes depleted by prolonged exposure to chloride-free saline takes place against the electrochemical gradient. 3. Chloride reuptake does not take place in sodium-free solution or in a sodium-substituted potassium-free solution. It is inhibited by bumetanide (10(-5) M) in the bathing medium. 4. The overall stoichiometry of the transport mechanism deduced from simultaneous measurements of intracellular sodium and chloride using ion-selective electrodes is 1Na+:1K+:2Cl-. 5. Ammonium ions substitute for potassium on the cotransporter. 6. In oocytes smaller than 0.9 mm in diameter, exposure to external ammonium causes an alkaline shift in intracellular pH as the NH3 enters and takes up H+ to form NH4+. We propose that chloride-dependent NH4+ transport contributes to the accumulation of NH4+ and causes the 'postexposure' acidification as the intracellular NH4+ releases H+ to form NH3 which is then lost from the cell. 7. In larger oocytes ammonium exposure produces a rapid reduction in pHi which may be explained in part by cotransport-mediated uptake of NH4+. Evidence is also provided for a second chloride-dependent NH4+ transport mechanism and a chloride-independent process.
Full text
PDF
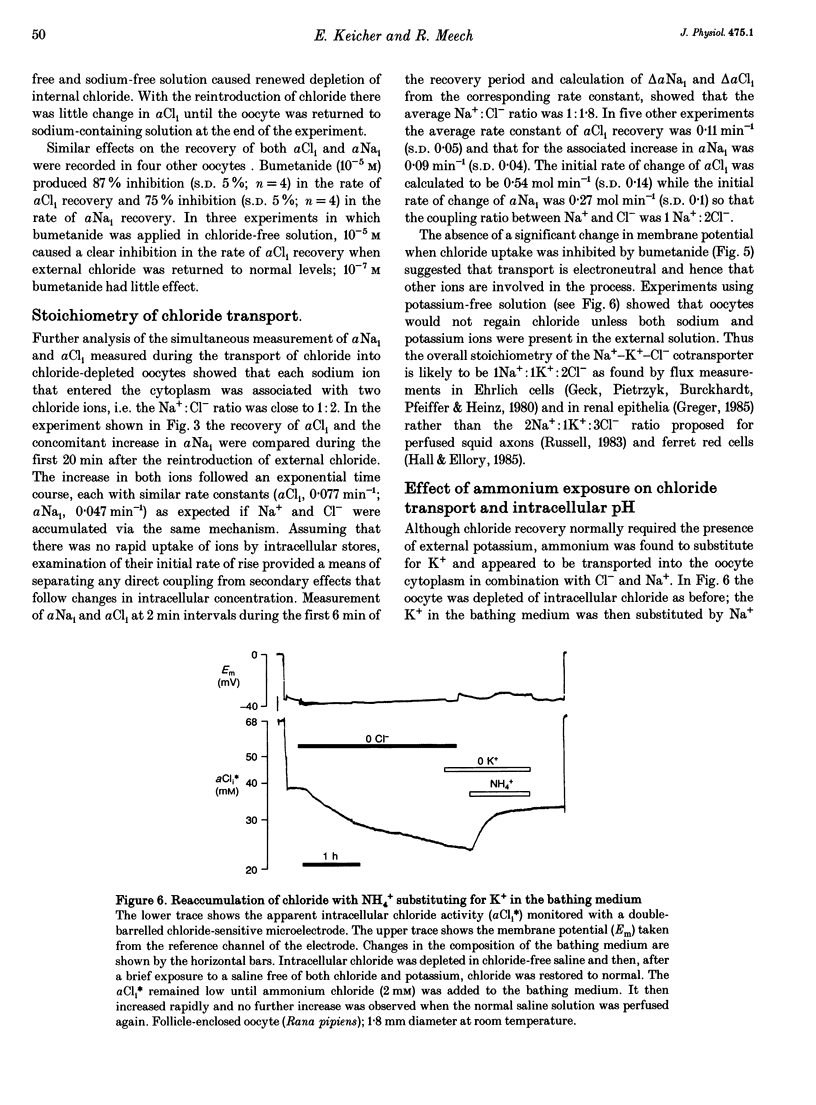
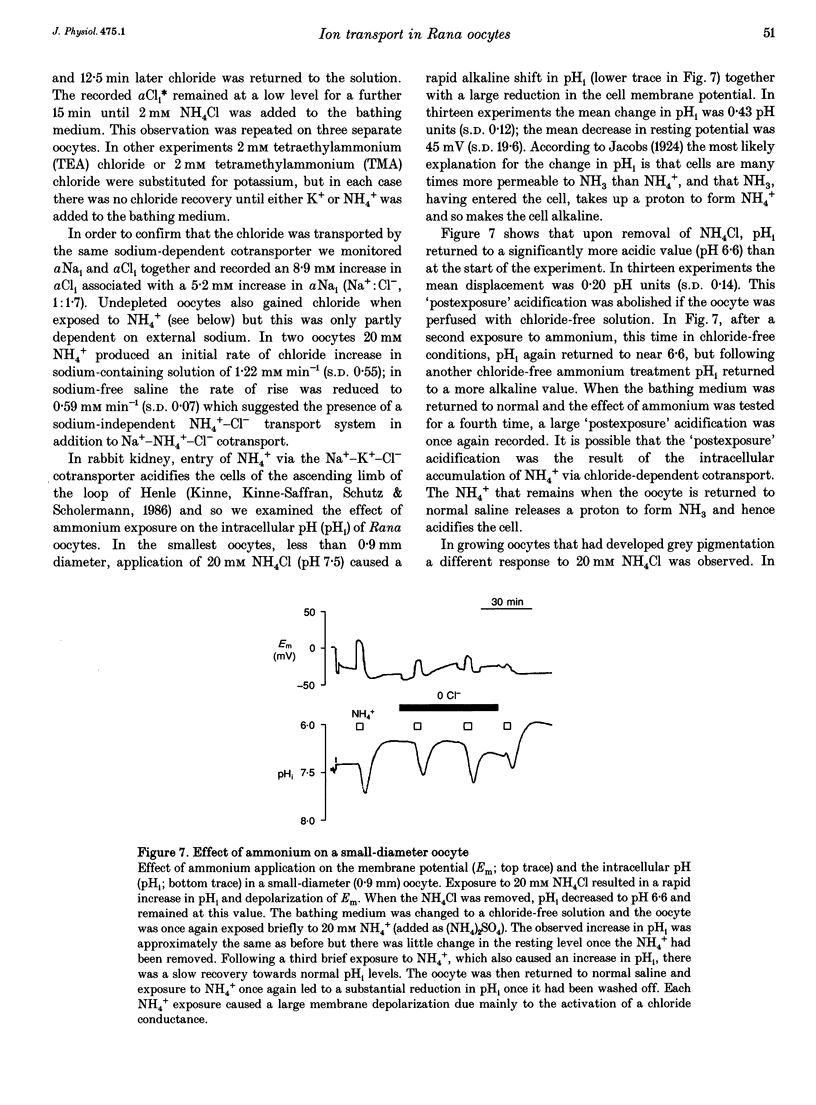
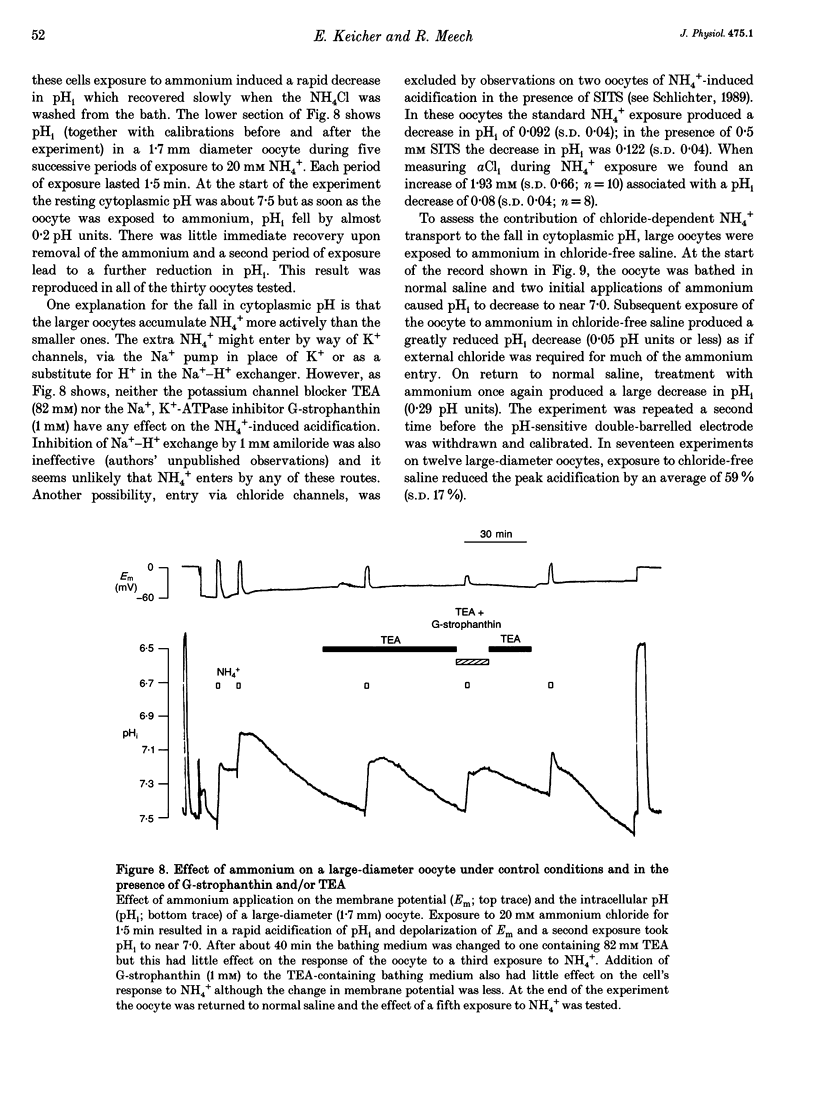
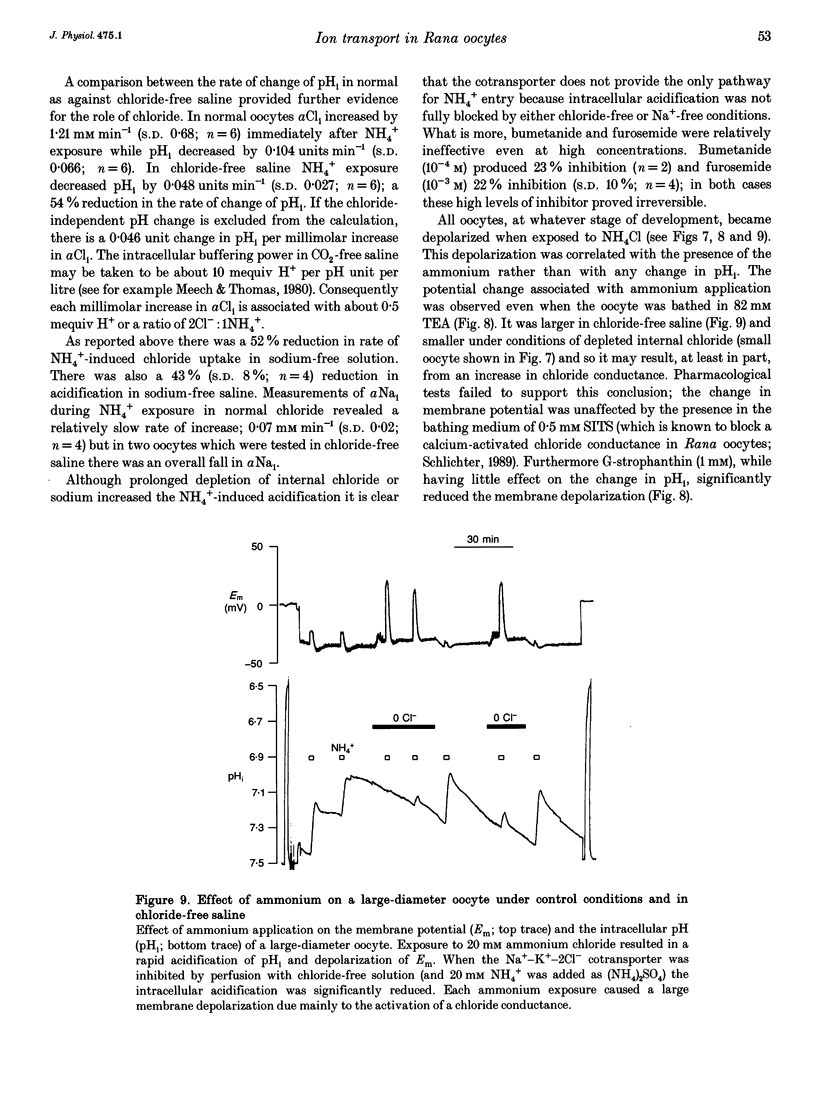




