Abstract
Background
Limited research has focused on the correlation between an external compression and the regeneration of ruptured Achilles tendons. The aim of this study was to evaluate the influence of a constricted paratenon with external compression on the regeneration process of separated rabbit common calcanean tendon stumps.
Methods
A transection, establishing a 4 mm gap, was created in the right common calcanean tendon of 24 young adult male New Zealand white rabbits. The animals were assigned to two groups: In the control group, only received cast immobilization. In the constricted paratenon (CP) group, the rabbits had a local 3-dimensional printed clasp applied to mimic external compression and same cast immobilization as the control group. Morphologic, histologic and immunohistochemistry examinations were performed at 2 and 4 weeks postoperative.
Results
Separated tendon stumps were connected by novel granulated tendon fibrils in the control group. However, the regenerated tendon fibrils appeared insufficient in the CP group, the tendon length and the adhesion grade of the CP group was significantly larger than that of the control group at 4 weeks (P < 0.05, P = 0.030). Disorganized collagen and round-shaped fibroblasts were demonstrated in the CP group. A prolonged expression of proliferating cell nuclear antigen (PCNA) and lower intensity in clusters of differentiation 146 (CD146) were also shown in the CP group. A prolonged existence of the vascular endothelial growth factor (VEGF) and lesser intensity of the transforming growth factor-beta 1 (TGF-β1) were confirmed within this group. Furthermore, the CP group’s expression had less collagen I than that of the control group at 4 weeks.
Conclusions
Sufficient regeneration can be obtained, even though there is an obvious gap between severed rabbit common calcanean tendon stumps. However, constricted paratenons with external compression can negatively influence the intrinsic regeneration process of the tendon fibrils and promotes the disorganization of regenerated collagen.
Keywords: Achilles tendon, Common calcanean tendon, Paratenon, Tendon regeneration, Microenvironment, Stem/progenitor cell recruitment, Animal study
Introduction
Although there are still discussions regarding surgical treatments for acute Achilles tendon ruptures, the relatively higher risks of re-rupture and elongation associated with conservative treatments remain a concern [1–5]. The mature tenocytes have low proliferation, metabolism and limited ability of regeneration, easily leading to scar tissue formation after tendon injuries [6]. The biomechanical strength of scar tissue is not adequate to meet the needs of strenuous exercise, it is difficult to regain the elasticity and tension, accompanied by weakness, persistent pain and a higher risk of re-rupture [3, 7].
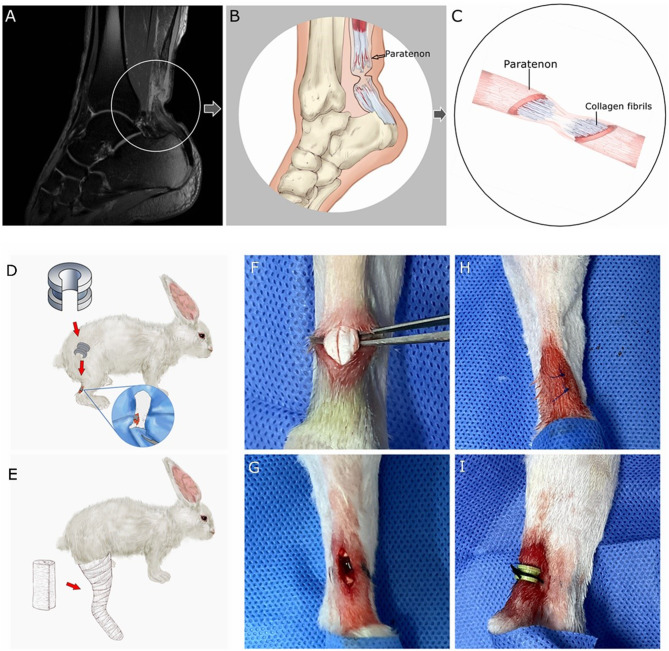
Tendon-derived stem/progenitor cells (TSPCs) were found in a specific niche within collagen fibrils, surrounded by the extracellular matrix, that allows for tenocyte differentiation [8]. The regenerative abilities of stem cells could be affected by the microenvironment, stem cells can also regulate the microenvironment [9]. The paratenon, surrounding the Achilles tendon, is an important structure to maintain the microenvironment for tendon regeneration [10–12]. Moreover, the paratenon is the source and reservoir of TSPCs and growth factors [13, 14]. In the damaged paratenon, progenitor cells may not be able to be recruited due to the low level of growth factors [1]. On some patients’ magnetic resonance imaging (MRI), with plantar flexion cast immobilization, the folded local soft tissue at the rupture site was shown (Fig. 1A, B). The regeneration process could be influenced by the local folded soft tissue. However, there are limited studies focusing on the correlation between the regeneration process of the ruptured tendon and constricted paratenon with external compression.
Fig. 1.
