Abstract
This study aimed to investigate the effects of concurrent aerobic and strength training (CT) in patients with type 2 diabetes and determine the most effective dose of CT. From the inception of the databases to March 2024, we conducted a systematic search of four electronic databases (PubMed, Embase, Web of Science, and Cochrane Library) to identify randomized controlled trials (RCTs) on CT intervention in patients with type 2 diabetes. Two independent authors assessed the risk of bias of the study using the Cochrane Risk of Bias Assessment Tools. Results analyzed included glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG), body mass index, body fat percentage, blood pressure, and VO2max. Pairwise and dose-response meta-analyses using Bayesian hierarchical random-effects modeling were performed to analyze the effects of CT in patients with type 2 diabetes. From the inception of the databases to March 2024, we conducted a systematic search of four electronic databases (PubMed, Embase, Web of Science, and Cochrane Library) to identify randomized controlled trials (RCTs) on CT intervention in patients with type 2 diabetes. Two independent authors assessed the risk of bias of the study using the Cochrane Risk of Bias Assessment Tools. Results analyzed included glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG), body mass index, body fat percentage, blood pressure, and VO2max. Pairwise and dose-response meta-analyses using Bayesian hierarchical random-effects modeling were performed to analyze the effects of CT in patients with type 2 diabetes. A total of 1948 participants (935 males) were included in 23 RCTs. The male/female ratio of participants was 52/48; the mean age range was 50–65 years. The results show that CT significantly reduced HbA1c levels (MD=−0.48%, 95% CrI: −0.55 to −0.40), with some heterogeneity among different levels (SD=0.31, 95% CrI: 0.17 to 0.51), and the model converged well. Similarly, FBG levels were also significantly improved (MD=−0.48 mmol/L, 95% CrI: −0.55 to −0.40), with greater heterogeneity (SD=17.73, 95% CrI: 11.23 to 28.09). Additionally, we found a non-linear dose-response relationship between CT and HbA1c levels, with an optimal dose of 1030 METs-min/week (MD=−0.47%, 95% CrI: –0.68 to –0.26, SE=0.11). CT significantly improves several health indicators in patients with type 2 diabetes. A non-linear dose-response relationship was observed between the training dose of CT and HbA1c, and it is recommended that 270 min of moderate-intensity CT or 160 min of vigorous-intensity CT be performed weekly.
PROSPERO registration number: CRD42024547119.
Keywords: meta-analysis; concurrent aerobic and strength training.
Keywords: Training; Type 2 Diabetes; Diabetes Mellitus, Type 2; Exercise
What is already known on this topic.
What this study adds
CT significantly improved glycosylated hemoglobin (HbA1c), fasting blood glucose, BMI, body fat percentage, blood pressure, and VO2max levels in patients with type 2 diabetes, effectively enhancing their overall health status and reducing the risk of related complications.
How this study might affect research, practice or policy
The findings of this study support the use of CT as a multi-benefit therapeutic strategy with the ability to flexibly adjust training doses to optimize outcomes.
Introduction
Type 2 diabetes is an epidemic characterized by chronic hyperglycemia, with approximately 537 million adults (10.5% of the global population) living with diabetes worldwide. This number is projected to grow to 783.2 million by 2045, with type 2 diabetes accounting for the majority of these cases.1 Studies have shown that patients with type 2 diabetes are two to four times more at risk of cardiovascular diseases, including stroke, heart failure, atrial fibrillation, and coronary and peripheral artery disease, which further contribute to an increased risk of cardiovascular and all-cause mortality.2 These complications cause significant psychological and physical suffering for patients and impose a huge economic burden on society. Global diabetes healthcare-related expenditure is expected to increase to 1054 billion by 2045.1
Currently, there are several ways to control the development of type 2 diabetes, including nutritional therapy, medication, health education, and exercise therapy.2 Exercise therapy is regarded as the basic treatment for glycemic control because of its safety and low cost. It is effective in improving blood glucose levels in patients with type 2 diabetes and has a positive impact on cardiovascular risk factors.3 Among the various types of exercise, concurrent aerobic and strength training (CT), a combination of aerobic and strength training, is gaining attention. According to recent studies, the CT modality has become widely popular among all types of patients with diabetes, such as middle-aged and older patients4 and overweight and obese patients,5 due to its outstanding potential. In addition, several studies have confirmed the significant effect of concurrent training on improving glycemic control, cardiovascular health, inflammation, and overall disease management in patients with type 2 diabetes.4,7 These studies emphasize the importance of combining aerobic and strength training, showing a wider range of health benefits than a single form of training. It is evident that CT is effective in helping people with diabetes reach their health goals through indirect or direct improvements in health-related outcomes. Furthermore, The European Society of Cardiology also recommends CT as a level 1 evidence.2
However, its overall effectiveness in patients with type 2 diabetes remains uncertain. Some studies have shown that CT can be effective in improving glycated hemoglobin (HbA1c) levels,4 7 suggesting its potential benefit in improving glycemic control. However, other studies have failed to show significant improvements in glycemic control with such training.8,10 In a network meta-analysis conducted by Pan et al, indirect evidence suggested that CT significantly reduced HbA1c levels, while its effect on fasting blood glucose (FBG) was more limited.6 Furthermore, the optimal dose of CT for most patients with type 2 diabetes is currently unclear. Although the study by Gallardo-Gómez et al11 examined the dose-response relationship between physical activity and HbA1c levels, it did not distinguish CT in detail. Additionally, high-quality, evidence-based research on the effects of CT on body composition, blood pressure, and oxygen capacity in patients with type 2 diabetes is lacking; further research is needed to clarify the specific effects.