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A., Lux H. D. Ammonium action on post-synaptic inhibition in crayfish neurones: implications for the mechanism of chloride extrusion. J Physiol. 1982 Aug;329:319–339. doi: 10.1113/jphysiol.1982.sp014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., Noguerón I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988 Dec;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Kunze D., Neild T. O. Chloride distribution in Aplysia neurones. J Physiol. 1976 Apr;256(2):441–464. doi: 10.1113/jphysiol.1976.sp011332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P. An intracellular analysis of gamma-aminobutyric-acid-associated ion movements in rat sympathetic neurones. J Physiol. 1985 Aug;365:41–58. doi: 10.1113/jphysiol.1985.sp015758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D., Lepke S., Layh-Schmitt G., Legrum B., Passow H. Anion transport in oocytes of Xenopus laevis induced by expression of mouse erythroid band 3 protein--encoding cRNA and of a cRNA derivative obtained by site-directed mutagenesis at the stilbene disulfonate binding site. EMBO J. 1989 Dec 1;8(12):3601–3609. doi: 10.1002/j.1460-2075.1989.tb08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Lecar H. Ammonium ion currents in the squid giant axon. J Gen Physiol. 1969 Mar;53(3):342–361. doi: 10.1085/jgp.53.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R. The (Na+-K+-Cl-) co-transport system. Clin Sci (Lond) 1986 Nov;71(5):465–476. doi: 10.1042/cs0710465. [DOI] [PubMed] [Google Scholar]