(A) The MRI of one patient with cast shows the local folded soft tissue (patient give consent to use this image). (B) The local paratenon could be constricted by the folded soft tissue. (C) The regeneration process of the collagen fibrils might be negatively influenced by the constricted paratenon. (D, E) The 3D printed clasp was applied in the CP group, while cast immobilization was utilized for both groups. (F, G) The rabbit common calcanean tendon was severed leaving a 4 mm gap. (H) The paratenon and incision were closed. (I) A 3D printed clasp was applied in the CP group; no skin necrosis was documented
The present study was designed to evaluate the impact of the constricted paratenon with external compression on growth factors and specific markers of the TSPCs recruitment during tendon stump regeneration in vivo. It is hypothesized that a constricted paratenon with external compression can negatively influence the regeneration process of the ruptured Achilles tendon fibrils (Fig. 1C). To prove this hypothesis, the constricted paratenon animal model was established by applying a customized 3-dimensional (3D) printed clasp, which could create external compression on the local soft tissue.
Materials and methods
This research has been approved by the Institutional Animal Care and Use Committee of General Hospital of Central Theater Command (No.2021030). The protocol followed guidelines of the Institutional Animal Care and Use Committee.
The design of the customized 3D printed clasp
A customized 3-dimensional (3D) printed clasp was made from Acrylonitrile Butadiene Styrene plastic (ABS) by a 3D printer (Up300, TierTime, China), and was utilized for the constricted paratenon (CP) group. The average diameter of the rabbit common calcanean tendons was measured with a caliper to be 4.0 ± 0.3 mm in the pre-experiment (five samples were taken from euthanized young adult male New Zealand white rabbits, aged 12–14 weeks, ranging in weight from 2.7 to 3.0 kg). The main parameters of the customized clasps are as follows: the inner diameter and width is 4 mm (considering the thickness of the rabbit skin, the constricted subspace of the paratenon was designed with a 1 mm adjustment), respectively; a 3 mm notch was designed for fixing it to the rabbit common calcanean tendon; a groove was designed to hold the clasp with sutures (Fig. 1D). This was an innovative design, as there was no data to reference from.
Animal model and surgical procedures
Twenty-four young adult male New Zealand white rabbits (age, 12–14 weeks), ranging in weight from 2.7 to 3.0 kg, were randomly assigned to two groups, each consisting of 12 rabbits. Each animal was housed in a standard condition with individual cages. Before surgery, the rabbits received preoperative antibiotics (cefazolin sodium). The rabbits were anaesthetized by an intramuscular injection of a mixture of a ZOLETIL 50 solution (Zoletil™, France; 0.1 ml/per 1 kg of animal body weight). Only the right common calcanean tendon underwent surgery for each rabbit. The surgical area was prepared using an antiseptic (iodine) and draped in sterile fashion. A caudal longitudinal incision (approx. 10 mm) was established, through the paratenon, until the common calcanean tendon was exposed. The tendon bundle was completely tenotomized at 2 cm proximal to the calcaneal insertion while trying to avoid paratenon damage (Fig. 1F). The gap between tendon stumps was designed to be 4 mm referenced from the Müller and Wellings’ studies [6, 12]. In rabbits, flexor digitorum superficialis follows the calcanean tendon (equivalent to the Achilles tendon in human being), forming a complex structure called the common calcanean tendon. To prevent interference of the flexor digitorum superficialis, it was also tenotomized with the same gap. The tendon stumps were fixed to local subcutaneous tissue with 5 − 0 polydioxanone sutures (Ethicon, Johnson & Johnson, Inc., USA) on both stumps, keeping the 4 mm gap (Fig. 1G). The paratenon and incision were carefully closed with 5 − 0 polydioxanone sutures (Fig. 1H). These limbs were immobilized from the toes to the thigh with casts at 150° ankle plantar flexion, which will keep a 4 mm gap between the tendon stumps. In the control group, these limbs were only immobilized with casts in the previously mentioned position (Fig. 1E). In the CP group, additional 3D printed clasps were applied to mimic the constricted paratenon before immobilizing them with casts (Fig. 1I). To avoid skin complications, 2 weeks postoperative, the clasps and casts were removed in all groups.
Morphologic observations
The rabbits were gradually sacrificed at 2 and 4 weeks postoperative (6 per group each time), the common calcanean tendon samples of both limbs were collected. The left intact tendons were used as the “native tendon” sample. The length from the calcaneal tuber to the musculotendinous junction was measured. The extent of peritendinous adhesion was graded using a validated adhesion grading scale as described by Tang et al. [15]: no adhesion (0), slight adhesion (2), moderate adhesion (3,4), and severe adhesion (5,6).
