Abstract
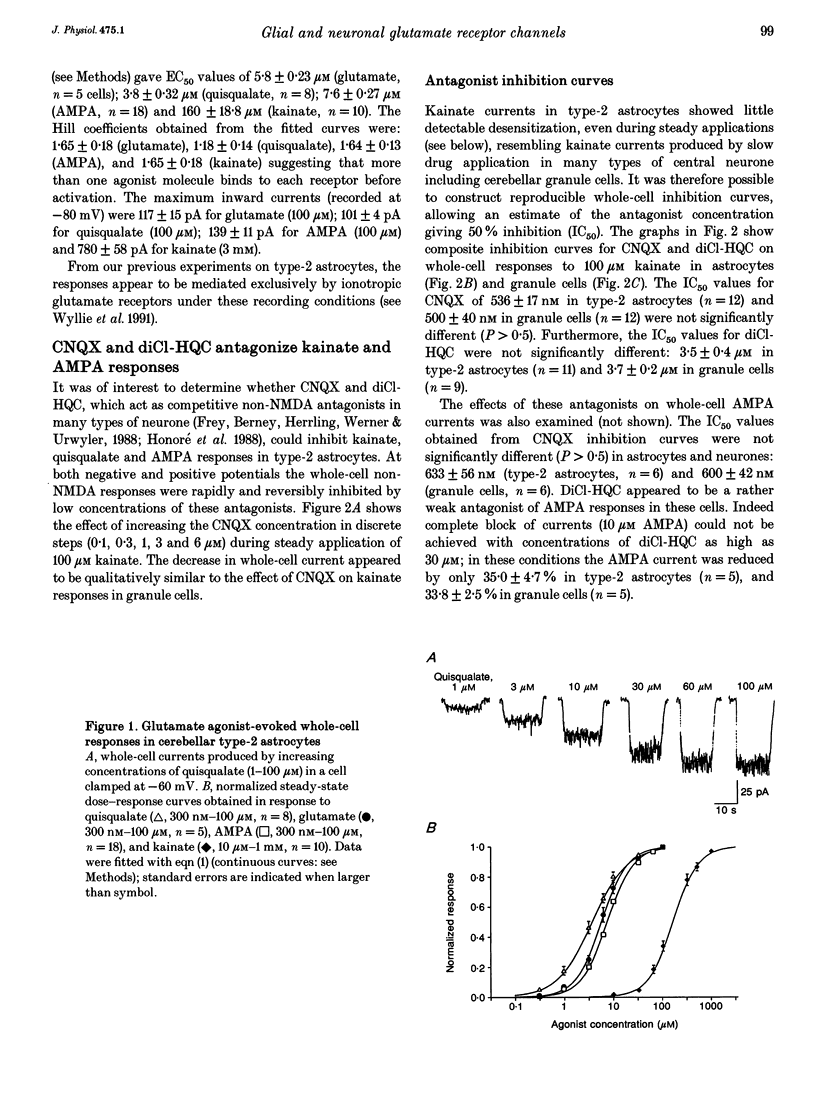
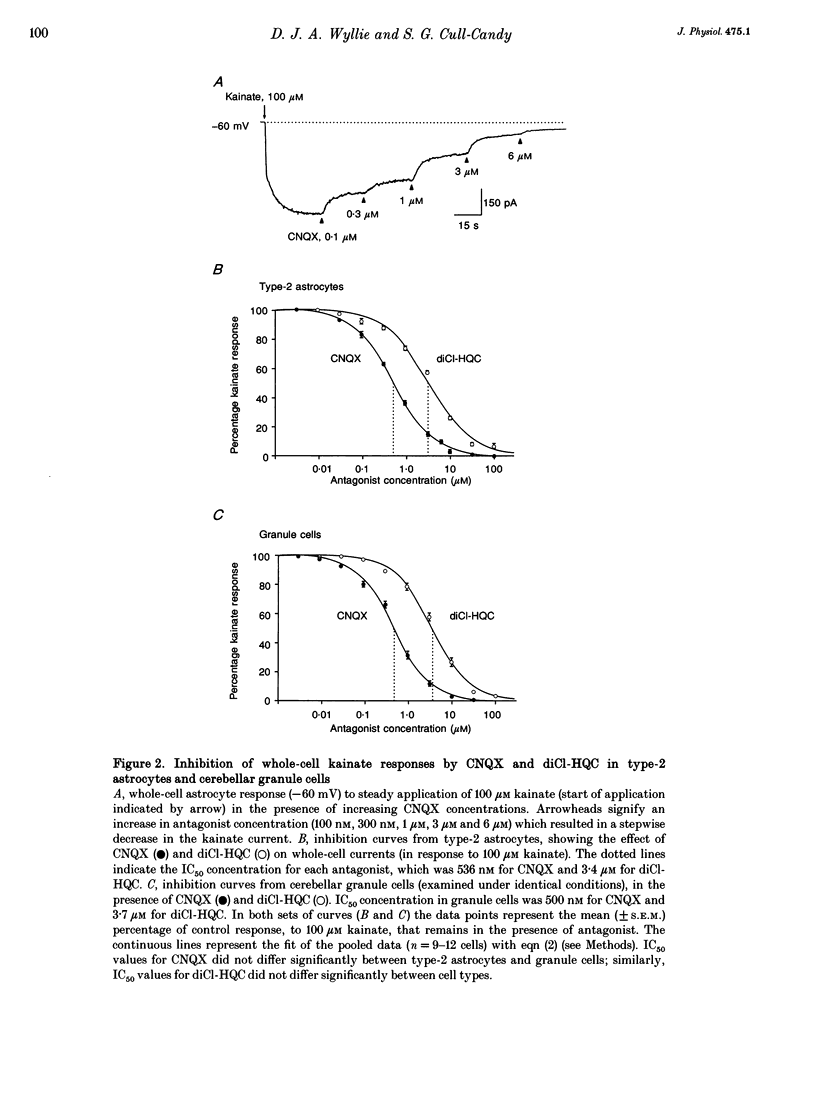
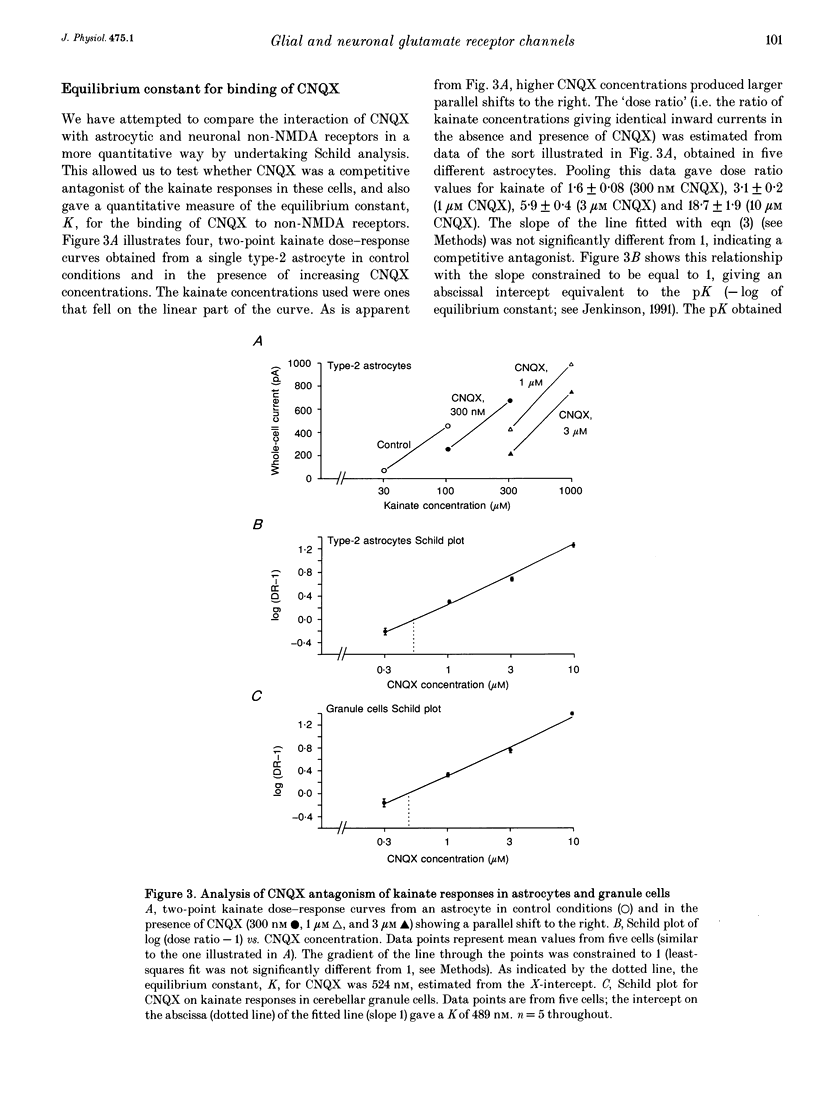
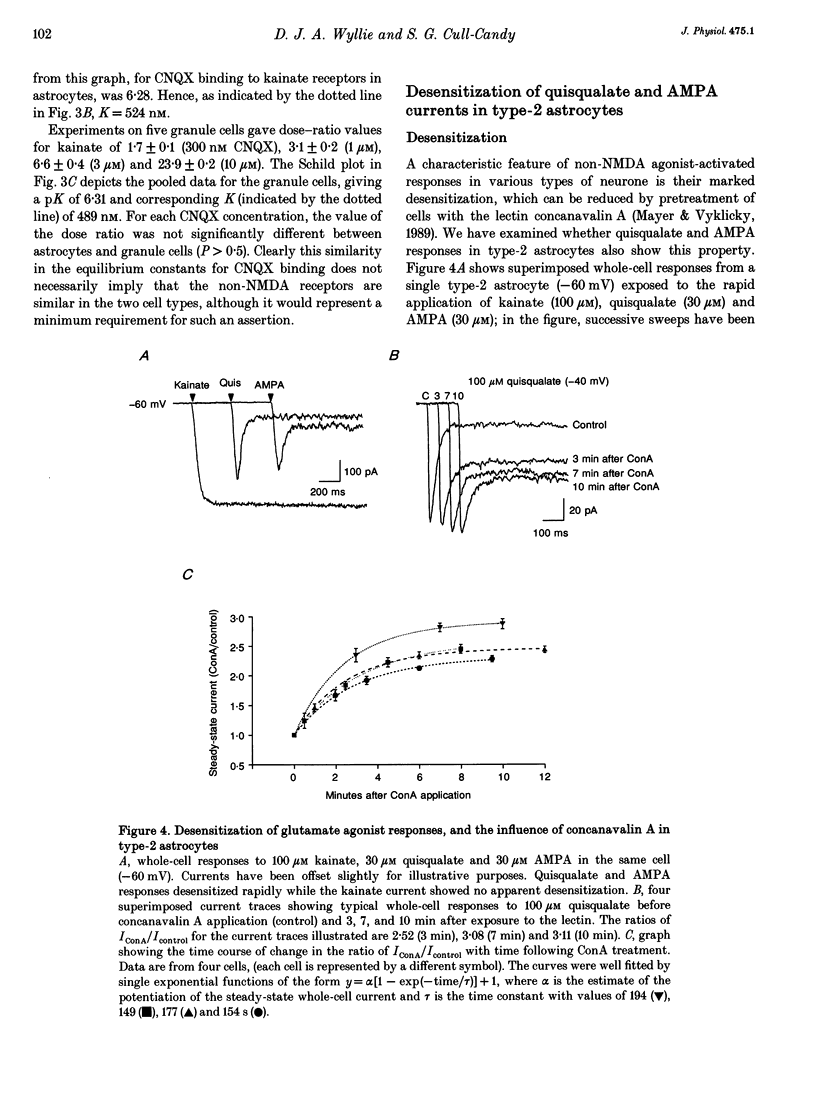
1. Patch-clamp recording methods have been used to compare the pharmacological properties and single-channel characteristics of non-NMDA receptor channels in cerebellar type-2 astrocytes and granule cells. 2. In type-2 astrocytes whole-cell concentration-response curves for glutamate, quisqualate, AMPA and kainate gave EC50 values of 5.8, 3.8, 7.6 and 160 microM and Hill slopes of 1.65, 1.18, 1.64 and 1.65, respectively, resembling estimates for granule cell receptors. 3. The non-NMDA receptor antagonists CNQX and diCl-HQC (see Methods) inhibited whole-cell kainate currents in both cell types. The IC50 for CNQX antagonism of the kainate response was 536 nM in type-2 astrocytes, and 500 nM in granule cells. The IC50 for diCl-HQC was 3.5 microM in astrocytes and 3.7 microM in granule cells. 