- Cross N. L., Elinson R. P. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Dev Biol. 1980 Mar;75(1):187–198. doi: 10.1016/0012-1606(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Dunham P. B., Logue P. J., Stewart G. W. Anion-dependent cation transport in erythrocytes. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):483–495. doi: 10.1098/rstb.1982.0146. [DOI] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Measurement and stoichiometry of bumetanide-sensitive (2Na:1K:3Cl) cotransport in ferret red cells. J Membr Biol. 1985;85(3):205–213. doi: 10.1007/BF01871515. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Schlichter L. C. Fertilization-induced ionic conductances in eggs of the frog, Rana pipiens. J Physiol. 1985 Jan;358:299–319. doi: 10.1113/jphysiol.1985.sp015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R., Kinne-Saffran E., Schütz H., Schölermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl(-)-cotransporter. J Membr Biol. 1986;94(3):279–284. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- Kinne R., Koenig B., Hannafin J., Kinne-Saffran E., Scott D. M., Zierold K. The use of membrane vesicles to study the NaCl/KCl cotransporter involved in active transepithelial chloride transport. Pflugers Arch. 1985;405 (Suppl 1):S101–S105. doi: 10.1007/BF00581788. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am J Physiol. 1981 Nov;241(5):C220–C226. doi: 10.1152/ajpcell.1981.241.5.C220. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Loracher C., Neher E. The action of ammonium on postsynaptic inhibition of cat spinal motoneurons. Exp Brain Res. 1970;11(5):431–447. doi: 10.1007/BF00233967. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- Richter H. P., Jung D., Passow H. Regulatory changes of membrane transport and ouabain binding during progesterone-induced maturation of Xenopus oocytes. J Membr Biol. 1984;79(3):203–210. doi: 10.1007/BF01871059. [DOI] [PubMed] [Google Scholar]
- Russell J. M. Cation-coupled chloride influx in squid axon. Role of potassium and stoichiometry of the transport process. J Gen Physiol. 1983 Jun;81(6):909–925. doi: 10.1085/jgp.81.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter L. C. Ionic currents underlying the action potential of Rana pipiens oocytes. Dev Biol. 1989 Jul;134(1):59–71. doi: 10.1016/0012-1606(89)90078-x. [DOI] [PubMed] [Google Scholar]
- Tseng H., Berk B. C. The Na/K/2Cl cotransporter is increased in hypertrophied vascular smooth muscle cells. J Biol Chem. 1992 Apr 25;267(12):8161–8167. [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Steinhardt R. A., Winkler M. M. Intracellular calcium release and the mechanisms of parthenogenetic activation of the sea urchin egg. Dev Biol. 1978 Aug;65(2):285–295. doi: 10.1016/0012-1606(78)90028-3. [DOI] [PubMed] [Google Scholar]


