Abstract
INTRODUCTION
It is unclear how midlife depression and anxiety affect dementia risk. We examined this in a Norwegian cohort followed for 30 years.
METHODS
Dementia status at age 70+ in the fourth wave of the Trøndelag Health Study (HUNT4, 2017–2019, N = 9745) was linked with anxiety and depression from HUNT1 (1984–1985), HUNT2 (1995–1997), HUNT3 (2006–2008), and HUNT4. Longitudinal anxiety and depression score, and prevalence trajectories during 1984–2019 by dementia status at HUNT4 were fitted using mixed effects regression adjusting for age, sex, education, and lifestyle and health factors.
RESULTS
Dementia at HUNT4 was associated with higher case prevalence at all waves, from 1.9 percentage points (pp) (95% CI: 0.1–3.7) higher at HUNT1 to 7.6 pp (95% CI: 5.7–9.6) higher at HUNT4.
DISCUSSION
Our findings show that depression and anxiety was more common more than 30 years before dementia onset in those who later developed dementia.
Highlights
Older individuals with dementia had a higher prevalence of mixed anxiety‐ and depressive symptoms (A + D), both concurrently with and more than three decades prior to their dementia diagnosis.
Older individuals with dementia had higher levels of anxiety, both concurrently and up to two decades prior to their dementia diagnosis.
Depressive symptoms increased by time among those who developed dementia, but not among others.
Results were similar for all cause dementia, Alzheimer's disease, and other types of dementia; however, for vascular dementia, the difference was not significant until dementia was present.
Keywords: Alzheimer's disease, anxiety, association between anxiety and dementia, association between depression and dementia, dementia, depression, depressive symptoms, mental distress, midlife anxiety, midlife anxiety and depression, midlife depression, risk factor for dementia, trajectories
1. BACKGROUND
Lowering risk of dementia is of increasing importance in the future with the aging population.
The Lancet Commission on Dementia Prevention, intervention and Care estimated that 3% of dementia cases are attributable to depression, one of the 12 common modifiable risk factors that, assuming causality, jointly account for 40% of dementia cases. 1 Depression is a major contributor to the burden of disease. Globally, 5% of all adults and 6% of adults 60 years and older are affected by depression. 2 The burden of depression and anxiety disorders increased by more than 25% in 2020, during the coronavirus disease 2019 (COVID‐19) pandemic. 3
In studies of older people, the evidence consistently shows increased incidence of dementia during the initial years after depression assessment. 4 , 5 , 6 , 7 , 8 Some studies have found an association between dementia and depression symptoms manifesting more than 10 years prior to dementia diagnosis, 7 , 9 whereas other studies show no such long‐term associations. 10 , 11 The evidence is mixed regarding whether depression before the age of 65 years is associated with increased risk of dementia in later life, 12 , 13 , 14 , 15 whether treatment of depression can reduce the risk, 16 and whether recovery from depression might attenuate 14 or even remove the excess risk. 17 Evidence on the relationship between depression and subtypes of dementia is also mixed: although some studies report an association between depression and Alzheimer's disease (AD), 6 others have found that depression was more strongly associated with vascular dementia (VaD) than AD, 18 , 19 and the association remained for VaD—but not for AD—when depression was assessed more than 20 years before dementia diagnosis. 7
Depression and anxiety frequently co‐occur. 20 Several studies have reported that anxiety is associated with an increased risk of dementia, 13 , 21 , 22 , 23 although a recent meta‐analysis found no overall association between anxiety and dementia. 24 In addition, the sum of anxiety and depressive symptoms in midlife has been linked to increased risk of dementia 25 and death related to dementia. 26
In this analysis of a prospective population‐based cohort study of Norwegian adults, we aimed to investigate whether depression, anxiety, and the prevalence of clinical relevant sum of anxiety‐ and depressive symptoms (A + D), as assessed up to 33 years before dementia diagnosis, were more common among individuals who later developed dementia compared to those who did not. Furthermore, we aimed to clarify whether the pattern was similar for all‐cause dementia, AD, VaD, and other dementias, and for men and women. We hypothesized that midlife A + D, depression, and anxiety were associated with an increased risk of dementia in later life, with stronger associations observed nearer to the dementia diagnosis.
2. METHODS
2.1. Study design
We used data from 9745 participants in the population‐based Trøndelag Health (HUNT) study, limited to the participants who underwent cognitive testing at HUNT4 (2017 A + D 2019) at age 70 years or older. Cognitive assessment at the end of follow‐up at HUNT4 was linked to four repeated assessments of depression and anxiety back to 1984, creating a longitudinal analysis. This study design enabled us to investigate potential differences in trajectories of mean depressive and anxiety scores between those who developed dementia and those who did not, prior and up to dementia diagnosis at HUNT4.
2.2. Study population and procedure
The HUNT study waves—HUNT1 (1984–1986), HUNT2 (1995–1997), HUNT3 (2006–2008), and HUNT4 (2017–2019)—have been described in detail, 27 , 28 , 29 , 30 including the characteristics of the participants versus non‐participants. 27 , 31 Of the participants invited to join HUNT4, 19,403 were 70 years of age or older. These participants were offered an extended examination, called HUNT4 70+, in which 9930 participated. The 9745 participants who provided enough information for a conclusive cognitive assessment were included in the current analyses.
The comprehensive clinical examination of HUNT4 70+ participants included vitals measurements (blood pressure, heart rate, height, and weight), cognitive tests, and questionnaires on subjective health, chronic disease, lifestyle and functioning, cognitive decline, symptom debut and course, neuropsychiatric symptoms, and performance in activities of daily living (ADLs).
The assessment and diagnostic procedure for dementia has been described in previous work. 32 Briefly, information from the Montreal Cognitive Assessment (MoCA), 33 the Word List Memory Task (WLMT), 34 the 8‐item Severe Impairment Battery, 35 and caregiver interview was collected. The final dementia diagnosis was determined independently by two medical doctors who were specialists in geriatrics, old‐age psychiatry, or neurology.
The dementia group was further categorized into AD, VaD, Lewy‐body dementias (dementia with Lewy bodies and Parkinson's disease with dementia), frontotemporal dementia, mixed dementia, other specified dementia, and unspecified dementia. In the present study, the latter five groups were combined into the group “other dementia,” due to their small numbers. All diagnoses were set according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5).
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature through traditional sources (e.g., PubMed, EMBASE, Cinahl, and PsycInfo), and found that despite firm evidence of positive associations between depression and risk of dementia, the evidence is conflicting regarding midlife anxiety and depression as risk factors for dementia.
Interpretation: Our results show that a higher prevalence of depression and anxiety is associated with dementia occurring more than 30 years later, but also that dementia might be a prodrome of dementia.
Future directions: Anxiety and depression earlier in life should be further investigated as risk factors for dementia. The difference between men and women in terms of anxiety and depression in midlife and the association with dementia should be further explored. An active approach to prevent anxiety and depression in midlife may reduce the risk of dementia in late life.