Therefore, in this study, pairwise and dose-response meta-analyses were performed using advanced Bayesian techniques to investigate the exact effects of CT on glycemic control, body composition, blood pressure, and VO2max in patients with type 2 diabetes, and to analyze the optimal dose of CT, providing a reference basis for the CT modality in these patients.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses12 12 and was conducted according to the Cochrane Handbook for Systematic Review of Interventions.13 The study protocol was registered in PROSPERO (CRD42024547119) before conducting the study.
Search strategy
A systematic search of the PubMed, Embase, Web of Science, and Cochrane Library databases was conducted from their inception to May 20, 2024. Searches were performed using a combination of medical subject terms and free words (online supplemental appendix 1). English search terms included “Diabetes Mellitus,” “Type 2,” “Type 2 diabetes,” “concurrent training,” “concurrent strength and endurance training,” “combined strength and endurance training,” “concurrent resistance and endurance training,” and “combined resistance and endurance training.” The language restriction was English, with no restrictions on regional publications. Previously published reviews and meta-analyses of relevant studies were also examined to prevent missing literature.
Study selection
The selection process for the studies was performed using Endnote software (Clarivate, Philadelphia, Pennsylvania, USA). Titles, abstracts, and main content of all studies were independently identified by two reviewers. Any disagreements were discussed and resolved by a third experienced reviewer.
Inclusion criteria
Populations
Adults (≥18 years old) with type 2 diabetes who maintained their previous medication and dietary habits during the intervention period.2
Interventions
Concurrent aerobic and strength training was performed, and the intervention lasted more than 4 weeks; the study included a control group (non-exercise inertia interventions such as waiting lists, usual care, and maintenance routine).3
Outcomes
At least one outcome of HbA1c, FBG, body mass index (BMI), body fat percentage (BFP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and VO2max were included.4
Studies
Randomized controlled trials (RCTs) were included in the study.
Exclusion criteria
Studies that compared CT to aerobic or resistance training alone.
Studies with no suitable outcomes.
Conference papers, reviews, and experimental protocols, and quasi-experimental studies.
Data extraction
Two independent reviewers extracted relevant data from eligible studies. In case of disagreement, a third experienced reviewer adjudicated. Extracted data included the first author, year of publication, patient demographic characteristics (age, sex, and sample size), intervention details (duration, frequency, and intensity of exercise), and appropriate outcomes (HbA1c, FBG, BMI, BFP, SBP, DBP, and VO2max). Because HbA1c reflects the average blood glucose level over the past 8–12 weeks, for HbA1c, only data from studies with CT interventions lasting at least 10 weeks were extracted.
Quality assessment and certainty of evidence
Two independent reviewers evaluated the quality of the included studies using the Cochrane Risk of Bias Assessment Tools.14 This tool includes seven entries: randomized sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases, which were rated as low, medium, and high risk, respectively. Owing to the unique nature of the study, complete blinding of the participants was not possible; therefore, both participant and implementer blinding were rated as high risk. Certainty of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation tool,15 considering the risk of bias, inconsistency, indirectness, risk of publication bias, and other factors. The mentioned entries were graded as high, moderate, low, and very low quality levels. However, if the two reviewers reported inconsistencies in their assessments, a third experienced author was consulted.
Pairwise meta-analysis
This study mainly extracted the mean difference (MD) and the SD change from baseline for various outcomes. Where the aforementioned data were not provided in the study, SE and quartiles were used to calculate the SD of the corresponding outcomes.13 We conducted a Bayesian meta-analysis to investigate the effect of CT on patients with type 2 diabetes using the “brms” package in R software. This package provides a more flexible modeling approach and enables an intuitive interpretation of results by reporting probabilities.16 Bayesian hierarchical modeling was developed using this package, which allowed us to nest effect sizes in the study and obtain posterior distributions for the estimates.16 17 A weakly informative prior was used for the intercept parameter (prior distribution of overall effect size μ (0,1), between-study heterogeneity Tau (0, 0.5)),18 and a half-Cauchy prior was employed for the sigma parameter. Inferences from all the analyses were drawn from the posterior distributions generated using the Hamiltonian Markov Chain Monte Carlo method.19 Uncertainty in the estimates is expressed using 95% credible intervals (CrIs). It was assumed that there was within-study or between-study variability in the observed estimates of intervention effects owing to variability in CT interventions and sampling. Therefore, we chose a randomized model. Heterogeneity at each level (within and between studies) in this meta-analysis was calculated using τ (SD). The convergence and validity of the model were assessed using the potential scale reduction factor (PSRF), which indicated that the model converged when PSRF was less than 1.01.20 The Ranef function was used to extract the “true” effect size for each study and to estimate the deviation from the combined effect. The elements included in the BARG Statement for reporting the results of the Bayesian analysis are shown in online supplemental appendix 9. Funnel plots, Egger’s test, and Begg’s test were used to test for publication bias across the outcomes. Finally, forest plots were generated using “tidybayes” and the “ggplot2” package.
Regression analysis
We developed meta-regression models to analyze the association between the effect of CT and HbA1c levels using the following potential moderator variables: participant age, baseline HbA1c level, BMI, and proportion of women. Additionally, we explored the moderating role of baseline HbA1c levels and intervention duration in dose-response analyses. In addition, we summarized the dropout rates for each study and explored the effect of dropout rates on CT efficacy in the primary outcomes (HbA1c and FBG).
Dose-response meta-analysis
HbA1c is the gold standard for assessing glycemic homeostasis. It is a combination of fasting and postprandial glycemic changes over 3 months.21 Therefore, we analyzed the dose-response relationship between CT dose and HbA1c level using a Bayesian random-effects model. Analyses were performed using contemporaneous training intensity (metabolic equivalent of tasks (METs)) × total weekly exercise time, expressed as METs-min/week, as provided in the original literature. Codes for exercise intensity were derived from the 2024 Adult Compendium of Physical Activities22 and the ACSM guidelines for exercise testing and prescription.23 A natural spline (Section 4), based on previous studies,11 was used to fit the nonlinear relationship between CT dose and HbA1c. The predicted response effects at different doses were reported as 95% CrI and were used to assess estimate certainty. Heterogeneity was reported as standard SD units. Dose-response was similarly analyzed using the “rms”package, and data were collated and visualized using the “tidybayes” and “ggplot2” packages (see online supplemental appendix 8 for R codes). Finally, a table of recommendations (based on HbA1c) on CT training doses was produced.