Histologic assessments
Each whole tendon was then fixed with two small pins on a slab to decrease shrinkage before being submerged in 4% paraformaldehyde. Then, the fixed tendons were embedded into paraffin wax. We obtained 24 paraffin blocks corresponding to 24 animals. The paraffin blocks were sliced into 5 μm thick longitudinal sections using a tissue microtome (RM2255, Leica, Germany). After deparaffinization, the specimens were stained with hematoxylin and eosin (H&E), Masson’s trichrome (MT) and Picrosirius red staining to assess collagen distribution and organization. Fifteen histologic slides were obtained per tendon sample. To provide the optimal qualitative and quantitative assessment of organization of collagen, all the specimen sections were kept in the same orientation at a 45° angle respective to the polarizers [16]. Another 6 native tendon samples were obtained randomly form the controlateral limbs. All specimens were analyzed under ×100 magnification or ×200 magnification.
Immunohistochemistry assessments
The paraffin blocks of the 24 tendon samples were also used for immunohistochemical analysis to evaluate the changes in the expressions of CD146, PCNA, VEGF and TGF-β1(all from Proteintech; 1:100 dilution). Anti-type I and type III collagen antibodies (Servicebio; 1:800 dilution) were used as evaluation indicators for tendon regeneration. Antigen retrieval was performed by heating the samples with a citrate buffer. Nonspecific reactive sites were masked with 3% bovine serum albumin (DAKO, antibody manufacturer based in Denmark) before the slides were incubated overnight at 4 °C with primary antibodies. A horseradish Peroxidase (HRP) conjugated goat antibody (SeraCare; 1:100 dilution or 1:200) was used as the secondary antibody. Slides were then incubated with a species-appropriate secondary antibody in the dark at room temperature for 50 min. Representative images are taken from five random sections per tendon sample. The percentages of CD146 + cells and PCNA cells were calculated using ImageJ software (National Institutes of Health, America). Immunohistochemical staining for VEGF and TGF-β1 were observed under a light microscope. Average optical density (AOD) was performed to evaluate the density of immune reactivity against VEGF and TGF-β1 using ImageJ. The collagen types I and III contents were evaluated using the methods describe by Chailakhyan with two researchers blinded to this experiment, 0-to-4 rating scale represents from the absent marker expression to the intensive diffuse marker expression [17].
Statistical analysis
All analyses were assessed with SPSS 22 (IBM Corp, Chicago, IL, USA). The tendon length, CD146+, PCNA, VEGF, and TGF-β1 were analyzed using the Student’s T test; adhesion grade and collagen ratings were analyzed with the Mann-Whitney U test. The level of significance was set at a two-sided P value of less than 0.05. Means ± standard deviations are indicated with corresponding P values.
Results
Morphologic findings
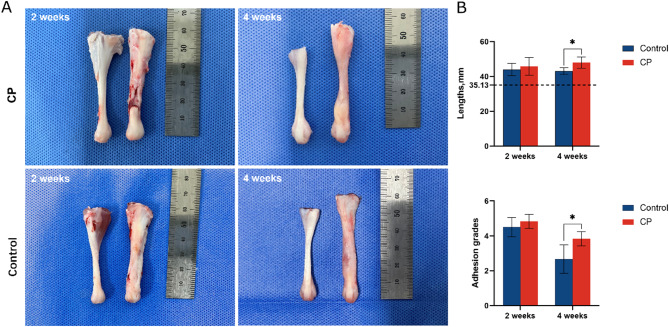
The gap of the severed tendon stumps was connected by novel granulated tendon fibrils in the control group at 2 weeks postoperative (Fig. 2). The local tissue is soft, milky white, with few pannus on the surface with slight adhesions. However, the gap did not completely integrate with appropriate granulated tendon fibrils in the CP group. Three samples with obvious defects in the CP group did not meet the adequate regeneration requirement, by completely filling the gap with fibrous connective tissue, the delayed regeneration rate was 50% (3/6 samples). A large amount of pannus on the surface and severe adhesions were also observed. After 4 weeks, the regenerated tendon of the control group became white, hard, and covered with rich blood vessels and membrane-like substances. The tenotomy site of the control group formed in a spindle shape. The granulated tissue of the CP group partially filled the gap, however, the tenotomy site transmuted to pink, soft, and semitransparent. The local site of the CP group formed in a dumbbell shape, the diameter of the regenerated site diminished noticeably. Two weeks postoperative, there is no significant difference in the average regenerated tendon length between the control group and CP group (44.0 ± 3.6 mm vs. 45.8 ± 5.1 mm, P = 0.486). However, the tendon length of the CP group was significantly longer than that of the control group (48.0 ± 3.2 mm vs. 43.2 ± 1.9 mm, P < 0.05) after 4 weeks. In both groups, the adhesion grades decreased from 2 weeks to 4 weeks postoperatively. In the CP group, the adhesion grades at 2 weeks (Z = 1.173, P = 0.241) and 4 weeks (Z = 2.345, P = 0.019) were more substantial than that of the control group.