2.3. Measurement of depression and anxiety
Depression and anxiety were measured using self‐report questionnaires at all four HUNT waves. HUNT2‐4 used the Hospital Anxiety and Depression Scale (HADS), with subscales for depression (HADS‐D), and anxiety (HADS‐A), each consisting of seven items. The response format for each item was a 0–3 Likert scale, yielding subscale scores in the range 0–21 and a HADS total score in the range of 0–42. As in previous research, a cutoff of 15 on the total scale (HADS‐T) was used to define caseness of sum of anxiety‐ and depressive symptoms (A + D) at HUNT2‐4. 36 The HADS questions were not included in the HUNT1 questionnaires.
At HUNT1 and HUNT2, anxiety and depression were measured using the 4‐item Anxiety and Depression Inventory (ADI‐4), which quantifies nervousness, calmness, mood, and vitality. This inventory does not distinguish sufficiently between depression and anxiety, but it is considered an acceptable indicator of psychiatric caseness of A + D in the total HUNT1 population with the right cutoff. 25 At HUNT2, a cutoff at 15 on the HADS total score matched a cutoff at the 88th percentile on ADI‐4; consequently, a cutoff at the 88th percentile on ADI‐4 were used to define caseness of A + D at HUNT1. In addition, four groups of course of A + D was classified based on the results from HUNT1‐3 into “No A + D,” “Transient A + D,” “Incident A + D,” and “Persistent A + D.”
2.4. Measurement of covariates
We selected covariates based on relevant literature, clinical experience, and a directed acyclic graph (DAG) diagram. 37 In addition to sex and age, self‐reported information from the questionnaires was used. Covariates included in the analysis are co‐living status, physical activity, alcohol consumption, loneliness, body mass index (BMI), systolic blood pressure (SBP), history of stroke, cardiac attack, diabetes, smoking habits, and hearing threshold level (HTL). A description of covariates may be found in the Supplementary Material.
Registry‐based information on education from Statistics Norway (SSB) for the year 2018 and household income after tax for the year of participation in HUNT2 were linked to participants, using the unique personal identification number given to all Norwegian residents. Education was categorized into five levels (unspecified/none, primary school, secondary school, university up to 4 years, and university more than 4 years), and income was grouped in quartiles.
2.5. Ethical approval
The study adhered to the guidelines for the protection of human data concerning safety and privacy at the Norwegian National Centre for Ageing and Health. The present analysis is part of the project “Depression, anxiety and social relationships as risk factors for dementia,” which was approved by the Norwegian Regional Ethics Committee for Medical Research (REC, reference number 182824). All participants gave written informed consent.
2.6. Statistical analyses
STATA 17 was used for all statistical analyses. To study whether trajectories of mean depression and anxiety scores (HUNT2–4) and the prevalence of A + D, differed according to dementia status at HUNT4, we used dementia status at HUNT4 as the exposure variable. We considered depression, anxiety, and A + D as outcome variables, with separate models for each of these outcomes. Multilevel mixed‐effects linear regression with random intercept and slope, and with independent (default) covariance structure, was used, also for the binary outcome after assessment (see Table S1). In the regression model, depression, anxiety, and A + D were the dependent outcome variables, with separate models for each outcome. Dementia status (dichotomized to “no dementia” and “dementia”), time (coded as 1–4 for HUNT1– HUNT4), and potential confounders were the independent variables. Interaction terms between time and dementia were used, and, in addition, interaction terms between sex and dementia status and between birth cohort and dementia status were included, to investigate the possible moderating effects of these variables. The fully adjusted model included the following time‐invariant variables: dementia status, sex, education, income, diabetes, HTL, stroke, history of heart attack, and smoking history; the model also included the following time‐dependent variables: time, age, physical activity, loneliness, BMI, and SBP. Time‐invariant variables were either stable or could not be exactly timed. Mean scores of HADS and prevalence of A + D by dementia status at HUNT4 were predicted from the mixed models post hoc using the margins command. The analyses were repeated using dementia type as the main independent variable. Each of the subtypes—AD, VaD, and other dementias—in comparison to no dementia was analyzed in separate models. Stratified regressions according to sex were performed.
Additional analyses of whether course of A + D and occurrence of A + D before and/or after age ≤65 is associated with dementia is described in the Table S2.
As recommended in the literature, 38 imputation by subjects’ mean was performed to handle missingness at the item level in our outcome variables HADS and ADI‐4. Mean HADS scores before and after imputation are shown in the appendix (Table S3). In addition, we used multiple imputation by chained equations (MICE) to impute missing data in covariates, using all study variables. Twenty imputed data sets were produced using Stata's “mi” procedure. Year of birth, sex, and dementia diagnosis were used as non‐missing variables in the prediction. The proportion of missing data are reported in Table 1.
TABLE 1a.
Time‐dependent characteristics of participants at waves 1 to 4 (1984–2008) by dementia status at wave 4 (2017–2019) in the Norwegian HUNT study.
| No dementia | Dementia | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HUNT1 | HUNT2 | HUNT3 | HUNT4 | HUNT1 | HUNT2 | HUNT3 | HUNT4 | |||||||||
