Abstract
Introduction
Trials of GLP-1 (glucagon-like peptide-1) medicines have changed the paradigm of obesity treatment. Diversity in trial participation is imperative considering that obesity disproportionately impacts marginalised populations worldwide. We performed a systematic review and meta-analyses to evaluate the representation of racialised and ethnically diverse populations in randomised controlled trials (RCTs) of GLP-1 medicines for obesity.
Methods
We searched PubMed/Embase/ClinicalTrials.gov. Prevalence of each racial/ethnic group was compared in relation to the USA, Canada, the UK, Brazil and South Africa. The geographical locations of the trial sites were extracted.
Results
27 RCTs were identified (n=21 547 participants). Meta-analyses of prevalence demonstrated the vast predominance of white/Caucasians (79%) with smaller proportion of blacks (9%), Asians (13%), Indigenous (2%) and Hispanics (22%). The gaps in representation were evidenced by the significantly under-represented proportion of non-white individuals in these RCTs as compared with the prevalence of non-white individuals in the general population of the USA (−23%, p=0.002) and Canada (−34%, p<0.0001), reaching an alarming gap of −58% in relation to Brazil and striking under-representation of −68% as compared with South Africa. Similar discrepancies in proportions of blacks, Asians and Indigenous peoples as compared with reference nations were found. Moreover, the trial sites (n=1859) were predominately located in high-income countries (84.2%), in sharp contrast to the global prevalence of obesity that is predominantly in low-income and middle-income countries.
Conclusion
There are discrepancies in representation of racialised and ethnically diverse populations in obesity trials as compared with multiethnic populations worldwide. These data highlight the need for broader reform in the research process in order to ultimately address health inequities.
Keywords: Global Health, Systematic review
WHAT IS ALREADY KNOWN ON THIS TOPIC
Obesity disproportionately impacts marginalised populations worldwide.
There is possibility of differential pharmacological responses in individuals from diverse racial/ethnic background.
The assessment of representation of racialized and ethnically diverse populations is of critical importance.
WHAT THIS STUDY ADDS
Our study represents a comprehensive portrayal of the representation of racialised and ethnically diverse populations in randomised controlled trials (RCTs) of glucagon-like peptide-1 medicines for obesity treatment obtained by a systematic review and meta-analyses (n=21 547 participants; 27 studies).
We showed a consistent pattern of under-representation of diverse populations in these RCTs as compared with multiethnic countries.
We demonstrated that the trial sites (n=1859) were predominately located in high-income countries (84.2%), in sharp contrast to data on global prevalence of obesity that is predominant in low-income and middle-income countries.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
There are discrepancies in representation of racialised and ethnically diverse populations in obesity trials as compared with multiethnic populations worldwide.
These data highlight the need for a broader reform in the research process in order to ultimately address health inequities.
Introduction
Obesity, defined by body mass index (BMI) ≥30 kg/m2, is a complex chronic disease that has become a major public health problem worldwide linked to a variety of obesity-associated diseases and increased mortality.1 2 Notably, even metabolically healthy obesity in which increased BMI occurs without metabolic disease is associated with 24% higher cardiovascular (CV) mortality over 10 years follow-up as compared with lean individuals.3 Nevertheless, the therapeutic toolkit for successful long-term management of obesity has represented one of the biggest challenges of modern medicine. Historically, adjunctive pharmacotherapy for weight loss promoted moderate weight reduction, with significant associated adverse events, and rebound weight gain once withdrawn.4 In addition, despite the fact that observational studies of bariatric surgery suggested increased life expectancy following these procedures,5 for decades there was a lack of trial evidence of the clinical benefit of obesity management on CV mortality.6 The bench-to-trial discovery of modern glucagon-like peptide-1 (GLP-1) medicines has transformed this landscape.
The results of recent randomised controlled trials (RCT) of GLP-1 medicines for weight loss have changed the paradigm of obesity treatment providing robust trial data to support the pharmacological management of obesity aiming to reduce the burden of this global epidemic.7 Specifically, GLP-1 receptor agonists (GLP-1 RA)-based therapies (including formulations with glucose-dependent insulinotropic polypeptide (GIP) and glucagon (GCG) receptor agonists) have demonstrated not only a powerful weight-reduction effect of ~15%–25% of body weight8,10 but also cardiorenal benefits in selected populations coupled with an acceptable safety profile.11 12 Moreover, trials published over the past months have expanded the potential efficacy of this class of medications to include a positive impact on diseases associated with obesity such as metabolic liver disease13 and obstructive sleep apnoea14 with several other ongoing trials being conducted on the use of GLP-1 RA medicines in other clinical settings.
In light of the growing evidence of the clinical benefits yielded from RCTs of GLP-1 medicines for obesity, it is relevant to evaluate the suitability of the broad generalisation of these trial results. Notably, the assessment of representation of racialised and ethnically diverse populations is of critical importance considering that obesity disproportionately impacts marginalised population worldwide1 15 and the possibility of differential pharmacological responses in individuals from diverse racial/ethnic backgrounds.16 17 In addition to the generation of biomedical knowledge relevant to global communities, key aspects of proper diversity and representativeness in human trials such as earning and building trust in medical research and fairness promotion support the relevance of diverse clinical trial participation.18 Thus, we sought to perform a systematic review and meta-analysis to evaluate the representation of racialised and ethnically diverse populations in RCTs of GLP-1 medicines for obesity treatment.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement19 and was registered at International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/prospero/; CRD42024527758).
Search strategy and selection criteria
We selected relevant studies published up to 8 July 2024, by searching Embase, PubMed and ClinicalTrials.gov. The following combined text and Medical Subject Heading terms were used: glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptor agonist and obesity. The complete search used for PubMed was (“glucagon-like peptide-1 receptor agonists”[All Fields] OR “glucagon-like peptide-1 receptor agonists”[MeSH Terms]) OR (“glucose-dependent insulinotropic polypeptide receptor agonist” [All Fields] AND agonist[All Fields]) OR (“tirzepatide” OR “liraglutide” OR “semaglutide” OR “retatrutide” OR “survodutide” OR “orforglipron”) AND (“Obesity”[Mesh] OR “Anti-Obesity Agents”[Mesh] OR “Obesity Management”[Mesh])) OR (“Weight Loss”[Mesh]). All potentially eligible studies were considered for review, regardless of the primary outcome or language. A manual search was also performed, using references of key articles published in English.
Studies were eligible for inclusion if they (1) evaluated the impact of any incretin-based therapy including GLP-1RA, GIP-GLP-1R co-agonists, GCG-GLP-1R co-agonists or GCG-GIP-GLP1R tri-agonists for weight management of overweight/obesity in adult patients (primary outcome) using injectable or oral formulations, (2) presented original data of an RCT either phase 2 or phase 3, (3) enrolled participants from more than one study centre (multicentre). Exclusion criteria were as follows: (1) studies conducted in paediatric population, (2) single centre RCTs, (3) studies that did not report data on race/ethnicity or (4) observational studies.
