Abstract
Background
Bacterial diseases caused by phytopathogenic Xanthomonas pose a significant threat to global agricultural production, causing substantial economic losses. Biofilm formation by these bacteria enhances their resistance to environmental stressors and chemical treatments, complicating disease control. The key to overcoming this challenge lies in the development of multifunctional green bactericides capable of effectively breaking down biofilm barriers, improving foliar deposition properties, and achieving the control of bacterial diseases.
Results
We have developed a kind of innovative green bactericide from small-molecule conception to eco-friendly supramolecular nanovesicles (DaPA8@β -CD) by host-guest supramolecular technology. These nanoscale assemblies demonstrated the ability to inhibit and eradicate biofilm formation, while also promoted foliar wetting and effective deposition properties, laying the foundation for improving agrochemical utilization. Studies revealed that DaPA8@β -CD exhibited significant biofilm inhibition (78.66% at 7.0 µ g mL− 1) and eradication (83.50% at 25.0 µ g mL− 1), outperforming DaPA8 alone (inhibition: 59.71%, eradication: 66.79%). These nanovesicles also reduced exopolysaccharide formation and bacterial virulence. In vivo experiments showed enhanced control efficiency against citrus bacterial canker (protective: 78.04%, curative: 50.80%) at a low dose of 200 µ g mL− 1, superior to thiodiazole-copper-20%SC and DaPA8 itself.
Conclusion
This study demonstrates the potential of DaPA8@β -CD nanovesicles as multifunctional bactericides for managing Xanthomonas -induced plant diseases, highlighting the advantages of using host-guest supramolecular technology to enhance agrochemical bioavailability.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-03028-9.
Keywords: Dansyl derivative, Host-guest interaction, Supramolecular nanovesicles, Biofilm disruptors, Foliar deposition, Effective prevention
Introduction
Bacterial diseases caused by phytopathogenic bacteria have led to a significant food shortage and an annual economic loss of approximately $40 billion worldwide [1–4]. Among these bacteria, Xanthomonas poses a particularly troublesome threat, contributing to about 400 crop diseases [5]. For instance, Xanthomonas axonopodis pv. citri (Xac) infects plant tissues via stomata or wounds [6, 7]. Subsequently, pustules and typical raised cankered lesions develop, leading to citrus fruit shedding and a decline in quality. Another example, Xanthomonas oryzae pv. oryzae (Xoo) reduces rice yields by causing leaves to yellow and dry [8, 9]. Currently, the primary method for controlling bacterial plant diseases is through the application of chemical bactericides, however, their efficacy is greatly hampered by bacterial resistance [10]. Biofilms, acting as autocrine polysaccharide matrices, shield bacteria from the host’s immune response and confer high tolerance to antibiotics [11–13], exhibiting nearly a 1000-fold increase in resistance compared to normal bacterioplankton [14, 15]. This resistance severely hampers efforts to control bacterial infections. The formation of biofilms by phytopathogenic bacteria plays a significant role in the spread of plant diseases [16]. Despite the availability of commercial bactericides, none of them are known to effectively eradicate pathogenic biofilms and eliminate bacterial colonies within them. Additionally, agrochemicals often form droplets on leaves after application, leading to bouncing and splashing due to insufficient adhesion forces [17]. Subsequently, rain erosion, drift, and soil leaching further contribute to the loss of traditional pesticides to the environment [18], with up to 60% being lost during application [19], resulting in low retention and utilization rate of the active ingredient [20, 21]. These factors diminish agrochemical efficiency and contribute to negative environmental impacts such as pollution. Thus, an urgent need to address these challenges exists for the development of new green bactericides with effective biofilm inhibition and eradication capabilities along with better foliar deposition properties and biocompatibility.
To this end, supramolecular chemistry offers a promising approach to tackle these issues, widely applying in analytical chemistry, food science, biomedicine, and environmental science [22]. Supramolecular chemistry focuses on molecular aggregation formed through non-covalent interactions containing host–guest interactions [23, 24], electrostatic interaction [25], hydrophobic effect [26], π–π stacking and hydrogen bonds [27]. The supramolecular self-assembly enables the arrangement of active ingredients to spontaneously form new morphologies such as vesicles, nanoflowers, micelles, fibers, etc [28–31]., differing from those of the parent drug and the simple mixed drugs. Based on this, the formed nanostructures exhibit excellent physicochemical and biological properties including water-solubility, high biocompatibility, multi-functionality, and improved bioavailability. For example, Ping et al. [32] constructed a kind of protein nanocarriers with higher solubility and systemic stability in aqueous solvents. Tang et al. [33] fabricated herbicide nano-formulations with improved physicochemical properties, providing a strong synergistic effect on the herbicidal activity and simultaneously lowering their adverse effects to the environment. The above-mentioned findings suggested that supramolecular chemistry offers a great opportunity in optimizing and adjusting the physicochemical and biological functions of active substrates, thereby amplifying their bioavailability.
Host-guest recognition, a key aspect of supramolecular chemistry, involves selective integration of host and guest molecules in an entirety. This specific encapsulation normally allows the active guest molecule to inherit many advantages of the macrocyclic host, resulting in the amplification and replenishment of diverse functions [34, 35]. Common host molecules include cyclodextrins, cucurbit[n]urils, and calixarene [22, 36–39]. Among them, β-cyclodextrin (β-CD), a cyclic oligosaccharide, is low cost, easily available, highly selective, chemically stable, and bioavailable, which can specifically recognize active small molecules and alter their original properties, such as solubility, biocompatibility and permeability [40, 41]. Dansyl derivative, known for its significant fluorescence properties [42], can be specifically recognized and encapsulated by cyclodextrin cavities to construct supramolecular assemblies [43, 44]. In addition, dansyl-derived compounds exhibit excellent biological activities containing antiviral [45, 46], antimicrobial [47], and potential anti-biofilm properties [48–50]. Therefore, optimizing the dansyl-functionalized compounds by β-CD-mediated host-guest supramolecular technology offers a feasible approach to discover new efficient bactericides for controlling biofilm-associated bacterial diseases [23, 51]. However, no such study has been reported in agrochemical science.
In this study, we flexibly manipulate the supramolecular technology to optimize a bioactive dansyl derivative (DaPA8) by β-cyclodextrin (β-CD)-involved host-guest recognition, creating biocompatible supramolecular nanovesicles (DaPA8@β-CD) (Scheme 1). These nanoscale assemblies not only inhibit the formation of biofilm, but also eradicate the established mature biofilm. The liquid-solid interface interactions reveal that DaPA8@β-CD droplets can efficiently spread out and deposit on the foliage surface, laying the foundation for improving agrochemical utilization. Mechanism research found that the fabricated material impaired the formation of exopolysaccharides (EPS), a key component of biofilms, also weakened the bacterial virulence and pathogenicity. Finally, DaPA8@β-CD showed an enhancive control efficiency against citrus bacterial canker, which was distinctly superior to the commercial bactericide thiodiazole-copper-20%SC and the single DaPA8. In brief, this study presents a significative inspiration for using host-guest supramolecular technology to optimize the active small molecule, eventually realizing multiple purposes and enhancing the agrochemical bioavailability in managing Xanthomonas infections.
Scheme 1.
Schematic illustration indicates the fabrication of supramolecular nanovesicles (DaPA8@β-CD) assembled by dansyl derivative (DaPA8) and β-CD-involved host-guest recognition. This smart material enhances foliar deposition and biofilm disruption in efficiently controlling bacterial infections
Materials and methods
Materials and instruments
All chemical reagents, including dansyl chloride, tert-butyl 1-piperazine-carboxylate, 1-bromo-2,3-epoxypropane, various substituted amines, and various substituted heterocyclic rings, were purchased from Energy Chemical Co., Ltd. (China), with a purity of ≥ 98%. The macrocyclic oligosaccharide—β-cyclodextrin (β-CD) was obtained from Energy-Chemical of Saen-Chemical-Technology (Shanghai) Co., Ltd., with a high-grade purity (≥ 99%, HPLC).
NMR spectra were acquired using a Bruker Biospin-AG-400 apparatus (Bruker Optics, Switzerland). The corresponding mass spectrum was recorded using a high-resolution mass spectrometer (Uiti-Mate 3000, Thermo Scientific). SEM images were obtained using an FEI Nova Nano SEM 450 (FEI). Ultraviolet − visible (UV − vis) spectra were acquired using a TU-1900 spectrophotometer (Beijing General Instrument Co., China), and fluorescence imaging was carried out on a ZEISS LSM 900 confocal microscope. Contact angles were measured using a JC-2000D1 apparatus (Shanghai Zhongchen Digital Technical Apparatus Co., Ltd., Shanghai, China). The splashing behavior of different solutions on plant leaves was recorded using a high-speed camera (C FOR Nikon Cyclona-2-2000-C). CLSM images were acquired using a Nikon A1R Confocal Microscope System (Nikon Instruments Inc., Melville, NY, USA). The in vivo experiments were conducted in an intelligent artificial climate box (RXZ-436 C, Ningbo Jiangnan Instrument Factory, China). The molecular particle size and Zeta potential were measured using a DLS analyzer (Delsa Nano C, Beckman Coulter, Inc.). The turbidity for the in vitro antibacterial evaluation of compounds was monitored using an enzyme plate analyzer (BioTek, Vermont).
