Abstract
The HEALTHYBACK trial is based on a multimodal intervention to determine the effectiveness of a supervised physical exercise, mindfulness, behaviour change and pain neuroscience education programme on several health variables in individuals with chronic primary low back pain (CPLBP). The study will be a randomised controlled trial among 70 individuals diagnosed with CPLBP (aged 18–65 years). The intervention will be conducted in person within a hospital setting for 16 weeks and comprises a first phase (16 sessions supervised physical exercise (2 days/week, 45 min/session), mindfulness (1 day/week, 2.5 hours/session), behaviour change (daily/24 hours via a wrist-worn activity prompting device) and pain neuroscience education (1 day/biweekly, 2 hours/session)) and a second phase (16 sessions functional full-body muscle strengthening exercise, 3 days/week, 50 min/session). The primary outcomes will include perceived acute pain, pain pressure threshold, conditioned pain modulation, temporal summation of pain and disability due to pain. Secondary measures will include physical fitness, body composition, gait parameters, device-measured physical activity and sedentary behaviour, haematological profile, self-reported sedentary behaviour, quality of life, pain catastrophising, mental health, sleep duration and quality, and symptoms related to central sensitisation. The groups will undergo pretest (before the intervention), post-test (after each phase of the intervention) and retest (at a 6-week detraining period after the intervention) measurements. The results will determine the effectiveness of multidimensional interventions on several health parameters in individuals with CPLBP. They will provide knowledge for pain management and functioning in affected individuals, which might diminish the need for primary healthcare services. Trial registration number: NCT06114264.
Keywords: Exercise, Exercise rehabilitation, Gait analysis, Lumbar spine, Physical activity
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
The HEALTHYBACK study aims to evaluate the effects of physical exercise, mindfulness, behaviour change and pain neuroscience education on overall health in individuals with CPLBP. Furthermore, this study explores which exercise modality is most effective in this population and includes blood biomarker measurements.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study may provide new knowledge for understanding the mechanisms of chronic pain, enabling healthcare professionals to make better decisions based on scientific evidence regarding the type of treatment required for CPLBP and providing tools for the management of patients’ symptoms.
Introduction
The estimated prevalence of people of all ages and sexes suffering from low back pain (LBP) in 2020 was 619 million worldwide. LBP is characterised by the pain experienced between the 12th rib and the gluteal folds, which lasts more than 12 weeks in cases of chronicity. When a pathoanatomical diagnosis is not provided, the condition is classified as non-specific chronic low back pain or as chronic primary low back pain (CPLBP), according to the terminology recently recommended by WHO. This condition is the leading cause of years lived with disability in the general population1 and results in physical and psychological impairments that diminish the quality of life.2 Furthermore, a systematic review has shown that individuals with CPLBP might present greater systemic inflammation than healthy controls. Additionally, neuropeptides such as neuropeptide Y, substance P and beta-endorphin, which act as signalling molecules within the nervous system, modulate various physiological processes, including pain perception.3 Alterations in these biomarkers have the potential to impact central sensitisation, an amplification of neural signalling within the central nervous system that leads to hypersensitivity to pain.4 High-quality studies, including inflammatory biomarkers (such as C-reactive protein, tumour necrosis factor-α and interleukin 6) and neuropeptides, are required as the levels of evidence are low and would help understand the mechanisms involved in chronic pain maintenance.5
In response to exercise, individuals with CPLBP develop muscle atrophy, decreased strength, proprioception, somatosensory alterations and reduced pain modulation. Conditioned pain modulation (CPM) assesses the function of endogenous pain inhibitory pathways, a mechanism that reduces the pain experience. Otherwise, temporal summation of pain (TSP) assesses neural mechanisms related to pain facilitation. Both mechanisms induce a process of endogenous pain modulation. Physical exercise might enhance pain modulation and relief in individuals with persistent pain.6 Addressing this challenge, core stabilisation exercises focusing on trunk and hip muscles have consistently shown effectiveness in these deficits. However, there is significant heterogeneity in exercise methodologies across randomised controlled trials (RCTs) in this population, complicating effective prescription. To enhance clinical applicability and reduce heterogeneity, future trials have been suggested to include trunk, lower body and upper body muscle strength measures to validate the efficacy of physical exercise programmes in the CPLBP population.7 This approach will enable healthcare professionals to tailor exercises according to the specific dose, intensity and type required for effective management.
The CPLBP population does not usually meet the WHO’s physical activity (PA) recommendations (>150 min/week of moderate PA intensity or at least 75 min/week of vigorous PA intensity). PA presents potential health benefits in individuals with chronic conditions and disability.8 Individuals with CPLBP have a sedentary lifestyle, remaining longer in sedentary behaviour compared with healthy controls.9 Implementing inactivity alarms to promote behaviour change and reduce sedentary behaviour may help individuals with CPLBP achieve the WHO’s PA recommendations.
The mindfulness-based stress reduction (MBSR) method is based on increasing awareness and acceptance of moment-to-moment experiences, alleviating distress, supporting interpersonal and extrapersonal communication and promoting neuroplastic changes. Previous studies have shown that the MBSR method improves mental outcomes, subjective pain and mobility in individuals with chronic physical impairments. Mindfulness could help individuals with chronic pain accept their pain perception and decrease the need to evade it, allowing positive emotions and qualities.10
Recent advances in neuroscience have led to a better understanding of pain mechanisms and highlighted the importance of maladaptive neuroplastic changes and central sensitisation (excessive response of central nervous system nociceptive neurons to normal and subumbral stimuli) in chronic pain. Pain neuroscience education (PNE) aims to educate individuals about their chronic pain experience and help them reconceptualise their pain and coping strategies. It addresses issues such as kinesiophobia (fear of movement), catastrophising (negative thinking), fear-avoidance behaviours and disability. PNE is effective in reducing these maladaptive behaviours and erroneous pain beliefs.11 In addition, clinical practice guidelines recommend using PNE combined with physical exercise for individuals with CPLBP, but not as a stand-alone therapy.12
Despite all this previous evidence, individuals with CPLBP typically receive single-dimensional and passive therapies, such as pharmacological treatment or manual therapy, as first-line interventions in primary healthcare settings. These therapies often yield short-term improvements and render patients passive observers of their condition, resulting in poorer long-term outcomes. Recent evidence and clinical practice guidelines highlight the significant challenge of implementing multidimensional and active treatment approaches in CPLBP.12 13 These approaches encompass physical, biopsychosocial and behavioural components.14 Integrating supervised physical exercise (physical factors) with MBSR (mental-emotional factors), behaviour change strategies and PNE comprehensively addresses the biopsychosocial aspects of human health. Evaluating the effectiveness of this integrated approach is crucial in determining its impact on pain reduction and overall health improvement in individuals with CPLBP.