| Variable | N | % | N | % | n | % | N | % | n | % | n | % | n | % | N | % |
| Participating (missing) | (8.6) | (11.5) | (11.9) | (8.6) | (13.3) | (24.9) | ||||||||||
| Did not participate | 941 | 12.9 | 413 | 5.4 | 686 | 8.7 | 1 | 0.0 | 165 | 12.2 | 107 | 7.5 | 328 | 22.3 | 0 | 0 |
| Participated | 6357 | 87.1 | 7271 | 94.6 | 7242 | 91.3 | 8219 | 100 | 1193 | 87.8 | 1322 | 92.5 | 1144 | 77.7 | 1525 | 100 |
| Physical activity (missing) | (53.7) | (44.0) | (29.1) | (22.4) | (54.0) | (53.1) | (46.8) | (63.4) | ||||||||
| Low grade | 2769 | 72.7 | 4248 | 92.2 | 3484 | 59.7 | 3642 | 57.1 | 566 | 80.6 | 669 | 93.4 | 562 | 69.2 | 446 | 79.9 |
| High grade | 1040 | 27.3 | 357 | 7.8 | 2347 | 40.3 | 2740 | 42.9 | 136 | 19.4 | 47 | 6.6 | 250 | 30.8 | 112 | 20.1 |
| Alcohol use (missing) | (23.3) | (27.8) | (14.0) | (4.7) | (24.0) | (39.1) | (30.6) | (32.4) | ||||||||
| Never/seldom | 3056 | 48.5 | 1666 | 28.1 | 2165 | 30.6 | 1579 | 20.2 | 699 | 60.3 | 363 | 39.1 | 500 | 47.2 | 458 | 44.4 |
| About once a month | 0 | 0 | 682 | 11.5 | 749 | 10.6 | 2065 | 26.4 | 0 | 0 | 118 | 12.7 | 114 | 10.8 | 299 | 29 |
| 2–3 times a month | 2873 | 45.6 | 1627 | 27.4 | 1383 | 19.6 | 2557 | 32.6 | 385 | 33.2 | 232 | 25 | 167 | 15.8 | 172 | 16.7 |
| About once a week | 0 | 0 | 1350 | 22.7 | 1426 | 20.2 | 0 | 0 | 0 | 0 | 148 | 15.9 | 161 | 15.2 | 0 | 0 |
| 2–3 times a week | 238 | 3.8 | 548 | 9.2 | 1061 | 15 | 1231 | 15.7 | 34 | 2.9 | 64 | 6.9 | 93 | 8.8 | 75 | 7.3 |
| >4 times a week | 137 | 2.2 | 63 | 1.1 | 288 | 4.1 | 400 | 5.1 | 41 | 3.5 | 4 | 0.4 | 24 | 2.3 | 27 | 2.6 |
| Living alone (missing) | (23.7) | (27.4) | (19.5) | (2.8) | (23.0) | (31.4) | (32.9) | (36.3) | ||||||||
| Not alone | 6096 | 97.2 | 5934 | 99.4 | 5474 | 82.7 | 5387 | 67.4 | 1108 | 94.4 | 987 | 94.4 | 689 | 67.4 | 470 | 48.4 |
| Alone | 173 | 2.8 | 33 | 0.6 | 1145 | 17.3 | 2605 | 32.6 | 66 | 5.6 | 59 | 5.6 | 334 | 32.6 | 501 | 51.6 |
| Feeling lonely (missing) | (23.4) | (19.0) | (17.6) | (11.5) | (23.0) | ((27.1) | (35.0) | (40.5) | ||||||||
| Not lonely | 4828 | 76.7 | 5629 | 84.6 | 5581 | 82.4 | 5486 | 75.4 | 834 | 71 | 877 | 78.9 | 721 | 72.8 | 473 | 52.1 |
| A little lonely | 1204 | 19.1 | 815 | 12.2 | 931 | 13.7 | 1413 | 19.4 | 287 | 24.4 | 182 | 16.4 | 194 | 19.6 | 273 | 30.1 |
| Lonely | 183 | 2.9 | 174 | 2.6 | 193 | 2.8 | 302 | 4.2 | 36 | 3.1 | 45 | 4 | 57 | 5.8 | 118 | 13 |
| BMI (kg/m2) (missing) | (11.3) | (11.7) | (12.2) | (1.3) | (11.2) | (14.0) | (25.6) | (22.8) | ||||||||
| Underweight | 38 | 0.5 | 17 | 0.2 | 22 | 0.3 | 66 | 0.8 | 8 | 0.6 | 9 | 0.7 | 8 | 0.7 | 28 | 2.4 |
| Normal weight | 4286 | 58.8 | 2386 | 32.9 | 1763 | 24.4 | 2413 | 29.7 | 639 | 47.2 | 362 | 27.6 | 288 | 25.4 | 431 | 36.6 |
| Pre‐obese | 2528 | 34.7 | 3715 | 51.2 | 3669 | 50.8 | 3747 | 46.2 | 576 | 42.5 | 666 | 50.8 | 534 | 47.1 | 473 | 40.2 |
| Obese class I | 377 | 5.2 | 917 | 12.6 | 1392 | 19.3 | 1456 | 18 | 103 | 7.6 | 211 | 16.1 | 237 | 20.9 | 187 | 15.9 |
| Obese class II | 54 | 0.7 | 196 | 2.7 | 305 | 4.2 | 345 | 4.3 | 23 | 1.7 | 50 | 3.8 | 54 | 4.8 | 50 | 4.2 |
| Age (years), mean SD | 43.7 | 5.7 | 55.3 | 5.8 | 66.4 | 5.6 | 76.9 | 5.7 | 50.5 | 7.5 | 62.1 | 7.5 | 72.6 | 7.1 | 83.7 | 7.4 |
| SBP (mmHg), mean SD | 128.2 | 15.2 | 137.5 | 18.4 | 137.1 | 18.5 | 140.1 | 19.9 | 134.7 | 18.2 | 146.1 | 21.7 | 140.8 | 20.7 | 135 | 21.6 |
| HTL (dB), mean SD | 4.5 | 7.8 | 4.5 | 7.8 | 4.5 | 7.8 | 4.5 | 7.8 | 8.6 | 9.6 | 8.6 | 9.6 | 8.6 | 9.6 | 8.6 | 9.6 |
Note: Percentage of categories is calculated from the observations at each item, whereas percentage of missing (in parentheses) is calculated from the study population of 9745 (8220 without dementia and 1525 with dementia at HUNT4).
Abbreviations: BMI, body mass index; HTL, hearing threshold level, best ear in decibel (dB); HUNT, Trøndelag Health study; SBP, systolic blood pressure.
Sensitivity analyses were performed with complete cases. In addition, we conducted stratified analyses by age cohort, with cutoff at age 80 years, to examine death as a competing risk. In all analyses, significance was set to 5% in two‐sided tests.
3. RESULTS
3.1. Descriptive characteristics
Mean age at HUNT4 was 78.0 years, standard deviation (SD) 6.45 (range 70–103), and 54.4% of participants were women. Of the 9745 participants, 8220 (84%) had no cognitive impairment and 1525 (16%) had dementia; among those with dementia, 870 (57%) had AD, 159 (10.4%) had VaD, and 522 (32.5%) had “other dementia.” Descriptive characteristics with respect to all covariates are presented in Table 1, according to dementia status at HUNT4.
TABLE 1b.
Time independent characteristics by dementia status at HUNT4.
| No dementia | Dementia | |||
|---|---|---|---|---|
| N | % | N | % | |
| Sex (missing) | (0) | (0) | ||
| Women | 4400 | 53.5 | 900 | 59.0 |
| Men | 3820 | 46.5 | 625 | 41.0 |
| Education (missing) | (0.3) | (0.3) | ||
| Unspecified/none | 5 | 0.1 | 0 | 0.0 |
| Primary school | 1820 | 22.2 | 674 | 44.3 |
| Secondary school | 4415 | 53.9 | 676 | 44.4 |
| University. <4 years | 1586 | 19.3 | 151 | 9.9 |
| University. ≥4 years | 372 | 4.5 | 20 | 1.3 |
| Income HUNT2 (missing) | (11.6) | (13.3) | ||
| Lower quartile | 3007 | 41.4 | 893 | 67.5 |
| Second quartile | 2381 | 32.7 | 279 | 21.1 |
| Third quartile | 1389 | 19.1 | 109 | 8.2 |
| Upper quartile | 494 | 6.8 | 42 | 3.2 |
| Smoking history (missing) | (0.2) | (3.0) | ||
| Never | 3158 | 38.5 | 620 | 41.9 |
| Ever | 4552 | 55.5 | 789 | 53.3 |
| Current | 493 | 6.0 | 71 | 4.8 |
| Diabetes (missing) | (0.6) | (8.6) | ||
| No diabetes | 7201 | 88.2 | 1142 | 81.9 |
| Diabetes | 967 | 11.8 | 252 | 18.1 |
| Apoplexia ever (missing) | (9.9) | (8.8) | ||
| No | 6641 | 89.6 | 1138 | 81.8 |
| Yes | 767 | 10.4 | 253 | 18.2 |
| Had heart attack (missing) | (0.4) | (1.6) | ||
| No | 7347 | 89.7 | 1310 | 87.3 |
| Yes | 843 | 10.3 | 191 | 12.7 |
Abbreviation: HUNT, Trøndelag Health study.