In vitro antibacterial evaluation
The in vitro bioactivity of compounds DaPA1-DaPA34 against two types of plant pathogens—Xanthomonas axonopodis pv. citri (Xac) and Xanthomonas oryzae pv. oryzae (Xoo)—was assessed using the turbidity method [52]. The commercial agricultural bactericides thiodiazole-copper (TC) and bismerthiazol (BT) were used as positive controls. A 40 µ L suspension of bacteria (Xac, Xoo) at the logarithmic growth phase was taken and added to a medium containing different compounds in a predetermined concentration gradient. The bacteria were then incubated until the control sample (0.4% DMSO) reached approximately 0.6 (OD595 nm). Each experiment was repeated three times, and the OD595 nm value was measured using a microplate reader by aspirating 200 µ L of the bacterial suspension into a 96-well plate. Correction values and suppression rates were calculated using the following formula:
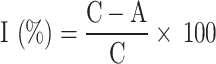 |
 |
I: Suppression rates; C: Correction values of control sample; A: Correction values of treat sample; Cv: Correction values.
Assembly of DaPA8@β-CD
Briefly, DaPA8 (12.0 µ L, 90.8 mM) in tetrahydrofuran (THF) solvent was added dropwise to 3.0 mL aqueous solution containing the macrocycle β-CD (0.36 mM), in which the final effective concentration of DaPA8 was 200 µ g mL− 1 (0.36 mM). Followed by natural evaporation of THF, the self-assembled supramolecular nanoparticles formed.
SEM of DaPA8 and DaPA8@β-CD
Before performing SEM, the preparation method for DaPA8 or DaPA8@β-CD follows the above section with a final concentration of 200 µ g mL− 1. Thirty microliters of the prepared water solution were dropped onto conductive glass, allowed to dry, and then gold-sprayed for 45 s. The resulting sample was photographed using scanning electron microscopy (SEM).
UV − vis and fluorescence spectra
UV − vis and fluorescence spectra of DaPA8 were measured using titration measurements with concentrations ranging from 60 µ M to 210 µ M (λex = 331 nm, slit = 3 nm). The concentration of DaPA8 was determined to be 170 µ M. UV − vis and fluorescence spectra of DaPA8@β-CD were obtained by continuously adding β-CD in different ratios. Based on this, a binding constant calculated from the formula as below. Besides, the Job’s plots were obtained by testing UV − vis spectra of mixture of DaPA8 and DaPA8@β-CD in different ratios.
 |
1H NMR spectra of DaPA8@β-CD
Five-point-five milligrams of DaPA8 was dissolved in CD3OD, and 17 mg of β-CD was dissolved in D2O. The 1H NMR spectra of β-CD, DaPA8, and DaPA8@β-CD were obtained by mixing different ratios of DaPA8 and β-CD (0:1; 1:0; 1:0.5; 1:1; 1:1.5; 1:2). The final percentage of CD3OD was 0.2%.
Interaction between active ingredients and plant leaves
The plant leaves were affixed to glass slides on a stage. Twelve microliters of DaPA8 and DaPA8@β-CD (200 µ g mL− 1) were applied to the leaves using a micro-injector. The contact angles of the droplets on the leaves were recorded using a JC-2000D1 apparatus, and accurate values were measured with Image-J [53]. This test was repeated at least three times for each component in different areas of the plant leaves. The largest droplet of each solution to be tested was suspended at the needle end of the micro-injector. The surface tension of the droplets and their corresponding values were obtained using the pendant drop method with a JC-2000D1 apparatus. This test was repeated at least 10 times for each droplet of different components. A droplet of 200 µ g mL− 1 of DaPA8 or DaPA8@β-CD was placed on a plant leaf affixed to a slide by a needle trap device. The contact angle and impact behavior of drug droplets on plant leaves were recorded by a JC-2000D1 apparatus and high-speed cameras, respectively.
Deposition behavior of active ingredients on plant leaf surface
Citrus leaves were punched into 1.0 cm diameter pieces and then immersed in an aqueous solution of drugs (200 µ g mL− 1) for 30 s. The weight of all pieces was recorded before and after soaking. The micro-structured morphologies of DaPA8 or DaPA8@β-CD on plant leaves were characterized using a SEM.
Penetration of DaPA8 and DaPA8@β-CD on lemon fruit
Twenty microliters of DaPA8 and DaPA8@β-CD were dropped into a hole in the peel of a lemon, drilled by a punch with a 0.5 cm inner diameter. The penetration of DaPA8 and DaPA8@β-CD was observed and recorded by slicing under the irradiation of a UV lamp (365 nm) on the second and seventh day.
Growth curves of Xac strains
Xac strains were incubated to the logarithmic phase of growth and resuspended to an OD595 nm of 0.1 using the sterilized medium. DaPA8 was added to the bacteria to create a predetermined concentration gradient (0.7, 1.4, 2.8, 7.0, 14 µ g mL− 1), and the incubation was continued on a shaker (220 rpm, 28 °C) for 36 h. Testing of the density of bacterial cultures at 595 nm was performed every 3 h. Each experiment was repeated three times, and the average value was calculated.
Anti-biofilm experiments
Inhibition of biofilm: Two hundred microliters of each bacterial suspension (OD595 nm = 0.1) containing different concentrations of the drug solution were placed into a 96-well plate [54]. The plates were sealed and placed in an incubator (28 °C) for static incubation. Forty-eight hours later, the density of bacterial cultures at 595 nm was measured to calculate the live cell inhibition rate. Bacterial biofilm staining was performed using 200 µ L crystal violet dye (10 mg mL− 1) for 10 min. Then, the density of bacterial biofilm at 570 nm was measured to calculate the biofilm inhibition rate. Each experiment was repeated three times, and the mean value was calculated.
Eradication of biofilm: Two hundred microliters of each bacterial suspension (OD595 nm = 0.1) were taken into a 96-well plate. Plates were sealed and placed in an incubator (28 ℃) for static incubation. Forty-eight hours later, the bacterial suspensions were discarded and 200 µ L fresh culture medium containing different concentrations of active ingredients were added into the plate. Plates were sealed and placed in an incubator (28 ℃) for static incubation again. Twenty-four hours later, staining of bacterial biofilm using 200 µ L crystal violet dye (10 mg mL− 1) for 10 min. Then, the density of bacterial biofilm at 570 nm were measured to calculate the biofilm eradication rate. Each experiment was repeated three times and the mean value was calculated.
CLSM of Xac bacterial biofilm
Inhibition of Biofilm: Ten milliliters of each bacterial suspension (OD595 nm=0.1), containing different concentrations of the drug solution, were added to a 6-well plate with a piece of conductive glass in each well. The plates were sealed and placed in an incubator (28 °C) for static incubation. After 48 h, the bacterial suspensions were discarded, and the bacterial biofilm was stained using 10 mL AO/PI (AO: PI = 1:1, 10 mg mL− 1) for 20 min. The excess dyes from outside the biofilm were washed with PBS solution. The conductive glass stained with dyes was mounted on glass slides after natural evaporation. The inhibition of DaPA8 and DaPA8@β-CD on biofilm could be observed under a Nikon A1R Confocal Microscope System.
Eradication of Biofilm: Ten milliliters of bacterial suspension (OD595 nm=0.1) and conductive glass were added to a 6-well plate. The plates were sealed and placed in an incubator (28 °C) for static incubation. After 48 h, the bacterial suspensions were discarded, and 10 mL fresh culture medium with different concentrations of the DaPA8 and DaPA8@β-CD solution were added to the plate. The plates were sealed again and placed in the incubator for static incubation. After 24 h, the bacterial suspensions were discarded, and staining of bacterial biofilm was performed using 10 mL AO/PI (AO: PI = 1:1, 10 mg mL − 1) for 20 min. The excess dyes from outside the biofilm were washed with PBS solution. The conductive glass stained with dyes was mounted on glass slides after natural evaporation. The eradication of DaPA8 and DaPA8@β-CD on biofilm could be observed under a Nikon A1R Confocal Microscope System.
Pathogenicity of Xac strains
Xac strains were incubated until they reached the logarithmic phase of growth and were resuspended to an OD595 nm of 0.1 using the sterilized medium. DaPA8 and DaPA8@β-CD were added to the bacterial culture for incubation on a shaker (220 rpm, 28 °C). After 4 h, the bacterial suspension was centrifuged at 10,000 rpm for 10 min, and the supernatant was discarded. The pellet fraction was then resuspended to an OD595 nm of 0.3 using PBS (phosphate-buffered saline) solutions. The treated bacterial solution was injected into the abaxial surface of citrus leaves with a syringe, following the pressure infiltration method, while taking care to avoid the leaf veins. Each treatment was repeated three times, and the mean value was calculated. The citrus trees were then placed in an incubator (28 °C, light: 16 h; dark: 8 h; humidity: 95%) for 7 days to observe leaf infection.
Determination of EPS contents (phenol-sulfuric acid method)
Ten milliliters of bacterial suspension (OD595 nm = 0.1) containing different concentrations of the drug solution were transferred into Erlenmeyer flasks. All flasks were placed on a shaker for incubation (220 rpm, 28 °C). After 48 h, the bacterial suspension was centrifuged at 10,000 rpm for 10 min. Then, 0.5 mL of the supernatant was transferred to a centrifuge tube, followed by the addition of 0.5 mL of phenol solution (50 g L− 1). After mixing, 2.5 mL of sulfuric acid solution (95%) was added under ice bath conditions. The mixture was allowed to react in the dark for 10 min, followed by shaking well and continued reaction for another 15 min. The absorbance of the samples at 490 nm was measured using spectrophotometry. Each treatment was repeated three times, and the mean value was calculated.