This study protocol aims to determine the effectiveness of a multimodal programme based on supervised physical exercise, MBSR, behaviour change and PNE compared with a control group on endogenous pain modulation, disability, muscle strength, gait parameters, levels of PA, quality of life, mental health and haematological profile in individuals with CPLBP.
Methods
Study design
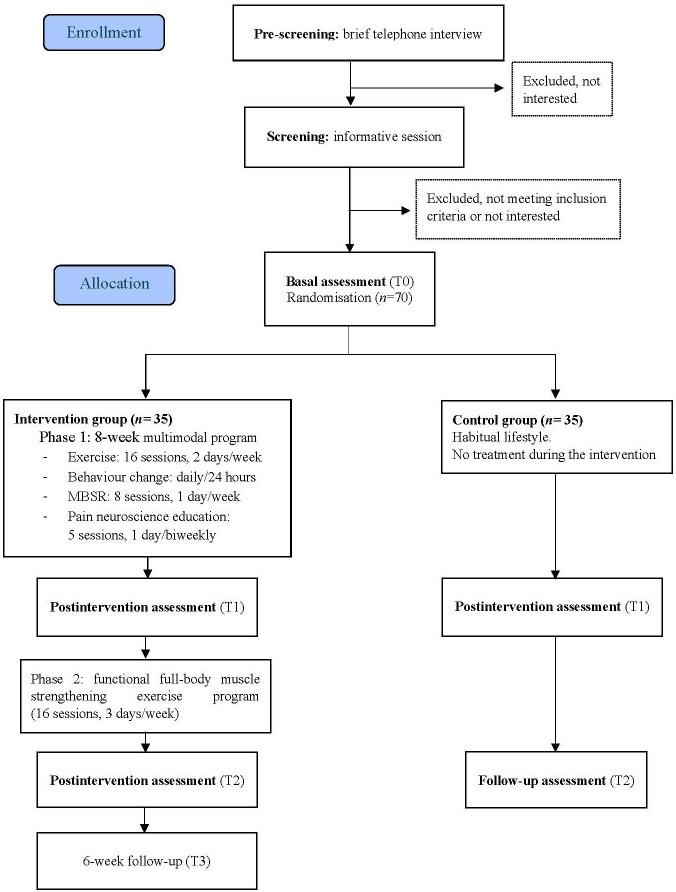
This protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials statements.15 It is a prospective, two-arm, randomised study registered on ClinicalTrials.gov (NCT06114264) and will commence in September 2023. The study will be conducted following the ethical guidelines of the Declaration of Helsinki.16 The trial flowchart is shown in figure 1, and the schedule is provided in online supplemental apendix 1.
Figure 1. Flowchart of the HEALTHYBACK project. MBSR, mindfulness-based stress reduction.
Participants
Individuals who meet the eligibility criteria (box 1) will be included. The Physical Activity Readiness Questionnaire will detect individuals who should undergo a medical examination before doing any type of PA. Setting the mean power at 0.80, the alpha error at 0.05 and the effect size at 0.70 (G∗power software), at least 26 participants for each group will be sufficient to find differences in pain after the intervention.17 Therefore, considering the dropout rate of 20%–30%,18 70 individuals will be randomly assigned to the control group (CG, n=35) or the intervention group (IG, n=35).
Box 1. Inclusion and exclusion criteria of the HEALTHYBACK project.
Inclusion criteria
Have been previously diagnosed with chronic primary low back pain by a healthcare professional.
Be ≥18 and ≤65 years old.
Able to read and understand the informed consent and the objective of the study.
Able to walk and move without external help.
Able to communicate possible problems emerging during the evaluation tests.
Exclusion criteria
Serious lumbar structural disorders: spondylolysis, spondylolisthesis, canal stenosis, degenerative disc disease and/or disc herniation, tumour, trauma or fracture of the lumbar and lower limbs, cauda equina syndrome, and radicular leg pain.
Acute or terminal illness.
Physical injury.
Physical or mental illness.
Engage in any additional physical exercise.
Participate in other treatments for low back pain.
Medical prescriptions that prevent participation in the study.
Recruitment
The recruitment will take place at the Physical Medicine and Rehabilitation Service from Virgen de las Nieves University Hospital in Granada, Spain. Individuals referred to the rehabilitation unit by their doctor and placed on a waiting list will receive an invitation to participate via telephone. Subsequently, those interested individuals will be invited to an informative session in which the study details will be explained, and any doubts that may arise will be answered. Furthermore, all individuals interested in participating will receive written information about the study and will be required to provide written informed consent after reading the information.
Randomisation
Participants will be randomly allocated to each group in a 1:1 allocation ratio by a computer-generated randomisation sequence stratified by two factors (age and sex). This randomisation sequence will be created by a blinded researcher external to the project using Statistical Package for the Social Sciences (SPSS) software.