3.2. All‐cause dementia
Results from the fully adjusted mixed regressions showed that compared to participants without dementia, the prevalence of A + D among those with dementia was 1.9 percentage points (pp) [95% confidence interval [CI] 0.1–3.7] higher at HUNT1, 1.9 pp [0.1–3.8] higher at HUNT2, 2.9 pp [0.9–4.8] higher at HUNT3, and 7.6 pp [5.7–9.6] higher at HUNT4. HADS scores were not available for HUNT1, but participants with dementia at HUNT4 had significantly higher HADS‐T scores at HUNT2 (mean difference 0.38 [95% CI: 0.05–0.70]), HUNT3 (0.74 [0.41–1.07]), and HUNT4 (1.86 [1.50–2.22]). In addition they had higher HADS‐D scores at HUNT3 (0.33 [0.15–0.52]) and HUNT4 (1.29 [1.09–1.48]), but there were no differences between groups at HUNT2. Correspondingly, for participants with dementia at HUNT4, HADS‐A scores were higher at HUNT2 (0.27 [0.08–0.45]), HUNT3 (0.41 [0.22–0.59]), and HUNT4 (0.57 [0.37–0.78]) (Table 2 and Figure 1).
TABLE 2.
Prevalence of cases with clinical signifcant sum of anxiety‐ and depressive symptoms (A + D) and mean score of HADS with 95% confidence interval (CI) by dementia status at HUNT4, adjusted for age and sex, and fully adjusted.
| No dementia | Dementia | Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | pp | 95% CI | ||||
| Imputed cases, adjusted for age and sex (N = 9745) | |||||||||
| A + D * | |||||||||
| HUNT1 | 11,7 | 11,0 | 12,4 | 15,0 | 13,3 | 16,8 | 3,3 | 1,4 | 5,3 * |
| HUNT2 | 11,9 | 11,2 | 12,6 | 15,1 | 13,3 | 16,9 | 3,2 | 1,2 | 5,1 * |
| HUNT3 | 9,0 | 8,3 | 9,7 | 13,8 | 11,9 | 15,7 | 4,8 | 2,8 | 6,9 * |
| HUNT4 | 8,9 | 8,2 | 9,6 | 22,0 | 20,1 | 24,0 | 13,1 | 11,0 | 15,2 * |
| Mean | 95% CI | Mean | 95% CI | Δ | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| HADS‐T | |||||||||
| HUNT2 | 7,96 | 7,83 | 8,09 | 8,57 | 8,26 | 8,88 | 0,61 | 0,27 | 0,95 * |
| HUNT3 | 7,07 | 6,94 | 7,20 | 8,23 | 7,90 | 8,56 | 1,16 | 0,80 | 1,51 * |
| HUNT4 | 7,01 | 6,88 | 7,14 | 10,01 | 9,67 | 10,35 | 3,00 | 2,63 | 3,36 * |
| HADS‐D | |||||||||
| HUNT2 | 3,77 | 3,70 | 3,84 | 4,00 | 3,83 | 4,17 | 0,23 | 0,04 | 0,41 * |
| HUNT3 | 3,48 | 3,41 | 3,55 | 4,03 | 3,85 | 4,22 | 0,56 | 0,36 | 0,75 * |
| HUNT4 | 3,47 | 3,40 | 3,54 | 5,37 | 5,19 | 5,56 | 1,91 | 1,71 | 2,11 * |
| HADS‐A | |||||||||
| HUNT2 | 4,19 | 4,11 | 4,26 | 4,57 | 4,40 | 4,75 | 0,39 | 0,19 | 0,58 * |
| HUNT3 | 3,60 | 3,52 | 3,67 | 4,19 | 4,01 | 4,38 | 0,60 | 0,40 | 0,80 * |
| HUNT4 | 3,55 | 3,47 | 3,62 | 4,63 | 4,44 | 4,82 | 1,09 | 0,88 | 1,29 * |
| % | 95% CI | % | 95% CI | pp | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Imputed cases, fully adjusted (N = 9745) | |||||||||
| A + D * | |||||||||
| HUNT1 | 10,4 | 9,6 | 11,1 | 12,3 | 10,6 | 14,0 | 1,9 | 0,1 | 3,7 * |
| HUNT2 | 12,2 | 11,5 | 12,9 | 14,2 | 12,5 | 15,9 | 1,9 | 0,1 | 3,8 * |
| HUNT3 | 10,4 | 9,7 | 11,1 | 13,2 | 11,4 | 15,1 | 2,9 | 0,9 | 4,8 * |
| HUNT4 | 10,2 | 9,4 | 10,9 | 17,8 | 15,9 | 19,7 | 7,6 | 5,7 | 9,6 * |
| Mean | 95% CI | Mean | 95% CI | Δ | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| HADS‐T | |||||||||
| HUNT2 | 7,89 | 7,76 | 8,02 | 8,27 | 7,97 | 8,56 | 0,38 | 0,05 | 0,70 * |
| HUNT3 | 7,26 | 7,13 | 7,38 | 8,00 | 7,70 | 8,30 | 0,74 | 0,41 | 1,07 * |
| HUNT4 | 7,18 | 7,05 | 7,31 | 9,04 | 8,70 | 9,38 | 1,86 | 1,50 | 2,22 * |
| HADS‐D | |||||||||
| HUNT2 | 3,73 | 3,66 | 3,80 | 3,84 | 3,67 | 4,00 | 0,11 | −0,07 | 0,29 |
| HUNT3 | 3,58 | 3,51 | 3,65 | 3,91 | 3,74 | 4,08 | 0,33 | 0,15 | 0,52 * |
| HUNT4 | 3,56 | 3,49 | 3,63 | 4,85 | 4,66 | 5,03 | 1,29 | 1,09 | 1,48 * |
| HADS‐A | |||||||||
| HUNT2 | 4,16 | 4,09 | 4,24 | 4,43 | 4,26 | 4,60 | 0,27 | 0,08 | 0,45 * |
| HUNT3 | 3,68 | 3,61 | 3,75 | 4,09 | 3,92 | 4,26 | 0,41 | 0,22 | 0,59 * |
| HUNT4 | 3,62 | 3,54 | 3,69 | 4,19 | 4,00 | 4,38 | 0,57 | 0,37 | 0,78 * |
Abbreviations: A + D, prevalence of cases with clinical significant sum of anxiety‐ and depressive symptoms; CI, confidence interval; HADS, Hospital Anxiety and Depression Scale; HADS‐T, total score of HADS; HADS‐D, HUNT, Trøndelag Health study; Depression subscore of HADS; HADS‐A, Anxiety subscore of HADS; pp, percentage points; Δ, difference of mean.
* p < 0.05.
FIGURE 1.
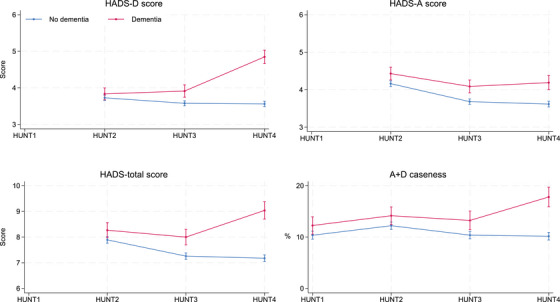
Mean HADS total score and sub‐scores, and prevalence of cases with clinical significant sum of anxiety‐ and depressive symptoms (A+D), by dementia status at HUNT4 over time (1984 = HUNT1, 1995 = HUNT2, 2006 = HUNT3, 2017 = HUNT4), fully adjusted. HADS, Hospital Anxiety and Depression Scale; HUNT, Trøndelag Health study.