Fig. 2.
(A) Native tendons (left) versus regenerated tendons (right). At 2 weeks, the tenotomy gap connected with insufficient granulated tendon fibrils in the CP group; the tenotomy gap was connected by appropriate granulated tendon fibrils in the control group. At 4 weeks, the tenotomy site of the CP group formed in a dumbbell shape. The tenotomy site of the control group formed in a spindle shape. (B) Length and adhesion grades. The dotted line indicates the mean length of native tendons. Data is represented as mean ± SD. *p < 0.05
Histologic findings
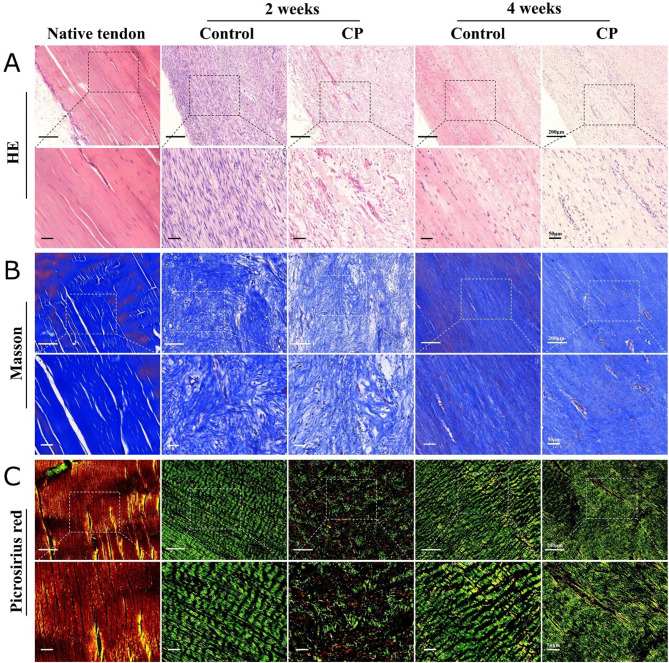
The H&E staining revealed the native tendon consisted of spindle-shaped tenocytes with flattened and mature nuclei and dense collagen fibrils. After surgery, fibrotic tissue filled the gap with various cells and disorganized collagen. In the CP group, the cells were found to be rounder, had lower density, and contain more unorganized collagen fibrils than in the control group (Fig. 3A). With Masson’s trichrome staining, the native tendons showed that collagen fibrils were parallel to each other with few nuclei and vessels. During the regeneration process, the arrangement of collagen fibrils gradually went from disorganized to organized in both groups. However, the granulated tissue in the CP group was found to contain fewer tenocytes and loose collagen fibrils (Fig. 3B). After the native tendons were stained with Picrosirius red and observed under polarized light microscopy, abundant type I collagen was displayed bright yellow, and were well defined. In the control group, the regenerated tissue appeared homogeneous with well-aligned type III collagen (green color) at 2 weeks postoperatively, then the collagen fibrils became denser at 4 weeks. In the CP group, the regenerated tissue looked unsystematic with scattered collagen at 2 weeks, for the type I collagen (yellow color blotches) appeared at 2 weeks. Consequently, the structure of the collagen fibrils showed thinner and irregularities at 4 weeks (Fig. 3C).
Fig. 3.
Hematoxylin and eosin (A), Masson’s trichrome (B), and Picrosirius red stain (C) with polarized light microscopy of native, control, and experimental Achilles tendons at 2 and 4 weeks postoperatively. All the specimen sections were kept in the same orientation at a 45° angle respective to the polarizers
Immunofluorescence findings
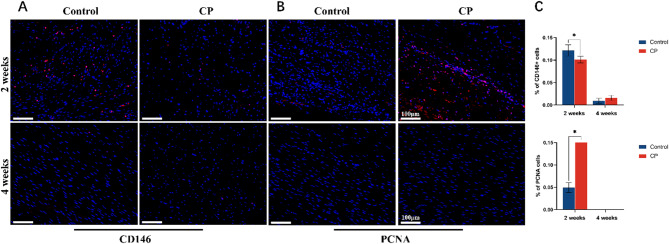
Regenerated tendon samples in the control group were recorded to express relatively obvious CD146 signals compared with the CP group at 2 weeks (0.12 ± 0.01 vs. 0.10 ± 0.01, P = 0.006). CD146 was rare in both groups at 4 weeks (0.01 ± 0.01 vs. 0.02 ± 0.01, P = 0.075). Cellular proliferation was traced by PCNA, a cell proliferative antigen. It showed prominent proliferation of the cells in the CP group than that of the control group, as PCNA signals were expressed more noticeably (0.05 ± 0.01 vs. 0.96 ± 0.02, P<0.01). It displayed that PCNA faded in both groups at 4 weeks. (Fig. 4)
Fig. 4.