Procedure
Participants of the IG will be invited to attend four assessments: before (T0), after the multimodal programme (T1), after the functional full-body muscle strengthening exercise training (T2) and following a 6-week detraining period (T3). Participants of the CG will attend three assessments: before (T0), after the IG completes the multimodal programme (T1) and following a 6-week detraining period (T2). First, participants will need to visit the hospital for a blood sample. Second, they will visit the Faculty of Sport Sciences (University of Granada, Spain), where sociodemographic and clinical information will be collected through an initial anamnesis. Subsequently, basal heart rate, blood pressure and primary and secondary outcomes will be evaluated. To avoid possible confounders in the pain-related outcomes, patients will be asked not to take analgesic drugs 24 hours before the evaluation. Participants will receive several health-related questionnaires to be completed at home on the same day as their evaluation, and an accelerometer will be worn for a whole consecutive week. They will be asked to return the accelerometer and the questionnaires 9 days following the assessment.
Intervention programme
The multimodal programme will include supervised physical exercise, MBSR, behaviour change and PNE, all of which are prescribed and designed by sports science professionals and physical therapy professionals. All the professionals participating in the intervention will have previous experience with this population. It will be conducted in the Physical Medicine and Rehabilitation Service of the hospital. Sessions will be scheduled in both morning and afternoon slots to accommodate the availability of the participants.
The IG will undergo a first-phase intervention comprising a 16-session multimodal programme, including physical exercise, MBSR, behavioural change strategy and PNE. They will proceed to a second-phase intervention involving a 16-session functional full-body muscle strengthening exercise programme. The overall information on the intervention programme is shown in table 1.
Table 1. Phase I, 8-week multimodal intervention programme.
| Physical exercise programme | |||||||
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | ||||
| RPE: ≥5 | RPE: ≥5 | RPE: ≥6 | RPE: ≥6 | ||||
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
| Sessions 1–2 | Sessions 3–4 | Sessions 5–6 | Sessions 7–8 | Sessions 9–10 | Sessions 11–12 | Sessions 13–14 | Sessions 15–16 |
| 5-min warm-up | 5-min warm-up | 5-min warm-up | 5-min warm-up | ||||
| 35-min mobility, isometric and motor control exercises | 35-min co-contraction exercises | 35-min functional exercises with load | 35-min functional exercises on an unstable surface | ||||
| 5-min cooldown | 5-min cooldown | 5-min cooldown | 5-min cooldown | ||||
| + | |||||||
| Mindfulness based stress reductionprogramme, 2.5 hours per session, 1 day per week (eight sessions) | |||||||
| + | |||||||
| Behaviour change programme, 24 hours/daily | |||||||
| + | |||||||
| Pain neuroscience education, 2 hours per session, 1 day biweekly (five sessions) | |||||||
RPErating of perceived exertion
In the CG, participants will be placed on a waiting list and instructed to continue their daily life habits. They will be advised not to engage in any additional physical exercise or other treatment for CPLBP except for their current medication for the whole duration of the intervention. Once the intervention is completed, the CG will receive the same multimodal programme as the IG.
Phase I: 8-week multimodal programme
The physical exercise programme
The physical exercise programme will be conducted under the supervision of professionals in sports science and physical therapy by adapting rehabilitation exercises previously used in the literature19 and according to the Consensus on Exercise Reporting Template guidelines (CERT).20 This guide consists of 16 items that describe information about the execution of an exercise intervention programme. Thus, it allows its development, guidance, evaluation, interpretation and assistance in the clinical setting. The programme will report the frequency, intensity, time and type (FITT) exercise principles to ensure a high-quality and detailed exercise intervention. Parallelly, it will follow the National Strength and Conditioning Association21 and American College of Sports Medicine recommendations,22 which suggest performing between 6 and 12 repetitions for each muscle-strengthening PA at a moderate-to-vigorous intensity that equals >5 (1 ‘no effort’ to 10 ‘maximal effort’) on the rating of perceived exertion (RPE) scale.23
The exercise programme will be divided into three sections: warm-up, muscle-strengthening and cool-down (stretching exercises). The intervention programme will focus on strengthening the core muscles. It will begin with low-intensity isometric contraction to stabilise the trunk, along with mobility exercises. The intensity will gradually increase by incorporating functional tasks and will be monitored using an RPE scale ranging from 0 to 10.23 Special attention will be given to ensuring that each exercise is executed without causing pain. The progression will follow stages outlined in previous literature19: (1) Stage 1: mobility, isometric and motor control exercises encompassing the full body, with special emphasis on the core muscles. Particular attention will be given to the isometric contraction of the transversus abdominis muscle; (2) Stage 2: co-contraction and functional tasks of the deep trunk muscles; (3) Stage 3: functional tasks including greater difficulty or intensity with load; (4) Stage 4: functional task with an unstable surface. The material used in the exercise intervention programme will include mats, foam rollers (for myofascial release), 2 kg dumbbells, elastic bands, fitballs and unstable surfaces.
Individuals will receive feedback focusing on technique and posture correction for each exercise. To facilitate familiarisation, an example will be provided before executing each exercise. During the sessions, strategies to improve exercise adherence will be employed, including (1) creating a positive and dynamic environment and (2) implementing an attendance monitoring system to prevent absences and dropouts.
The MBSR programme
The MBSR programme will follow the protocol developed by Jon Kabat Zinn.10 Each session will include three activities: the presentation of a topic, moments of dialogue and exploration in the group (using appreciative inquiry) and a mindfulness practice. Participants will receive homework prescriptions such as workbooks and audio with guided meditations. The MBSR sessions will be taught by a physical therapist accredited by Brown University.
The behaviour change programme
The behaviour change programme will use a wrist-worn smartwatch (Redmi Smart Band 2, Xiaomi, China) worn continuously by participants. This device will alert participants to take breaks from sedentary behaviour whenever it detects that they have been sitting for 1 hour. Participants will be instructed to engage in active breaks, such as walking or dancing, for 2 min upon receiving the alert. The alarms will be programmed to be active for 12 hours each day. Specifically, the device will not issue alarms during sleep hours (22:00–08:00) and nap times (15:00–17:00).