An analysis of dementia risk among those with A + D in midlife only versus those with persistent symptoms showed no significant difference. However, those with A + D in midlife only had a higher risk than those who never had A + D, and lower risk than those with late‐life A + D only (Table S2). Similarly, participants with incident and persistent A + D had a higher risk of dementia than participants without A + D (Table S2).
3.3. Type of dementia
Table 3 and Figure 2 present the differences between participants with and without dementia by type (AD, VaD, and other dementias) at HUNT4 in terms of depression and anxiety levels and prevalence of A + D at HUNT1–HUNT4. The difference between participants with AD compared to those without dementia was similar to the difference between all‐cause dementia and no‐dementia groups. For those with VaD, there was no significant difference from those without dementia until HUNT4, whereas the other dementia group differed from those without dementia in the same pattern as in the all‐cause dementia analysis in HUNT3–HUNT4–.
TABLE 3.
Prevalence of cases with clinical significant sum of anxiety‐ and depressive symptoms (A + D) and mean scores of HADS with 95% CI by type of dementia, in groups with and without dementia at HUNT4, and differences between dementia type and group without dementia, fully adjusted.
| No dementia | AD (n = 870) | Difference AD vs. noD | VaD (n = 159) | Difference VaD vs noD | Other (n = 496) | Difference other vs. noD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | Δ | 95% CI | p | % | 95% CI | Δ | 95% CI | P | % | 95% CI | Δ | 95% CI | p | ||||||||
| A + D | ||||||||||||||||||||||||
| HUNT1 | 10.4 | 9.6 | 11.1 | 12.7 | 10.5 | 14.9 | 2.3 | 0.0 | 4.7 | 0.05 | 12.2 | 6.9 | 17.4 | 1.8 | −3.5 | 7.1 | 0.50 | 11.5 | 8.7 | 14.3 | 1.2 | −1.7 | 4.1 | 0.42 |
| HUNT2 | 12.2 | 11.5 | 12.9 | 14.0 | 11.8 | 16.3 | 1.8 | −0.6 | 4.1 | 0.13 | 13.0 | 7.9 | 18.2 | 0.8 | −4.4 | 6.0 | 0.76 | 14.8 | 11.9 | 17.7 | 2.6 | −0.4 | 5.6 | 0.09 |
| HUNT3 | 10.4 | 9.7 | 11.1 | 13.3 | 10.9 | 15.6 | 2.9 | 0.4 | 5.3 | 0.02 | 13.3 | 7.9 | 18.7 | 2.9 | −2.6 | 8.4 | 0.30 | 13.2 | 10.4 | 16.0 | 2.8 | −0.1 | 5.7 | 0.06 |
| HUNT4 | 10.2 | 9.4 | 10.9 | 18.3 | 15.9 | 20.8 | 8.2 | 5.7 | 10.7 | 0.00 | 19.1 | 13.9 | 24.2 | 8.9 | 3.7 | 14.0 | 0.00 | 16.5 | 13.2 | 19.8 | 6.3 | 3.0 | 9.6 | 0.00 |
| Mean | 95% CI | Mean | 95%CI | Δ | 95% CI | p | Mean | 95%CI | Δ | 95% CI | p | Mean | 95%CI | Δ | 95% CI | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HADS‐T | ||||||||||||||||||||||||
| HUNT2 | 7.89 | 7.76 | 8.02 | 8.33 | 7.94 | 8.72 | 0.44 | 0.03 | 0.85 | 0.03 | 7.62 | 6.73 | 8.50 | −0.28 | −1.17 | 0.62 | 0.55 | 8.36 | 7.86 | 8.86 | 0.47 | −0.05 | 0.99 | 0.08 |
| HUNT3 | 7.26 | 7.13 | 7.38 | 8.06 | 7.66 | 8.45 | 0.80 | 0.38 | 1.21 | 0.00 | 7.85 | 6.88 | 8.82 | 0.59 | −0.40 | 1.58 | 0.24 | 7.95 | 7.43 | 8.47 | 0.69 | 0.16 | 1.22 | 0.01 |
| HUNT4 | 7.18 | 7.05 | 7.31 | 9.15 | 8.72 | 9.58 | 1.97 | 1.52 | 2.42 | 0.05 | 8.52 | 7.59 | 9.44 | 1.34 | 0.41 | 2.27 | 0.00 | 9.01 | 8.43 | 9.59 | 1.83 | 1.24 | 2.42 | 0.00 |
| HADS‐D | ||||||||||||||||||||||||
| HUNT2 | 3.73 | 3.66 | 3.80 | 3.86 | 3.65 | 4.07 | 0.13 | −0.09 | 0.35 | 0.24 | 3.62 | 3.14 | 4.10 | −0.11 | −0.60 | 0.38 | 0.65 | 3.86 | 3.58 | 4.14 | 0.13 | −0.16 | 0.42 | 0.37 |
| HUNT3 | 3.58 | 3.51 | 3.65 | 3.90 | 3.68 | 4.12 | 0.32 | 0.09 | 0.55 | 0.01 | 4.01 | 3.49 | 4.52 | 0.43 | −0.09 | 0.95 | 0.11 | 3.91 | 3.63 | 4.19 | 0.33 | 0.04 | 0.62 | 0.03 |
| HUNT4 | 3.56 | 3.49 | 3.63 | 4.85 | 4.62 | 5.09 | 1.29 | 1.05 | 1.53 | 0.00 | 4.74 | 4.24 | 5.24 | 1.18 | 0.68 | 1.68 | 0.00 | 4.87 | 4.56 | 5.18 | 1.31 | 1.00 | 1.63 | 0.00 |
| HADS‐A | ||||||||||||||||||||||||
| HUNT2 | 4.16 | 4.09 | 4.24 | 4.47 | 4.25 | 4.69 | 0.31 | 0.07 | 0.54 | 0.01 | 4.00 | 3.49 | 4.51 | −0.16 | −0.68 | 0.35 | 0.53 | 4.50 | 4.21 | 4.78 | 0.34 | 0.04 | 0.63 | 0.00 |
| HUNT3 | 3.68 | 3.61 | 3.75 | 4.16 | 3.93 | 4.39 | 0.48 | 0.24 | 0.72 | 0.00 | 3.84 | 3.27 | 4.42 | 0.16 | −0.42 | 0.74 | 0.59 | 4.04 | 3.74 | 4.34 | 0.36 | 0.05 | 0.66 | 0.02 |
| HUNT4 | 3.62 | 3.54 | 3.69 | 4.29 | 4.05 | 4.54 | 0.68 | 0.42 | 0.93 | 0.00 | 3.78 | 3.24 | 4.32 | 0.16 | −0.38 | 0.70 | 0.56 | 4.14 | 3.81 | 4.46 | 0.52 | 0.18 | 0.85 | 0.00 |
Note: Analyses are fully adjusted.