Data analysis
Two independent investigators (YJ and CKK) reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full-text evaluation. Studies selected for detailed analysis and data extraction were analysed by two investigators with an agreement value (κ) of 95·5%; disagreements were resolved by a third investigator (RR). The following data were extracted for each study: source of study funding, incretin-based therapy evaluated, study population, total number of participants, number and geographical location of study sites and baseline characteristics of participants by race/ethnicity (table 1). The risk for bias was evaluated according to the revised Cochrane tool for assessing risk of bias in RCTs (RoB 2)20 (online supplemental table 1).
Table 1. Characteristics of included studies.
| Author (programme) | Year | Sponsor | Incretin therapy | Study population | No of sites | Sample size (n) | Females (%) | Individual race/ethnic groups included(self-reported) | ||||
| White | Black/African American | Asian | Indigenous | Hispanic | ||||||||
| Wadden et al30 (SCALE maintenance) | 2013 | Novo Nordisk | Liraglutide | BMI ≥30 or ≥ 27 with comorbidities who lost ≥5% body weight with diet | 36 | 422 | 81.5 | Yes | Yes | No | No | No |
| Davies et al31 (SCALE diabetes) | 2015 | Novo Nordisk | Liraglutide | BMI ≥27 and T2DM | 126 | 846 | 49.8 | Yes | Yes | Yes | No | Yes |
| Pi-Sunyer et al32 (SCALE) | 2015 | Novo Nordisk | Liraglutide | BMI ≥30 or ≥ 27 with comorbidities without diabetes | 191 | 3731 | 78.5 | Yes | Yes | Yes | Yes | Yes |
| O'Neil et al33 (phase 2) | 2018 | Novo Nordisk | Liraglutide and semaglutide | BMI ≥30 kg/m2 without diabetes | 71 | 957 | 64.7 | Yes | Yes | No | No | No |
| Garvey et al34 (SCALE insulin) | 2020 | Novo Nordisk | Liraglutide | BMI ≥27 and T2DM treated with insulin | 54 | 396 | 53 | Yes | Yes | Yes | No | Yes |
| Wadden et al35 (SCALE IBT) | 2020 | Novo Nordisk | Liraglutide | BMI ≥30K without diabetes | 17 | 282 | 83.3 | Yes | Yes | Yes | No | Yes |
| Alba et al36 (phase 2) | 2021 | Janssen | JNJ-64565111 and liraglutide | BMI ≥35 and ≤50 without diabetes | 51 | 474 | 75.1 | Yes | Yes | Yes | No | No |
| Davies et al37 (STEP 2 diabetes) | 2021 | Novo Nordisk | Semaglutide | BMI ≥27 and T2DM | 149 | 1210 | 50.1 | Yes | Yes | Yes | No | Yes |
| Nahra et al38 (phase 2) | 2021 | AstraZeneca | Cotadutide and liraglutide | BMI ≥25 and T2DM | 120 | 834 | 53.7 | Yes | Yes | Yes | No | No |
| Rubino et al39 (STEP 4) | 2021 | Novo Nordisk | Semaglutide | BMI ≥30 or ≥ 27 with comorbidities without diabetes | 73 | 803 | 78.9 | Yes | Yes | Yes | No | Yes |
| Wadden et al40 (STEP 3) | 2021 | Novo Nordisk | Semaglutide | BMI ≥30 or ≥27 with comorbidities without diabetes | 41 | 611 | 81.0 | Yes | Yes | Yes | Yes | Yes |
| Wilding et al8 (STEP 1) | 2021 | Novo Nordisk | Semaglutide | BMI ≥30 or ≥27 with comorbidities without diabetes | 129 | 1961 | 74.0 | Yes | Yes | Yes | No | Yes |
| Garvey et al41 (STEP 5) | 2022 | Novo Nordisk | Semaglutide | BMI ≥30 or ≥27 with comorbidities without diabetes | 41 | 304 | 77.6 | Yes | Yes | Yes | Yes | Yes |
| Kadowaki et al42 (STEP 6) | 2022 | Novo Nordisk | Semaglutide | BMI ≥27 with ≥2 comorbidities or BMI ≥30 ≥1 comorbidity | 28 | 401 | 37 | No | No | Yes | No | No |
| Jastreboff et al9 (SURMOUNT 1) | 2022 | Eli Lilly | Tirzepatide | BMI ≥30 or ≥ 27 with comorbidities without diabetes | 119 | 2539 | 67.5 | Yes | Yes | Yes | Yes | Yes |
| Rubino et al43 (STEP 8) | 2022 | Novo Nordisk | Semaglutide and liraglutide | BMI ≥30 or ≥ 27 with comorbidities without diabetes | 19 | 338 | 78.4 | Yes | Yes | Yes | No | Yes |
| Garvey et al44 (SURMOUNT 2) | 2023 | Eli Lilly | Tirzepatide | BMI ≥27 and T2DM | 77 | 938 | 51 | Yes | Yes | Yes | Yes | Yes |
| Jastreboff et al10 (phase 2) | 2023 | Eli Lilly | Retatrutide | BMI ≥30 or ≥ 27 with comorbidities without diabetes | 25 | 338 | 48 | Yes | Yes | Yes | Yes | Yes |
| Kosiborod et al51 (STEP HFpEF) | 2023 | Novo Nordisk | Semaglutide | BMI ≥30 with heart failure with preserved ejection | 96 | 529 | 56.1 | Yes | Yes | No | No | Yes |
| Knop et al46 (OASIS) | 2023 | Novo Nordisk | Oral semaglutide | BMI 30 or ≥27 with comorbidities without diabetes | 50 | 667 | 73 | Yes | Yes | Yes | No | Yes |