In vivo trial against citrus bacterial canker
In this study, the commercial agricultural bactericide thiodiazole-copper-20%SC was used as a positive control [52]. Xac cells were incubated until reaching the logarithmic phase. Prior to the experiment, citrus leaves were swabbed with absorbent cotton soaked in distilled water and then left to dry in the air. All citrus leaves were punctured with a sterile disposable syringe. For the protection test, a filter paper soaked in compounds (200 µ g mL− 1) was applied to the wounds. After 24 h, a new filter paper dipped in Xac cells (OD595 nm = 0.01) was applied to the wounds. For the curative test, a filter paper dipped in Xac cells (OD595 nm = 0.01) was applied to the wounds. After 24 h, a new filter paper soaked in compounds (200 µ g mL− 1) was applied to the wounds. Meanwhile, distilled water was used as the blank control. The treated plants were cultivated for 14 days in a climate chamber (28 °C, light: 16 h; dark: 8 h; humidity: 95% RH). Each sample has three repetitions. Control efficiency (I%) was calculated using the formula:
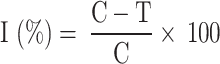 |
C: the losses of total chlorophyll content of the negative control group; T: the losses of total chlorophyll content of the treatment group.
In vivo trial against rice bacterial leaf blight
In this experiment, the commercial agricultural bactericides thiodiazole-copper-20%SC and bismerthiazol were used as positive controls. Xoo cells were incubated until reaching the logarithmic phase. Typically, Xoo inoculation was performed on rice plants (Seed variety: Feng you xiang zhan, cultured for 8 weeks) using a disinfecting scissor dipped in Xoo cells (OD595 nm = 0.6 ~ 0.8) by the leaf cutting method [52]. For the protection test, the corresponding compounds (200 µ g mL− 1, effective components) were uniformly sprayed on the leaves. After 24 h, Xoo cells were inoculated. For the curative test, the inoculation of Xoo cells was performed first, followed by uniform spraying of the corresponding compounds (200 µ g mL− 1, effective components) on the leaves. The treated plants were then cultivated for 14 days in a climate chamber (28 °C, light: 16 h; dark: 8 h; humidity: 95% RH). Each treatment group has two repetitions. After cultivation, the disease index (C or T) of the inoculated rice leaves was measured. Control efficiencies (I %) for protection and curative activities were calculated using the formula:
 |
I: control efficient; C: the disease index of the negative control; T: the disease index of the treatment group.
Statistical analysis
All experimental data were presented as mean ± standard deviation (SD) values. Statistical analysis was conducted using IBM SPSS Statistics 27 and ANOVA, assuming equal variances (P > 0.05) or unequal variances (P < 0.05).
Results and discussion
Access to highly active dansyl-modified derivatives as the guest molecule
Based on the versatile biological profiles of dansyl compounds aforementioned, a facile modification that introduced an active amino-isopropanol fragment was carried out to acquire new bactericidal structures. As displayed in Scheme 2, we designed a simple synthetic route to prepare the final dansyl-modified structures (DaPA1-DaPA34). In brief, the starting material dansyl chloride was subjected to nucleophilic substitution to produce a key intermediate product with a piperazine-linked propylene oxide pendant. This intermediate was then ring-opened by various substituted amines to afford new structures, which were characterized using 1H NMR, 13C NMR, 19F NMR and HRMS (Fig. S14-S13). The bactericidal efficacy of compounds DaPA1-DaPA34 was tested through the commonly used turbidimetric approach and illustrated in Table 1.
Scheme 2.
Synthetic route for new dansyl isopropanolamine derivatives
Table 1.
Inhibition effect of target compounds DaPA1-DaPA34 against Xac
| Comp. | Regression equation | EC 50a (µg mL− 1) | Comp. | Regression equation | EC 50a (µg mL− 1) |
|---|---|---|---|---|---|
| DaPA1 | y = 2.843x + 3.281 | 5.94 ± 0.21 | DaPA18 | y = 6.333x − 0.234 | 6.71 ± 0.32 |
| DaPA2 | y = 4.671x + 1.410 | 5.87 ± 0.11 | DaPA19 | y = 1.272x + 4.079 | 5.13 ± 0.12 |
| DaPA3 | y = 6.230x + 1.595 | 3.52 ± 0.04 | DaPA20 | y = 1.834x + 3.581 | 5.94 ± 0.21 |
| DaPA4 | y = 3.180x + 3.376 | 3.24 ± 0.21 | DaPA21 | y = 2.187x + 3.507 | 4.81 ± 0.57 |
| DaPA5 | y = 2.601x + 3.446 | 5.10 ± 0.05 | DaPA22 | y = 1.578x + 3.273 | 12.4 ± 0.6 |
| DaPA6 | y = 2.935x + 3.077 | 4.52 ± 0.13 | DaPA23 | y = 3.589x + 1.066 | 12.5 ± 0.7 |
| DaPA7 | y = 3.271x + 2.448 | 6.03 ± 0.20 | DaPA24 | y = 1.549x + 3.874 | 5.33 ± 0.89 |
| DaPA8 | y = 3.808x + 3.034 | 2.80 ± 0.06 | DaPA25 | y = 0.965x + 3.867 | 14.9 ± 0.2 |
| DaPA9 | y = 5.444x + 0.852 | 4.47 ± 0.10 | DaPA26 | y = 1.633x + 2.942 | 18.2 ± 2.7 |
| DaPA10 | y = 2.967x + 3.320 | 3.68 ± 0.19 | DaPA27 | y = 1.027x + 4.003 | 9.35 ± 0.97 |
| DaPA11 | y = 3.309x + 3.236 | 3.51 ± 0.27 | DaPA28 | y = 10.56x − 10.19 | 27.5 ± 0.6 |
| DaPA12 | - | > 50 | DaPA29 | y = 2.946x + 1.210 | 19.3 ± 1.3 |
| DaPA13 | y = 5.378x + 0.921 | 5.73 ± 0.11 | DaPA30 | y = 3.002x + 1.152 | 19.1 ± 0.8 |
| DaPA14 | y = 11.31x − 6.884 | 11.2 ± 0.2 | DaPA31 | - | > 50 |
| DaPA15 | y = 1.902x + 3.233 | 8.49 ± 0.14 | DaPA32 | - | > 50 |
| DaPA16 | y = 2.855x + 2.522 | 7.38 ± 0.29 | DaPA33 | y = 2.253x + 2.164 | 18.1 ± 0.4 |
| DaPA17 | y = 1.859x + 4.048 | 3.25 ± 0.39 | DaPA34 | y = 2.967x + 0.851 | 25.0 ± 0.8 |
| BT | y = 7.859x − 8.607 | 53.9 ± 0.9 | TC | y = 4.738x − 4.082 | 82.6 ± 1.3 |
a EC50 values of bactericidal activity indicated as means ± SD (standard deviation)
For the anti-Xac activity, most of the designed compounds (except DaPA12, DaPA31, DaPA32) displayed strong bactericidal effects with EC50 values ranging from 2.80 to 27.5 µ g mL-1, which were observably better than commercial bactericides thiodiazole-copper (TC, EC50 = 82.6 µ g mL-1) and bismerthiazol (BT, EC50 = 53.9 µ g mL-1). Among them, DaPA8 afforded the minimum EC50 value of 2.80 µ g mL-1, revealing the successful discovery of highly active dansyl compounds. The molecular structure and anti-Xac efficacy relationship was preliminarily deduced. (1) A trifluoromethyl group located at the meta-position of benzyl (DaPA8, EC50 = 2.80 µ g mL-1) exhibited better bactericidal effects than those at the ortho-position (DaPA6, EC50 = 4.52 µ g mL-1) or para-position (DaPA7, EC50 = 6.03 µ g mL-1). (2) A strong electron withdrawing trifluoromethyl group on the benzene ring (3-CF3, DaPA8, EC50 = 2.80 µ g mL-1) afforded superior anti-Xac activity to those of compounds with weak electron withdrawing groups (3-F, DaPA4, EC50 = 3.24 µ g mL-1; 3-Cl, DaPA10, EC50 = 3.68 µ g mL-1) and electron-donating group (3-CH3, DaPA2, EC50 = 5.87 µ g mL-1). (3) Ring-opening the oxirane with a suitable alkyl chain improves the antibacterial efficacy, comparing EC50 values of DaPA21 (4.81 µ g mL-1) and DaPA22-DaPA27 (5.33 ~ 18.2 µ g mL-1). (4) Addition of a heterocyclic group to the amino-isopropanol fragment decreased bioactivity, as seen in DaPA31-DaPA32. Intriguingly, the designed small molecules (except DaPA12, DaPA24, DaPA25, DaPA29, DaPA31, and DaPA32) also showed broad-spectrum bioactivity against Xoo, with EC50 values ranging from 2.08 to 40.6 µ g mL-1 (Table S1). In particular, DaPA8 again presented the best anti-Xoo activity with the smallest EC50 value of 2.08 µ g mL-1. However, DaPA8 itself has some inherent problems, such as low solubility in water, easy aggregation and precipitation, and undesirable biocompatibility, influencing the final utilization rate. Therefore, using the supramolecular technology to further optimize this bioactive ingredient (DaPA8) is imperative.