The PNE programme
The PNE programme will include original content delivered via slides, blackboard and written materials, using relatable language and metaphors. It will be led by two physical therapists specialising in chronic pain neurobiology and its management. Validated analogies, illustrations, online audiovisual resources and interactive questions will be used to engage participants.11 The main contents will be (1) pain as a protective alarm system; (2) differences between pain, nociception and damage; (3) acute versus chronic pain; (4) predictive neuroimmune system: evaluative failure and dis/adaptative responses to threats; (5) neuroplastic changes and maladaptive learning in persistent pain, including a hypervigilant system, learnt sensitisation and further shifts mediated by iatrogenesis, kinesiophobia, fear-avoidance behaviours, social isolation, maladaptive cognitions and emotions; (6) potential reversal of functional and structural changes, addressing perpetual factors by active coping tools, such as graded exposure movement techniques (motor imagery, laterality and sensitivity training, dual tasks, neurodynamics, motor variability exploration and games) and methods for identifying and managing maladaptive cognitions, emotions and behaviours.24 The contents of each session are detailed in online supplemental appendix 2.
Phase II: 16-session functional full-body muscle strengthening exercise.
Participants will perform additional moderate-to-high-intensity functional full-body muscle strengthening exercise training. This phase aims to introduce a greater exercise intensity, as this may be a critical factor in achieving therapeutic outcomes. This study investigates whether participants will experience greater improvements in their condition when engaged in exercises of higher intensity. By systematically introducing and monitoring moderate-to-high intensity levels during the training sessions, we aim to evaluate the efficacy of this approach and its potential benefits in enhancing the outcomes. This programme will focus on strengthening the entire body’s musculature, with a primary emphasis on applying movements based on activities of daily living. It will also follow the CERT20 and will report the FITT exercise principles. The intensity will be moderate-to-high and controlled following the repetition in reserve (RIR) theory, which represents the number of repetitions a person can perform while leaving a specified number of final repetitions uncompleted.25 26 The load (resistance) for each exercise shall be determined as previously described and as presented in table 2. The number of repetitions and the RIR for each session will be adapted progressively to increase the intensity of the sessions (table 3). Each session will include: (1) warm-up with mobility exercises, (2) resistance training, progressively developing each exercise based on the RIR25 and (3) cool-down at a low intensity with stretching and relaxation exercises. The functional full-body muscle strengthening exercises are detailed in online supplemental appendix 3.
Table 2. Rating of perceived exertion based on repetitions in reserve.
| Range | Description |
| 10 | Maximum effort |
| 9.5 | Could not perform another repetition, but could add more load |
| 9 | 1 repetition in reserve |
| 8.5 | 1–2 repetitions in reserve |
| 8 | 2 repetitions in reserve |
| 7.5 | 2–3 repetitions in reserve |
| 7 | 3 repetitions in reserve |
| 5–6 | 4–6 repetitions in reserve |
| 3–4 | Light effort |
| 1–2 | Light to no effort |
Table 3. Summary of the phase II, 16-session functional full-body muscle strengthening exercise programme.
| Frequency | 3 days/week | 3 days/week | |||
| Session duration | 45–50 min | 55–60 min | |||
| Session structure | Type | Sessions1–4 | Sessions5–8 | Sessions9–12 | Sessions13–16 |
| Warm-up | Mobility | T: 10 min | T: 10 min | T: 10 min | T: 10 min |
| Conditioning | Muscle-strengthening | T: 30 min | T: 35 min | T: 40 min | T: 40 min |
| V: 2 sets, 12–15 rep | V: 2 sets, 12–15 rep | V: 2 sets, 10–12 rep | V: 2–3 sets, 10 rep | ||
| M: 6 exercises: whole-body | M: 7 exercises: whole-body | M: 8 exercises: whole-body | M: 8 exercises: whole-body | ||
| I:5–6 RPE/5–6 RIR | I: 6–7 RPE/3–4 RIR | I: 7–8 RPE/2–3 RIR | I: 8 RPE/2 RIR | ||
| Cool-down | Stretching and relaxation | T: 5 min | T: 5 min | T: 5 min | T: 5 min |
A 5–6 RPE score indicates that one could perform 4–6 repetitions; a 7 RPE means you could do three more repetitions; and an 8 RPE means you could complete two more repetitions.
IintensityMmodereprepetitionRIRrepetition in reserveRPErating of perceived exertionTtimeVvolume
Primary outcomes
Perceived acute pain
A visual analogue scale will be used to measure subjective changes in back pain. This scale was previously validated among individuals with chronic pain.27 Testing will be performed upon arrival and immediately after performing each physical fitness test during the assessment. A numerical rating scale (0–10) will assess the pain before and after each intervention session.
Pain pressure threshold (PPT)
A hand-held standard pressure algometer (FPK 20, Wagner Instruments, Greenwich, Connecticut, USA) will be used to measure PPT.28
Conditioned pain modulation (CPM)
The conditioning painful stimulus will be applied with a 12-cm wide pressure cuff (Riester minimus III, Jungingen, Germany). The CPM will be calculated as the difference between the PPT of the conditioning painful stimulus minus the initial PPT. Positive values will represent an increase in pain intensity.29
Temporal summation of pain
For painful stimuli, the individual mean of PPT+1 kg will be used with the hand-held standard pressure algometer.28
Pain catastrophising
The Pain Catastrophising Scale will be used to assess pain catastrophising.30
Disability due to pain
The Oswestry Low Back Pain Scale will be used to measure pain disability (limitations in daily life activities) due to LBP.31
Secondary outcomes
Sociodemographic and clinical characteristics
Sociodemographic and clinical information will be collected before the intervention through face-to-face interviews.