4. CNQX acted as a competitive antagonist of whole-cell kainate responses in type-2 astrocytes and granule cells giving Schild plots with a slope near 1. The equilibrium constant, K, for CNQX binding was 524 nM in astrocytes and 489 nM in granule cells. 5. Quisqualate and AMPA responses showed rapid desensitization in type-2 astrocytes with a ratio of steady-state to peak response of 0.09. Concanavalin A reduced this desensitization. 6. Non-NMDA channels in type-2 astrocytes and granule cells showed a low permeability to Ca2+ ions with a reversal potential, for kainate-activated whole-cell currents in isotonic Ca2+, of approximately -25 mV for astrocytes and -45 mV for granule cells. 7. Outside-out patches from type-2 astrocytes exhibited a range of single-channel conductances that were superficially similar to the glutamate-activated conductances in granule cells. However, the type-2 astrocytes were devoid of NMDA receptors, hence all of these conductances originated from non-NMDA channels. Their slope conductances were approximately 11, 21, 32, 42 and 52 pS. Amplitudes were verified with mean low-variance plots and single-channel current-voltage curves, which were linear. 8. There was also evidence of lower conductance kainate-activated channels in astrocyte patches. From noise analysis their estimated mean conductance was 1.9 pS, as described for the 'low-conductance' type kainate responses in cerebellar neurones. 9. Apparent open times, shut times and burst lengths of AMPA-activated (3-10 microM) channels were examined in patches from type-2 astrocytes, and kinetic properties of the 40 and 50 pS levels were compared with the lower levels. 10. Our results indicate some marked pharmacological similarities between non-NMDA receptor channels in type-2 astrocytes and granule cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
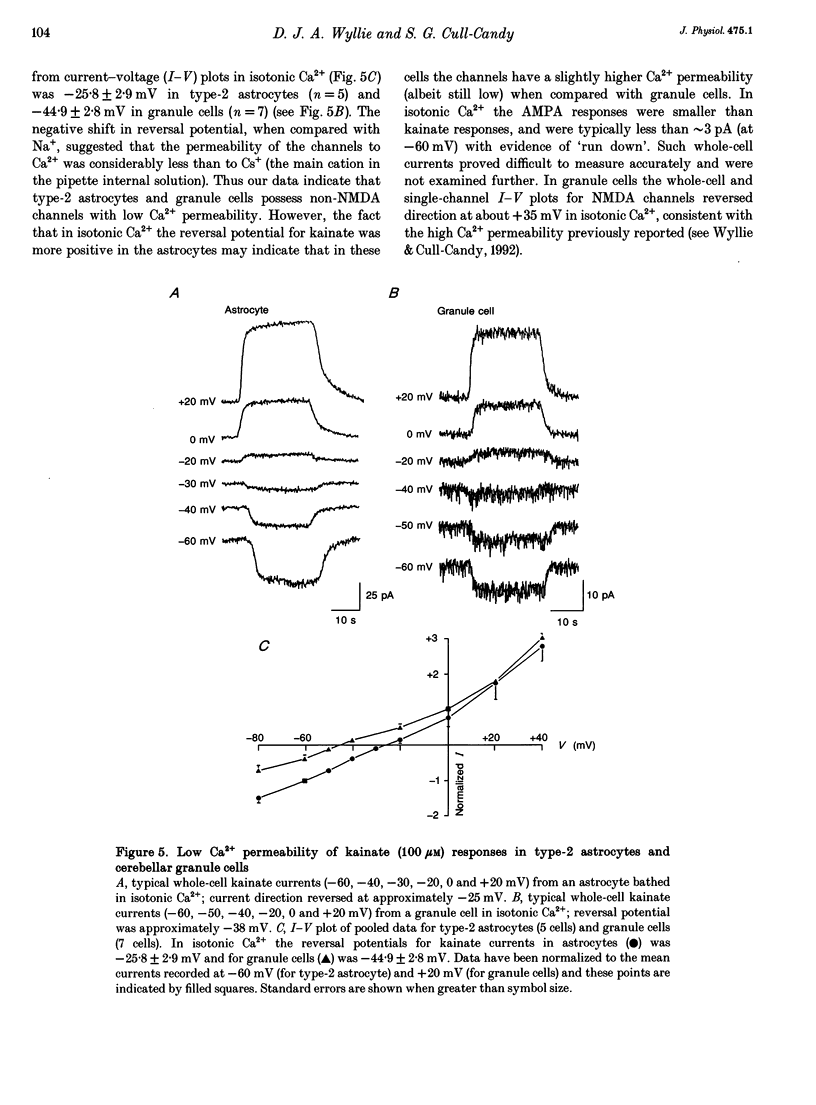
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
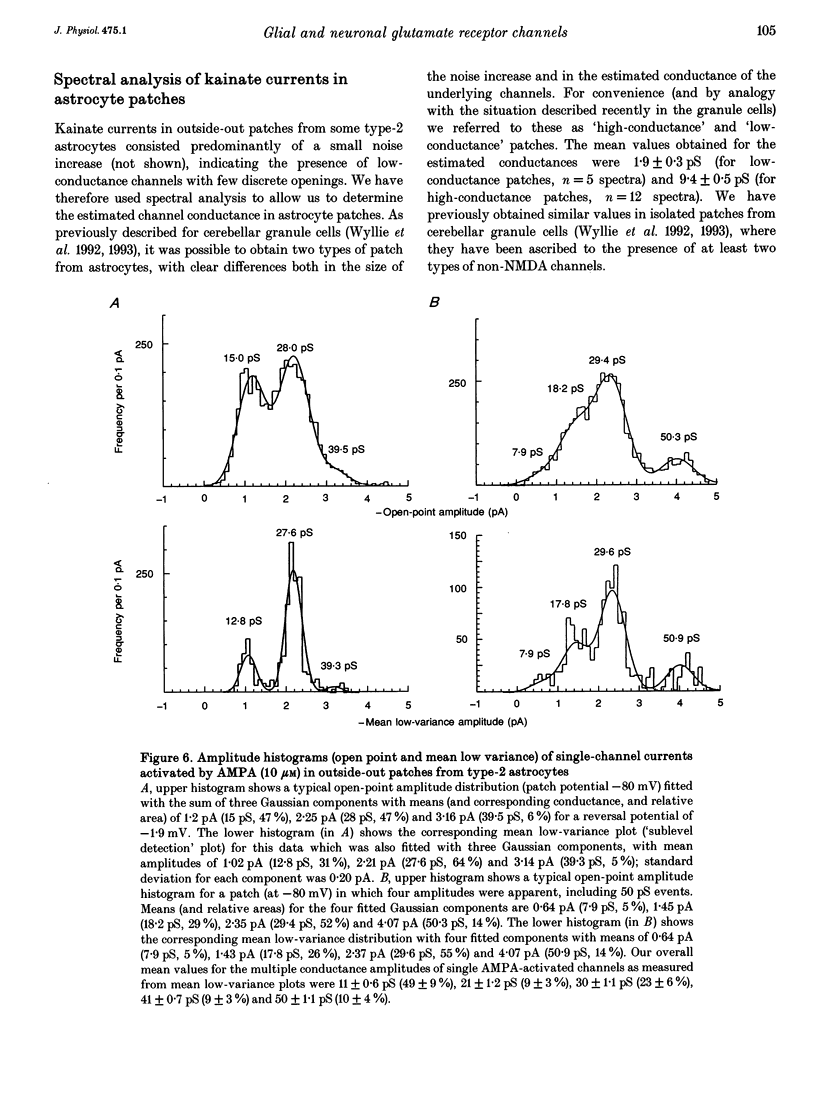
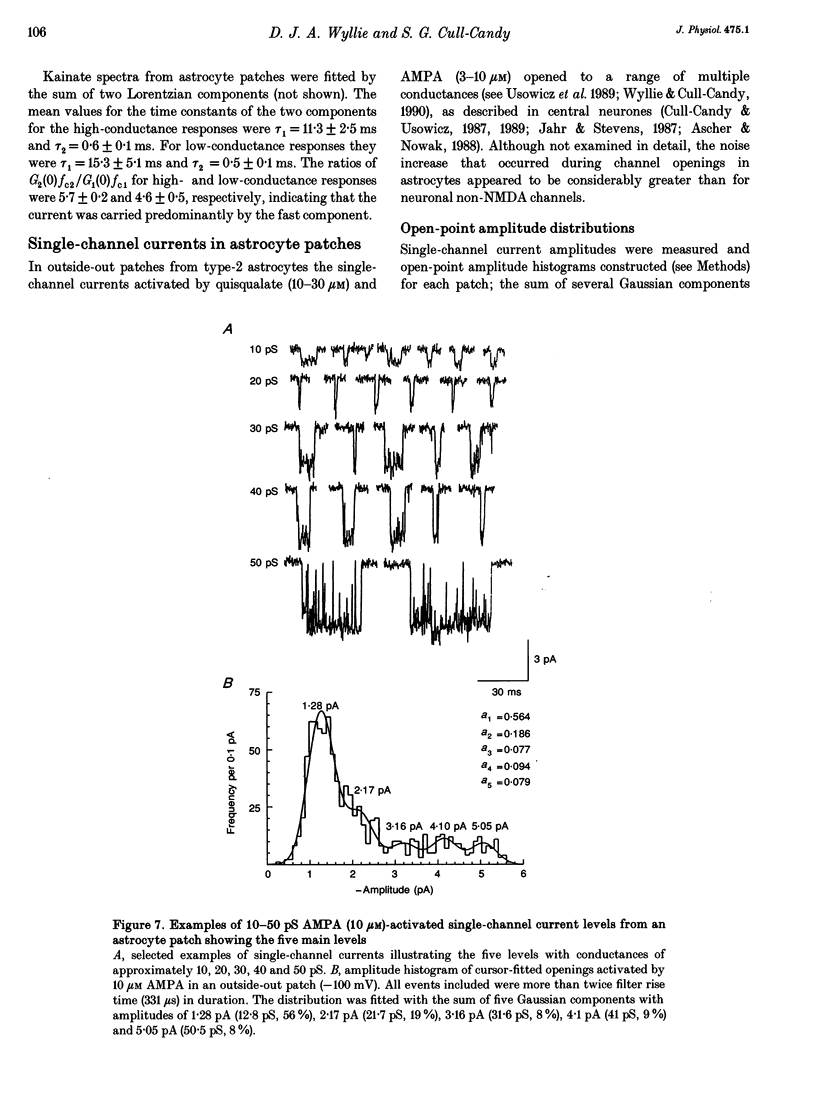
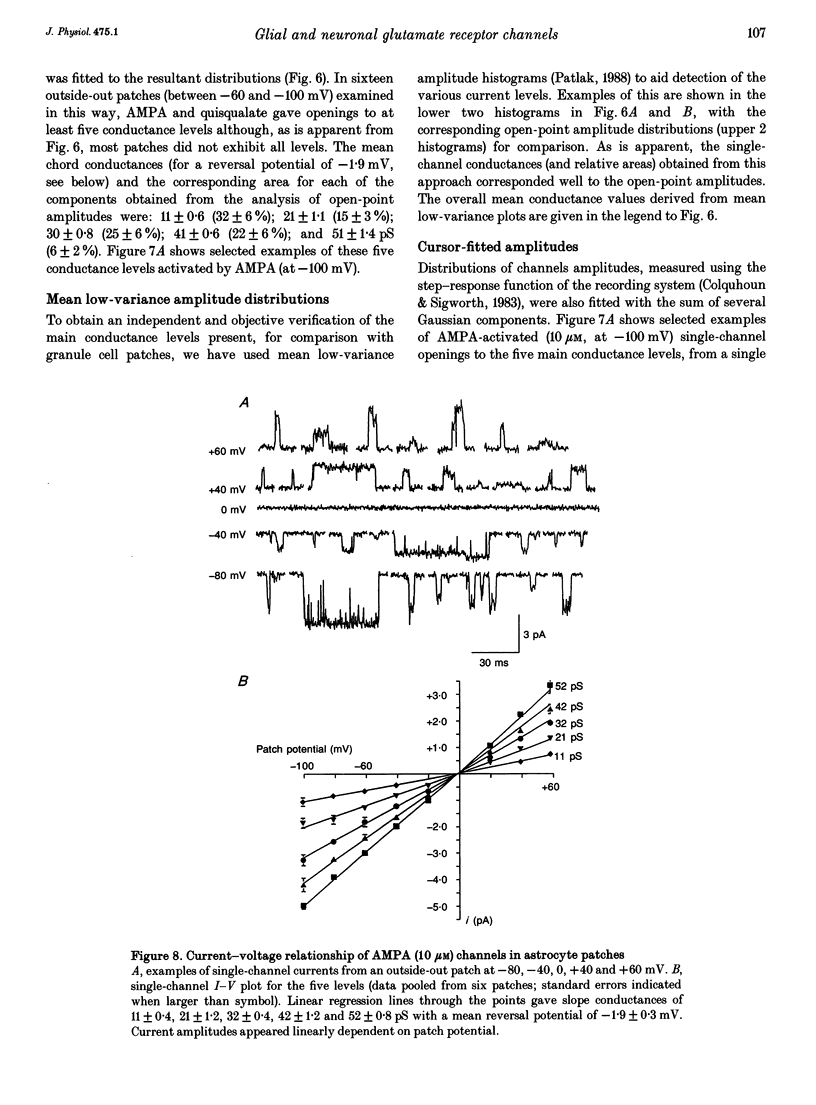