Abbreviations: A + D, prevalence of cases with clinical significant sum of anxiety‐ and depressive symptoms; AD = Alzheimer's dementia; CI, confidence interval; HADS, Hospital Anxiety and Depression Scale; HADS‐T, total score of HADS; HADS‐D, Depression‐subscore of HADS; HADS‐A, Anxiety subscore of HADS; noD, no dementia; HUNT, Trøndelag Health study; other, other dementias; pp, percentage points; Δ, difference of mean; VaD, vascular dementia.
FIGURE 2.
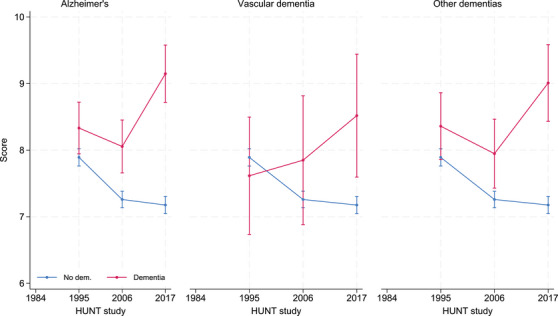
Mean HADS‐total (HADS‐T) score by types of dementia over time, fully adjusted, vertical plots indicating confidence intervals. HADS, Hospital Anxiety and Depression Scale.
3.4. Sex‐specific analysis
The results for men and women were analyzed separately and are shown in the appendix (Table S4). Among women, those with dementia at HUNT4 had a higher prevalence of A + D at HUNT1, HUNT3, and HUNT4, higher HADS‐T scores at HUNT2 –HUNT4, higher HADS‐D scores at HUNT3 and HUNT4, and higher HADS‐A scores at HUNT2–HUNT4. Among men, those with dementia at HUNT4 had higher prevalence of A + D at HUNT4 along with higher HADS‐T, HADS‐D, and HADS‐A scores at HUNT3 and HUNT4.
3.5. Sensitivity analyses
A complete case analysis showed the same pattern of results with increasing differences in HADS‐T, HADS‐A, and HADS‐D scores between participants with and without dementia from HUNT2 to HUNT4. Regarding prevalence of A + D, no significant difference was found between the groups at assessments prior to HUNT4 (Table S5). In a stratified analysis by age (with the cutoff at age 80 years), a difference in anxiety and depression between those with and without dementia was found only in the older age group (Table S6). In a multilevel logistic regression analysis on a simplified imputed data set, A + D‐trajectories had the same trends as in the linear regression (Table S1).
4. DISCUSSION
In this population‐based study of 9745 men and women aged 70+ years, those who had developed dementia at the cognitive assessment showed a higher mean total HADS‐T score and higher A + D prevalence compared to those who did not develop dementia more than two decades earlier. The differences were marginal at the first waves and grew larger as participants drew closer to dementia diagnosis. For A + D, the difference between the groups was already evident three decades before the cognitive assessment.
These findings are consistent with a previous prospective follow‐up study reporting that those with mental distress (labeled A + D in the present study), as measured using ADI‐4 at HUNT1, had a 35% greater risk of developing dementia, as identified from medical records in hospitals and nursing homes, over a 27‐year period. 25
We found higher levels of depressive symptoms 11 years (but not 22 years) prior to dementia diagnosis. A study with a similar design found higher levels of depressive symptoms among participants who develop dementia 10 years before their diagnosis. 11 A recent systematic review found that associations between depression and dementia were stronger in studies with shorter follow‐up periods. 24 Studies of the association between dementia risk and course of depression have found that recurrent, 12 persistent, 17 and treatment‐resistant 6 depressive episodes are associated with dementia, whereas remitting, decreasing, or mild depressive episodes (i.e., low symptom scores) are not associated with increased dementia risk. 39 We found no significant difference between participants with A + D only in midlife versus those with persistent symptoms regarding association with dementia in our material. However, when all ages were included, those with incident and persistent symptoms had higher risk of dementia, whereas the risk among those with transient symptoms was not higher than among those without symptoms (Table S2). This is in line with the UK Biobank study, suggesting that improved depression does not indicate a higher dementia risk. 16 In our study, the higher level of depressive symptoms in the dementia group at HUNT3 may be driven partially by individuals already being in a prodromal stage of dementia, as we found no significant difference in the previous wave. However, a Swedish study found that even if the risk of dementia declined sharply with time, patients with major depressive episodes had higher risk of dementia over a follow‐up period of up to 35 years. 7
We also found that the level of anxiety was higher in the dementia group both 22 and 11 years before the diagnosis of dementia, as well as at the time of dementia diagnosis at HUNT4. This is in line with other studies, suggesting that anxiety is associated with increased dementia risk. 40 , 41 However, a recent systematic review found mixed results and no overall association between anxiety and risk of dementia in a meta‐analysis of five prospective cohort studies. 24
It has been suggested that anxiety is also a prodrome of dementia, 21 , 42 and that anxiety could be a manifestation of an underlying neurodegenerative process leading to dementia. 43 Another suggestion is that anxiety is part of a personality associated with increased dementia risk. 44 Caution is warranted when comparing studies on anxiety and dementia, given the considerable variation in sampling, diagnostic criteria, and categories used.
Among men, no difference in A + D was found between the dementia and non‐dementia groups until 11 years before dementia, whereas in women the difference between the groups was significant already 33 years before dementia diagnosis. For women, a difference was found in the anxiety scores 22 years before dementia, but not in the depression scores. Although we adjusted the analyses for several covariates, there may remain other unmeasured risk factors for dementia that were more common among women and related to anxiety. Furthermore, certain female‐related factors, such as parity, 45 or menopause, 46 or the greater role in caring and lower income, may influence susceptibility to anxiety, which was found to be significantly higher among women compared to men, even in those who did not develop dementia. Our findings suggest that, for women in particular, addressing A + D from midlife onward should be a priority for reducing the risk of dementia and improving mental health. In addition, factors that trigger or sustain A + D in women should be explored further to better understand the potential associations with dementia.
The complex processes on the pathway from anxiety and depression toward dementia are shown in a DAG, but the main direction of the arrows is not always obvious (Figure S1), and a diversity of mechanisms is likely to be involved. Biologically, mechanisms involving chronic stress and activated hypothalamic‐pituitary‐adrenal (HPA)‐ axis, chronic inflammation involving interleukins, altered tryptophan metabolic pathways, and changes in neurotransmitter systems have been reported to play a role in the pathway between anxiety, depression, and dementia. 47 However, especially when the follow‐up of individuals with anxiety and depression is long, psychosocial pathways may be important, as these disorders might increase the risk of unhealthy lifestyle, like social isolation, lack of exercise, and self‐neglect. 48 It has been found that psychologically distressed patients who participated in medium to hard physical activity had a reduced risk of dementia. 49
When stratifying by age, we found that anxiety and depression affected the risk of dementia only in the older group (≥80 years at the dementia assessment). Participants with depression and anxiety are likely more prone to drop out of the study, 31 which could attenuate any true association with dementia. Therefore, our estimates may be conservative.