(A, B)Immunofluorescence of CD146(red) and PCNA(red) was evaluated at 2 and 4 weeks postoperatively, magnification×200. Nuclei were visualized with DAPI staining(blue). (C) The percentages of CD146 + cells and PCNA cells were calculated with ImageJ. Representative images are taken from five random sections per rabbit tendon sample. (*p < 0.05)
Immunohistochemistry findings
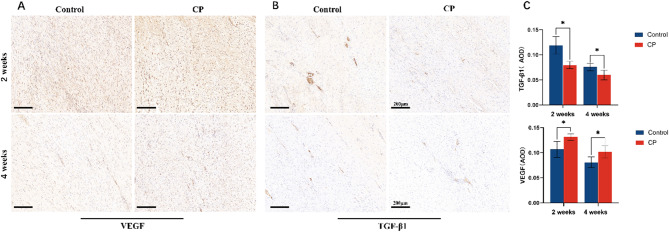
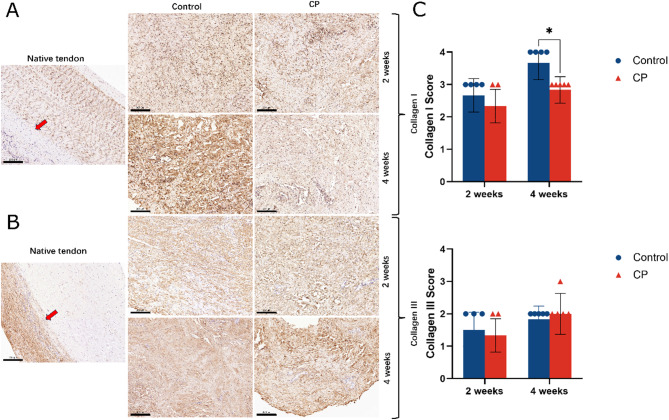
High levels of VEGF were detected in both groups at 2 weeks (0.11 ± 0.02 vs. 0.13 ± 0.01), then decreased at 4 weeks. However, compared with the control group, the VEGF expression existed at a higher level (0.08 ± 0.01 vs. 0.11 ± 0.01) and the stained neo blood vessels appeared richer in the CP group. From 2 weeks to 4 weeks, TGF-β1 signals decreases in both groups. Furthermore, it found that the regenerated tendon in the control group expressed TGF-β1 more intensely than that of the CP group at 2 weeks (0.12 ± 0.02 vs. 0.08 ± 0.01). (Fig. 5) The expression of type I collagen increased from 2 weeks to 4 weeks in both groups. At 4 weeks, the control group contained more type I collagen than the CP group (Z = 2.373, P = 0.018). Compared with the control group, the expression of type III collagen in the CP group was slightly lower at 2 weeks (Z = 0.561); but it is litter denser at 4 weeks (Z=-0.527). No statistical difference was encountered (P = 0.575, P = 0.598). (Fig. 6)
Fig. 5.
(A, B) Immunohistochemical analysis of VEGF and TGF-β1 was assessed at 2 and 4 weeks postoperatively, magnification×200. (C) Average optical density (AOD) measurements of the immunostaining against VEGF and TGF-β1 are presented. (*p < 0.05)
Fig. 6.
(A, B) Immunohistochemical analysis of the type I collagen and type III collagen was assessed at 2 and 4 weeks postoperatively, magnification×200. The native tendon was formed by type I collagen, moreover, the paratenon (red arrow) was formed by type III collage (left). After regeneration, type III collagen was noticeable in the sample. (C) Semi-quantitative analysis of the content of collagens types I and III (*p < 0.05). Type I collagen increased gradually over time
Discussion
The main finding of this study is that sufficient regeneration, by completely filling the gap with novel granulated tendon fibrils, was obtained, even though there is an obvious gap between severed common calcanean tendon stumps. However, a constricted paratenon with external compression has a negative influence on the regeneration process. According to previous studies, the poor regeneration of the ruptured Achilles tendon was observed in some cases with conservative treatments, resulting in a little higher risk of re-rupture and elongation of the tendon [1]. Furthermore, intrinsic regeneration of tendons shows better biomechanics, whereas extrinsic regeneration leads to the formation of scar tissue [18]. As mentioned previously, on MRI of some patients with plantar flexion cast, the local folded soft tissue was found. The paratenon could be compressed by the local folded soft tissue. It is difficult to accurately mimic this external compression in rabbit models using a cast alone. So, a 3D printed clasp was applied to simulate this compression, while a cast was used to immobilize the limbs and reduce interference. In the present study, even though the tenotomy site was not sutured, type I collagen was found in the novel regenerated tissue in both groups. Moreover, better regeneration of tendon fibrils was observed in the control group. Well-aligned type III collagen was also observed in this group. Contrarily, in the CP group, an insufficient regeneration of tendon fibrils, an imbalanced ratio of collagen I and III, and a lack of fibril alignment was present. Actually, biomechanical stimulation has an influence on tendon regeneration through changing extracellular matrix proteins, growth factors, and transcription factors [19]. Tendons are exposed to mechanical stress, as this is known to greatly interfere with the physiological regeneration process [20]. Our results show that the constricted paratenon with external compression resulted in poor quality of regenerated tendon fibrils, varying morphologic characteristics and high grade adhesions. Both groups exhibited histopathological differences in cellular morphology, collagen orientation, and vascular distribution, as the previous studies had mentioned [21]. In our immunohistochemistry, type I collagen is still visible at 4 weeks in the CP group, but far less than type III collagen. This could mean that type I collagen regenerated earlier, and then formed a disorganized structure in the constricted paratenon with external compression. Additionally, the collagen semiquantitative analysis from the immunohistochemistry revealed that the control group expressed more type I collagen than that of the CP group. The turnover of the tendon extracellular matrix is also affected by physical stimuli, both transcriptional and post-translational modifications, and the release of growth factors, which are enhanced after exercise [22].Furthermore, biological activity of tenocytes and collagen fibril arrays adapt to mechanical stimulation [23]. Our results also confirmed that a constricted paratenon with external compression has a negative effect on the differentiation of collagen.