Driving forces, stoichiometric ratio, and self-assembly process of Supramolecular Nanovesicles (DaPA8@β-CD)
The biocompatible macrocyclic host molecule β-CD was used to optimize the guest molecule DaPA8 via the specific host-guest encapsulation. Briefly, DaPA8 (12.0 µ L, 90.8 mM) in tetrahydrofuran (THF) solvent was added dropwise to 3.0 mL aqueous solution containing the macrocycle β-CD (0.36 mM), in which the final effective concentration of DaPA8 was 200 µ g mL− 1 (0.36 mM). Followed by natural evaporation of THF, the self-assembled supramolecular nanoparticles formed. During this assembly process, fluorescence/UV-vis titrimetry, 1H NMR titrimetry, high-resolution mass spectrometry (HRMS), particle size and Zeta-potential measurements, and scanning electron microscopy (SEM) imaging were performed to expound the relevant driving forces, binding stoichiometry, systemic stability, and topological structures.
Fluorescence/UV-vis titrations were firstly presented to explore the driving forces and stoichiometric ratio. As shown in Fig. 1A, the gradual addition of β-CD caused a progressive decrease in the fluorescence intensity of DaPA8 solution and coincided with an obvious bathochromic-shift (from 500 nm to 550 nm). This phenomenon indicates the probable formation of host-guest complexes (DaPA8@β-CD) through packaging the fluorescent dansyl moiety of DaPA8 inside the hydrophobic cavity of β-CD. Certainly, this specific host-guest envelopment breaks the original molecular arrangement (DaPA8 itself forms H-aggregates with a distinct blue-shift, Fig. S1), thereby leading to the resumption of its native fluorescence emission at 550 nm. Similarly, upon the supplement of β-CD, the UV absorption at 331 nm also presents a downward trend (Fig. 1B), revealing the selective encapsulation of the dansyl moiety inside the cavity of β-CD. Another interesting finding was that a sharp decline in fluorescence and UV intensity was observed, when 0.5 equiv. (red curves) and 1.0 equiv. (orange curves) β-CD were added, implying the possible binding ratio was 1:1 (DaPA8: β-CD). This inference agrees with the Job’s plots experiment between DaPA8 and β-CD (Fig. 1C), confirming the 1:1 stoichiometric ratio with a binding constant of Ka = 5.156 × 103 M− 1 (Fig. 1D), consistent with literature reports [55].
Fig. 1.
(A) Fluorescence spectra of DaPA8 upon the addition of β-CD in H2O ([DaPA8] = 1.7 × 10− 4 M and [β-CD] = 0 − 8.5 × 10− 4 M). (B) UV − vis spectra of DaPA8 upon the addition of β-CD in H2O ([DaPA8] = 1.7 × 10− 4 M and [β-CD] = 0 − 8.5 × 10− 4 M). (C) Job’s plots for ΔA at 331 nm with the total concentration of DaPA8 and β-CD at 1.7 × 10− 4 M in H2O. (D) Tsien equation plot of colorimetric 1/[ΔA]. (E) 1H NMR spectra of DaPA8 (1.7 × 10− 4 M), β-CD, and DaPA8/β-CD with different molar ratios (1:0.5, 1:1, 1:1.5, 1:2) in D2O. (F) HRMS spectra of DaPA8@β-CD. (G) DLS of DaPA8 and DaPA8@β-CD in H2O (200 µ g mL− 1). (H) Zeta potential of DaPA8 and DaPA8@β-CD in H2O (200 µ g mL− 1). Statistical analysis of particle sizes of (I) DaPA8 and (J) DaPA8@β-CD by Image-J. (K) SEM images of DaPA8 and DaPA8@β-CD (200 µ g mL− 1)
The driving force between DaPA8 and β-CD was elucidated through 1H NMR titration experiments (Fig. 1E). Upon the introduction of 1.0 equiv. β-CD, the proton peaks H1, H7 and H10 belonging to the dansylated naphthalene ring were shifted to the low field (Δδ1 = 0.06 ppm, Δδ7 = 0.05 ppm, Δδ10 = 0.14 ppm), while the proton peaks H2 − 3 and H9 were shifted to the high field (Δδ2−3 = − 0.06 ppm, Δδ9 = − 0.01 ppm). This outcome indicates that protons H1, H7 and H10 are located in the β-CD ports due to the deshielding effect, while protons H2 − 3 and H9 are located inside the cavity of β-CD. By contrast, protons H4 − 6 on the trifluoromethylbenzene ring had less chemical shifts after introducing 1.0 equivalent of β-CD, suggesting the enfolding location is mainly on the dansyl fragment. In addition, protons H13 and H15 belonging to the piperazine are displaced to the lower field due to the deshielding effect (Δδ13 = 0.10 ppm, Δδ15 = 0.11 ppm). Whereas, the fluctuation in chemical shifts ceased upon addition of more than 1.0 equiv of β-CD, again revealing a probable 1:1 binding mode. Further validation of the successful encapsulation was provided by HRMS (Fig. 1F), which showed molecular weights of 1685.6016 and 1707.5656 (m/z) assigned as [DaPA8 + β-CD + H+] and [DaPA8 + β-CD + Na+].
Dynamic light scattering (DLS) measurements revealed that DaPA8 itself exhibited two kinds of size distributions with averaged particle sizes of 259 nm and 2350 nm at 200 µ g mL− 1 (Fig. 1G). This observation was attributed to the inherent instability of DaPA8, which led to particle agglomeration and formation of larger agminated particles. In contrast, DaPA8@β-CD solution gave an average particle size of 555 nm, suggesting that the introduction of host-guest supramolecular technology can significantly optimize the current system. As expected, DaPA8@β-CD solution afforded an enhancive Zeta potential of 45.56 mV (Fig. 1H), which was quite better than that of DaPA8 (16.00 mV). This favourable result reveals that loading DaPA8 by a biocompatible oligosaccharide (β-CD) can markedly enhance the solution stability and particle dispersions. The following SEM imaging (Fig. 1K) at an effective concentration of 200 µ g mL− 1, illustrated that DaPA8@β-CD presented relatively uniform vesicles with statistical average particle size of 1694 nm (Fig. 1J). Compared with DLS data, this difference is due to the possible aggregation of pellets during sample preparation and drying on the silicon wafer. At the same conditions, the guest molecule DaPA8 afforded large, cross-linked, and irregular aggregates with a statistical particle size distribution of 790–7692 nm (Fig. 1I). These findings align with the trends observed in DLS and Zeta potential results, demonstrating the inherent instability of DaPA8. Overall, these meticulous investigations demonstrate the successful construction of biocompatible supramolecular nanovesicles by flexibly manipulating the host-guest recognition strategy. The stability of this supramolecular system at different storage periods and temperatures was further assessed by HPLC measurements. As shown in Fig. S2, after seven days of storage at different temperatures (15 ~ 35 ℃), the peak area of DaPA8@β-CD did not change significantly, confirming the stability of the current supramolecular system. This assertion was agreeing with its thermogravimetric analysis (TGA) from Fig. S3, verifying the successful formation of host-guest complex—DaPA8@β-CD with a relative stability. The release experiments using dialysis membranes with two intercepted molecular weights (1000 Da and 3500 Da) were carried out, in which the molar ratio of DaPA8: β-CD was set as 1:0.5 and 1:1, respectively. As shown in Fig. S4C, at the molar ratio of 1:0.5 (DaPA8: β-CD), insufficient β-cyclodextrins cannot completely encapsulate all the guest molecules, resulting in about 42.5% cumulative release of DaPA8 (Mw = 550.64) with an intercepted molecular weight of 1000 Da. However, at the molar ratio of 1:1 (DaPA8: β-CD), β-cyclodextrins just completely encapsulate all the guest molecules to form host-guest binary complexes (DaPA8@β-CD, Mw = 1685.63), so the guest molecules cannot be released from an intercepted molecular weight of 1000 Da (Fig. S4E). For another dialysis membrane (intercepted molecular weight 3500 Da), there was about 78.1% cumulative release of supramolecular complexes (DaPA8@β-CD) at 12 h (Fig. S4F). This outcome revealed that nearly all the monomers (DaPA8) were involved in the formation of host-guest supramolecular complexes (DaPA8@β-CD), confirming the construction of a relatively stable system.
Based on the surveyed driving force, we speculated a possible assembly mechanism for forming nanovesicles (Scheme 1). Firstly, a new binary building block (DaPA8@β-CD) was constructed through the selective encapsulation of dansyl moiety of DaPA8 by the macrocyclic molecule β-CD. Given the bulked and hydrophilic β-CD/dansyl part, these building units would be assembled in a staggered arrangement, affording a curved layered structure to avoid the exposure of hydrophobic areas to the aqueous environment. Then, with the help of hydrogen bonding interactions, all the layered structures were integrated together to afford the final supramolecular nanovesicles. Therefore, we hypothesize that these supramolecular assemblies not only inherit the properties of cyclodextrins and small molecules, but also achieve further biological performance enhancement.