Both anxiety and depressive disorders are distressing when present and should be treated appropriately. Furthermore, treating these conditions may reduce the risk of dementia. Our findings suggest that lowering anxiety and depression symptom scores in the general population could potentially reduce the risk of dementia. This makes it plausible that universal efforts to improve well‐being from midlife onward may have a significant impact on reducing the dementia risk across the population. 16
4.1. Strengths and limitations
Important strengths of the present study are its large sample size, its long follow‐up period, and a robust method for diagnosis of dementia and its subtypes. The sample was population based, inviting all residents in a specific area, and—to increase inclusion—clinical examinations were offered at the participant's home or at the nursing home. Another study strength is its inclusion of both anxiety and depression in the analysis.
The study also has its limitations. At HUNT4, nearly half of the people 70 years and older invited to join did not participate. Those who did not participate or had missing values on covariates might differ from the (full) participants in terms of prevalence of dementia and the investigated risk factors, 31 potentially introducing selection bias. To account for some of this bias, missing values were imputed; unfortunately; however, we did not have access to population weights at each survey wave to correct for selective drop‐out from the study. In addition, the HADS tool, despite being well‐validated and used in many population studies, has some limitations. However, the lack of assessment of somatic symptoms 50 can be argued to be a strength, as older people often have other illnesses with somatic symptoms. Self‐report instruments may be less reliable among individuals with dementia at HUNT4 due to memory issues, which can affect their ability to accurately report their feelings over the past week. In addition, self‐reported data on anxiety and depression generally have limitations compared to clinical assessment. Furthermore, given the long intervals between waves, episodes of depression and anxiety may have occurred without being identified by the study. Moreover, the HUNT cohort is representative of the Norwegian population but with slightly lower education levels, no large city, and few immigrants. The lack of imaging and biomarker data in the diagnostic process may have caused diagnostic misclassification, especially with respect to subtypes of dementia; such misclassifications might also attenuate any true association. Moreover, many people have more than one pathology. 51 The number of dementia cases was insufficient to study the different subtypes of dementia. Finally, our results should be interpreted with caution due to multiple testing.
5. CONCLUSION
Our study shows that anxiety and depression in midlife are associated with increased risk of dementia, and it finds that the levels of depression and anxiety symptoms 20 and 30 years before diagnosis are higher among those who subsequently develop dementia. The difference in mean anxiety scores between the groups with and without dementia was small and increased only slightly nearer the dementia diagnosis, whereas the difference in mean depression scores increased markedly over time. Our results indicate that particularly depression in late life should alert health services to monitor whether dementia is potentially in progress. Treating depression and anxiety and taking a more active approach to preventing mental distress in midlife may reduce the risk of dementia in late life.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants gave written informed consent.
Supporting information
Supporting Information
ICMJE Disclosure Form
ACKNOWLEDGMENTS
The Trøndelag Health Study is a collaboration between the Trøndelag Health Study Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology), the Trøndelag County Council, the Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. R.H.A. was supported by the Foundation Dam (2020/FO297384) through The Norwegian Health Association. M.K. was supported by Wellcome Trust (221854/Z/20/Z), Medical Research Council (R024227), National Institute on Aging (R01AG062553), and Academy of Finland, Finland (350426).
Aunsmo RH, Strand BH, Anstey KJ, et al. Associations between depression and anxiety in midlife and dementia more than 30 years later: The HUNT Study. Alzheimer's Dement. 2024;16:e70036. 10.1002/dad2.70036
REFERENCES
- 1. Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet Standing Commission. Lancet North Am Ed. 2024; 404(10452):572–628. doi: 10.1016/S0140-6736(24)01296-0 [DOI] [PubMed] [Google Scholar]
- 2. WHO WHO . Depression. WHO. March 31, 2023. Accessed July 22, 2022, https://www.who.int/news‐room/fact‐sheets/detail/depression [Google Scholar]
- 3. Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID‐19 pandemic. Lancet North Am Ed. 2021; 398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry. 2017; 7(5):e1117. doi: 10.1038/tp.2017.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heser K, Fink A, Reinke C, Wagner M, Doblhammer G. The temporal association between incident late‐life depression and incident dementia. Acta Psychiatr Scand. 2020; 142(5):402–412. doi: 10.1111/acps.13220 [DOI] [PubMed] [Google Scholar]
- 6. Chan Y‐LE, Chen M‐H, Tsai S‐J. Treatment‐resistant depression enhances risks of dementia and alzheimer's disease: a nationwide longitudinal study. J Affect Disord. 2020; 274:806–812. doi: 10.1016/j.jad.2020.05.150 [DOI] [PubMed] [Google Scholar]
- 7. Holmquist S, Nordstrom A, Nordstrom P. The association of depression with subsequent dementia diagnosis: a Swedish nationwide cohort study from 1964 to 2016. PLoS Med. 2020; 17(1):1003016. doi: 10.1371/JOURNAL.PMED.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim H, Jeong W, Kwon J, Kim Y, Park EC, Jang SI. Association between depression and the risk of Alzheimer's disease using the Korean National Health Insurance Service‐Elderly Cohort. Sci Rep. 2021; 11(1):22591. doi: 10.1038/s41598-021-02201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010; 75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim D, Wang R, Kiss A, et al. Depression and increased risk of Alzheimer's dementia: longitudinal analyses of modifiable risk and sex‐related factors. Am J Geriatr Psychiatry. 2021; 29(9):917–926. doi: 10.1016/j.jagp.2020.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh‐Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28‐year follow‐up study. JAMA Psychiatry. 2017; 74(7):712–718. doi: 10.1001/Jamapsychiatry.2017.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late‐life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012; 69(5):493–498. doi: 10.1001/archgenpsychiatry.2011.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuring JK, Mathias JL, Ward L. Risk of dementia in persons who have previously experienced clinically‐significant depression, anxiety, or PTSD: a systematic review and meta‐analysis. J Affect Disord. 2020; 274:247–261. doi: 10.1016/j.jad.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 14. Yang W, Li X, Pan KY, et al. Association of life‐course depression with the risk of dementia in late life: a nationwide twin study. Alzheimers Dement. 2021; 17(8):1383–1390. doi: 10.1002/alz.12303 [DOI] [PubMed] [Google Scholar]
- 15. Byers AL, Yaffe K. Depression and risk of developing dementia. NatRevNeurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L, Deng YT, Leng Y, et al. Depression, depression treatments, and risk of incident dementia: a prospective cohort study of 354,313 participants. Biol Psychiatry. 2023; 93(9):802–809. doi: 10.1016/j.biopsych.2022.08.026 [DOI] [PubMed] [Google Scholar]