Results
Study selection
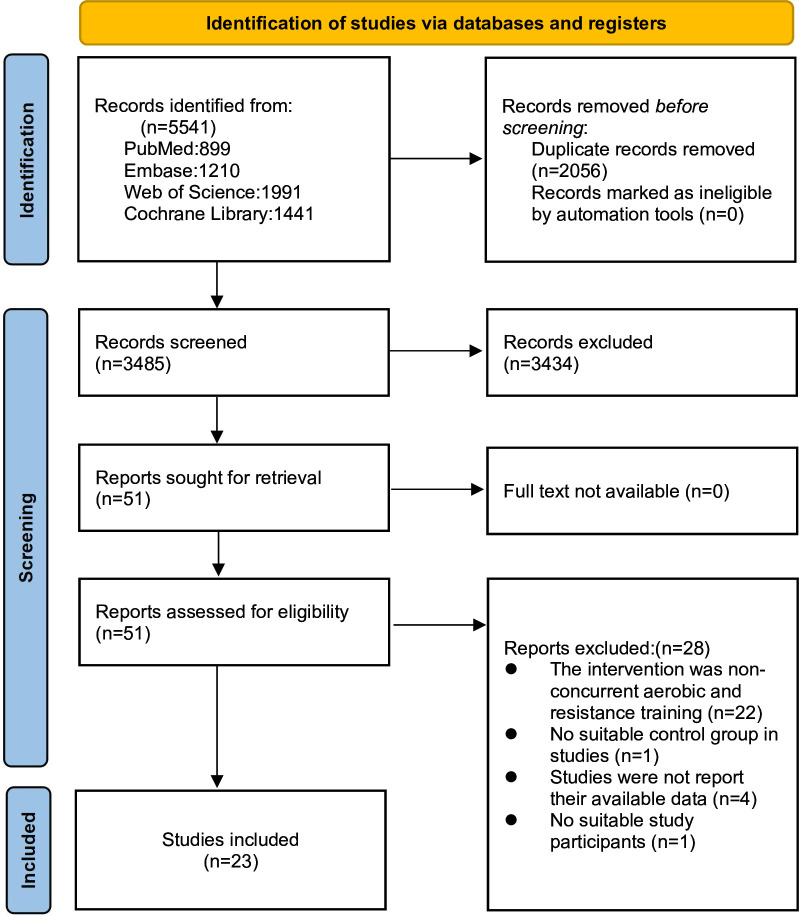
A total of 5541 publications were obtained from the databases, including 899 from PubMed, 1210 from Embase, 1991 from Web of Science, and 1441 from the Cochrane Library. After excluding 2056 duplicates and screening 3485 documents for titles and abstracts, 51 full-text documents were identified. Finally, 23 RCTs were included in the meta-analysis (online supplemental appendix 7). Reasons for exclusion were non-concurrent strength and aerobic training (n=22), no relevant outcome (n=4), intervention subject non-compliance (n=1), and control group non-compliance (n=1) (figure 1).
Figure 1. Literature review flowchart.
Study characteristics
A total of 1948 participants (935 males) were included in 23 publications, with 1022 participants in the CT group and 926 participants in the control group. The mean age range of the participants was 49.5–65 years, the mean BMI was 23.9–35, and the range of baseline HbA1c was 6.4–9.5%. Most of the studies included participants from the Americas (n=9), followed by those from Europe (n=6). The total duration of the intervention ranged from 4 to 104 weeks, and the frequency of the intervention ranged from 2 to 5 times per week. The detailed characteristics of the literature are shown in online supplemental appendix 2.
Risk of bias and certainty of evidence
Of the 23 included studies, 14 were at low risk of bias in reporting methods of randomized sequence generation, 9 studies performed allocation concealment and were at low risk of bias, 8 studies used blinding in performing outcome assessment and were also at low risk of bias, 1 study was at high risk of bias in completeness of outcomes, and among the other biases, 13 studies were at high risk of bias. Details of the quality ratings in the literature are shown in online supplemental appendix 3. The quality of evidence was rated as low to very low, owing to the risk of bias, imprecision, and indirectness (table 1).
Table 1. Grading of Recommendations Assessment, Development, and Evaluation.
| Outcomes | Studies (n) | Certainty assessment | Patients (n) | Certainty | ||||||
| Study design | Risk of bias | Inconsistency | Indirectness | Other considerations | CT | CG | ||||
| HbA1c (%) | 17 | RCT | Serious (−1) | Serious (−1) | Serious (−1) | No | 823 | 758 | ⊕㊀㊀㊀ | Very Low |
| FBG | 13 | RCT | Serious (−1) | Very serious (−2) | Serious (−1) | No | 657 | 644 | ⊕㊀㊀㊀ | Very Low |
| BMI | 14 | RCT | Serious (−1) | Not serious | Serious (−1) | No | 817 | 796 | ⊕⊕㊀㊀ | Low |
| BFP | 9 | RCT | Serious (−1) | Not serious | Serious (−1) | No | 238 | 224 | ⊕⊕㊀㊀ | Low |
| SBP | 12 | RCT | Serious (−1) | Serious (−1) | Serious (−1) | No | 466 | 441 | ⊕㊀㊀㊀ | Very Low |
| DBP | 12 | RCT | Serious (−1) | Serious (−1) | Serious (−1) | No | 745 | 720 | ⊕㊀㊀㊀ | Very Low |
| VO2max | 13 | RCT | Serious (−1) | Not serious | Serious (−1) | No | 488 | 446 | ⊕⊕㊀㊀ | Low |
GRADE Working Group grades of evidence: High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.HbA1c(%), Glycosylated hemoglobin.