Body composition
Weight (kg), body fat and skeletal muscle (kg and %) will be measured by bioelectrical impedance analysis (InBody R20, Biospace Gateshead, UK). Height will be measured with a height rod (Seca 22) and waist32 and neck circumferences33 with a measuring tape (Holtain).
Field-based muscular, cardiorespiratory and motor fitness testing.
Muscular fitness
The Biering-Sørensen test34 will evaluate back-extensor muscles' static and isometric endurance (erector spinae, multifidus and quadratus lumborum). The Prone Bridging test35 will measure back-flexor muscle strength (transversus and rectus abdominis). The 30-s chair stand test will be used to evaluate the strength of the quadriceps, hamstrings, and gluteus muscles.36 It is a reliable37 test used in populations with chronic pain.38 Hand dynamometry (5101 TKK handgrip dynamometer) will be used to measure maximal voluntary hand force. Previous research has shown a significant association between handgrip strength and CPLBP.36
Cardiorespiratory fitness
The YMCA 3 min test will be used to evaluate the maximal oxygen uptake.39
Motor fitness
Motor agility
The 8-foot up-and-go test is a safe and validated measure of motor agility.37
Spatiotemporal gait parameters
The optical sensor OptoGait (OptoGait; Microgate, Bolzano, Italy) is a valid and reliable method and will be used to evaluate spatiotemporal gait parameters, that is, gait speed, contact time, cadence, stride length and double support.40
Laboratory-based muscular testing
Participants will perform isokinetic and isometric strength testing of the back extensor, flexor and oblique muscles using a functional electromechanical dynamometer (FEMD) (Myoquality M1, Myoquality Solutions SL, Granada, Spain). The reliability of the FEMD for trunk strength measurement in healthy people has been previously established.41
Device-measured PA and sedentary behaviour
The triaxial ActiGraph GT3X+accelerometer (Actigraph, Fort Walton Beach, Florida, USA) will objectively assess PA and sedentary time.42
Self-reported sedentary behaviour
The Sedentary Behaviour Questionnaire will be used to assess self-reported sedentary behaviour.43
Quality of life and mental health
The 36-item Short-Form Health Survey will be used to evaluate health-related quality of life.44 The Beck Depression Inventory-II will be used to assess depression severity,45 and the State-Trait Anxiety Inventory-I will be used to measure state anxiety.46
Sleep duration and quality
The Pittsburgh Sleep Quality Index will assess sleep duration and quality.47
Central sensitisation
The Central Sensitisation Inventory will be used to evaluate symptoms related to the central sensitisation phenotype.48
Dietary assessment
The Mediterranean Diet Adherence Screener will be used to evaluate the diet quality.49
Haematological profile
Each participant will undergo a phlebotomy to collect 15 mL of blood samples. The haematological variables are described in table 4.
Table 4. Haematological variables.
| Haematological profile | Analytical techniques |
| Complete blood count | Automatic cell counter: XN-10/XN-20 (Sysmex) |
| Erythrocyte sedimentation rate | Westergren method: Ves-matic Cube 30 (Menarini) |
| Haemostasis/fibrinolysis | Analytical techniques |
| Prothrombin time | Clot-based assay: ACLTOP 750 (Werfen) |
| Partial activated thromboplastin time | |
| Fibrinogen (coagulative) | Clot-based assay, Clauss method: ACLTOP 750 (Werfen) |
| Biochemical variables | Analytical techniques |
| Glucose | Spectrophotometry, enzyme assay: Alinity c (Abbott) |
| Urea | |
| Uric acid | |
| Cholesterol | |
| HDL cholesterol | |
| Triglycerides | |
| Lactate dehydrogenase | |
| Gamma glutamyltransferase | |
| Aspartate transaminase | |
| Alanine transaminase | |
| Creatine kinase | |
| Alkaline phosphatase | |
| Albumin | Spectrophotometry, colorimetric assay: Alinity c (Abbott) |
| Calcium | |
| Phosphorus | |
| Iron | |
| Creatinine | Spectrophotometry modified Jaffé method: Alinity c (Abbott) |
| Alpha-amylase | Spectrophotometry, enzyme/colourimetric assay: Alinity c (Abbott) |
| LDL cholesterol (calculated) | Friedewald equation |
| Bilirubin total | Spectrophotometry, diazo reaction: Alinity c (Abbott) |
| Bilirubin direct | |
| Specific proteins | Analytical techniques |
| C reactive protein | Spectrophotometry, immunoturbidimetry assay: Alinity c (Abbott) |
| Interleukin 6 | Immunochemistry, sandwich principle: Cobas e402 (Roche) |
| Ferritin | Chemiluminescence: Alinity i (Abbott) |
| Hormones | Analytical techniques |
| Thyrotropin | Chemiluminescence: Alinity i (Abbott) |
| Somatotropin | Chemiluminiscence: Maglumi 2000 Plus (Snibe) |
| Cortisol | Chemiluminescence: Alinity i (Abbott) |
| Vitamins | Analytical techniques |
| Vitamin D (25 hydroxy) | Chemiluminescence: Alinity i (Abbott) |
| Immunological profile | Analytical techniques |
| Adiponectin | Immunoassay: LUMINEX 200 System (Bio-Rad Laboratories) |
| Complement factor C3 | Nephelometry: IMMAGE 800 (Beckman Coulter) |
| Complement factor C4 | |
| Human Neuropeptide Y | ELISA: GENLISA, Varioskan LUX multimode microplate (Thermo Fisher) |
| Substance P | |
| Human gamma-aminobutyric acid (GABA) | |
| Human Cystatin C | ELISA: CST3 ELISA Kit PicoKine, Varioskan LUX multimode microplate (Thermo Fisher) |
| Human tumour necrosis factor-alpha | ELISA: TNF ELISA Kit PicoKine, Varioskan LUX multimode microplate (Thermo Fisher) |
| Interferon gamma | Immunoassay: LUMINEX 200 System (Bio-Rad Laboratories) kit LUMINEX HU ACDC SIMPLEX |
| IL-1 alpha | Immunoassay: LUMINEX 200 System (Bio-Rad Laboratories) |
| IL-1 beta | |
| IL-8 (CXCL8) | |
| IL-10 | |
| Leptin | |
| Matrix metalloproteinase-1 | |
| Nerve growth factor beta |
CXCL8motif chemokine ligand 8HDLhigh-density lipoprotein cholesterolILinterleukinLDLlow-density lipoprotein cholesterol
Rate of perceived exertion
At the end of each physical exercise session and after performing each physical fitness test during assessments, the participants will determine their subjective exertion using the RPE scale based on Borg’s category ratio scale (CR-10).50
Further details about the procedures for measuring outcomes are described in online supplemental appendix 4.