| Mok et al47 (BARI-OPTIMISE) | 2023 | Novo Nordisk | Liraglutide | <20% body weight loss after metabolic surgery | 2 | 70 | 74 | Yes | Yes | Yes | No | No |
| Wadden et al48 (SURMOUNT 3) | 2023 | Eli Lilly | Tirzepatide | BMI ≥30 or ≥27 with comorbidities without diabetes | 62 | 579 | 62.8 | Yes | Yes | Yes | Yes | Yes |
| Wharton et al49 (phase 2) | 2023 | Eli Lilly | Oral orforglipron | BMI ≥30 or ≥27 with comorbidities without diabetes | 35 | 272 | 59.2 | Yes | Yes | Yes | Yes | No |
| Aronne et al50 (SURMOUNT 4) | 2024 | Eli Lilly | Tirzepatide | BMI ≥30 or ≥27 with comorbidities without diabetes | 70 | 670 | 70.6 | Yes | Yes | Yes | Yes | Yes |
| Kosiborod et al51 (STEP HFpEF diabetes) | 2024 | Novo Nordisk | Semaglutide | BMI ≥30 with heart failure with preserved ejection and T2DM | 108 | 616 | 44.3 | Yes | Yes | Yes | No | No |
| Le Roux et al53 (phase 2) | 2024 | Boehringer Ingelheim | Survodutide | BMI ≥27 without diabetes | 43 | 384 | 68 | Yes | Yes | Yes | Yes | No |
| Mu et al52 (STEP 7) | 2024 | Novo Nordisk | Semaglutide | BMI ≥30 or ≥27 with comorbidities | 23 | 375 | 45 | Yes | Yes | Yes | No | Yes |
BARI-OPTIMISEThe Evaluation of Liraglutide 3; mg in Patients With Poor Weight Loss and a Suboptimal Glucagon-Like Peptide-1 Response.BMI, body mass index; HFpEF, heart failure with preserved ejection fraction; IBT, intensive behavioural therapy; OASIS, Oral Semaglutide Treatment Effect in People with Obesity; SCALESatiety and Clinical Adiposity - Liraglutide Evidence in Nondiabetic and Diabetic IndividualsSTEP, Semaglutide Treatment Effect for People with obesity; SURMOUNT, A Study of Tirzepatide in Participants With Obesity or Overweight; T2DM, type 2 diabetes mellitus
Data on race and ethnicity were self-reported by participants using standardised categories and extracted as described by each individual trial. Data on biological sex (binary) were obtained as reported by the studies. We evaluated the proportion of each of the following race/ethnic groups: white/Caucasian, Non-white, Black/African-American, Asian, Indigenous (including Native Hawaiian or other Pacific Islander, American Indian or Alaska Native) and other (any other racial/ethnic group or multiple). Data on self-identification as Hispanic/Latino were also assessed. We calculated pooled estimates of the prevalence of each ethnic/race group by using a random-effects model (DerSimonian-Laird method) (figure 1). The I2 value was used to evaluate the magnitude of heterogeneity between studies, with values greater than 50% indicating moderate-to-high heterogeneity.21 The possibility of publication bias was evaluated using a funnel plot of effect size against the SE for each trial. Funnel plot asymmetry was evaluated by Begg’s and Egger’s tests, with significant publication bias defined as a p<0.10.22
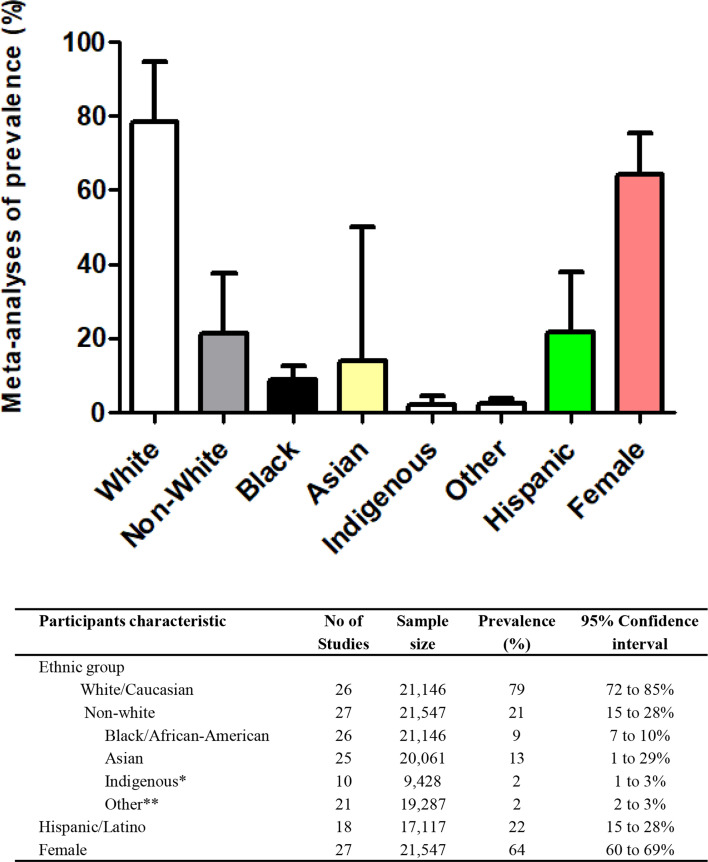
Figure 1. Meta-analysis of proportion of specific ethnic/race groups and females in randomised controlled trials of incretin-based therapies for obesity (data self-reported by study participants). Mean and 95% CIs are shown. *Includes Native Hawaiian or other Pacific Islander, American Indian or Alaska Native. **Any other ethnic group or multiple.
In order to evaluate the representation of racial and ethnically diverse populations in relation to worldwide populations, we meta-analysed the differences in prevalence of each race/ethnic group between study population and general population of the USA,23 Canada,24 the UK,25 Brazil26 and South Africa27 (figure 2). These nations/countries were selected as they are multiethnic nations/countries. Next, we assessed the geographical location of each clinical trial site by continent: (1) North America, (2) South America, (3) Europe, (4) Asia, (5) Africa and (6) Australasia (figures3A 4A), and by World Bank income groups28: (1) low-income and middle-income countries (LMICs) and (2) high-income countries (figure 3B). Global prevalence and projected estimates of obesity worldwide were obtained from the World Obesity Federation.29 All analyses were performed by using Stata V.14.0 (Stata).