BFPbody fat percentageBMIbody mass indexCGcontrol groupCTconcurrent aerobic combined resistance trainingDBPdiastolic blood pressureFBGfasting blood glucoseHbA1c (%)glycosylated hemoglobinMDmean differenceRCTrandomized controlled trialSBPsystolic blood pressureVO2maxmaximal oxygen uptake
Pairwise meta-analysis
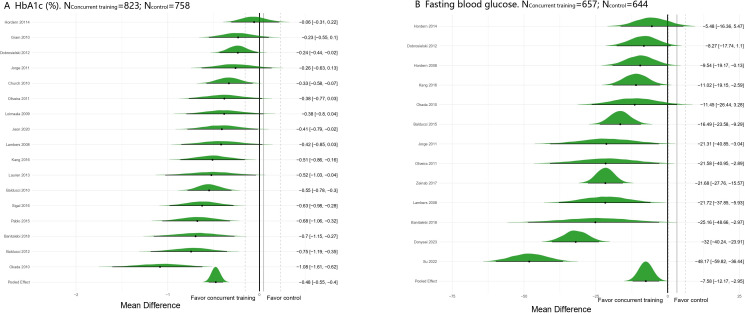
Table 2 and figure 2 show that CT significantly reduced HbA1c levels in patients with type 2 diabetes (MD=−0.48%, 95% CrI: −0.55 to −0.40), with some heterogeneity among different levels (SD=0.31, 95% CrI: 0.17 to 0.51), and the model converged well. Similarly, FBG levels also improved (MD=−0.48 mmol/L, 95% CrI: −0.55 to −0.40), with greater heterogeneity (SD=17.73, 95% CrI: 11.23 to 28.09). CT showed significant improvements in BMI, FBG, BFP, SBP, DBP, and VO2max (table 2, online supplemental appendix 4). The Egger and Begg tests for all outcomes were not significant (p>0.05), suggesting low publication bias (online supplemental appendix 5). In addition, all the models converged well (PSRF <1.01) (online supplemental appendix 6).
Table 2. Pairwise comparison of all outcomes.
| Outcomes | Estimate (MD) | Studies (n) | Patients (n) | l-95% CrI | u-95% CrI | RSRF | SD(Intercept) | sd-l-95% CrI | |
| CT | CG | ||||||||
| HbA1c (%) | −0.48 | 17 | 823 | 758 | −0.55 | −0.4 | 1 | 0.31 | 0.17 |
| FBG | −7.58 | 13 | 657 | 644 | −12.17 | −2.95 | 1 | 17.73 | 11.23 |
| BMI | −0.61 | 14 | 817 | 796 | −1.03 | −0.19 | 1 | 0.17 | 0.01 |
| BFP | −1.1 | 9 | 238 | 224 | −1.64 | −0.58 | 1 | 0.27 | 0.01 |
| SBP | −2.19 | 12 | 466 | 441 | −3.87 | −0.54 | 1 | 1.57 | 0.03 |
| DBP | −1.36 | 12 | 745 | 720 | −2.74 | −0.06 | 1 | 2.75 | 1.24 |
BFPbody fat percentageBMIbody mass indexCGcontrol groupCTconcurrent aerobic combined resistance trainingDBPdiastolic blood pressureFBGfasting blood glucoseHbA1c (%)glycosylated hemoglobinl-95% CIlower limit of 95% credible intervalMDmean differenceSBPsystolic blood pressureSD(Intercept)standard deviation of the random interceptu-95% CIupper limit of 95% credible intervalVO2maxmaximal oxygen uptake
Figure 2. Forest plot for pairwise comparison. (A) Glycosylated hemoglobin (HbA1c). (B) Fasting blood glucose.
Regression analysis
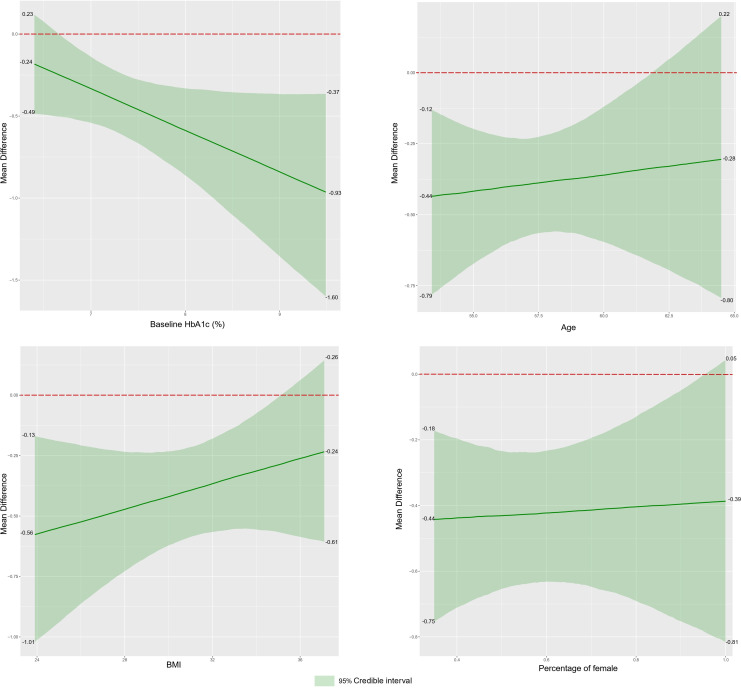
The intervention effect of CT may have been moderated by baseline HbA1c, with higher values of R2 (R2=0.66) for the interaction with CT that included baseline HbA1c compared with the model with only CT (R2=0.61). No significant increase in R2 was observed for the model where CT interacted with age (R2=0.58), BMI (R2=0.57), or proportion of women (R2=0.60). Additionally, analysis of the regression images revealed that a higher baseline HbA1c level might be associated with a better intervention effect; a low BMI resulted in better outcomes than a high BMI (figure 3). However, we found no clear evidence that dropout rates affect the effectiveness of CT interventions (online supplemental appendix 10).