- 17. Lee ATC, Fung AWT, Richards M, et al. Risk of incident dementia varies with different onset and courses of depression. J Affect Disord. 2021; 282:915–920. doi: 10.1016/j.jad.2020.12.195 [DOI] [PubMed] [Google Scholar]
- 18. Hickey M, Hueg TK, Priskorn L, et al. Depression in mid‐ and later‐life and risk of dementia in women: a prospective study within the Danish nurses cohort. J Alzheimer's Dis. 2023; 93(2): 779–789. doi: 10.3233/jad-230091 [DOI] [PubMed] [Google Scholar]
- 19. Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd. Late‐life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta‐analysis of community‐based cohort studies. Br J Psychiatry. 2013; 202(5):329–335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lallukka T, Mekuria GB, Nummi T, Virtanen P, Virtanen M, Hammarström A. Co‐occurrence of depressive, anxiety, and somatic symptoms: trajectories from adolescence to midlife using group‐based joint trajectory analysis. BMC Psychiatry. 2019; 19(1):236. doi: 10.1186/s12888-019-2203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta‐analysis. Am J Geriatr Psychiatry. 2016; 24(10):823–842. doi: 10.1016/j.jagp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 22. Gimson A, Schlosser M, Huntley JD, Marchant NL. Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open. 2018; 8(4):e019399–e019399. doi: 10.1136/bmjopen-2017-019399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becker E, Orellana Rios CL, Lahmann C, Rücker G, Bauer J, Boeker M. Anxiety as a risk factor of Alzheimer's disease and vascular dementia. Br J Psychiatry. 2018; 213(5):654–660. doi:10.1192/bjp.2018.173 [DOI] [PubMed] [Google Scholar]
- 24. Stafford J, Chung WT, Sommerlad A, Kirkbride JB, Howard R. Psychiatric disorders and risk of subsequent dementia: Systematic review and meta‐analysis of longitudinal studies. Int J Geriatr Psychiatry. 2022; 37(5). doi: 10.1002/gps.5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skogen JC, Bergh S, Stewart R, Knudsen AK, Bjerkeset O. Midlife mental distress and risk for dementia up to 27 years later: the Nord‐Trøndelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC Geriatrics. 2015; 15:23–23. doi: 10.1186/s12877-015-0020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosness TA, Strand BH, Bergem AL, et al. Association of psychological distress late in life and dementia‐related mortality. Aging Ment Health. 2016; 20(6):603–610. doi: 10.1080/13607863.2015.1031639 [DOI] [PubMed] [Google Scholar]
- 27. Holmen J, Midthjell K, Forsén L, Skjerve K, Gorseth M, Oseland A. A health survey in Nord‐Trøndelag 1984‐86. Participation and comparison of attendants and non‐attendants. Tidsskr Nor Laegeforen. 1990; 110(15):1973–1977. [PubMed] [Google Scholar]
- 28. Holmen J, Midthjell K, Krüger Ø, et al. The Nord‐Trøndelag Health Study 1995‐97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiologi. 2003; 13(1): 19–32. [Google Scholar]
- 29. Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: The HUNT study, Norway. Int J Epidemiol. 2013; 42(4):968–977. [DOI] [PubMed] [Google Scholar]
- 30. Åsvold BO, Langhammer A, Rehn TA, et al. Cohort profile update: The HUNT Study, Norway. Int J Epidemiol. 2023;52(1):e80–e91. doi: 10.1093/ije/dyac095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Method. 2013; 12(1):143. doi: 10.1186/1471-2288-12-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GjØra L, Strand BH, Bergh S, et al. Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population‐based sample of people 70 years and older in Norway: The HUNT Study. J Alzheimer's Dis. 2021; 79(3):1213–1226. doi: 10.3233/jad-201275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 34. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39(9):1159–1165. doi: 10.1212/wnl.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 35. Schmitt FA, Saxton J, Ferris SH, Mackell J, Sun Y. Evaluation of an 8‐item Severe Impairment Battery (SIB‐8) vs. the full SIB in moderate to severe Alzheimer's disease patients participating in a donepezil study. Int J Clin Pract. 2013; 67(10):1050–1056. doi: 10.1111/ijcp.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vodermaier A, Millman RD. Accuracy of the hospital anxiety and depression scale as a screening tool in cancer patients: a systematic review and meta‐analysis. Support Care Cancer. 2011; 19(12):1899–1908. doi: 10.1007/s00520-011-1251-4 [DOI] [PubMed] [Google Scholar]
- 37. Tennant PWG, Murray EJ, Arnold KF, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. 2021; 50(2):620–632. doi: 10.1093/ije/dyaa213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shrive FM, Stuart H, Quan H, Ghali WA. Dealing with missing data in a multi‐question depression scale: a comparison of imputation methods. BMC Med Res Method. 2006; 6(1):57. doi: 10.1186/1471-2288-6-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mirza SS, Wolters FJ, Swanson SA, et al. 10‐year trajectories of depressive symptoms and risk of dementia: a population‐based study. Lancet Psychiatry. 2016; 3(7): 628–635. doi: 10.1016/s2215-0366(16)00097-3 [DOI] [PubMed] [Google Scholar]
- 40. Gulpers BJA, Oude Voshaar RC, van Boxtel MPJ, Verhey FRJ, Köhler S. Anxiety as a risk factor for cognitive decline: a 12‐year follow‐up cohort study. Am J Geriatr Psychiatry. 2019; 27(1):42–52. doi: 10.1016/j.jagp.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 41. Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimers Dement. 2016; 12(4):399–406. doi: 10.1016/j.jalz.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Bruijn RF, Direk N, Mirza SS, et al. Anxiety is not associated with the risk of dementia or cognitive decline: the Rotterdam Study. Am J Geriatr Psychiatry. 2014; 22(12): 1382‐90. doi: 10.1016/j.jagp.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 43. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community‐dwelling men. Int Psychogeriatr. 2017; 29(7):1137–1145. doi: 10.1017/s104161021700045x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Terracciano A, Sutin AR, An Y, et al. Personality and risk of Alzheimer's disease: new data and meta‐analysis. Alzheimers Dement. 2014; 10(2):179–186. doi: 10.1016/j.jalz.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bae JB, Lipnicki DM, Han JW, et al. Does parity matter in women's risk of dementia? A COSMIC collaboration cohort study. BMC Medicine. 2020; 18(1):210. doi: 10.1186/s12916-020-01671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mulhall S, Andel R, Anstey KJ. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas. 2018; 108:7‐12. doi: 10.1016/j.maturitas.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 47. Dafsari FS, Jessen F. Depression‐an underrecognized target for prevention of dementia in Alzheimer's disease. Transl Psychiatry. 2020; 10(1):160. doi: 10.1038/s41398-020-0839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geraets AFJ, Leist AK, Deckers K, Schram MT, Verhey FRJ, Köhler S. Contributions of modifiable risk factors to increased dementia risk in depression. Psychol Med. 2023; 53(14):6583–6591. doi: 10.1017/S0033291722003968 [DOI] [Google Scholar]
- 49. Zotcheva E, Bergh S, Selbæk G, et al. Midlife physical activity, psychological distress, and dementia risk: The HUNT Study. J Alzheimer's Dis. 2018; 66(2):825–833. doi: 10.3233/jad-180768 [DOI] [PubMed] [Google Scholar]
- 50. Coyne JC, van Sonderen E. No further research needed: abandoning the Hospital and Anxiety Depression Scale (HADS). J Psychosom Res. 2012; 72(3):173–174. doi: 10.1016/j.jpsychores.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 51. Nichols E, Merrick R, Hay SI, et al. The prevalence, correlation, and co‐occurrence of neuropathology in old age: harmonisation of 12 measures across six community‐based autopsy studies of dementia. Lancet Healthy Longev. 2023; 4(3):e115–e125. doi: 10.1016/S2666-7568(23)00019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
ICMJE Disclosure Form