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992 Jun 12;256(5063):1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Wyllie D. J. Glutamate-receptor channels in mammalian glial cells. Ann N Y Acad Sci. 1991;633:458–474. doi: 10.1111/j.1749-6632.1991.tb15636.x. [DOI] [PubMed] [Google Scholar]
- Dawson T. L., Nicholas R. A., Dingledine R. Homomeric GluR1 excitatory amino acid receptors expressed in Xenopus oocytes. Mol Pharmacol. 1990 Dec;38(6):779–784. [PubMed] [Google Scholar]
- Farrant M., Cull-Candy S. G. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci. 1991 Jun 22;244(1311):179–184. doi: 10.1098/rspb.1991.0067. [DOI] [PubMed] [Google Scholar]
- Frey P., Berney D., Herrling P. L., Mueller W., Urwyler S. 6,7-Dichloro-3-hydroxy-2-quinoxalinecarboxylic acid is a relatively potent antagonist at NMDA and kainate receptors. Neurosci Lett. 1988 Aug 31;91(2):194–198. doi: 10.1016/0304-3940(88)90767-7. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Burne J. F., Raff M. C. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992 Dec;12(12):4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Giovannini C., Suergiu R., Levi G. Expression of excitatory amino acid receptors by cerebellar cells of the type-2 astrocyte cell lineage. J Neurochem. 1989 Jan;52(1):1–9. doi: 10.1111/j.1471-4159.1989.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson T. A., Scobey R., Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science. 1991 Mar 29;251(5001):1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Gu Y. P., Huang L. Y. Block of kainate receptor channels by Ca2+ in isolated spinal trigeminal neurons of rat. Neuron. 1991 May;6(5):777–784. doi: 10.1016/0896-6273(91)90174-x. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Cull-Candy S. G., Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991 Jan;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol Sci. 1991 Feb;12(2):53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- Jonas P., Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992 Sep;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Levison S. W., Goldman