Figure 3. Meta-regression analysis on HbA1c. BMI, body mass index; HbA1c, glycosylated hemoglobin.
Dose-response meta-analysis
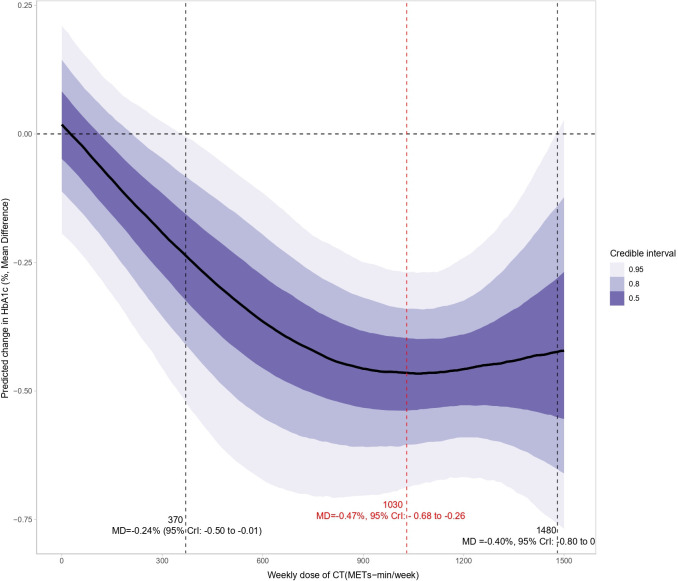
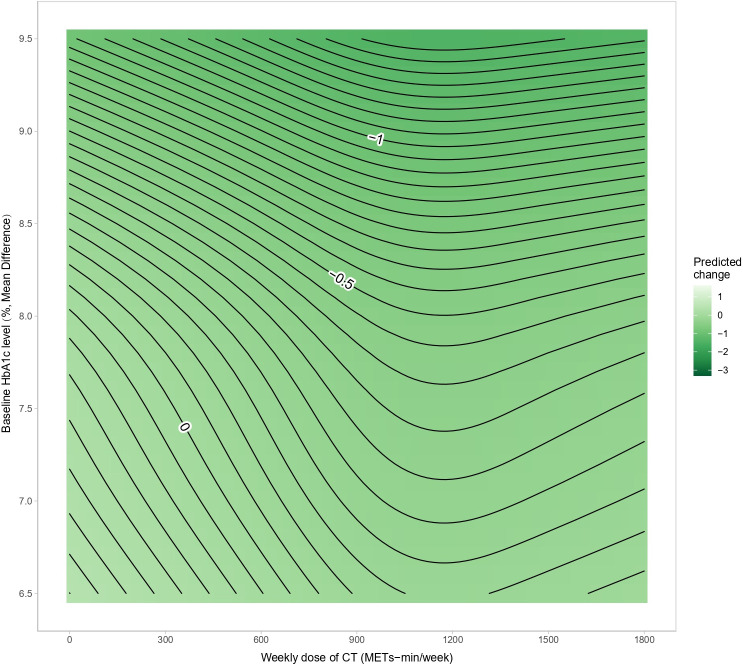
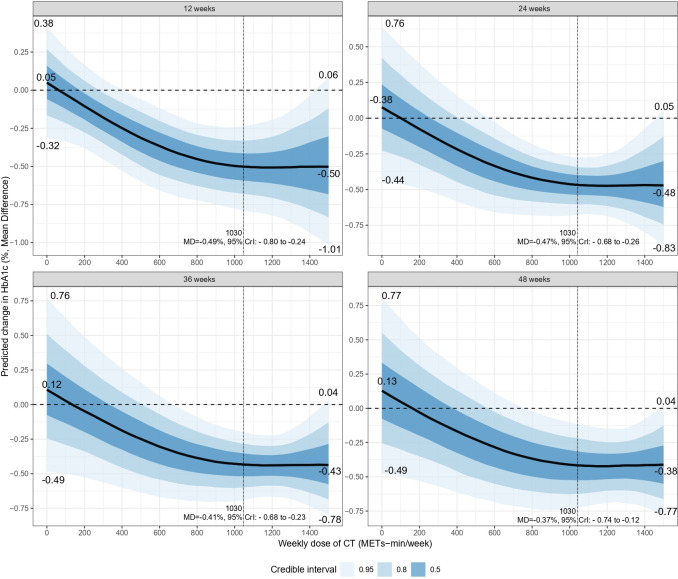
Figure 4 shows the dose-response relationship between CT and HbA1c, revealing a non-linear “J”-shaped relationship. The minimal significant dose for improving HbA1c was 370 METs-min/week, with a pooled effect size of MD=−0.24% (95% CrI: −0.50 to −0.01, SE=0.13). The optimal significant dose was larger, at 1030 METs-min/week, with an effect size of MD=−0.47%, 95% CrI: −0.68 to −0.26, SE=0.11. Tolerance was reached at a CT dose of 1480 (MD=−0.40%, 95% CrI: −0.80 to 0, SE=0.20). Combining the effects of baseline HbA1c and dose revealed that the higher the baseline HbA1c, the greater the effect of CT (figure 5). After adjusting for the total intervention duration, the exercise dose produced a consistent response across weeks (figure 6).
Figure 4. Dose-response relationship between CT and HbA1c levels in patients with type 2 diabetes. CrI, credible interval; CT, concurrent aerobic and strength training; HbA1c, glycosylated hemoglobin; MD, mean differerence; METs, metabolic equivalent of tasks.