DaPA8@β-CD droplets improve liquid-solid interface interactions—showing good wettability and deposition on citrus foliage
Given the optimized physicochemical and biocompatible properties as well as the actual foliar spraying, the correlative liquid-solid interface interactions between DaPA8@β-CD droplets and citrus foliage should be investigated. Thus, the critical parameters, including contact angle, surface tension, droplet slip test, liquid holding capacity (LHC) and topological morphology on blade surface, were performed. Figure 2A indicated that the dynamic contact angles of water, β-CD and DaPA8 were 93°, 96° and 80°, respectively, while that of DaPA8@β-CD was 74°. This means that the self-assembled DaPA8@β-CD prefers wetting and spreading on the citrus leaf surface. Surface tension for DaPA8 significantly dropped from 62.12 to 45.57 mN/m after assembling with β-CD (Fig. 2B), further proving that the smart supramolecular optimization strategy can improve the biocompatibility and subsequent liquid-solid interface interactions of active substrates. Next, the impacting droplet slip experiment on an inclined citrus blade (60°) from a height of 10 cm was executed and recorded by a high-speed camera. As displayed in Fig. 2C and D and Movie S1, the impacting water and β-CD droplets reached the maximum slip lengths (11.94 mm and 11.12 mm, respectively) at 15 ms and stabilized soon without any retraction.
Fig. 2.
(A) Dynamic contact angles and (B) Surface tension of water, β-CD, DaPA8, and DaPA8@β-CD droplets (200 µ g mL− 1) on citrus leaves. (C) Droplets of water, β-CD, DaPA8, and DaPA8@β-CD impacting on inclined citrus blade (60°) from a height of 10 cm. (D) Sliding and retention lengths of water, β-CD, DaPA8, and DaPA8@β-CD during the impact process. (E) Citrus leaves irradiated by a UV lamp (365 nm) after soaking in DaPA8 and DaPA8@β-CD (200 µ g mL− 1). (F) The ΔWeight of citrus leaves before and after soaking in DaPA8 and DaPA8@β-CD solutions (200 µ g mL− 1). (G) SEM images for topological morphologies of DaPA8 and DaPA8@β-CD after deposition on the citrus leaf surface (200 µ g mL− 1)
In comparison, DaPA8@β-CD droplets provided a visibly reduced slip length of 9.59 mm, which was shorter than that of the single DaPA8 (9.94 mm). Another discovery was that this supramolecular material (DaPA8@β-CD) afforded the increased wetting and spreading after contact with the leaf surface, with a maximum length of 8.64 mm, markedly outstriping those of water (3.89 mm), β-CD (3.86 mm), and DaPA8 (6.48 mm) droplets. This outcome was in agreement with the above measured contact angle and the following LHC determination. Briefly, citrus leaves were immersed in the active ingredient (200 µ g mL-1) for 0.5 min to measure the deposition of different droplets through the difference in weight of leaves before and after dipping. The fluorescent properties of the dansyl derivatives were utilized to visualize the deposition effect of active ingredients under the irradiation of a UV lamp (365 nm). In Fig. 2E, the surface of circular pieces processed by DaPA8@β-CD exhibited more fluorescent spots than DaPA8. The ultimate mass of these leaves immersed in the aqueous solution of DaPA8@β-CD was significantly greater than that soaked in DaPA8 solution (Fig. 2F), demonstrating greatly enhanced retention and deposition behaviors of the supramolecular complex. Finally, the topological morphology on blade surface was characterized by SEM imaging after active ingredient deposition. As shown in Fig. 2G, DaPA8@β-CD sufficiently covered the leaves in a film-like manner, while DaPA8 offered large irregular and non-dispersed precipitates on the leaf surface, indicating that the constructed biocompatible supramolecular assemblies have better foliar dispersion and spreading properties, potentially setting the stage for enhancing bioavailability. To verify the penetrating capacity, the congeneric lemon fruit was used to do this test. As shown in Fig. S5, on the second day, the diffusion of active ingredient DaPA8@β-CD exhibited a diffusive pattern, while DaPA8 displayed as a punctate pattern. By the 7th day, both active ingredients had penetrated the rind at the other end of the lemon, but DaPA8@β-CD showed superior lateral diffusion compared to DaPA8. This suggests that the supramolecular strategy can improve the permeability and bioavailability by enhancing the solid-liquid interface interactions between agrochemical droplets and plant leaves. The abovementioned studies demonstrate that the host-guest strategy is a simple method to improve the physicochemical property, thereby eventually benefiting to the liquid-solid interface interaction and agrochemical utilization.
DaPA8@β-CD shows an enhanced efficacy to inhibit the formation of bacterial biofilms
Considering the excellent water dispersible and biocompatible properties of DaPA8@β-CD, we hypothesize that these self-assembled nanovesicles will have high probability to disrupt bacterial biofilms. To verify this inference, the effects of DaPA8@β-CD on Xac growth were investigated at various concentrations before the precise determination of biofilm content. As depicted in Fig. 3A. The growth rate of Xac achieves a balance at about 30 h. Comparing to the control group, low doses (below 7.0 µ g mL-1) of DaPA8@β-CD exhibited no significant effect on bacterial growth, whereas the growth of Xac was slightly inhibited as the concentration reached 7.0 µ g mL-1. At 14 µ g mL-1, DaPA8@β-CD gave an intensive inhibition towards Xac, and the final bacterial optical density (OD595 nm) value were approximately 30% lower than that of the control. Based on this result, the potential bactericidal mechanism of DaPA8@β-CD against Xac was further investigated by setting suitable concentrations.
Fig. 3.
(A) Growth curve of Xac at various concentrations of DaPA8@β-CD. (B) Inhibition effects of biofilm formation by different concentrations of DaPA8 and DaPA8@β-CD (From top to bottom: 28, 14, 7.0, 2.8, 1.4, 0.7 µ g mL− 1, β-CD, the control sample) against Xac. Each concentration of agents was tested in five wells. (C) Quantitative assays for DaPA8 and DaPA8@β-CD against Xac biofilm formation by measuring OD570 nm values. Error bars indicate the mean ± SD. (D) Influence of Xac biofilms formation in different concentrations of DaPA8 and DaPA8@β-CD observed by CLSM via live (AO: green)/dead (PI: red) staining, scale bars: 100 µ m
Biofilms were stained with crystal violet to investigate the effects of tested constituents on biofilm formation during bacterial growth. The corresponding results are shown in Fig. 3B and C. Under the same assay conditions, the biofilms of the samples incubated with DaPA8@β-CD (OD570 nm, dark blue columns) were significantly decreased compared to those treated with DaPA8, while more dense and thick bacterial biofilms were observed following incubation with the control or β-CD. The growth of bacterial biofilms was virtually reduced in a concentration-dependent manner under the impact of DaPA8@β-CD. At lower concentrations of 2.8 and 7.0 µ g mL-1, DaPA8@β-CD inhibited Xac bacterial biofilm formation by 59.58% and 78.66% (Fig. S6), quite superior to those of the guest molecule DaPA8 (51.40% and 59.71%). The significantly enhanced efficacy (8.18% and 18.95%) confirms that the biological function and bioavailability of active ingredients can be improved using a flexible supramolecular optimization strategy. Continuing to increase the concentration to 14.0 µ g mL-1, DaPA8@β-CD strongly disrupted the formation of biofilms with an inhibition ratio of 82.37%, again better than that of DaPA8 (76.60%).
The Xac biofilm structure was further observed by confocal laser scanning microscopy (CLSM) in Fig. 3D. Biofilms treated with various concentrations of different components were stained with propidium iodide (PI, staining for dead bacteria) and acridine orange (AO, staining for live bacteria). Fluorescence staining showed that tightly arranged and dense biofilms were formed by Xac in the absence of bactericides, resulting in a strong green fluorescent substrate. At 5.6 µ g mL-1, cells treated with DaPA8@β-CD exhibited faint red fluorescence, indicating a small amount of cell death in the process of co-incubation. However, DaPA8 hardly affected the normal growth of the cells. As the concentration of DaPA8@β-CD increased to 14.0 µ g mL-1, the cells incubated with DaPA8@β-CD showed a more robust red fluorescence and faint green fluorescence than those incubated with DaPA8 at the same conditions (Fig. S7). The biofilm became significantly looser and thinner due to the intervention of active ingredients, suggesting that the integrity of bacterial biofilms was affected. Perhaps due to the stronger disruption of biofilm growth by DaPA8@β-CD, thus leads to more bactericidal ingredients contact with pathogens and eventually induces more dead cells. These results indicate that the supramolecular material DaPA8@β-CD has a superior ability to inhibit biofilm formation compared to the single guest molecule, DaPA8.
DaPA8@β-CD possesses an enhanced eradication efficacy on mature xac-biofilms and reduces the EPS production and bacterial virulence
Thereafter, the same methods were used to evaluate the eradicative efficacy of DaPA8@β-CD for mature/established Xac-biofilms. In this experiment, sufficient Xac-biofilms were grown in 96-well microplates for 48 h and then continuously exposed to diverse dosages of DaPA8@β-CD and DaPA8 for another 24 h. As seen in Fig. 4A, the navy-blue color stained with crystal violet decreases with rising the drug concentrations, and cells incubated with DaPA8@β-CD performed a lighter color than those incubated with DaPA8. The related OD570 nm values were measured and shown in Fig. 4B, DaPA8@β-CD was effective in eradicating mature biofilm with eradication rates of 58.54%, 83.50%, and 91.69% at 12.5, 25.0, and 50.0 µ g mL-1, respectively (Fig. S8). Those eradicative effects were quite better than co-incubation with the single guest DaPA8 (51.62%, 66.79%, and 77.53%) at the same conditions, again proving the advisable supramolecular optimization method towards the elevated biological function of active ingredients. The CLSM imaging was also used in biofilm eradication experiments (Fig. 4C). Clearly, with increasing the doses of active ingredients, the green fluorescence of the cells decreased while the red fluorescence increased. The only difference was that DaPA8@β-CD had a markedly better eradication effect than that of DaPA8 (Fig. S9), basing on the statistical fluorescence intensity by Image-J software. All the above-mentioned results demonstrate that DaPA8@β-CD shows better biofilm inhibition and eradication effects than DaPA8 at the same conditions, which can be explained by the host-guest optimization strategy reinforcing the anti-biofilm properties of bioactive substrates.