Statistical analysis and sample size
Since this study aims to determine the potential efficacy and effectiveness of the multimodal intervention, the statistical analysis will be performed on both per-protocol and intention-to-treat analyses (≥70% of attendance).
The programme effects will be reported as between-group changes in the primary and secondary outcomes and will be assessed with a one-way analysis of covariance. The mean change (post minus baseline values) will be included as the dependent variable, the group will be the fixed factor, and the corresponding baseline value of the outcome will be included as a covariate. The same procedure will be used to analyse the persistence of the changes at follow-up. All the analyses will be adjusted for any potential confounder that is not well balanced at baseline, and the results from both models will be reported. Sex will be used as a potential covariate in all analyses to see if the results differ between men and women. Whenever possible, the sample will be stratified by sex. Cohen’s d will be used to calculate the standardised effect size. The statistical significance will be set at α=0.05. The SPSS software V.27.0. (Armonk, New York, USA: IBM Corp) will be used.
Discussion
Supervised physical exercise has been recommended as a first-line treatment to reduce pain in individuals with CPLBP.9 Field-based and laboratory muscular fitness tests in this study will help healthcare professionals understand if physical fitness mediates the relationship between physical exercise and pain and several health parameters. The WHO’s clinical practice guidance for individuals with CLBP elucidates the essential role of remaining physically active for overall health.1 However, individuals with CLBP showed low PA levels during their leisure time. A systematic review and meta-analysis also recommended engaging in moderate-to-vigorous PA intensity as a protective measure against CPLBP. Consequently, increasing PA levels in this population could improve their daily life activity performance and prevent disease relapse.9 51 This evidence demonstrates the relevance of strategies focused on increasing PA intensity levels, decreasing sedentary behaviour and objectively measuring these activities using tools such as a triaxial accelerometer.
Psychological factors can also influence the brain’s danger perception and modulate its responses through facilitation or inhibition. A study showed that MBSR can improve pain severity and physical and mental quality of life compared with usual medical care in women with CPLBP.52 Due to the reduction of catastrophic thoughts, the repeated practice of mindfulness can enhance the tolerance of negative emotions through exposure to painful sensations.53 Reducing negative thinking is crucial to mitigating heightened pain experience.2
Notably, a pragmatic RCT showed that a combination of PNE and physical exercise improved quality of life and reduced pain intensity and catastrophism in the population with spinal pain.11 Despite these findings, well-designed physical exercise interventions combined with MBSR and PNE have not been implemented in individuals with CPLBP. Most recent evidence elucidates the relevance of a multidimensional approach to address physical, biopsychosocial and behavioural factors that affect individuals with CPLBP.14 Unimodal approaches seem less effective than multimodal ones, including physical exercise.13 In fact, multimodal interventions have been recommended as a first-line treatment for CPLBP to improve quality of life, reduce pain, catastrophism, kinesiophobia and disability, and influence maladaptive nociplastic changes.11 According to a systematic review, inflammatory biomarkers are elevated in populations with neuropathic pain and lead to a ‘sickness response’.5 By measuring specific biomolecules in the blood, researchers can gain insights into the underlying mechanisms and pathophysiology of CPLBP.
The HEALTHYBACK RCT will implement a novel multimodal approach to determine the effects of combining various individually effective modalities on pain intensity, disability,54 overall health, quality of life, haematological profile, PA,9 sleep quality and active coping, among other factors, in individuals with CPLBP.47 This multimodal programme will provide them with tools for self-management and might reduce their need for healthcare, which translates into a decrease in future costs in primary healthcare settings.
Some limitations should be considered. The design will not allow the individual definition of the effectiveness of each intervention modality due to the design requiring a larger sample size. Also, it is important to carry out interventions for a longer duration in future studies to verify the permanence of the intervention’s effects on health outcomes. In addition, due to the nature of the intervention, neither the participants nor the healthcare professionals can be blinded.
supplementary material
Acknowledgements
The authors greatly appreciate the participants’ involvement and ongoing dedication throughout the study process.
Footnotes
Funding: This study was funded by Instituto de Salud Carlos III through the fellowships CP20/00178 and PI22/01791, co-funded by the European Social Fund. GT was supported by the Instituto de Salud Carlos III through the PFIS contract (FI23/00034), co-funded by the European Social Fund+. BD was supported by the 'Margarita Salas' postdoctoral grant UCOR01MS, convened by the University of Córdoba (UCO) and funded by the Ministry of the University of Spain and the European Union-Next Generation EU.
Provenance and peer review: Not commissioned; internally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by the Clinical Research Ethics Committee of Granada, Government of Andalusia, Spain (code: 0097-N-23). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Gavriella Tsiarleston, Email: gabi.tsia@gmail.com.
María Dolores López-Fernández, Email: e.mlopezf@go.ugr.es.
Rodrigo Pavón-Muñoz, Email: rodripamu@gmail.com.
Iván Aguilera-García, Email: ivanag0023@gmail.com.