Figure 5. Contour plot between CT and HbA1c levels in patients with type 2 diabetes. CT, concurrent aerobic and strength training; HbA1c, glycosylated hemoglobin; METs, metabolic equivalent of tasks.
Figure 6. Dose-response prediction at different weeks between CT and HbA1c levels in patients with type 2 diabetes. CrI, credible interval; CT, concurrent aerobic and strength training; HbA1c, glycosylated hemoglobin; MD, mean difference; METs, metabolic equivalent of tasks.
Discussion
Principal findings
This study is the first meta-analysis detailing the effects of CT in patients with type 2 diabetes and analyzing the effects of CT on multiple aspects of glycemic control, body composition, blood pressure, and VO2max. Long-term CT significantly reduced HbA1c and FBG levels compared with the control group, and the optimal intervention effect was achieved at a dose of 1030 METs-min/week; in addition, CT significantly improved BMI, BFP, blood pressure, and VO2max levels in patients with type 2 diabetes. In addition, the intervention effect of CT may be moderated by baseline HbA1c levels, with a low BMI yielding better results than a high BMI.
Comparison with previous studies
The results of this study show consistency with recent related studies4,7 on several key points. In particular, our findings support the notion that CT has a positive impact on patients with type 2 diabetes, such as glycemic control, blood pressure, and BMI, which is similar to the findings of the study by Al-Mhanna et al,5 which reported similar improvements in the obese type 2 diabetes population. In addition, in contrast to these previous studies, our results further emphasize the potential benefits of different CT doses in the comprehensive management of diabetes and provide an optimal CT dose for clinical practice as a preliminary reference.
HbA1c, a key indicator of glycemic control, is widely used in clinical practice for screening, diagnosis, glycemic control, and efficacy confirmation in patients with diabetes.2 Our study found that CT significantly reduced HbA1c and FBG levels. Several previous studies4,7 have confirmed the benefits of CT in improving HbA1c levels, potentially due to the upregulation of glucose transporter type 4 expression at the cellular level.24 By increasing the cellular uptake of glucose, CT effectively increases the overall metabolic efficiency of glucose in the body,25 ultimately leading to lower blood glucose levels. Therefore, by lowering the average blood glucose concentration, CT indirectly lowered HbA1c levels. Benham et al26 found a dose-response relationship between exercise frequency and HbA1c levels. Gallardo-Gómez et al11 observed a similar relationship between total physical activity and HbA1c levels, identifying an optimal dose of 1100 METs-min/week. The present study confirmed a dose-response relationship between CT intensity and HbA1c levels, identifying an optimal dose of 1030 MET-min/week, which is equivalent to 270 min of moderate-intensity CT or 150 min of high-intensity CT per week. Notably, exercise at only 370 MET-min/week significantly lowered HbA1c levels, well below the minimum weekly exercise dose (600 MET-min) recommended by the WHO.27 Therefore, CT is an important intervention in managing type 2 diabetes and effective for achieving the HbA1c target (6.5%) set by the International Expert Committee on Diabetes.28 Furthermore, Bassi et al24 showed that HbA1c improved significantly in a shorter intervention cycle (12 weeks), consistent with our finding that CT showed stable effects across the intervention durations (12/24/36/48 weeks) (figure 6).
The meta-analysis results showed that CT effectively reduced BMI levels in patients with type 2 diabetes, which is consistent with the findings from two previous RCTs.4 5 In addition, we found that CT had a greater benefit for BFP. CT combines the advantages of aerobic and strength training, effectively improving body fat status by increasing insulin sensitivity and decreasing leptin secretion and fat accumulation in adipose tissue.29 Strength training helps maintain and increase lean body mass, enhancing upper and lower extremity strength and improving the resting metabolic rate.30 Therefore, CT can overcome the limitations of aerobic or strength training alone and improve body composition more comprehensively in patients with type 2 diabetes. However, Dobrosielski et al31 noted that although CT reduced the percentages of total body fat, abdominal fat, and subcutaneous fat, it had no significant effect on visceral fat reduction. Therefore, future studies are required to explore the specific effects of CT on different areas of body fat.
In patients without type 2 diabetes, both aerobic and strength training are effective treatment strategies for lowering blood pressure.26 However, the evidence is less consistent in patients with type 2 diabetes. According to a joint statement by the American College of Sports Medicine and the American Diabetes Association,32 exercise may slightly reduce systolic blood pressure (SBP) in patients with type 2 diabetes, but the reduction in diastolic blood pressure (DBP) is not significant. Previous studies have suggested that diabetes-related metabolic abnormalities may impair vascular function by limiting vasodilatory capacity and exacerbating vasoconstrictor responses, which may lead to the remodeling and hardening of arterial structures, subsequently increasing SBP.33 Nonetheless, the results of the present study confirmed that CT significantly reduced SBP and DBP in patients with type 2 diabetes, possibly because CT intervention increased vascular elasticity and improved metabolic profiles, which may be effective in reversing these unfavorable vascular changes. These findings emphasize the potential value of CT in managing type 2 diabetes, particularly for improving blood pressure control.
Both epidemiological and clinical studies have shown that patients with long-term type 2 diabetes tend to experience a decline in cardiorespiratory fitness, which is negatively correlated with mortality in patients with diabetes.34 In addition, Bassi et al24 confirmed that CT significantly increases VO2max, which is closely associated with a decrease in HbA1c, consistent with our findings. This process reduces the affinity of hemoglobin for oxygen, thereby making oxygen more readily available to tissues during exercise, significantly enhancing the VO2max of patients.35
Clinical implications
The findings of this study support the use of CT as a multi-benefit therapeutic strategy with the ability to flexibly adjust training doses to optimize outcomes based on patient-specific needs and abilities according to the exercise dose recommendation table (based on HbA1c) (table 3). It was also highlighted that patients with high HbA1c levels and low BMI achieved more significant intervention effects on CT. This suggests that CT is particularly effective in enhancing glycemic control and body composition in these patients. Therefore, CT should be considered a key component of a comprehensive type 2 diabetes management program that helps improve patients’ overall health and reduces the risk of associated complications. These findings have important implications for clinical practitioners supporting patients with type 2 diabetes. Our findings emphasize that the systematic inclusion of concurrent training in treatment regimens, with precise dosage adjustments tailored to individual characteristics, is critical to combating this chronic metabolic disease and improving patients’ quality of life and health outcomes.36 Clinical practitioners can use these results to optimize treatment regimens and, more effectively, help patients achieve their health goals. In addition, these findings provide valuable clinical guidance to advance personalized medicine and ensure more efficient treatment protocols.