Fig. 4.
(A) Disruption of mature Xac-biofilms by various concentrations of DaPA8@β-CD and DaPA8 (From top to bottom: 100, 50, 25, 12.5, 6.25, 3.125 µ g mL-1, β-CD, control sample). Each concentration of agents was tested in eight wells. (B) Quantitative assays for DaPA8 and DaPA8@β-CD against mature Xac-biofilms by measuring OD570 nm values. Error bars indicate the mean ± SD. (C) CLSM imaging for the Xac-biofilms eradication in different concentrations of DaPA8 and DaPA8@β-CD based on live (AO: green)/dead (PI: red) staining, scale bars: 100 µ m. (D) EPS production tested by using the phenol-sulfuric acid method after treating Xac cells with DaPA8 and DaPA8@β-CD at various concentrations. (E) Absorbance of EPS by colorimetric in 490 nm. (F) Effect of DaPA8 and DaPA8@β-CD on the pathogenicity of Xac. (G-H) Diameter of citrus bacterial canker symptoms after treating with DaPA8 and DaPA8@β-CD at (G) 2.8 µ g mL-1 and (H) 7.0 µ g mL-1
In order to further decipher the underlying mechanism of DaPA8@β-CD on biofilm eradication, the essential component exopolysaccharide (EPS) in the biofilm maturation process was tested by using the phenol-sulfuric acid method and subsequently measuring the absorbance at 490 nm. Figure 4D indicated that EPS production was certainly inhibited by DaPA8@β-CD, basing on the gradually discolored solutions. In Fig. 4E, EPS in Xac biofilms decreased by 28.23% (0.7 µ g mL-1), 50.37% (1.4 µ g mL-1), 65.31% (2.8 µ g mL-1), 79.95% (5.6 µ g mL-1), and 85.67% (8.4 µ g mL-1) after treatment with DaPA8@β-CD compared to the control without active ingredients. The reductions were approximately 24.60% (0.7 µ g mL-1), 34.60% (1.4 µ g mL-1), 37.64% (2.8 µ g mL-1), 30.91% (5.6 µ g mL-1), and 12.14% (8.4 µ g mL-1) greater than the guest molecule DaPA8. In other words, DaPA8@β-CD presented a significantly enhanced efficacy in inhibiting the expression of Xac-EPS than DaPA8, again validating the necessary molecular optimization through this smart host-guest supramolecular strategy. To explore the underlying reasons, the transcriptional level of the correlative gum gene cluster that can regulate the synthesis and transport of EPS in Xac, was monitored by qRT-PCR experiments. As illustrated in Fig. S10 and Table S2, upon the treatment of DaPA8@β-CD at 5.6 µ g mL-1, the expression levels of certain gum genes (gumB, gumC, gumD, gumG, gumH, gumK, gumL, gumN) were markedly downregulated compared to treatment with DaPA8 alone, which explains that the biofilm decrease was probably attributed to the obvious downregulation of EPS expression by DaPA8@β-CD. Subsequently, we also assessed the membrane permeability by measuring the relative conductivity. As presented in Fig. S11, upon the co-incubation of Xac strains with various concentrations of DaPA8@β-CD, the relative electric conductivity was increased gradually, especially at 6.25 µ g mL-1, indicating that DaPA8@β-CD has superior membrane permeability to perform its anti-biofilm function. Given these superiorities of the constructed DaPA8@β-CD, the bacterial virulence in pathogenicity was assessed by pressure osmosis inoculation method. As displayed in Fig. 4F, the control groups exhibited significantly larger lesions of approximately 1.1 cm2. At concentrations of 2.8 and 7.0 µ g mL-1, DaPA8@β-CD notably reduced the bacterial pathogenicity by 53.73% and 71.72%, respectively, while DaPA8 resulted in the attenuation of disease symptoms on citrus leaves by 26.45% and 58.84%, respectively (Fig. 4G and H). This means that DaPA8@β-CD exhibits a greater reduction in pathogenicity to Xac than DaPA8 at the same dosages. The above experimental results demonstrate that the supramolecular bactericide (DaPA8@β-CD) constructed by host-guest recognition strategy can serve as a potent biofilm disruptor for effectively controlling bacterial infections.
DaPA8@β-CD exhibits superior in vivo control efficacy against bacterial infections
Given the enhanced wetting and deposition properties as well as the efficient disruption efficacy on bacterial biofilms, the in vivo antibacterial experiments against citrus bacterial canker were further investigated. The result indicates that DaPA8@β-CD can effectively reduce canker lesions in citrus leaves at a lower dose of 200 µ g mL-1 (Fig. 5A). The protective efficacy of DaPA8@β-CD was 78.04% (Fig. 5B), showing a superior effect by 20.83% compared to the commercial bactericide thiadiazole-copper (TC, 57.21%), also better than that of DaPA8 (65.58%). The curative efficacy of DaPA8@β-CD was 50.80%, which was quite superior to those of TC (27.60%) and DaPA8 (41.92%). In contrast to DaPA8@β-CD, the frequently applied thiodiazole-copper can not be used in sensitive crops and young fruit period of crop flowering. Moreover, its quick effect and internal absorption are weak, so it is difficult to meet the needs of agriculture. Besides, the long-term application of TC has led to strong resistance in bacteria. The above well-pleasing outcomes confirm that the constructed supramolecular assemblies (DaPA8@β-CD) were effective to manage alarming bacterial diseases.
Fig. 5.
(A) Disease symptoms and (B) control efficacies of DaPA8@β-CD and DaPA8 against citrus bacterial canker at 200 µ g mL− 1. (C, E) Disease symptoms and (D) curative and (F) protective efficacies of DaPA8@β-CD and DaPA8 against rice bacterial blight at 200 µ g mL− 1
To broaden the broad-spectrum bactericidal application, in vivo pot experiments against rice bacterial blight (RBB) disease were shown in Fig. 5C and D (curative efficacy) and Fig. 5E and F (protective efficacy). Clearly, DaPA8@β-CD exhibited excellent control effects (curative: 47.03%, protective: 54.88%) against RBB, which were considerably higher than those of commercial bactericides TC (curative: 33.33%, protective: 38.62%) and bismerthiazol (BT, curative: 31.83%, protective: 42.48%), also exceeding that of DaPA8 (curative: 36.71%, protective: 48.89%). Moreover, the achieved control efficiency is better than our previous work with a moderate efficacy (40.3%~43.6%) at the same dosage [56]. In summary, the fabricated supramolecular bactericide DaPA8@β-CD that efficiently targets bacterial biofilms is a promising material in addressing the significant threat of plant bacterial diseases to agricultural production. To preliminarily assess the long-term influences of using DaPA8@β-CD in agricultural settings, the related phytotoxicity to citrus/rice plants and the citrus/rice chlorophyll contents and conductivity were tested. As illustrated in Fig. S12, after culturing citrus/rice plants with DaPA8@β-CD and DaPA8 for 14 days at an effective concentration of 200 µ g mL-1, all tested plants were healthy and did not produce phytotoxicity. Moreover, the designed bactericides also did not produce remarkable effects on chlorophyll contents and conductivity. And beyond that, DaPA8@β-CD is harmfulless to the representative non-target organisms—earthworms (Fig. S13). These findings reveals that the fabricated bactericides are safe to target plants and non-target organisms.
DaPA8@β-CD exhibits good wettability and deposition on hydrophobic rice foliage
Given the good antibacterial efficacy of DaPA8@β-CD against rice bacterial blight, the liquid-solid interface interactions referring to the bouncing, wetting and deposition properties between DaPA8@β-CD droplets and rice leaves were assessed by high-speed cameras (Movie S2). In Fig. 6A, driven by inertia, the impacting droplet first spread to a maximum area after contact with the rice leaf surface. Then, it sharply retracted and partially rebounded, eventually settling into a hemispherical shape. During the retraction process, water and β-CD droplets bounced to a hovering point before falling down to their final state, while the DaPA8@β-CD droplet remained adherent to the leaves throughout the changes. The rebound height of DaPA8@β-CD droplets was less than those of others. This process allowed the DaPA8@β-CD droplet to be pinned, thereby reducing the receding velocity through the wettability transition at the peripheral area of maximum spreading. Consequently, the high-speed impacting DaPA8@β-CD droplets could quickly deposit on rice leaf surfaces. To describe the impact processes of different droplets on the hydrophobic interface in detail, the normalized spreading diameter (Dt/D0) and rebound height (Ht/D0) were displayed in Fig. 6B and C. The normalized spreading diameter (Dt/D0) of DaPA8@β-CD droplets was almost 1.6 times than that of DaPA8 droplets, yet lower than water and β-CD droplets. Moreover, the normalized maximum height (Ht/D0) of water droplets was almost 1.4 times to that of DaPA8 droplets, while it was almost 2.4 times than that of DaPA8@β-CD droplets. In general, the distinct change in droplet spreading diameters and rebound heights further indicates that the enhancive liquid-solid interface interactions between biocompatible supramolecular nanovesicles and blade surfaces, thus reducing splashing and bouncing of droplets. This outcome was consistent with the following dynamic contact angle test. As seen in Fig. 6D, the contact angle of DaPA8@β-CD (θ = 96°) on rice leaves was smaller than that of DaPA8 (θ = 108°). Rice leaves were also observed by SEM after active ingredient deposition (Fig. 6E). Clearly, DaPA8@β-CD formed a dense covering layer by filling the pits entirely on the surface of leaf blade, superior to that of DaPA8. This indicates that the host-guest strategy was effective in enhancing liquid deposition behavior and inhibiting the bouncing and splashing of liquid droplets on plant leaf surfaces.