The second finding is that the constricted paratenon with external compression can cause a longer duration of the inflammatory process. Persistent inflammation can disturb the microenvironment and have a negative impact on the tendon regeneration process. Our results show that the VEGF expression lasts longer and TGF-β1 was found to have lower levels in the CP group. During tissue regeneration, the new blood vessels which are induced by the vascular endothelial growth factor (VEGF) are essential for providing oxygen, nutrients, and ensuring metabolism [24]. However, some scholars have found that sustained high expression of VEGF may limit tendon regeneration at a later stage [25]. High levels of VEGF lead to blood vessel over-growth, which has been shown to promote scar tissue formation [26]. Especially for non-vascular tissues (such as tendons, ligaments, cartilage, etc.), limiting rather than promoting VEGF during the tissue regeneration process, may be beneficial [25]. On the other hand, TGF-β1 can direct cell differentiation, proliferation, and even tendon ECM production, playing an important role in tendon regeneration [27]. Although TGF-β1 ligands are often used to induce chondrogenesis in vitro, they are also effective inducers of tendon markers such as Scx [28]. Hou et al. implanted bone marrow mesenchymal stem cells transfected with the TGF-β1 gene into experimentally injured Achilles tendons, showing higher concentration of type I collagen, faster matrix remodeling and larger fiber bundles [29]. Heinemeier et al. showed that the mechanical load of a human tendon during exercise would increase TGF-β1 and stimulate the production of type I collagen in the tendon [30]. The TGF-β1 has been proven to direct mesenchymal progenitors in the direction of tenogenic differentiation [31]. If the content of TGF-β1 is reduced, the mesenchymal progenitors cell migration, differentiation and proliferation could be astricted, resulting in scar tissue formation (mostly type III collagen). In the present study, the regenerated tendon in the control group expressed TGF-β1 more intensely. Finally, the control group contained more type I collagen than the CP group (P = 0.018).
How the formation of external mechanical pressure induced local scar tissue is still unclear. One possible reason for scar tissue formation at the ruptured tendon site is that the recruitment of tendon stem/progenitor cells (TSPCs) was limited with an altered microenvironment in the constricted paratenon with external compression. Increasing TSPC recruitment and reducing inflammation could be a promising approach to speed up the intrinsic regeneration process [13, 14, 32]. Chailakhyan’s research showed that the application of bone marrow-derived mesenchymal stem cells (BM-MSCs) in a tissue-engineered tendon construct leads to the restitution of the tendon tissue [17]. Endogenous tendon CD146+ cells migrate to the injury site, which plays a critical role in tendon regeneration. Shi declared that an increased expression of CD146 related to tendon matrix formation and tendon differentiation, as well as a decrease in apoptotic cells and pro-inflammatory macrophages [33]. In addition, proliferating cell nuclear antigen (PCNA), as an indicator of cell proliferation involved in DNA replication, is utilized to assess the proliferation capacity of fibroblasts [34]. Leong observed that the proliferation of fibroblasts peaked and subsequently decreased, as evidenced by the location of PCNA [35]. In this study, stem cells tagged CD146 were denser in the control group, however, the cell proliferation landmark PCNA was more noticeable in the CP group at 2 weeks. Both types of the fluorescence dye started to dissipate by 4 weeks. Our results indicated that the intrinsic regeneration was dominated by TSPCs in the control group, however, the scar tissue repair process was dominated by proliferating fibroblasts in the CP group. Based on these results, the concept of “Paratenon Guided Regeneration (PGR)” was proposed: Maintaining the integrity and sufficient sub-space of the paratenon could be a key factor for the intrinsic regeneration of the ruptured Achilles tendon fibrils. In clinical practice, external compression should be avoided during immobilization after acute Achilles tendon rupture. The paratenon should be protected carefully during Achilles tendon repair surgery.