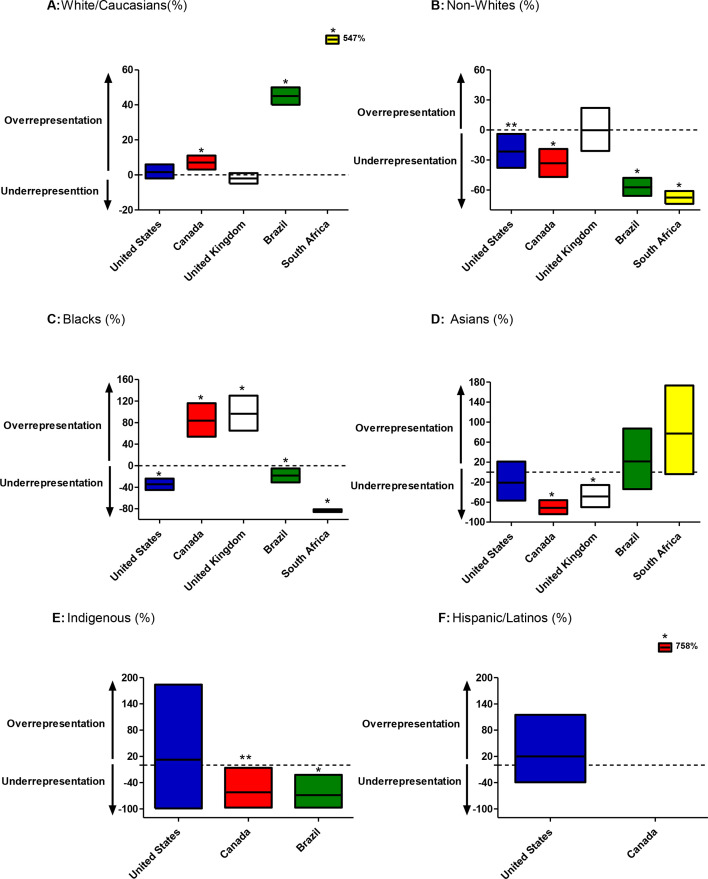
Figure 2. Meta-analyses of differences in proportion (%) of specific ethnic/race groups between study populations and general population of reference countries. (A) White/Caucasians, (B) Non-whites, (C) Blacks, (D) Asians, (E) Indigenous and (F) Hispanic/Latinos. Dash line represents null differences (0%). Mean and 95% CIs are shown (squares). *p<0.0001, **p<0.05.
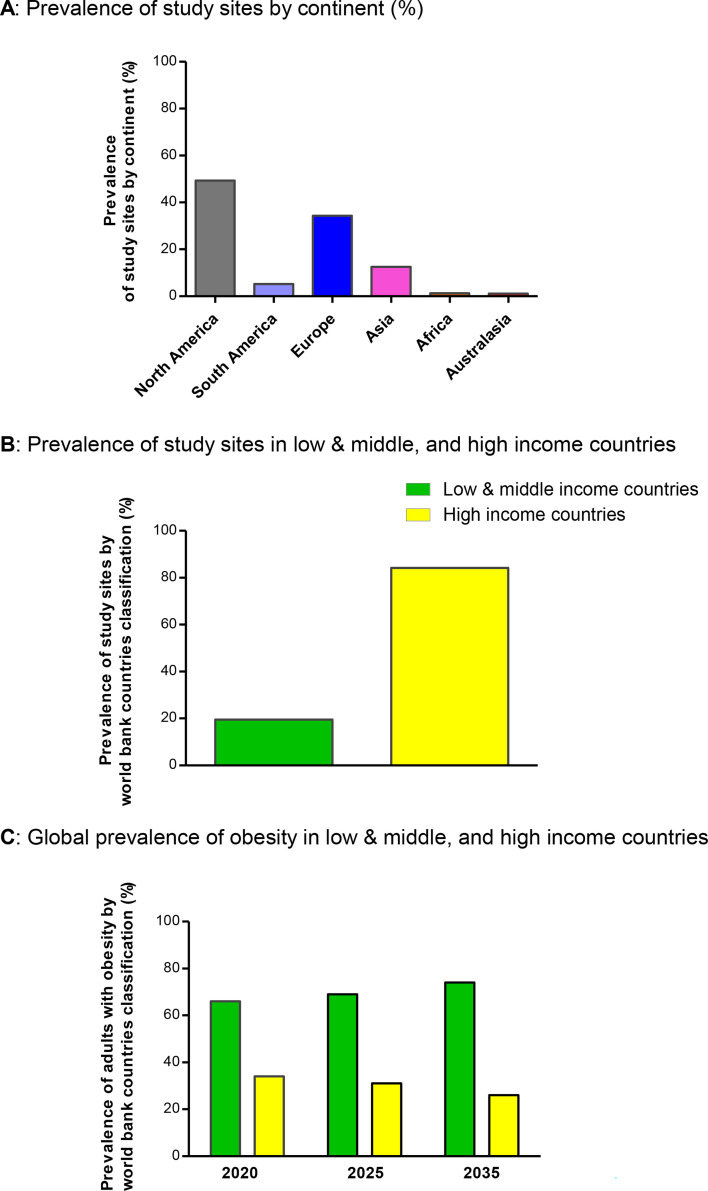
Figure 3. Geographical location of study sites of incretin-based clinical trials for obesity. (A) Prevalence of study sites by continent. (B) Prevalence of study sites in low and middle, and high-income countries according to World Bank. (C) Global prevalence and projected estimates of obesity in low and middle, and high-income countries according to World Bank (years 2020, 2025 and 2035).
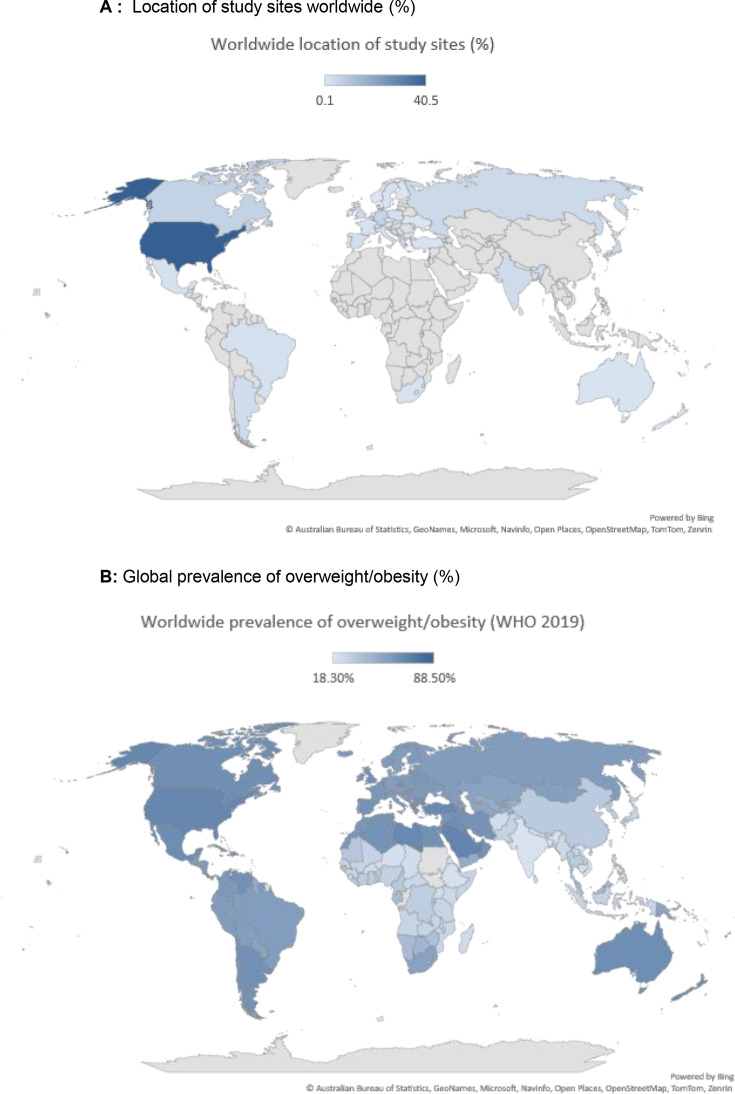
Figure 4. Population enrolled in clinical trials for obesity vs global prevalence of excess weight. (A) Location of study sites worldwide (%) (B) Global prevalence of overweight/obesity (%).