J. E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993 Feb;10(2):201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Möller T., Berger T., Schnitzer J., Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992 Jun 12;256(5063):1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M., Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991 Jul;66(1):2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Winters C. A., Mayer M. L., Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993 Dec;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991 May;6(5):785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990 Jul;10(7):2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Akeson R. L., Racke M. M., Wilburn J. L. Agonist-activated cobalt uptake identifies divalent cation-permeable kainate receptors on neurons and glial cells. Neuron. 1991 Sep;7(3):509–518. doi: 10.1016/0896-6273(91)90302-g. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Kettenmann H., Backus K. H., Schachner M. Glutamate opens Na+/K+ channels in cultured astrocytes. Glia. 1988;1(5):328–336. doi: 10.1002/glia.440010505. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991 Feb;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Dingledine R. Excitatory amino acid receptors expressed in Xenopus oocytes: agonist pharmacology. Mol Pharmacol. 1988 Sep;34(3):298–307. [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. N-methyl-D-aspartate/glycine and quisqualate/kainate receptors expressed in Xenopus oocytes: antagonist pharmacology. Mol Pharmacol. 1989 Mar;35(3):360–368. [PubMed] [Google Scholar]
- Wyllie D. J., Mathie A., Symonds C. J., Cull-Candy S. G. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. doi: 10.1113/jphysiol.1991.sp018383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie D. J., Traynelis S. F., Cull-Candy S. G. Evidence for more than one type of non-NMDA receptor in outside-out patches from cerebellar granule cells of the rat. J Physiol. 1993 Apr;463:193–226. doi: 10.1113/jphysiol.1993.sp019591. [DOI] [PMC free article] [PubMed] [Google Scholar]