María López-Corchón, Email: marialopez.corchon@usc.es.
Manuel Delgado-Fernández, Email: manueldf@ugr.es.
María Yolanda Castellote-Caballero, Email: mycastel@ujaen.es.
Belén Donoso, Email: belendonosoperez@gmail.com.
Antonio Manuel Mesa-Ruiz, Email: antoniom.mesa.sspa@juntadeandalucia.es.
Rocío Pozuelo-Calvo, Email: rocio_pozuelo@hotmail.com.
Ángela María Ríos-Ortiz, Email: angela.rios.sspa@juntadeandalucia.es.
Gemma Álvarez-Corral, Email: gemma.alvarez.sspa@juntadeandalucia.es.
Nuria Marín-Jiménez, Email: nuriaproyecto@gmail.com.
Dario Martinez-Garcia, Email: damaga1991@gmail.com.
Ignacio Jesús Chirosa Ríos, Email: ichirosa@ugr.es.
Víctor Segura-Jiménez, Email: vsegura@ibsgranada.es.
Data availability statement
No data are available.
References
- 1.World Health Organization . WHO Guideline for Non-Surgical Management of Chronic Low Back Pain in Adults in Primary and Community Care Settings. Geneva: World Health Organization; 2023. [PubMed] [Google Scholar]
- 2.Ogunlana MO, Odole AC, Adejumo A, et al. Catastrophising, pain, and disability in patients with nonspecific low back pain. Hong Kong Physiother J. 2015;33:73–9. doi: 10.1016/j.hkpj.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos MS, Santos P de J, Vasconcelos ABS, et al. Neuroendocrine effects of a single bout of functional and core stabilization training in women with chronic nonspecific low back pain: A crossover study. Physiol Rep. 2022;10:e15365. doi: 10.14814/phy2.15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris P, Ali K, Merritt M, et al. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet Disord. 2020;21:142. doi: 10.1186/s12891-020-3154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Gong Y, Liu J, et al. Peripheral and Central Pathological Mechanisms of Chronic Low Back Pain: A Narrative Review. J Pain Res . 2021;14:1483–94. doi: 10.2147/JPR.S306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen PJ, Miller CT, Mundell NL, et al. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br J Sports Med. 2020;54:1279–87. doi: 10.1136/bjsports-2019-100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–62. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hock M, Járomi M, Prémusz V, et al. Disease-Specific Knowledge, Physical Activity, and Physical Functioning Examination among Patients with Chronic Non-Specific Low Back Pain. Int J Environ Res Public Health. 2022;19:12024. doi: 10.3390/ijerph191912024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane RS, Brewer J, Feldman C, et al. What defines mindfulness-based programs? The warp and the weft. Psychol Med. 2017;47:990–9. doi: 10.1017/S0033291716003317. [DOI] [PubMed] [Google Scholar]
- 11.Galan-Martin MA, Montero-Cuadrado F, Lluch-Girbes E, et al. Pain Neuroscience Education and Physical Therapeutic Exercise for Patients with Chronic Spinal Pain in Spanish Physiotherapy Primary Care: A Pragmatic Randomized Controlled Trial. J Clin Med. 2020;9:1201. doi: 10.3390/jcm9041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791–803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 13.George SZ, Fritz JM, Silfies SP, et al. Interventions for the Management of Acute and Chronic Low Back Pain: Revision 2021. Journal of Orthopaedic & Sports Physical Therapy . 2021;51:CPG1–60. doi: 10.2519/jospt.2021.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bemani S, Sarrafzadeh J, Dehkordi SN, et al. Effect of multidimensional physiotherapy on non-specific chronic low back pain: a randomized controlled trial. Adv Rheumatol . 2023;63:57. doi: 10.1186/s42358-023-00329-9. [DOI] [PubMed] [Google Scholar]
- 15.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray BH, Cooke RA, Tannenbaum AS. World Medical Association Declaration of Helsinki. JAMA. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 17.Mlekusch S, Neziri AY, Limacher A, et al. Conditioned Pain Modulation in Patients With Acute and Chronic Low Back Pain. Clin J Pain. 2016;32:116–21. doi: 10.1097/AJP.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 18.Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi: 10.1136/bmj.h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.In T-S, Jung J-H, Jung K-S, et al. Effects of the Multidimensional Treatment on Pain, Disability, and Sitting Posture in Patients with Low Back Pain: A Randomized Controlled Trial. Pain Res Manag. 2021;2021:1–8. doi: 10.1155/2021/5581491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br J Sports Med. 2016;50:1428–37. doi: 10.1136/bjsports-2016-096651. [DOI] [PubMed] [Google Scholar]
- 21.Essentials of Strength Training and Conditioning, 4th Edition. Med Sci Sports Exerc. 2016;48:2073. doi: 10.1249/MSS.0000000000001081. [DOI] [Google Scholar]
- 22.Haskell WL, Lee I-M, Pate RR, et al. Physical Activity and Public Health. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 23.Nelson ME, Rejeski WJ, Blair SN, et al. Physical Activity and Public Health in Older Adults. Med Sci Sports Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 24.Moseley GL, Butler DS. Fifteen Years of Explaining Pain: The Past, Present, and Future. J Pain. 2015;16:807–13. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zourdos MC, Klemp A, Dolan C, et al. Novel Resistance Training-Specific Rating of Perceived Exertion Scale Measuring Repetitions in Reserve. J Strength Cond Res. 2016;30:267–75. doi: 10.1519/JSC.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 26.Ormsbee MJ, Carzoli JP, Klemp A, et al. Efficacy of the Repetitions in Reserve-Based Rating of Perceived Exertion for the Bench Press in Experienced and Novice Benchers. J Strength Cond Res. 2019;33:337–45. doi: 10.1519/JSC.0000000000001901. [DOI] [PubMed] [Google Scholar]