Role of funding source
The study was supported by intramural funds, with no commercial entity involved. The funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Patient and public involvement
There was no patient involvement due to the study design.
Results
We identified 1281 studies through electronic searches, of which 1242 were excluded based on title and abstract. 39 studies were retrieved for detailed assessment, 12 of which were excluded (online supplemental figure 1). 27 RCTs met our inclusion study, providing data for 21 547 participants.8,1030 Table 1 summarises the characteristics of the included trials. These studies were published from 2013 to 2024 and were funded by the pharmaceutical companies Novo Nordisk, Janssen, AstraZeneca, Eli Lilly and Boehringer Ingelheim. The most common incretin-therapy studied was semaglutide (n=12 studies),833 37 39,43 45 46 51 52 followed by liraglutide (n=10 studies)30,3638 43 47 and tirzepatide (n=4 studies).9 44 48 50 The majority of the studies included participants with obesity (BMI ≥30 kg/m2) and/or overweight/obesity (BMI ≥27 kg/m2) with the presence of obesity-related co-morbidities excluding type 2 diabetes. Dedicated RCTs including individuals with type 2 diabetes and BMI ≥27 kg/m2 were also included.31 37 38 44 51 The sample size studied varied between 70 and 3731 participants. These RCTs were performed worldwide and the number of study sites varied from 2 to 191; 2 RCTs were performed in East Asia and included mainly individuals with Asian background.42 52 All included studies had low risk of bias according to RoB 2 (online supplemental table 1).
The meta-analysis of the proportion of individual racial/ethnic groups within the population enrolled in RCTs evaluating GLP-1 medicines for obesity demonstrated a vast predominance of participants self-identified as white/Caucasian. Figure 1 shows the prevalence of each group. Specifically, 26 of the 27 studies included white/Caucasian participants (n=21 146), with a mean prevalence of 79% (95% CI 72% to 85%; I2=99.4%). All studies included non-white participants (n=21 547), with a mean prevalence of 21% (95% CI 15% to 28%; I2=99.3%). Representing the non-white group, the mean prevalence of black/African-American participants was 9% (95% CI 7% to 10%), Asian was 13% (95% CI 1% to 29%) and the proportion of Indigenous participants was 2% (95% CI 1% to 3%) (figure 1). Notably, only 10 studies reported data on Indigenous participants (table 1 and figure 1).910 32 40 41 44 48,50 53 18 of the studies included Hispanic/Latino participants, with a mean prevalence of 22% (95% CI 15% to 28%). The funnel plot for prevalence of white-Caucasian and non-white demonstrated no publication bias (Ps>0.10) (data not shown). Regarding biological sex, the majority of the included participants were female (65% (95% CI 60% to 69%; I2=98.3%)).
Next, we completed a meta-analysis of differences in proportions of racial and ethnically diverse peoples between study participants and the general population of the USA, Canada, the UK, Brazil and South Africa (figure 2). We demonstrated that white individuals were well represented in these RCTs as compared with general population of the USA (mean difference +1%, 95% CI −1% to+6%, p=0.19) and the UK (mean difference −2%, 95% CI −5% to+1%, p=0.24) but significantly over-represented as compared with general population of Brazil (mean difference +45%, 95% CI +40% to +50%, p<0.0001) and South Africa (mean difference +547%, 95% CI +524% to +570%, p<0.0001) (figure 2A). Echoing these results, the proportion of non-white individuals accurately represent the prevalence of non-whites in the UK (p=0.91) (figure 2B). However, this racial/ethnic group is significantly under-represented in the RCTs as compared with general population of USA (mean difference −23%, 95% CI −38% to −4%, p=0.002) and Canada (mean difference −34%, 95% CI −47% to −19%, p<0.0001), reaching an alarming gap of −58% (95% CI −66%to −48%, p<0.0001) in relation to Brazil and a striking under-representation of −68% (95% CI −74% to −61%, p<0.0001) as compared with the general population of South Africa (figure 2B).
Addressing each racial and ethnically diverse group individually, we demonstrated that black/African-Americans participants were over-represented in these obesity trials as compared with nations that have reduced proportion of individuals of African descent such as Canada (mean difference +84%, 95% CI +54% to +116%, p<0.0001) and UK (mean difference +94%, 95% CI +64% to +130%, p<0.0001) (figure 2C). On the other hand, black/African-Americans were under-represented in the study population as compared with general population of USA (mean difference −35%, 95% CI −45% to −24%, p<0.0001), Brazil (mean difference −19%, 95% CI −31% to −5%, p=0.011) and South Africa (mean difference −83%, 95% CI −86% to −91%, p<0.0001) (figure 2C). Regarding representation of participants with Asian background, we showed that Asian population was well represented in these RCTs as compared with general population from the USA (p=0.220), Brazil (p=0.695) and South Africa (p=0.068). Conversely, the Asian population was significantly under-represented in relation to Canada (−74%, 95% CI −84% to 56%, p<0.001) and the UK (−50%, 95% CI −70% to −26%, p=0.009) (figure 2D).
The proportion of Indigenous participants in the incretin-based RCTs for obesity was well represented in relation to the USA (p=0.45), but there was a gap of −83% in comparison to prevalence of Indigenous population in Canada (95% CI −97% to −6%, p=0.04) with an even wider discrepancy of −86% in relation to the prevalence of Indigenous population in Brazil (95% CI −97% to −22%, p=0.025) (figure 2E). Regarding the ethnically diverse population of Hispanics/Latinos, the proportion of these groups enrolled in the trials reflects the Hispanic population in the USA (mean difference −16%, 95% CI −39% to +115%, p=0.28) but is over-represented in relation to the proportion of Hispanic/Latinos living in Canada (mean difference +758%, 95% CI +528% to +1007%, p<0.001) (figure 2F).