Fig. 6.
(A) Impacting process of water, DaPA8, β-CD, and DaPA8@β-CD droplets (200 µ g mL-1) on rice leaf surface at a height of 10 cm. (B) Spreading diameter of droplets normalized by the initial diameter (Dt/D0). (C) Droplets rebounded height normalized by the initial diameter (Ht/D0). (D) Dynamic contact angles of water, β-CD, DaPA8, and DaPA8@β-CD droplets (200 µ g mL-1) on rice leaf surface. (E) SEM images of DaPA8@β-CD and DaPA8 after depositing on rice leaf surface
Conclusions
Targeted bactericide for biofilm eradication is seldomly found in agrochemicals and most agrochemical molecules have poor water-solubility, dispersibility and biocompatibility in water environments. In this study, a supramolecular bactericide DaPA8@β-CD was constructed through a simple and inexpensive method, along with various structural characterizations and antibacterial potential studies. The supramolecular bactericide effectively inhibited the growth of plant bacteria at low concentrations and could inhibit and eradicate bacterial biofilms. In planta experiments indicated that DaPA8@β-CD had better control efficiency than commercial bactericides. The superior bioactivity of DaPA8@β-CD helped alleviate situations where bacteria continued to increase resistance under the application of traditional bactericides. Furthermore, DaPA8@β-CD significantly enhanced the foliar deposition performance while reducing the bouncing ability during droplet spraying. This event not only alleviates the loss of pesticides during application, but also minimizes environmental pollution caused by agrochemical loss.
In conclusion, the supramolecular bactericide fabricated through the host-guest strategy is a green bactericide with good biocompatibility, offering prospects for reducing bacterial resistance and improving active ingredient bioavailability. Additionally, the preparation of DaPA8@β-CD involves fewer steps, resulting in lower costs, and high potential for field application. The construction of novel green bactericides through supramolecular strategies opens avenues for the development of green agriculture. If other types of bactericides or pesticides can form relatively stable supramolecular compositions driven by non-covalent interactions, we think this strategy is applicable in sustainable agriculture practices.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge funds from the National Key Research and Development Program (2022YFD1700300), Innovation Program for High-level Talents of Guizhou Province (No. GCC[2023]008), Guizhou Provincial S&T Project (ZK[2022]017), Research and Innovation Team of Guizhou University (Guidakechuangtuan[2023]03), Natural Science Special Project of Guizhou University (Guidazhuanjihe[2024]02), Central Government Guides Local Science and Technology Development Fund Projects (Qiankehezhongyindi (2023) 001), and the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023).
Author contributions
Hui-Ling Zhang: Data curation, Formal analysis. Hong-Wei Wang: Data curation. Jing-Han Yang: Writing – review & editing. Jia-Jia Chen: Data curation, Formal analysis. Juan Liu: Writing – review & editing. Qing-Chuan Shi and Hai-Cong Zhao: Data curation, Investigation. Mo-Xian Chen: Writing – review & editing. Run Yang and Qing-Tian Ji: Data curation. Pei-Yi Wang: Conceptualization, Methodology, Writing – review & editing, Supervision, Validation.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui-Ling Zhang and Hong-Wei Wang contributed equally to this work.
Contributor Information
Mo-Xian Chen, Email: cmx2009920734@gmail.com.
Pei-Yi Wang, Email: pywang@gzu.edu.cn, Email: pywang888@126.com.
References
- 1.Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3:430–9. [DOI] [PubMed] [Google Scholar]
- 2.Zeng L, Xu JF, Zhang X. Degradable bactericide constructed using a charge-reversal surfactant against Plant pathogenic Bacteria. ACS Appl Mater Interfaces. 2022;14:10134–41. [DOI] [PubMed] [Google Scholar]
- 3.Daniel AI, Keyster M, Klein A. Biogenic zinc oxide nanoparticles: a viable agricultural tool to control plant pathogenic fungi and its potential effects on soil and plants. Sci Total Environ. 2023;897:165483–92. [DOI] [PubMed] [Google Scholar]
- 4.Zhenyu Z, Meiyun L, Peihua S, Alanine-Dependent TCA. Cycle Promotion restores the zhongshengmycin-susceptibility in Xanthomonas oryzae. Int J Mol Sci. 2023;24:3004–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayinga R, Makumi A, Tumuhaise V, Tinzaara W. Xanthomonas bacteriophages: a review of their biologBiocontrolontrol applications in agriculture. BMC Microbiol. 2021;21:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wang N. Foliar Application of Biofilm formation–inhibiting compounds enhances control of Citrus Canker caused by Xanthomonas citri subsp. Citri. Phytopathology. 2014;104:134–42. [DOI] [PubMed] [Google Scholar]
- 7.Suárez-Acevedo S, Chaves-Bedoya G, Guariz-Pinheiro D, Cristina-Lopes A, Mari-Murata M, Hirochi-Herai R, et al. Comparative transcriptional analyzes of Xanthomonas citri subsp. citri reveal mechanisms of adaptation and bacterial virulence in the early stage of citrus canker disease. Eur J Plant Pathol. 2022;163:557–72. [Google Scholar]
- 8.Chen J, Luo Y, Wei C, Wu S, Wu R, Wang S, et al. Novel sulfone derivatives containing a 1,3,4-oxadiazole moiety: design and synthesis based on the 3D‐QSAR model as potential antibacterial agent. Pest Manag Sci. 2020;76:3188–98. [DOI] [PubMed] [Google Scholar]
- 9.Vishakha K, Das S, Ganguli A. Photodynamic antibacterial and antibiofilm activity of riboflavin against Xanthomonas oryzae Pv. Oryzae: an ecofriendly strategy to combat bacterial leaf blight (BLB) rice disease. Arch Microbiol. 2022;204:566. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Zhou H, Wang YR, Zhang BQ, Zhang ZJ, Peng D, et al. Short pathways to highly active antimicrobial: structurally diverse polyamines derivativesfrom amino-aldehyde condensation strategy. Pest Manag Sci. 2023;79:5321–32. [DOI] [PubMed] [Google Scholar]
- 11.Gurevich D, Dor S, Erov M, Dan Y, Moy JC, Mairesse O, et al. Directed enzyme evolution and encapsulation in peptide nanospheres of quorum quenching lactonase as an antibacterial treatment against plant pathogen. ACS Appl Mater Interfaces. 2021;13:2179–88. [DOI] [PubMed] [Google Scholar]
- 12.Joseph R, Naugolny A, Feldman M, Herzog IM, Fridman M, Cohen Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J Am Chem Soc. 2016;138:754–7. [DOI] [PubMed] [Google Scholar]
- 13.Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, et al. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieltjens L, Appermans K, Lissens M, Lories B, Kim W, Van der Eycken EV, et al. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat Commun. 2020;11:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vishwakarma A, Dang F, Ferrell A, Barton HA, Joy A. Peptidomimetic polyurethanes inhibit bacterial biofilm formation and disrupt surface established biofilms. J Am Chem Soc. 2021;143:9440–9. [DOI] [PubMed] [Google Scholar]
- 16.He X, Yang Y, Guo Y, Lu S, Du Y, Li JJ, et al. Phage-guided targeting, discriminative imaging, and synergistic killing of bacteria by AIE bioconjugates. J Am Chem Soc. 2020;142:3959–69. [DOI] [PubMed] [Google Scholar]
- 17.Wen Q, Huang J, Tang H, He F, Yuan J, Wan S, et al. Fabricating network-link acetamiprid-loading micelles based on dopamine-functionalized alginate and alkyl polyglucoside to enhance folia deposition and retention. J Agric Food Chem. 2022;70:3596–607. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Xiong Q, Li J, Gan C, Zhang Y, Mo Q, et al. Enhancing systemic translocation of insecticides via nanoformulations incorporating β-cyclodextrin octadecarboxylate as a carrier. J Agric Food Chem. 2024;72:3374–87. [DOI] [PubMed] [Google Scholar]
- 19.Zhao K, Hu J, Ma Y, Wu T, Gao Y, Du F. Topology-regulated pesticide retention on plant leaves through concave Janus carriers. ACS Sustainable Chem Eng. 2019;7:13148–56. [Google Scholar]
- 20.Song M, Ju J, Luo S, Han Y, Dong Z, Wang YW, et al. Controlling liquid splash on superhydrophobic surfaces by a vesicle surfactant. Sci Adv. 2017;3:e1602188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang S, Li G, Qu H, Xu W, Song Q, et al. Dynamic spreading of insecticidal pesticide droplets on superhydrophobic plant leaves through host-guest chemistry. ACS Agric Sci Technol. 2023;3:158–64. [Google Scholar]