Table 3. Exercise dose recommendation (based on HbA1c).
| Dose of CT (MET-min/week) | Intensity | Dose intensity and codes* | Recommended training time† (min/week) | Recommended training time (reps×min/week)‡ | ||
| Minimal§ | 370 | Moderate | 3.3 (17160, 02054) | ~110 | 3×~35 | 4×~28 |
| 3.5 (01010, 02054) | ~105 | 3×~35 | 2×~53 | |||
| 3.8 (12027, 02054) | ~100 | 3×~33 | 2×~50 | |||
| High intensity | 6.5 (01014, 02050) | ~60 | 2×~30 | 1×~60 | ||
| 6.9 (12029, 02050) | ~55 | 1×~55 | 2×~30 | |||
| Optimal¶ | 1030 | Moderate | 3.3 (17160, 02054) | ~310 | 5×~60 | 6×~50 |
| 3.5 (01010, 02054) | ~295 | 5×~59 | 6×~49 | |||
| 3.8 (12027, 02054) | ~270 | 4×~68 | 5×~54 | |||
| High intensity | 6.5 (01014, 02050) | ~160 | 4×~40 | 5×~32 | ||
| 6.9 (12029, 02050) | ~150 | 4×~38 | 5×~30 | |||
17160: Walking for pleasure.
02054: Resistance (weight) training, multiple exercises, 8–15 reps at varied resistance.
01010: Bicycling, <10 mph, leisure, to work or for pleasure.
12027: Jogging on a mini-tramp.
01014: Bicycling, general.
12029: Running 4.3 to 4.8 mph.
02050: Resistance (weight lifting - free weight, nautilus or universal-type), power lifting or body building, vigorous effort.
Dose intensity is the average of the dose intensities of the codes in parentheses, the codes are from the 2024 Adult Compendium of Physical Activities22 and the intensity magnitude is referenced to Gallardo-Gómez et al.11
Exercise time does not include warm-up and relaxation.
The number of training sessions per week and the length of each exercise session, generally equally divided between aerobic and strength training time.
Minimum dose to improve HbA1c.
Improvement in HbA1c optimal dose.
CTconcurrent trainingHbA1cglycosylated hemoglobinMETsmetabolic equivalent of tasks
Strengths and limitations
The present study identified several strengths through meta-analysis. First, the use of advanced Bayesian meta-analysis offers flexibility in dealing with complex data structures, accurately modeling multi-arm studies, crossover designs, and nested data in a hierarchy. Additionally, it allows the synthesis of information from different sources, including prior knowledge and experimental data, to enhance analysis accuracy and robustness. This approach also outperforms traditional methods in managing and exploring data heterogeneity, estimating its magnitude and exploring potential sources, such as differences in study design and sample characteristics. These properties make multilevel Bayesian meta-analysis a powerful tool for dealing with complex and variable data.37 Second, by combining pairwise meta-analysis and dose-response analysis based on natural spline modeling, it is possible to gain a more comprehensive understanding of how the study results are obtained at different dose levels rather than just at the level of the combined effect. This approach enhances the credibility of the evidence by providing a contemporaneous training prescription, which can serve as a guideline for both clinical practitioners and people with diabetes.
This study had some limitations. First, the literature’s quality was low because of the limitations of blinded assessment in the included RCTs, potentially affecting the overall quality of the study. Second, the limited number of included studies prevented the analysis of key metrics such as insulin levels and insulin resistance indices, thereby limiting our ability to fully assess CT’s impact on glycemic control in patients with type 2 diabetes. Finally, owing to the limitations of the included literature, we did not categorize for different ages, genders, and levels of obesity when presenting exercise dosage recommendations, potentially reducing the generalizability and replicability of the study results.
Conclusion
This study used advanced Bayesian pairwise and dose-response meta-analysis methods. The results showed that CT significantly improved HbA1c, FBG, BMI, BFP, blood pressure, and VO2max levels in patients with type 2 diabetes, effectively enhancing their overall health status and reducing the risk of related complications. Additionally, the study revealed a “J”-shaped dose-response relationship between CT and HbA1c, with the most significant effects observed with 270 min of moderate-intensity CT or 150 min of high-intensity CT per week. Notably, patients with high HbA1c levels and low BMI levels may be able to obtain more benefits from CT. Nonetheless, given the study’s limitations, future studies should adopt more rigorous methodological criteria and consider a wider range of population characteristics to enhance the reliability and applicability of their findings. These findings provide an essential basis for future clinical practice and guidance, emphasizing the importance of personalized interventions in diabetes management.
supplementary material
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Funding: 1.Jiangsu Province Graduate Student Research and Practice Innovation Program (KYCX24_3509).2.General Program of Social Science Foundation of Jiangsu Province (23TYB004).3.Jiangsu Shuangchuang Talent Program (JSSCRC2021544).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Contributor Information
Han Xue, Email: xhXH258022@yeah.net.
Yuehui Zou, Email: 1154449346@qq.com.