Further to these analyses, we sought to evaluate the geographical location of study sites worldwide. The 27 RCTs on incretin-based therapies for the treatment of obesity had a total of 1859 study sites located across the globe (table 1). The study sites were distributed predominately in North America (49.3%; USA, Canada, Puerto Rico and Mexico) and Europe (34.3%; Belgium, Bulgaria, Denmark, Germany, France, Finland, Poland, Russia, UK, Hungary, Netherlands, Greece, Spain, Italy, Sweden, Turkey, Czech Republic, Slovakia, Austria, Serbia and Montenegro, Switzerland, Ireland, Norway, Portugal and Ukraine), with the lowest proportion in Australasia (1.1%; Australia and New Zealand), Africa (1.3%; South Africa), South America (5.2%; Brazil and Argentina) and Asia (12.6%; India, Japan, Taiwan, Mainland China and Honk Kong, Israel, United Arab Emirates and South Korea) (figure 3A). Collectively, 84.2% of the study sites were based in high-income countries and only 19.5% in LMIC (figure 3B). These results are in sharp contrast to data on global prevalence of obesity that demonstrates that 66% of adults living with obesity were residing in LMIC in 2020 (ie, only 34% in high-income countries) (figure 3C). Moreover, the projected estimates of obesity for 2025 and 2035 are dominant in LMIC as compared with its prevalence in high-income countries (figure 3C). As demonstrated in figure 4, while the global prevalence of overweight/obesity (WHO) is greatest in North America, South America, Australasia, North Africa (Morocco, Algeria, Libya, Egypt) and countries in Western Asia (Saudi Arabia, Iraq and Iran), the greatest prevalence of study sites in this meta-analysis was in the USA (40.5%), with other countries being vastly under-represented.
Discussion
This comprehensive evaluation of the representation of racialised and ethnically diverse populations in GLP-1 medicine trials for the treatment of obesity showed the predominance of females and a consistent pattern of under-representation of diverse groups such as Non-whites, Black/African-Americans, Asians, Indigenous and Hispanic/Latinos as compared with multiethnic nations (the USA, Canada, the UK, Brazil and South Africa). Individuals self-identified as white represent the vast majority of the population enrolled in these RCTs (79%) which translates to a significant over-representation that is particularly pronounced when compared with lower-income countries (Brazil and South Africa). Importantly, the location of trial sites was predominantly in high-income countries (84.2%) in sharp contrast to the greater and increasing prevalence of obesity in LMICs.
The over-representation of females in our analyses (65%) is supported by previous reports that have demonstrated a historical preponderance of women enrolled in obesity trials54 55 which is in contrast to the under-representation of female participants in RCTs in other medical fields (ie, cardiology and paediatrics).56 While this result could reflect the increased prevalence of women seeking care in obesity clinics, sex differences in the effect of weight loss interventions have been reported with women presenting with greater weight reduction and improvement in physical function in response to weight-loss interventions than men.57 58 In the phase 2 trial of the GCG-GIP-GLP1-R triagonist, retatrutide, women had significantly augmented weight reduction compared with men (−28.5% vs −19.8% with retatrutide 12 mg).10 Similarly, in the post hoc analyses of the semaglutide trial STEP-1, the estimated treatment difference in weight reduction in the semaglutide group compared with the group receiving placebo was −14.0% in women vs −8.0% in men.8 59 A possible explanation for the enhanced clinical response to incretin-therapies in women could be related to a favourable pharmacokinetic profile (ie, increased plasma concentration) observed in females as compared with males.60 In addition, sex differences in the regulation of feeding behaviour due to the impact of sex hormones have been described. Specifically, superior efficacy of GLP-1 RA medications on satiety regulation in females could be explained by the promotion of enriched incretin-based reduction in food intake and food reward in the presence of oestrogen.59 61 These data suggest that equal representation of men and women in obesity trials is important for the appropriate generalisation of trial results to the general population where the prevalence of men and women is equivalent.
The reduced participation of Non-whites, Black/African-Americans, Asians, Indigenous and Hispanic/Latinos in the included studies was consistent throughout the comparisons with multiethnic nations, reaching striking gaps as demonstrated in figure 2. The lack of diversity in obesity trial dated back to three decades in previous analyses of all classes of antiobesity drugs.55 62 Our study exposed a persistent structural problem in the clinical research enterprise that yields consequences relevant to clinical care. Although previous post hoc analyses of liraglutide and semaglutide trials did not demonstrate treatment effect differences in the magnitude of weight reduction between the racial subgroups white, black/African-American, Asian and Hispanic/Latinos,63 64 individuals from diverse backgrounds present with important variations in the risk profile of obesity-related diseases. Particularly, illustrative of this aspect is the evidence that for the equivalent age-adjusted and sex-adjusted incidence of type 2 diabetes at a BMI of 30.0 kg/m2 in white populations, the BMI cutoffs for other groups are 23.9 kg/m2 in south Asian, 28.1 kg/m2 in black, 26.9 kg/m2 in Chinese and 26.6 kg/m2 in Arab populations.17 Indigenous populations represent another group with unique risk for obesity and its complications as evidenced by studies of Indigenous peoples to South America15 and North America.65 Yet, in our systematic review and meta-analyses, only 10 studies reported data on Indigenous peoples. In addition, analysis of racial disparities in obesity-related CV mortality in the USA demonstrates increased mortality rate among black individuals as compared with other groups.66 These findings of racial and ethnic-specific profiles of obesity-related cardiometabolic risk suggest the possibility of differential treatment effect driven by diversity and its complex interrelation with biological and social determinants of health. Notably, the small sample size of non-white groups in these clinical trials may impact the statistical power of post hoc stratified analyses by racial/ethnic groups which should be interpreted with caution.
We recognise that our analyses have limitations that are inherent to large multicentre RCTs design. Specifically, data on race and ethnicity were based on participant self-reporting by using standardised categorises that were used by these trials, and these information were missing for a few study locations. Thus, the possibility of reporting bias cannot be excluded, being particularly relevant for individuals with mixed racial backgrounds. In addition, the epidemiological nature of our analyses could have oversimplified the population diversity within each racial/ethnicity category. For instance, the group categorised as ‘Asian’ included individuals with Japanese, Chinese, South Asian and Korean background among others and the ‘Indigenous’ group included Native Hawaiian or other Pacific Islander, American Indian or Alaska Native among others. However, the comparison of similar racial/ethnic categories between study population and reference nations located in diverse continents and representing high and LMICs likely mitigated this possible limitation, yielding a consistent pattern of under-representation of diverse groups. Another aspect of relevance is that all included trials were funded by pharmaceutical industry. It is possible that greater efforts to ensure diverse trial participation are practised by non-profit lead research.