- 27.Alghadir AH, Anwer S, Iqbal A, et al. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–6. doi: 10.2147/JPR.S158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira FCL, Cossette C, Mailloux C, et al. Within-Session Test-Retest Reliability of Pressure Pain Threshold and Mechanical Temporal Summation in Chronic Low Back Pain. Clin J Pain. 2023;39:217–25. doi: 10.1097/AJP.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy DL, Kemp HI, Ridout D, et al. Reliability of conditioned pain modulation: a systematic review. Pain. 2016;157:2410–9. doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–32. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 31.Alcántara-Bumbiedro S, Flórez-García MT, Echávarri-Pérez C, et al. Escala de incapacidad por dolor lumbar de Oswestry. Rehab. 2006;40:150–8. doi: 10.1016/S0048-7120(06)74881-2. [DOI] [Google Scholar]
- 32.Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177–89. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroll C, Mastroeni S, Czarnobay SA, et al. The accuracy of neck circumference for assessing overweight and obesity: a systematic review and meta-analysis. Ann Hum Biol. 2017;44:667–77. doi: 10.1080/03014460.2017.1390153. [DOI] [PubMed] [Google Scholar]
- 34.Biering-Sørensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine (Phila Pa 1976) 9:106–19. doi: 10.1097/00007632-198403000-00002. n.d. [DOI] [PubMed] [Google Scholar]
- 35.Schellenberg KL, Lang JM, Chan KM, et al. A clinical tool for office assessment of lumbar spine stabilization endurance: prone and supine bridge maneuvers. Am J Phys Med Rehabil. 2007;86:380–6. doi: 10.1097/PHM.0b013e318032156a. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Piñero J, Marin-Jimenez N, Fernandez-Santos JR, et al. Criterion-Related Validity of Field-Based Fitness Tests in Adults: A Systematic Review. J Clin Med. 2021;10:3743. doi: 10.3390/jcm10163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–67. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 38.Carbonell-Baeza A, Aparicio VA, Ortega FB, et al. Does a 3-month multidisciplinary intervention improve pain, body composition and physical fitness in women with fibromyalgia? Br J Sports Med. 2011;45:1189–95. doi: 10.1136/bjsm.2009.070896. [DOI] [PubMed] [Google Scholar]
- 39.Kieu NTV, Jung SJ, Shin SW, et al. The Validity of the YMCA 3-Minute Step Test for Estimating Maximal Oxygen Uptake in Healthy Korean and Vietnamese Adults. J Lifestyle Med. 2020;10:21–9. doi: 10.15280/jlm.2020.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weart AN, Miller EM, Freisinger GM, et al. Agreement Between the OptoGait and Instrumented Treadmill System for the Quantification of Spatiotemporal Treadmill Running Parameters. Front Sports Act Living. 2020;2:571385. doi: 10.3389/fspor.2020.571385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes-Ferrada W, Chirosa-Ríos L, Chirosa-Ríos I, et al. A new reliable device to assess trunk extensors strength. Acta Bioeng Biomech. 2022;24:49–57. [PubMed] [Google Scholar]
- 42.Montoye AHK, Clevenger KA, Pfeiffer KA, et al. Development of cut-points for determining activity intensity from a wrist-worn ActiGraph accelerometer in free-living adults. J Sports Sci. 2020;38:2569–78. doi: 10.1080/02640414.2020.1794244. [DOI] [PubMed] [Google Scholar]
- 43.Munguia-Izquierdo D, Segura-Jiménez V, Camiletti-Moirón D, et al. Spanish adaptation and psychometric properties of the Sedentary Behaviour Questionnaire for fibromyalgia patients: the al-Andalus study. Clin Exp Rheumatol. 2013;31:S22–33. [PubMed] [Google Scholar]
- 44.Alonso J, Prieto L, Antó JM. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): an instrument for measuring clinical results. Med Clin (Barc) 1995;104:771–6. [PubMed] [Google Scholar]
- 45.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger CD. The Corsini Encyclopedia of Psychology. 2010. State‐Trait anxiety inventory. [Google Scholar]
- 47.Roseen EJ, Gerlovin H, Femia A, et al. Yoga, Physical Therapy, and Back Pain Education for Sleep Quality in Low-Income Racially Diverse Adults with Chronic Low Back Pain: a Secondary Analysis of a Randomized Controlled Trial. J Gen Intern Med. 2020;35:167–76. doi: 10.1007/s11606-019-05329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neblett R, Hartzell MM, Mayer TG, et al. Establishing Clinically Relevant Severity Levels for the Central Sensitization Inventory. Pain Pract. 2017;17:166–75. doi: 10.1111/papr.12440. [DOI] [PubMed] [Google Scholar]
- 49.Schröder H, Fitó M, Estruch R, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–5. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 50.Shariat A, Cleland JA, Danaee M, et al. Borg CR-10 scale as a new approach to monitoring office exercise training. Work. 2018;60:549–54. doi: 10.3233/WOR-182762. [DOI] [PubMed] [Google Scholar]
- 51.Gordon R, Bloxham S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare (Basel) 2016;4:22. doi: 10.3390/healthcare4020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banth S, Ardebil MD. Effectiveness of mindfulness meditation on pain and quality of life of patients with chronic low back pain. Int J Yoga. 2015;8:128–33. doi: 10.4103/0973-6131.158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikemoto T, Miki K, Matsubara T, et al. Psychological Treatment Strategy for Chronic Low Back Pain. Spine Surg Relat Res . 2019;3:199–206. doi: 10.22603/ssrr.2018-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasimis K, Apostolou T, Kallistratos I, et al. Effects of Manual Therapy Plus Pain Neuroscience Education with Integrated Motivational Interviewing in Individuals with Chronic Non-Specific Low Back Pain: A Randomized Clinical Trial Study. Medicina (Kaunas) 2024;60:556. doi: 10.3390/medicina60040556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.