Shijie Zhang, Email: 1916551441@qq.com.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043–140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 3.Sluik D, Buijsse B, Muckelbauer R, et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern Med. 2012;172:1285–95. doi: 10.1001/archinternmed.2012.3130. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Tam WWS, Hounsri K, et al. Effectiveness of Combined Aerobic and Resistance Exercise on Cognition, Metabolic Health, Physical Function, and Health-related Quality of Life in Middle-aged and Older Adults With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2024;105:1585–99. doi: 10.1016/j.apmr.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mhanna SB, Batrakoulis A, Wan Ghazali WS, et al. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ. 2024;12:e17525. doi: 10.7717/peerj.17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan B, Ge L, Xun Y-Q, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15:72. doi: 10.1186/s12966-018-0703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Z, Zhang M, Wang C, et al. The Best Exercise Modality and Dose to Reduce Glycosylated Hemoglobin in Patients with Type 2 Diabetes: A Systematic Review with Pairwise, Network, and Dose–Response Meta-Analyses. Sports Med . 2024;54:2557–70. doi: 10.1007/s40279-024-02057-6. [DOI] [PubMed] [Google Scholar]
- 8.Hordern MD, Coombes JS, Cooney LM, et al. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart . 2009;95:1343–9. doi: 10.1136/hrt.2009.165571. [DOI] [PubMed] [Google Scholar]
- 9.Gram B, Christensen R, Christiansen C, et al. Effects of nordic walking and exercise in type 2 diabetes mellitus: a randomized controlled trial. Clin J Sport Med . 2010;20:355–61. doi: 10.1227/NEU.0b013e3181e56e0a. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Lu B, Su W, et al. Comprehensive assessment of the effects of concurrent strength and endurance training on lipid profile, glycemic control, and insulin resistance in type 2 diabetes: A meta-analysis. Medicine (Baltimore) 2024;103:e37494. doi: 10.1097/MD.0000000000037494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallardo-Gómez D, Salazar-Martínez E, Alfonso-Rosa RM, et al. Optimal Dose and Type of Physical Activity to Improve Glycemic Control in People Diagnosed With Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2024;47:295–303. doi: 10.2337/dc23-0800. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J. Cochrane handbook for systematic reviews of interventions version 6.4. 2023
- 14.JP H. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. 2: The Cochrane Collaboration. 2009. https://training.cochrane.org/handbook/archive/v5.0.2/ Available. [Google Scholar]
- 15.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bürkner P-C. Advanced Bayesian Multilevel Modeling with the R Package brms. R J. 2018;10:395. doi: 10.32614/RJ-2018-017. [DOI] [Google Scholar]
- 17.Etzioni RD, Kadane JB. Bayesian statistical methods in public health and medicine. Annu Rev Public Health. 1995;16 doi: 10.1146/annurev.pu.16.050195.000323. [DOI] [PubMed] [Google Scholar]
- 18.Williams DR, Rast P, Bürkner P-C. Bayesian meta-analysis with weakly informative prior distributions. PsyArXiv . 2018 doi: 10.31234/osf.io/7tbrm. Preprint. [DOI]
- 19.M. J. Betancourt MG. Hamiltonian monte carlo for hierarchical models. 2013
- 20.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–55. [Google Scholar]
- 21.Sacks DB, Bruns DE, Goldstein DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436–72. [PubMed] [Google Scholar]
- 22.Herrmann SD, Willis EA, Ainsworth BE, et al. 2024 Adult Compendium of Physical Activities: A third update of the energy costs of human activities. J Sport Health Sci. 2024;13:6–12. doi: 10.1016/j.jshs.2023.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson B. ACSM’s guidelines for exercise testing and prescription. J Can Chiropr Assoc. 2014;58:328 [Google Scholar]
- 24.Bassi D, Mendes RG, Arakelian VM, et al. Potential Effects on Cardiorespiratory and Metabolic Status After a Concurrent Strength and Endurance Training Program in Diabetes Patients - a Randomized Controlled Trial. Sports Med Open. 2015;2:31. doi: 10.1186/s40798-016-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores-Opazo M, McGee SL, Hargreaves M. Exercise and GLUT4. Exerc Sport Sci Rev. 2020;48:110–8. doi: 10.1249/JES.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 26.Benham JL, Booth JE, Dunbar MJ, et al. Significant Dose-Response between Exercise Adherence and Hemoglobin A1c Change. Med Sci Sports Exerc. 2020;52:1960–5. doi: 10.1249/MSS.0000000000002339. [DOI] [PubMed] [Google Scholar]
- 27.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–62. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The International Expert Committee International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinas PC, Markati AS, Carrillo AE. Exercise-Induced Biological and Psychological Changes in Overweight and Obese Individuals: A Review of Recent Evidence. ISRN Physiol. 2014;2014:1–11. doi: 10.1155/2014/964627. [DOI] [Google Scholar]
- 30.Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11:209–16. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- 31.Dobrosielski DA, Gibbs BB, Ouyang P, et al. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med. 2012;27:1453–9. doi: 10.1007/s11606-012-2103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–6. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 34.Röhling M, Strom A, Bönhof GJ, et al. Cardiorespiratory Fitness and Cardiac Autonomic Function in Diabetes. Curr Diab Rep. 2017;17 doi: 10.1007/s11892-017-0959-z. [DOI] [PubMed] [Google Scholar]
- 35.Larose J, Sigal RJ, Khandwala F, et al. Associations between physical fitness and HbA₁(c) in type 2 diabetes mellitus. Diabetologia. 2011;54:93–102. doi: 10.1007/s00125-010-1941-3. [DOI] [PubMed] [Google Scholar]
- 36.Batrakoulis A, Jamurtas AZ, Fatouros IG. Exercise and Type II Diabetes Mellitus: A Brief Guide for Exercise Professionals. Str & Cond J. 2022;44:64–72. doi: 10.1519/SSC.0000000000000731. [DOI] [Google Scholar]
- 37.Part I. In: Doing bayesian data analysis. Kruschke JK, editor. Boston: Academic Press; 2015. Introduction; p. 13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.