To increase diversity in clinical trials, it is important to recognise the barriers to equitable recruitment. The preponderance of trial sites in high-income countries reflects the underlying global structural inequalities in healthcare research facilities. As an example, African researchers often experience political, logistic and economic challenges to conduct competitive research as compared with the rest of the world as a result of the high cost of laboratory supplies, indirect access to manufactures, poor logistic infrastructure and inefficient institutional support for research.67 Large RCTs funded by the pharmaceutical industry could represent an opportunity for capacity building in these locations in partnership with local governments, scientists and communities. Another important aspect pertains to the history of research abuses and medical experimentation against vulnerable populations (including Black and Indigenous populations, prisoners, pregnant women, and individuals living in LMICs),68 69 that have prompted deep-rooted mistrust in disenfranchised groups. After the American Tuskegee syphilis study in which black participants with syphilis were withheld treatment even after the treatment of choice, penicillin, was becoming widely available, black patients were less likely to trust physicians and seek healthcare.69 Similarly, in the dark past of Canada, horrendous nutrition experiments done in residential schools exposed young Indigenous children to chronic malnutrition and its complications,70 a historical wound that certainly contributes to the negligible trial participation of Indigenous peoples in diverse RCT settings.71 While the ongoing lack of diversity in modern RCTs may perpetuate the mistrust that marginalised groups have in medical institutions, inclusive and culturally safe enrolment practices should be pursued. In an important recognition of this problem, the American National Academies of Sciences, Engineering and Medicine recently made system-level recommendations to improve diversity in clinical trials.72 These recommendations included mandating study sponsors the submission of equitable recruitment plans to ensure that the trial population reflects the demographics of the disease studied, encouraging the submission of demographic characteristics to ClinicalTrials.gov, changing journal requirements for authors to include information on the representativeness of their trials in manuscripts and providing adequate compensation for trial participants, among others.72
In summary, our findings suggest there is under-representation of racialised and ethnically diverse populations in obesity trials evaluating GLP-1 medicines in relation to multiethnic populations worldwide. This under-representation, including from populations who experience high rates of obesity and its metabolic complications and would likely benefit significantly from obesity treatment, impacts the generalisability of study results, exacerbates historical gaps and promotes inequities in healthcare. Greater efforts to allow for more equitable recruitment of under-represented populations in clinical trials for obesity must be implemented globally in a collaborative initiative among governments, pharmaceutical sponsors, study investigators, patients and affected communities.
supplementary material
Acknowledgements
Dr Yaanu Jeyakumar identifies as a physician from a visible minority (Sri Lankan Tamil). Dr Lisa Richardson identifies as mixed Anishinaabe descent. Dr Shohinee Sarma identifies as a physician of visible minority. Dr Ravi Retnakaran holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital. Dr Caroline K Kramer is a Brazilian scholar, has mixed Indigenous ancestry (Tupi-Guarani) from Rio Grande do Sul/Brazil.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Fi Godlee
Patient consent for publication: Not applicable.
Data availability free text: Datasets generated during this study are not publicly available but are available from the corresponding author on reasonable request.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: The study is exempt from ethic approval since it does not involve human’s participants directly.
Contributor Information
Yaanu Jeyakumar, Email: yaanu.jeyakumar@mail.utoronto.ca.
Lisa Richardson, Email: lis.richardson@utoronto.ca.
Shohinee Sarma, Email: shohinee.sarma@gmail.com.
Ravi Retnakaran, Email: Ravi.Retnakaran@sinaihealth.ca.
Caroline K Kramer, Email: caroline.kramer@sinaihealth.ca.
Data availability statement
Data are available on reasonable request.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024:4031027–50. doi: 10.1016/S0140-6736(23)02750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 4.Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016;315:2424–34. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson LMS, Carlsson B, Jacobson P, et al. Life expectancy after bariatric surgery or usual care in patients with or without baseline type 2 diabetes in Swedish Obese Subjects. Int J Obes (Lond) 2023;47:931–8. doi: 10.1038/s41366-023-01332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet. 2016;387:1947–56. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 7.Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2022;399:259–69. doi: 10.1016/S0140-6736(21)01640-8. [DOI] [PubMed] [Google Scholar]
- 8.Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 9.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387:205–16. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 10.Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med. 2023;389:514–26. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 11.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N Engl J Med. 2023;389:2221–32. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ. Efficacy and Safety of GLP-1 Medicines for Type 2 Diabetes and Obesity. Diabetes Care. 2024;47:1873–88. doi: 10.2337/dci24-0003. [DOI] [PubMed] [Google Scholar]
- 13.Loomba R, Hartman ML, Lawitz EJ, et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N Engl J Med. 2024;391:299–310. doi: 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N Engl J Med. 2024;391:1193–205. doi: 10.1056/NEJMoa2404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer CK, Leitão CB, Viana LV. The impact of urbanisation on the cardiometabolic health of Indigenous Brazilian peoples: a systematic review and meta-analysis, and data from the Brazilian Health registry. Lancet. 2022;400:2074–83. doi: 10.1016/S0140-6736(22)00625-0. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Mariño-Ramírez L, Jordan IK. Race, Ethnicity, and Pharmacogenomic Variation in the United States and the United Kingdom. Pharmaceutics. 2023;15:1923. doi: 10.3390/pharmaceutics15071923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caleyachetty R, Barber TM, Mohammed NI, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9:419–26. doi: 10.1016/S2213-8587(21)00088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz AL, Alsan M, Morris AA, et al. Why Diverse Clinical Trial Participation Matters. N Engl J Med. 2023;388:1252–4. doi: 10.1056/NEJMp2215609. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Census USA. [22-May-2024]. https://www.census.gov/quickfacts/fact/table/US/PST045221 Available. Accessed.
- 24.Census Canada. [22-May-2024]. https://www12.statcan.gc.ca/census-recensement/index-eng.cfm Available. Accessed.
- 25.Census UK. [22-May-2024]. https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/bulletins/ethnicgroupenglandandwales/census2021 Available. Accessed.
- 26.Census Brazil. [22-May-2024]. https://censo2022.ibge.gov.br/panorama Available. Accessed.
- 27.Census South Africa. [22-May-2024]. https://www.gov.za/about-sa/south-africas-people Available. Accessed.
- 28.World bank income groups. [22-May-2024]. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html Available. Accessed.