- 22.Schmidt BVKJ, Barner-Kowollik C. Dynamic macromolecular material design—the versatility of cyclodextrin-based host-guest chemistry. Angew Chem Int Ed Engl. 2017;56:8350–69. [DOI] [PubMed] [Google Scholar]
- 23.Yu Q, Deng T, Lin FC, Zhang B, Zink JI. Supramolecular assemblies of heterogeneous mesoporous silica nanoparticles to co-deliver antimicrobial peptides and antibiotics for synergistic eradication of pathogenic biofilms. ACS Nano. 2020;14:5926–37. [DOI] [PubMed] [Google Scholar]
- 24.McGuire K, He S, Gracie J, Bryson C, Zheng D, Clark AW, et al. Supramolecular click chemistry for surface modification of quantum dots mediated by cucurbit[7]uril. ACS Nano. 2023;17:21585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DX, Wang MX. Exploring anion-π interactions and their applications in supramolecular chemistry. Acc Chem Res. 2020;53:1364–80. [DOI] [PubMed] [Google Scholar]
- 26.Borsdorf L, Herkert L, Bäumer N, Rubert L, Soberats B, Korevaar PA, et al. Pathway-controlled aqueous supramolecular polymerization via solvent-dependent chain conformation effects. J Am Chem Soc. 2023;145:8882–95. [DOI] [PubMed] [Google Scholar]
- 27.Shi H, Lu X, Liu Y, Song J, Deng K, Zeng Q, et al. Nanotribological study of supramolecular template networks induced by hydrogen bonds and Van Der Waals forces. ACS Nano. 2018;12:8781–90. [DOI] [PubMed] [Google Scholar]
- 28.Xi Y, Wang Y, Gao J, Xiao Y, Du J. Dual corona vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis. ACS Nano. 2019;13:13645–57. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Cui Y, Wei X, Zang Y, Chen X, Cheng L, et al. CuCo2O4 nanoflowers with multiple enzyme activities for treating bacterium-infected wounds via cuproptosis-like death. ACS Nano. 2024;18:15845–63. [DOI] [PubMed] [Google Scholar]
- 30.Xiao F, Cao B, Wang C, Guo X, Li M, Xing D, et al. Retraction of Pathogen-specific polymeric antimicrobials with significant membrane disruption and enhanced photodynamic damage to inhibit highly opportunistic bacteria. ACS Nano. 2020;14:6357–6357. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, van der Mei HC, et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10:4779–89. [DOI] [PubMed] [Google Scholar]
- 32.Ping Y, Ding D, Ramos RANS, Mohanram H, Deepankumar K, Gao J, et al. Supramolecular β-sheets stabilized protein nanocarriers for drug delivery and gene transfection. ACS Nano. 2017;11:4528–41. [DOI] [PubMed] [Google Scholar]
- 33.Tang G, Tian Y, Gao Y, Zhou Z, Chen X, Li Y, et al. Supramolecular self-assembly of herbicides with reduced risks to the environment. ACS Nano. 2022;16:4892–904. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Gui S, Gui R, Li J, Huang R, Hu M, et al. Multifunctional clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9-based nanobomb against carbapenem-resistant Acinetobacter baumannii infection through cascade reaction and amplification synergistic effect. ACS Nano. 2023;17:24632–53. [DOI] [PubMed] [Google Scholar]
- 35.Ma R, Zheng YD, Tian HW, Chen MM, Yue YX, Bian Q, et al. A general supramolecular adjuvant for pesticides based on host-guest recognition. Pest Manag Sci. 2023;79:3133–40. [DOI] [PubMed] [Google Scholar]
- 36.Sayed M, Pal H. An overview from simple host-guest systems to progressively complex supramolecular assemblies. Phys Chem Chem Phys. 2021;23:26085–107. [DOI] [PubMed] [Google Scholar]
- 37.Ravi A, Pathigoolla A, Balan H, Gupta R, Raj G, Varghese R, et al. Adamantoid scaffolds for multiple cargo loading and cellular delivery as β-cyclodextrin inclusion complexes. Angew Chem Int Ed Engl. 2023;62:e202307324. [DOI] [PubMed] [Google Scholar]
- 38.Xiong RY, Ruan YR, Zhou N, Wang XQ, Li L, Wang W. A pH-responsive supramolecular antibacterial agent based on host-guest chemistry. New J Chem. 2023;47:18295–301. [Google Scholar]
- 39.Xia L, Tian J, Yue T, Cao H, Chu J, Cai H, et al. Pillar[5]arene-based acid-triggered supramolecular porphyrin photosensitizer for combating bacterial infections and biofilm dispersion. Adv Healthc Mater. 2021;11:e2102015–2102015. [DOI] [PubMed] [Google Scholar]
- 40.Jiang JX, Li QY, Zhu DY, Xiao W, Chen ZP, Lan MH, et al. Host-guest assembly-crosslinked nanoemulsions with dual-antimicrobial mechanism for treating multidrug-resistant bacterial biofilms. ACS Appl Mater Interfaces. 2023;15:27046–55. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Xu W, Zhang S, Li J, Zhou J, Tian D, et al. Supramolecular biopharmaceutical carriers based on host-guest interactions. J Agric Food Chem. 2022;70:12746–59. [DOI] [PubMed] [Google Scholar]
- 42.Yang YY, Chen LS, Sun M, Wang CY, Fan Z, Du JZ. Biodegradable polypeptide-based vesicles with intrinsic blue fluorescence for antibacterial visualization. Chin J Polym Sci. 2021;39:1412–20. [Google Scholar]
- 43.Jiang B, Liu Y, Zhao L, Zhao L, Wang C, Liu C, et al. Construction of a pH-sensitive self-assembly in aqueous solutions based on a dansyl-modified β-cyclodextrin. Soft Matter. 2021;17:7516–23. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Pham DT, Kee TW, Clafton SN, Guo X, Clements P, et al. Aggregation and host-guest interactions in dansyl-substituted poly(acrylate)s in the presence of β-cyclodextrin and a β-cyclodextrin dimer in aqueous solution: a UV-Vis, fluorescence, 1H NMR, and rheological study. Macromolecules. 2011;44:9782–91. [Google Scholar]
- 45.Iwan D, Kamińska K, Denel-Bobrowska M, Olejniczak AB, Wojaczyńska E. Chiral sulfonamides with various N-heterocyclic and aromatic units—synthesis and antiviral activity evaluation. Biomed Pharmacother. 2022;153:113473–113473. [DOI] [PubMed] [Google Scholar]
- 46.Richard S, Dickson RB, Mark CW, Pastan IH. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of α2-macroglobulin. Proc Natl Acad Sci U S A. 1982;79:2291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skrzypczak N, Przybylski P. Modifications, biological origin and antibacterial activity of naphthalenoid ansamycins. Nat Prod Rep. 2022;39:1653–77. [DOI] [PubMed] [Google Scholar]
- 48.Melander RJ, Basak AK, Melander C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat Prod Rep. 2020;37:1454–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurnaz LB, Barman S, Yang X, Fisher C, Outten FW, Nagarkatti P, et al. Facial amphiphilic naphthoic acid-derived antimicrobial polymers against multi-drug resistant gram-negative bacteria and biofilms. Biomaterials. 2023;301:122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan W, Bai R, Wang S, Tian X, Li Y, Wang S, et al. Antibiotic resistance genes are increased by combined exposure to sulfamethoxazole and naproxen but relieved by low-salinity. Environ Int. 2020;139:105742. [DOI] [PubMed] [Google Scholar]
- 51.Machelart A, Salzano G, Li X, Demars AS, Debrie AS, Menendez-Miranda M, et al. Intrinsic antibacterial activity of nanoparticles made of β-cyclodextrins potentiates their effect as drug nanocarriers against tuberculosis. ACS Nano. 2019;13:3992–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu HW, Ji QT, Ren GG, Wang F, Su F, Wang PY, et al. Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J Agric Food Chem. 2020;68:12558–68. [DOI] [PubMed] [Google Scholar]
- 53.Dai H, Yang J, Fan L, Luo M, Wang P. Excellent foliar deposition of cyclodextrin-encapsulated furyl/thienyl-engineered ingredients: novel effective supramolecular agrochemicals for combating plant bacterial and fungal infections. Adv Funct Mater. 2024;34:2403823. [Google Scholar]
- 54.Tabassum N, Khan F, Jeong GJ, Oh D, Kim YM. Antibiofilm and antivirulence activities of laminarin-gold nanoparticles in standard and host-mimicking media. Appl Microbiol Biot. 2024;108:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mocanu S, Matei I, Leonties A, Tecuceanu V, Hanganu A, Minea Z, et al. New flexible molecular probes bearing dansyl and TEMPO moieties for host-guest interactions in solution and gels. New J Chem. 2019;43:11233–40. [Google Scholar]
- 56.Ji QT, Mu XF, Hu DK, Fan LJ, Xiang SZ, Ye HJ, Gao XH, Wang PY. Fabrication of host-guest complexes between adamantane-functionalized 1,3,4-oxadiazoles and β-cyclodextrin with improved control efficiency against intractable plant bacterial diseases. ACS Appl Mater Interfaces. 2022;14:2564–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.