There are some limitations in this study. First, we have only conducted limited testing in terms of gene expression. Second, no mechanical testing was performed in the present study, which results in a partial conclusion. A mechanical test will be carried out in sequential research. Thirdly, the 3D-printed clasp also exerts external compression on the entire soft tissue area, encompassing the skin, subcutaneous tissue, and paratenon. Accurately simulating the actual conditions of the constricted paratenon under external compression remains a challenge. The final limitation of this study is that an animal model was used, and further control studies are required to mimic the constricted paratenon of the repair process of the ruptured Achilles tendon in human beings.
Conclusion
Sufficient regeneration can be obtained, even though there is an obvious gap between severed rabbit common calcanean tendon stumps with an intact paratenon. However, a constricted paratenon with external compression could negatively influence the intrinsic regeneration process and promote disorgnaztion of regenerated collagen due to a longer duration of the inflammatory process and the limitation of tendon-resident stem/progenitor cell recruitment.
Acknowledgements
We would like to express gratitude to John Valerius, an English native speaker, for taking the time to edit the English language.
Abbreviations
- CP
Constricted paratenon
- 3D
3-dimensional
- PCNA
Proliferating cell nuclear antigen
- CD146
Clusters of differentiation 146
- VEGF
Vascular endothelial growth factor
- TGF-β1
Transforming growth factor-beta 1
- TSPCs
Tendon-derived stem/progenitor cells
- MRI
Magnetic resonance imaging
- ABS
Acrylonitrile Butadiene Styrene
- H&E
Hematoxylin and eosin
- MT
Masson’s trichrome
- HRP
Horseradish Peroxidase
Author contributions
Wu Helin, Dai Xiaojing and Li Xiaoyu prepared the manuscript preparation, and Wei Shijun and Huang Lixia designed this study. All authors reviewed the manuscript.
Funding
This study received funding from Advantageous Disciplinary Construction Project for Universities in Hubei Province. (No.202105).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Helin Wu, Xiaojing Dai and Lixia Huang contributed equally to this work and should be considered co-first authors.
References
- 1.Maffulli N, Peretti GM. Treatment decisions for acute Achilles tendon ruptures. Lancet. 2020;395:397–8. [DOI] [PubMed] [Google Scholar]
- 2.Godoy-Santos AL, Bruschini H, Cury J, Srougi M, De Cesar NC, Fonseca LF, Maffulli N. Fluoroquinolones and the risk of Achilles Tendon disorders: Update on a neglected complication. Urology 2017:S0090429517311007. [DOI] [PubMed]
- 3.Gajhede-Knudsen M, Ekstrand J, Magnusson H, Maffulli N. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47:763–8. [DOI] [PubMed] [Google Scholar]
- 4.Maffulli N, Irwin AS, Kenward MG, Smith F, Porter RW. Achilles tendon rupture and sciatica: a possible correlation. BRIT J SPORT MED. 1998;1998(2):174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffulli N. Current concepts in the management of subcutaneous tears of the Achilles tendon. Bulletin. 1998;57:152–8. [PubMed] [Google Scholar]
- 6.Wellings EP, Huang TC, Li J, Peterson TE, Hooke AW, Rosenbaum A, Zhao CD, Behfar A, Moran SL, Houdek MT. Intrinsic Tendon Regeneration after application of purified Exosome product: an in vivo study. Orthop J Sports Med. 2021;9:23259671211062929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amadio PC, Leopold SS. Editor’s spotlight/Take 5: CORR® ORS Richard A. brand award for outstanding Orthopaedic Research: Engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res. 2014;472:2564–8. [DOI] [PMC free article] [PubMed]
- 8.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. [DOI] [PubMed] [Google Scholar]
- 9.Marr N, Zamboulis DE, Werling D, Felder AA, Dudhia J, Pitsillides AA, Thorpe CT. The tendon interfascicular basement membrane provides a vascular niche for CD146 + cell subpopulations. Front Cell Dev Biol. 2022;10:1094124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walia B, Huang AH. Tendon stem progenitor cells: understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res. 2019;37:1270-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller SA, Quirk NP, Müller-Lebschi JA, Heisterbach PE, Dürselen L, Majewski M, Evans CH. Response of the injured tendon to growth factors in the Presence or absence of the Paratenon. Am J Sports Med. 2019;47:462–7. [DOI] [PubMed] [Google Scholar]
- 12.Müller SA, Evans CH, Heisterbach PE, Majewski M. The role of the Paratenon in Achilles Tendon Healing: a study in rats. Am J Sports Med. 2018;46:1214–9. [DOI] [PubMed] [Google Scholar]