- 29.World Obesity Federation . London: World Obesity Federation; 2024. World obesity Atlas 2024.https://data.worldobesity.org/publications/?cat=22 Available. [Google Scholar]
- 30.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int J Obes . 2013;37:1443–51. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 31.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 33.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–49. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 34.Garvey WT, Birkenfeld AL, Dicker D, et al. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care. 2020;43:1085–93. doi: 10.2337/dc19-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obesity (Silver Spring) 2020;28:529–36. doi: 10.1002/oby.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alba M, Yee J, Frustaci ME, et al. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: A randomized dose-ranging study. Clin Obes. 2021;11:e12432. doi: 10.1111/cob.12432. [DOI] [PubMed] [Google Scholar]
- 37.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. The Lancet. 2021;397:971–84. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 38.Nahra R, Wang T, Gadde KM, et al. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021;44:1433–42. doi: 10.2337/dc20-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubino D, Abrahamsson N, Davies M, et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414–25. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadden TA, Bailey TS, Billings LK, et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA. 2021;325:1403–13. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28:2083–91. doi: 10.1038/s41591-022-02026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10:193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 43.Rubino DM, Greenway FL, Khalid U, et al. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022;327:138–50. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613–26. doi: 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- 45.Kosiborod MN, Petrie MC, Borlaug BA, et al. TEP-HFpEF DM Trial Committees and Investigators. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N Engl J Med. 2024;390:1394–407. doi: 10.1056/NEJMoa2313917. [DOI] [PubMed] [Google Scholar]
- 46.Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402:705–19. doi: 10.1016/S0140-6736(23)01185-6. [DOI] [PubMed] [Google Scholar]
- 47.Mok J, Adeleke MO, Brown A, et al. Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial. JAMA Surg. 2023;158:1003–11. doi: 10.1001/jamasurg.2023.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023;29:2909–18. doi: 10.1038/s41591-023-02597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wharton S, Blevins T, Connery L, et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N Engl J Med. 2023;389:877–88. doi: 10.1056/NEJMoa2302392. [DOI] [PubMed] [Google Scholar]
- 50.Aronne LJ, Sattar N, Horn DB, et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA. 2024;331:38–48. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N Engl J Med. 2024;390:1394–407. doi: 10.1056/NEJMoa2313917. [DOI] [PubMed] [Google Scholar]
- 52.Mu Y, Bao X, Eliaschewitz FG, et al. Efficacy and safety of once weekly semaglutide 2·4 mg for weight management in a predominantly east Asian population with overweight or obesity (STEP 7): a double-blind, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2024;12:184–95. doi: 10.1016/S2213-8587(23)00388-1. [DOI] [PubMed] [Google Scholar]
- 53.le Roux CW, Steen O, Lucas KJ, et al. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024;12:162–73. doi: 10.1016/S2213-8587(23)00356-X. [DOI] [PubMed] [Google Scholar]
- 54.Pagoto SL, Schneider KL, Oleski JL, et al. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring) 2012;20:1234–9. doi: 10.1038/oby.2011.140. [DOI] [PubMed] [Google Scholar]
- 55.Alsaqaaby MS, Cooney S, le Roux CW, et al. Sex, race, and BMI in clinical trials of medications for obesity over the past three decades: a systematic review. Lancet Diabetes Endocrinol. 2024;12:414–21. doi: 10.1016/S2213-8587(24)00098-6. [DOI] [PubMed] [Google Scholar]
- 56.Steinberg JR, Turner BE, Weeks BT, et al. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw Open . 2021;4:e2113749. doi: 10.1001/jamanetworkopen.2021.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kantowski T, Schulze Zur Wiesch C, Aberle J, et al. Obesity management: sex-specific considerations. Arch Gynecol Obstet. 2024;309:1745–52. doi: 10.1007/s00404-023-07367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beavers KM, Neiberg RH, Kritchevsky SB, et al. Association of Sex or Race With the Effect of Weight Loss on Physical Function: A Secondary Analysis of 8 Randomized Clinical Trials. JAMA Netw Open . 2020;3:e2014631. doi: 10.1001/jamanetworkopen.2020.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensterle M, Rizzo M, Janež A. Semaglutide in Obesity: Unmet Needs in Men. Diabetes Ther. 2023;14:461–5. doi: 10.1007/s13300-022-01360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overgaard RV, Petri KCC, Jacobsen LV, et al. Liraglutide 3.0 mg for Weight Management: A Population Pharmacokinetic Analysis. Clin Pharmacokinet. 2016;55:1413–22. doi: 10.1007/s40262-016-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel H, Wolf S, Rabasa C, et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016;110:396–406.:S0028-3908(16)30335-5. doi: 10.1016/j.neuropharm.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 62.Johnson-Mann CN, Cupka JS, Ro A, et al. A Systematic Review on Participant Diversity in Clinical Trials-Have We Made Progress for the Management of Obesity and Its Metabolic Sequelae in Diet, Drug, and Surgical Trials. J Racial Ethn Health Disparities . 2023;10:3140–9. doi: 10.1007/s40615-022-01487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ard J, Cannon A, Lewis CE, et al. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: subgroup analysis across the SCALE and phase II randomized trials. Diabetes Obes Metab. 2016;18:430–5. doi: 10.1111/dom.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubino D, Angelene H, Fabricatore A, et al. Efficacy and safety of semaglutide 2.4 mg by race and ethnicity: A post hoc analysis of three randomized controlled trials. Obesity (Silver Spring) 2024;32:1268–80. doi: 10.1002/oby.24042. [DOI] [PubMed] [Google Scholar]
- 65.Pirisi A. Country in Focus: health disparities in Indigenous Canadians. Lancet Diabetes Endocrinol. 2015;3:319. doi: 10.1016/S2213-8587(15)00080-7. [DOI] [PubMed] [Google Scholar]
- 66.Raisi-Estabragh Z, Kobo O, Mieres JH, et al. Racial Disparities in Obesity-Related Cardiovascular Mortality in the United States: Temporal Trends From 1999 to 2020. J Am Heart Assoc. 2023;12:e028409. doi: 10.1161/JAHA.122.028409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukhwana A, Shorinola O, Ndlovu D. Slow, difficult and expensive: how the lab supplies market is crippling african science. https://www.nature.com/nature-index/news/slow-difficult-expensive-how-lab-supplies-market-crippling-african-science n.d. Available.
- 68.Angell M. The ethics of clinical research in the Third World. N Engl J Med. 1997;337:847–9. doi: 10.1056/NEJM199709183371209. [DOI] [PubMed] [Google Scholar]
- 69.Paul C, Brookes B. The Rationalization of Unethical Research: Revisionist Accounts of the Tuskegee Syphilis Study and the New Zealand “Unfortunate Experiment.”. Am J Public Health. 2015;105:e12–9. doi: 10.2105/AJPH.2015.302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mosby I, Galloway T. “Hunger was never absent”: How residential school diets shaped current patterns of diabetes among Indigenous peoples in Canada. CMAJ. 2017;189:E1043–5. doi: 10.1503/cmaj.170448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarma S, Richardson L, Neary J. One hundred years of solitude-underrepresentation of Indigenous and minority groups in diabetes trials. Lancet Glob Health. 2022;10:e1383–4. doi: 10.1016/S2214-109X(22)00356-4. [DOI] [PubMed] [Google Scholar]
- 72.Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research . Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington (DC): National Academies Press (US); 2022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.