- 13.Mienaltowski MJ, Adams SM, Birk DE. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A. 2013;19:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mienaltowski MJ, Adams SM, Birk DE. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res Ther. 2014;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang JB, Ishii S, Usui M, Aoki M. Dorsal and circumferential sheath reconstructions for flexor sheath defect with concomitant bony injury. J Hand Surg Am. 1994;19:61–9. [DOI] [PubMed] [Google Scholar]
- 16.De López CM, Coenen MJ, Tovar A, De la Vega RE, Evans CH, Müller SA. Picrosirius Red Staining: revisiting its application to the qualitative and Quantitative Assessment of Collagen Type I and type III in Tendon. J Histochem Cytochem. 2021;69:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chailakhyan RK, Kon E, Shekhter AB, Ivannikov SV, Telpukhov VI, Grosheva AG, Suslin DS, Vorobieva NN, Gerasimov YV, Churbanov SN, Kotova S, Fayzullin AL, Lychagin AV, Lipina MM, Timashev PS. Autologous bone marrow-derived mesenchymal stem cells provide complete regeneration in a rabbit model of the Achilles tendon bundle rupture. Int Orthop. 2021;45:3263–76. [DOI] [PubMed] [Google Scholar]
- 18.Tang JB, Wu YF, Cao Y, Chen CH, Zhou YL, Avanessian B, Shimada M, Wang XT, Liu PY. Basic FGF or VEGF gene therapy corrects insufficiency in the intrinsic healing capacity of tendons. Sci Rep. 2016;6:20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elb Surg. 2012;21:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel PA, Kronenberg D, Neu G, Stolberg-Stolberg J, Frank A, Pap T, Langer M, Fehr M, Raschke MJ, Stange R. Microsurgical reconstruction affects the outcome in a translational mouse model for Achilles tendon healing. J Orthop Translat. 2020;24:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JH, Kang C, Han SY, Park WH, Kim MH, Moon JH, Tae JY, Park HY, Yoo IH, Park JH, Yeo YH, Kim DY. Comparative analysis of Achilles tendon healing outcomes after open tenotomy versus percutaneous tenotomy: An experimental study in rats. J Orthop Res. 2022;40:1446-56. [DOI] [PubMed]
- 22.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. [DOI] [PubMed] [Google Scholar]
- 23.Franchi M, Torricelli P, Giavaresi G, Fini M. Role of moderate exercising on Achilles tendon collagen crimping patterns and proteoglycans. Connect Tissue Res. 2013;54:267–74. [DOI] [PubMed] [Google Scholar]
- 24.Lee T. Stem cell therapy independent of stemness. World J Stem Cells. 2012;4:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zhu B, Li Y, Liu X, Guo S, Wang C, Li S, Wang D. The role of vascular endothelial growth factor in Tendon Healing. Front Physiol. 2021;12:766080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wietecha MS, DiPietro LA. Therapeutic approaches to the regulation of Wound Angiogenesis. Adv Wound Care (New Rochelle). 2013;2:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin MJ, Bonod-Bidaud C, Ruggiero F, Schweitzer R, Duprez D. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141:3683–96. [DOI] [PubMed] [Google Scholar]
- 29.Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, Fu X, Zhang J, Yu C. The roles of TGF-beta1 gene transfer on collagen formation during Achilles tendon healing. Biochem Biophys Res Commun. 2009;383:235–9. [DOI] [PubMed] [Google Scholar]
- 30.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol (1985). 2003;95:2390–7. [DOI] [PubMed] [Google Scholar]
- 31.Arimura H, Shukunami C, Tokunaga T, Karasugi T, Okamoto N, Taniwaki T, Sakamoto H, Mizuta H, Hiraki Y. TGF-β1 improves Biomechanical Strength by Extracellular Matrix Accumulation without increasing the number of Tenogenic Lineage Cells in a Rat Rotator Cuff Repair Model. Am J Sports Med. 2017;45:2394–404. [DOI] [PubMed] [Google Scholar]
- 32.Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS ONE. 2014;9:e96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Kang X, Wang Y, Bian X, He G, Zhou M, Tang K. Exosomes Derived from Bone Marrow Stromal cells (BMSCs) enhance Tendon-Bone Healing by regulating macrophage polarization. Med Sci Monit. 2020;26:e923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu SC, Cheuk YC, Chan KM, Hung LK, Wong MW. Is cultured tendon fibroblast a good model to study tendon healing? J Orthop Res. 2008;26:374-83. [DOI] [PubMed] [Google Scholar]
- 35.Leong NL, Wu J, Greskovich KE, Li Y, Jiang J. Pdgfrβ(+) lineage cells transiently increase at the site of Achilles tendon healing. J Orthop Res. 2023;41:1882-89. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.